Background: High insulin in T2D is associated with low testosterone, but the role of insulin has not been fully studied in testis.
Results: Insulin directly inhibits testicular steroidogenesis via induction of DAX-1 in Leydig cells.
Conclusion: Insulin induces DAX-1 in Leydig cells, and DAX-1 inhibits LRH-1-mediated testicular steroidogenesis.
Significance: Elevated insulin level in insulin-resistant states such as T2D suppresses the synthesis of testicular steroidogenesis.
Keywords: Diabetes, Insulin, Insulin resistance, Nuclear receptors, Steroidogenesis, DAX-1
Abstract
Testosterone level is low in insulin-resistant type 2 diabetes. Whether this is due to negative effects of high level of insulin on the testes caused by insulin resistance has not been studied in detail. In this study, we found that insulin directly binds to insulin receptors in Leydig cell membranes and activates phospho-insulin receptor-β (phospho-IR-β), phospho-IRS1, and phospho-AKT, leading to up-regulation of DAX-1 (dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1) gene expression in the MA-10 mouse Leydig cell line. Insulin also inhibits cAMP-induced and liver receptor homolog-1 (LRH-1)-induced steroidogenic enzyme gene expression and steroidogenesis. In contrast, knockdown of DAX-1 reversed insulin-mediated inhibition of steroidogenesis. Whether insulin directly represses steroidogenesis through regulation of steroidogenic enzyme gene expression was assessed in insulin-injected mouse models and high fat diet-induced obesity. In insulin-injected mouse models, insulin receptor signal pathway was activated and subsequently inhibited steroidogenesis via induction of DAX-1 without significant change of luteinizing hormone or FSH levels. Likewise, the levels of steroidogenic enzyme gene expression and steroidogenesis were low, but interestingly, the level of DAX-1 was high in the testes of high fat diet-fed mice. These results represent a novel regulatory mechanism of steroidogenesis in Leydig cells. Insulin-mediated induction of DAX-1 in Leydig cells of testis may be a key regulatory step of serum sex hormone level in insulin-resistant states.
Introduction
Type 2 diabetes (T2D)4 is associated with low testosterone level and frequently results in hypogonadotropic hypogonadism in men (1). Moreover, low testosterone level can affect insulin sensitivity (2–4) and increase the risk for diabetes (5). In contrast, testosterone treatment improves insulin sensitivity by changing the body composition of subjects with hypogonadism due to T2D (6, 7). However, excess androgen can increase oxidative stress and induce beta cell failure in rodent models (8). Furthermore, elevated leptin levels, presumably due to leptin resistance, inhibit basal and human chorionic gonadotropin-induced testosterone secretion in rat testes and human obese males (9, 10).
In association with low testosterone level, gonadal dysfunction contributes to abdominal obesity in men due to changes in luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels (11). Although the involvement of reduced LH and FSH levels in testicular dysfunction in T2D is evident from these studies, there is also evidence that insulin regulates testosterone production in obese subjects and inhibits sex hormone-binding globulin level in T2D patients (12, 13). Indeed, overexpression of insulin in Leydig cells reduces germ cells and causes infertility in mice (14), suggesting the possibility that the elevated insulin level characteristic of T2D may have detrimental effects on testicular Leydig cells.
Testicular steroidogenesis is mediated by LH through multiple signaling pathways including the steroidogenic acute regulatory protein (StAR), the cholesterol side chain cleavage enzyme (P450scc), and 3β-hydroxysteroid dehydrogenase (3β-HSD) (15). Dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1 (DAX-1; NR0B1) acts as a corepressor and negative regulator of nuclear receptors to decrease gluconeogenesis and steroidogenesis in Leydig cells (16, 17). DAX-1 gene expression is repressed by LH and subsequently increases steroidogenesis in Leydig cells (17). Our previous study has shown that DAX-1 gene expression is induced in liver by insulin and that this suppresses hepatic gluconeogenesis by inhibiting expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (16). However, it remains unclear whether DAX-1 gene expression is induced in testis by insulin or whether insulin-induced suppression of testicular steroidogenic gene expression is mediated by DAX-1.
In the present study, we investigated the direct effect of insulin on testicular steroidogenesis. Our findings demonstrate that insulin induces the expression of DAX-1 in Leydig cells and that DAX-1 inhibits testicular steroidogenesis both in vivo and in vitro. These results suggest that the induction of DAX-1 by hyperinsulinemia in T2D may contribute to the reduction in testicular steroidogenesis and therefore the lowering of testosterone.
EXPERIMENTAL PROCEDURES
Animal Experiments
Male 8-week-old C57BL/6J mice, maintained at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, South Korea), were fed either a high fat diet (HFD) (D12492; Research Diets, New Brunswick, NJ) or a normal chow diet for 12 weeks. Moreover, male, 8-week-old C57BL/6J mice were divided into two groups: (a) vehicle (citrate buffer, pH 4.5) only and (b) 180 mg/kg dose of streptozotocin. For investigating the direct effect of insulin on testicular steroidogenesis, male 8-week-old C57BL/6J mice were intraperitoneally injected with insulin or vehicle (citrate buffer, pH 4.5). After 30 min of intraperitoneal insulin injection, all mice were euthanized with CO2, and testis tissues were collected. Testes were used for radioimmunoassay and immunohistochemical analyses. Plasma glucose concentrations were measured using Glucostix Accu-Chek (Roche Diagnostics, Mannheim, Germany) and a mouse insulin ELISA (ALPCO Diagnostics, Salem, NH) in accordance with the manufacturer's protocols, respectively. All animal studies and protocols were approved by the Institutional Animal Care and Use Committee of the Korea Research Institute of Bioscience and Biotechnology.
Chemicals
Insulin (Novolin R, Green Cross, Seoul, South Korea), wortmannin, Compound C, SP600125, SB203580, and U0126 were purchased from Calbiochem and then dissolved in dimethyl sulfoxide (DMSO) and added directly to the culture medium for incubation. Control cells were treated only with DMSO. The final DMSO concentration was always <0.2%.
Plasmids and DNA Construction
The StAR-Luc, 3β-HSD-Luc, P450c17-Luc, DAX-1-Luc, and Sft4-Luc reporter plasmids, nuclear receptor liver receptor homolog-1 (LRH-1), and DAX-1 were described previously (18–21).
Cell Culture and Transient Transfection Assay
MA-10 and HeLa cells were maintained as described previously (20, 21). Transient transfections were carried out using the FUGENE HD (Roche Applied Science) transfection reagent with the indicated reporter plasmids together with expression vectors encoding various transcription factors or treated with chemicals.
Immunohistochemistry and Immunostaining
Collected testes from mice of each group were fixed in Bouin's fixative and transferred to 70% ethanol before paraffin embedding. Sections (5 μm) were prepared from paraffin blocks with a rotary microtome and mounted on SuperFrost Plus glass slides (Fisher Scientific, Nepean, Ontario, Canada) after exposure to the appropriate antibodies of luteinizing hormone receptor (LH-R), insulin receptor-β (IR-β), and DAX-1, as described previously (16). MA-10 cells were cultured on uncoated glass coverslips for immunostaining and exposed to the appropriate antibodies (LH-R and IR-β) as described previously (21).
Preparation of Recombinant Adenovirus
Adenoviruses encoding LRH-1 and siRNA DAX-1 have been described previously (16, 21, 22). The multiplicity of infection (MOI) is representative of the ratio of infectious agent to infection target.
mRNA Measurements
Total RNA was isolated from each sample and utilized for reverse transcription-polymerase chain reaction, as described previously (16). Expression of all transcripts was normalized to β-actin expression.
Western Blot Analysis
Mouse Leydig cell and mouse testes were prepared, and Western blot analyses were performed using phospho-AKT (Ser-473), AKT, phospho-IR-β (Tyr-1162/1163), IR-β, DAX-1 (Cell Signaling Technology, Danvers, MA), phospho-insulin receptor substrate 1 (IRS1) (Tyr-989), IRS1, StAR, 3β-HSD, β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), and P450scc (Millipore Corp., Bedford, MA) and developed using an ECL Western blot detection kit (Amersham Biosciences).
LH and FSH Assay
LH and FSH enzyme-linked immunosorbent assay kits were purchased from BioVendor (RSHAKRLH-010 and RSHAKRFS-010; Brno, Czech Republic). The standards and samples were incubated in anti-LHα or anti-FSH antibody-coated wells, following the manufacturer's protocol.
Radioimmunoassay
Serum and testes from HFD-fed and insulin-injected mice or medium from insulin treatment with or without inhibitors in MA-10 cells were prepared, and testosterone and progesterone concentrations were measured by radioimmunoassay, as described previously (18).
Statistical Analysis
Data are expressed as means ± S.E. Differences between groups were evaluated by two-tailed unpaired Student's t test. One-way analysis of variance (ANOVA) was used to determine the significance of differences among treatment groups. The Newman-Keuls test was used for multigroup comparisons. Values of p < 0.05 were considered to be statistically significant.
RESULTS
Insulin Is Involved in Inhibition of Steroidogenesis in Leydig Cells
Previous studies have shown that IRS are expressed in human and rat testes (23, 24). However, the role of insulin receptor signaling in testicular steroidogenesis has not been defined. Therefore, we examined whether insulin regulates IR signaling in MA-10 cells, a Leydig tumor cell line. Antibodies against LH-R and IR-β stained the plasma membrane of MA-10 cells (Fig. 1A, arrowheads). Insulin treatment activated phospho-IR-β, phospho-IRS1 (Tyr), and phospho-AKT in a time- and dose-dependent manner in MA-10 cells when compared with the control (Fig. 1B). To further investigate the regulation of steroidogenesis by insulin, we evaluated the effect of insulin on steroidogenic gene expression in these cells. As expected, insulin inhibited cAMP-induced StAR, P450scc, and 3β-HSD gene expression (Fig. 1C). Furthermore, we found that inhibitors of the insulin receptor signaling pathway negatively affected the inhibition of steroidogenesis by insulin. Wortmannin (PI3K inhibitor), U0126 (MEK1,2 inhibitor), Compound C (AMP-activated protein kinase inhibitor), and SP600125 (JNK inhibitor) significantly reversed insulin-mediated inhibition of the stimulation of progesterone and testosterone synthesis by 8-bromo-cAMP (Fig. 1D). Of the compounds tested, only SB203580 (MAPK inhibitor) was ineffective in preventing the inhibitory effect of insulin on steroidogenesis.
FIGURE 1.
Insulin is involved in inhibition of steroidogenesis in Leydig cells. A, MA-10 cells were used for immunohistochemistry of the LH-R and IR-β. The arrowheads show the LH-R or IR-β stain in MA-10 cells. B, MA-10 cells were treated with insulin (Ins, 40 nm) for the indicated time period (left) or MA-10 cells were treated with insulin in a dose-dependent manner for 15 min (right) and harvested for Western blot analysis using antibodies against the phospho-IR-β (p-IR-β), IR-β, phospho-IRS1 (p-IRS1), IRS1, phospho-AKT (p-AKT), and AKT. C, MA-10 cells were treated with 8-Br-cAMP (100 μm) with or without insulin (40 nm). Total RNA was isolated for real-time PCR. StAR, P450scc, and 3β-HSD expression was measured. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from 8-Br-cAMP group by two-tailed t test. D, MA-10 cells were pretreated with inhibitors: SB203580 (SB, p38-MAPK inhibitor), 25 μm; U0126 (MEK1,2 inhibitor), 10 μm; wortmannin (WM, PI3K inhibitor), 0.1 μm; Compound C (Com. C, AMP-activated protein kinase inhibitor), 10 μm; SP600125 (SP, JNK inhibitor), 25 μm for 30 min and then treated with 8-Br-cAMP (100 μm) or 8-Br-cAMP (100 μm) + insulin (40 nm). Control (CON) cells were treated only with DMSO. The medium were collected for progesterone (left) and testosterone (right) measurement by radioimmunoassay. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test.
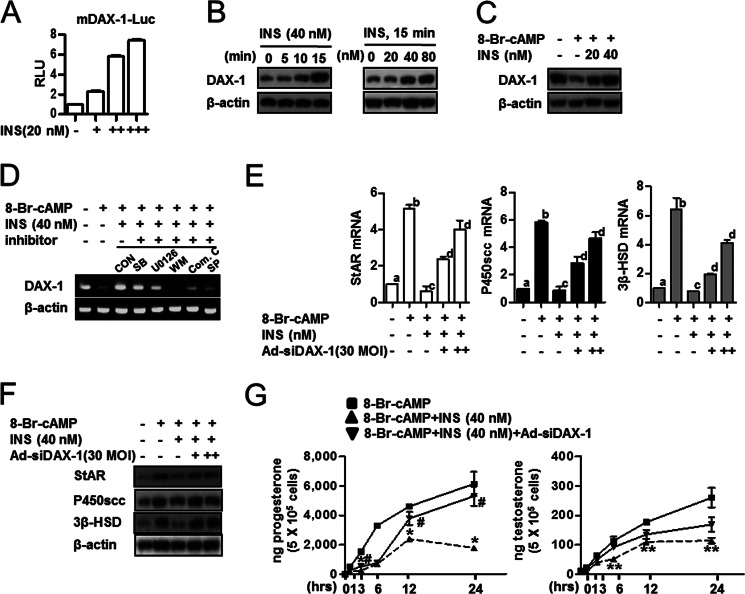
DAX-1 Is Involved in Insulin-mediated Inhibition of Steroidogenesis in Leydig Cells
To further confirm whether the regulation of steroidogenesis by insulin is mediated by DAX-1, we evaluated the role of DAX-1 on steroidogenic gene expression in Leydig cells. As expected, insulin increased DAX-1 promoter activity, and protein expression was also increased in a time- and dose-dependent manner (Fig. 2, A and B). Western blot analysis confirmed that the decrease of DAX-1 protein by cAMP was restored with insulin treatment (Fig. 2C). Moreover, insulin-mediated induction of DAX-1 gene expression was inhibited by wortmannin, Compound C, and SP600125 (Fig. 2D). Insulin also inhibited the mRNA and protein levels of StAR, P450scc, and 3β-HSD, which are induced by cAMP treatment, whereas DAX-1 knockdown reversed those of StAR, P450scc, and 3β-HSD (Fig. 2, E and F). In addition, Leydig cells were treated with insulin in the presence or absence of DAX-1 siRNA to identify the crucial role of insulin-induced DAX-1 in the regulation of steroidogenesis. Consistent with the data presented in Fig. 1, cAMP treatment significantly increased progesterone and testosterone levels, whereas these effects were significantly inhibited by insulin (Fig. 2F). In contrast, knockdown of DAX-1 gene expression increased insulin-inhibited progesterone and testosterone levels (Fig. 2F). Overall, these results demonstrate that insulin induced DAX-1 gene expression by altering the IR-dependent pathway and negatively regulated steroidogenesis by controlling steroidogenic gene expression in MA-10 cells.
FIGURE 2.
DAX-1 is involved in insulin-mediated inhibition of steroidogenesis in Leydig cells. A, MA-10 cells were transfected with DAX-1-Luc (200 ng) and treated with insulin (INS, 20, 40, or 60 nm). MA-10 cells were treated with insulin at the indicated concentrations 24 h after transfection. B, MA-10 cells were treated with insulin (40 nm) for the indicated time period (left) or MA-10 cells were treated with insulin in a dose-dependent manner for 15 min (right) and harvested for Western blot analysis using the DAX-1 and β-actin antibodies. C, MA-10 cells were treated with 8-Br-cAMP (100 μm) and insulin (20 or 40 nm) and harvested for Western blot analysis using DAX-1 and β-actin antibodies. D, MA-10 cells were pretreated with inhibitors: SB203580 (SB, p38-MAPK inhibitor), 25 μm; U0126 (MEK1,2 inhibitor), 10 μm; wortmannin (WM, PI3K inhibitor), 0.1 μm; Compound C (Com. C, AMP-activated protein kinase inhibitor), 10 μm; SP600125 (SP, JNK inhibitor), 25 μm for 30 min and then treated with 8-Br-cAMP (100 μm) or 8-Br-cAMP (100 μm) + insulin (40 nm). Control (CON) cells were treated only with DMSO. Total RNA was isolated for semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis. E, MA-10 cells were infected with Ad-siDAX-1 (30 or 60 MOI) and treated with 8-Br-cAMP (100 μm) or 8-Br-cAMP (100 μm) + insulin (40 nm). Total RNA was isolated for real-time PCR analysis. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. F, MA-10 cells were infected with Ad-siDAX-1 (30 or 60 MOI) and treated with 8-Br-cAMP (100 μm) or 8-Br-cAMP (100 μm) + insulin (40 nm). MA-10 cells were harvested for Western blot analysis using StAR, P450scc, 3β-HSD, and β-actin antibodies. G, MA-10 cells were treated with 8-Br-cAMP (100 μm), insulin (40 nm), or insulin with Ad-siDAX-1 (30 MOI) for the indicated time period, and medium was collected for progesterone (left) and testosterone (right) measurement by radioimmunoassay. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from 8-Br-cAMP group by the Newman-Keuls test; #, p < 0.05, significantly different from 8-Br-cAMP + insulin group by the Newman-Keuls test; **, p < 0.05, significantly different from 8-Br-cAMP group by the Newman-Keuls test.
Insulin-induced DAX-1 Inhibits Steroidogenesis in Leydig Cells
Although steroidogenic enzyme gene induction by cAMP is well characterized (21), regulation of steroidogenesis by insulin is not. Therefore, we examined the effects of insulin on the expression steroidogenic enzyme genes with promoter/reporter constructs of StAR, P450c17, and 3β-HSD in MA-10 cells. As expected, the promoter activities of these genes were greatly increased by cAMP. Insulin reversed the effects of cAMP in a dose-dependent manner (20 or 40 nm) (Fig. 3A). It is well known that the nuclear receptor LRH-1 plays a critical role in the regulation of steroidogenesis and that its transcriptional activity is inhibited by interaction with DAX-1 (25, 26). Based on the results of DAX-1 protein level regulated by insulin and cAMP (Fig. 2, B and C), we determined whether insulin affects stimulation of steroidogenesis by LRH-1. Increased Sft4-Luc promoter activity with LRH-1 overexpression was markedly decreased by insulin treatment or DAX-1 overexpression (Fig. 3B). Furthermore, insulin-induced DAX-1 inhibited LRH-1-mediated steroidogenesis. As a result, StAR, P450c17, and 3β-HSD promoter activities were increased by LRH-1 overexpression, whereas insulin treatment significantly reduced LRH-1-mediated increase of steroidogenic enzyme gene promoter activity (Fig. 3C). Insulin treatment also inhibited the increase of StAR, P450scc, and 3β-HSD mRNA levels by Ad-LRH-1, and these effects were reversed by knockdown of DAX-1 (Fig. 3D). Overall, these results indicate that insulin-induced DAX-1 decreased the stimulation of the steroidogenic enzyme gene expression by LRH-1 in MA-10 cells.
FIGURE 3.
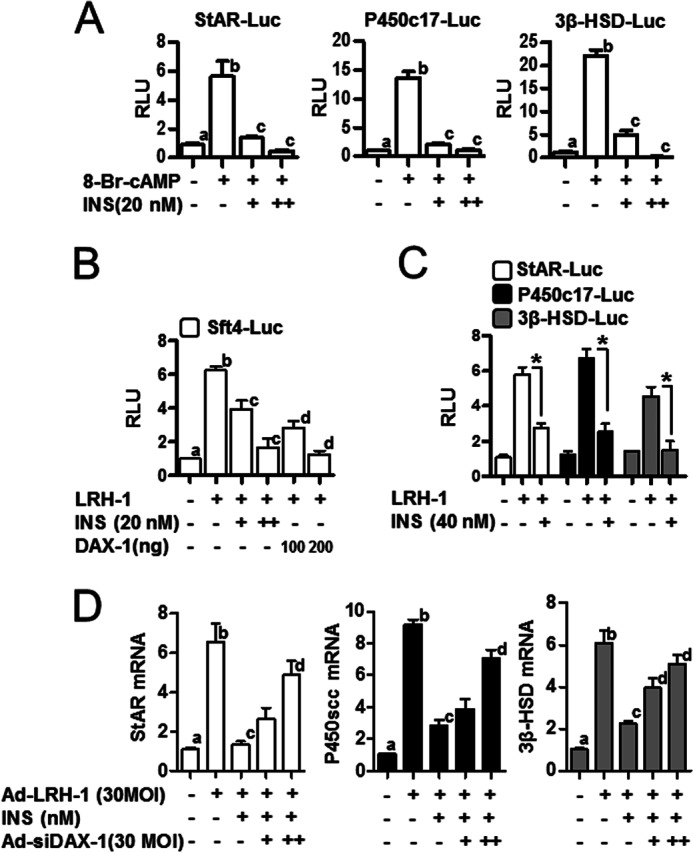
Inhibition of steroidogenic enzyme gene expression via insulin-induced DAX-1 in Leydig cells. A, MA-10 cells were transfected with StAR-Luc (200 ng), P450c17-Luc (200 ng), and 3β-HSD-Luc (200 ng) and treated with 8-Br-cAMP (100 μm) with or without insulin (INS, 20 nm). Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. B, HeLa cells were transfected with LRH-1 (200 ng), DAX-1 (100 or 200 ng), and Sft4-Luc (200 ng), respectively. Cells were treated with insulin (20 or 40 nm) 24 h after transfection. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. C, MA-10 cells were transfected with LRH-1 (200 ng), StAR-Luc (200 ng), P450c17-Luc (200 ng), and 3β-HSD-Luc (200 ng) and treated with insulin (20 nm). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from LRH-1group by two-tailed t test. D, MA-10 cells were infected with the Ad-LRH-1 (30 MOI) and/or Ad-siDAX-1 (30 or 60 MOI) for 24 h followed by insulin (20 or 40 nm) treatment. Total RNA was isolated for real-time PCR analysis. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test.
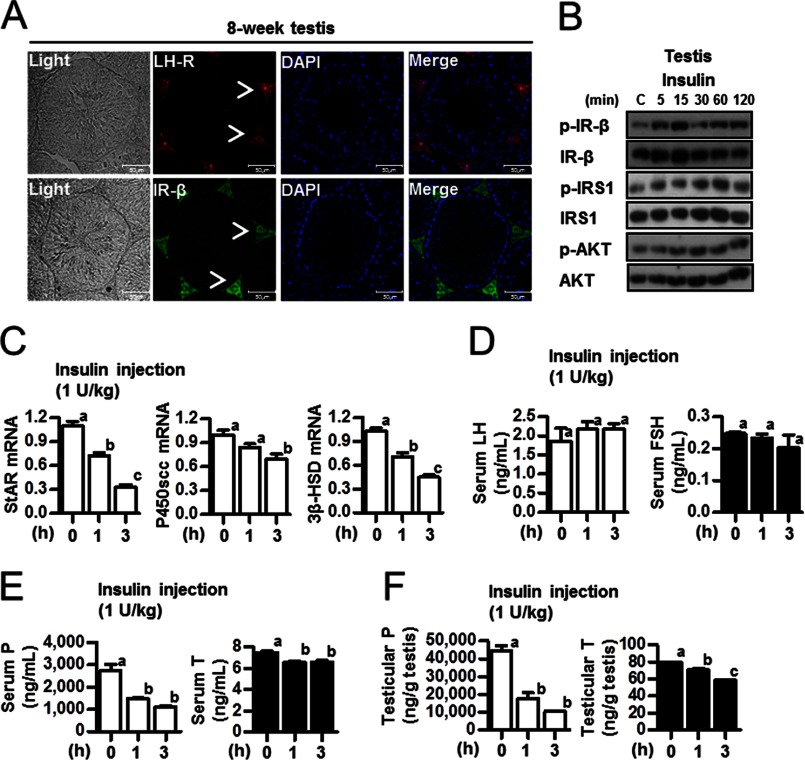
Insulin Inhibits Steroidogenesis by Activating Insulin Receptor Signaling Pathway in Normal Mice Testes
Based on the decreased mRNA levels of testicular steroidogenic enzymes and the low concentrations of testosterone and progesterone in serum and testes (Figs. 1 and 2), we hypothesized that insulin, glucose, or both could be involved in the regulation of testicular steroidogenesis in HFD-fed mice. Insulin induces a positive effect on de novo steroidogenesis in prostate cancer cells (27). On the hand, directed overexpression of insulin in Leydig cells causes infertility in a mouse model (14). As a first step in determining whether insulin regulates sex hormone level, immunohistochemical studies were conducted to compare the distribution patterns of the LH-R and IR-β in the testes of males at puberty (8 weeks old). Antibodies against LH-R and IR-β exclusively stained Leydig cells in the testis (Fig. 4A, arrowheads). Western blot analysis was performed to determine whether injecting insulin activates IR signaling in mouse testis. The results showed that insulin increased phospho-IR-β, phospho-IRS1, and phospho-AKT in the testis (Fig. 4B). In association with insulin receptor signal pathway and testicular steroidogenesis, mRNA levels of StAR, P450scc, and 3β-HSD, key enzymes for testicular steroidogenesis, were assessed in the testes of mice that had been intraperitoneally injected with insulin. The mRNA level of steroidogenic enzyme gene was significantly decreased by insulin injection (Fig. 4C). In contrast, serum levels of LH and FSH remained unchanged 3 h after the injection of insulin (Fig. 4D). Testicular and serum levels of progesterone and testosterone were decreased following insulin injection (Fig. 4, E and F). These results suggest that insulin negatively regulates steroidogenic enzyme gene expression and steroidogenesis.
FIGURE 4.
Effect of insulin on testicular steroidogenesis. A, testes were collected from 8-week-old C57BL/6J mice for immunohistochemistry. The LH-R and IR-β are shown in Leydig cells of the testis. The arrowheads show the LH-R or IR-β stain in 8-week testis. B, 8-week-old C57BL/6J mice were injected with insulin (1 unit/kg). Testes of C57BL/6J mice were prepared for Western blot assay. Samples were extracted, and Western blot analyses were performed using the phospho-IR-β (p-IR-β), IR-β, phospho-IRS1 (p-IRS1), IRS1, phospho-AKT (p-AKT), and AKT antibodies. C, total RNA was isolated for real-time PCR from insulin-injected 8-week-old mouse testes (n = 5). StAR, P450scc, and 3β-HSD expression was measured at the indicated time periods. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. D, 8-week-old C57BL/6J mice injected with insulin (1 unit/kg) were used to measure serum LH and FSH levels in insulin-injected 8-week-old mice (n = 5). Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. E, serum from panel C was used for radioimmunoassay (n = 5). Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. F, testes from panel C were used for radioimmunoassay (n = 5). Each point represents the average concentration of progesterone (P) and testosterone (T). Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test.
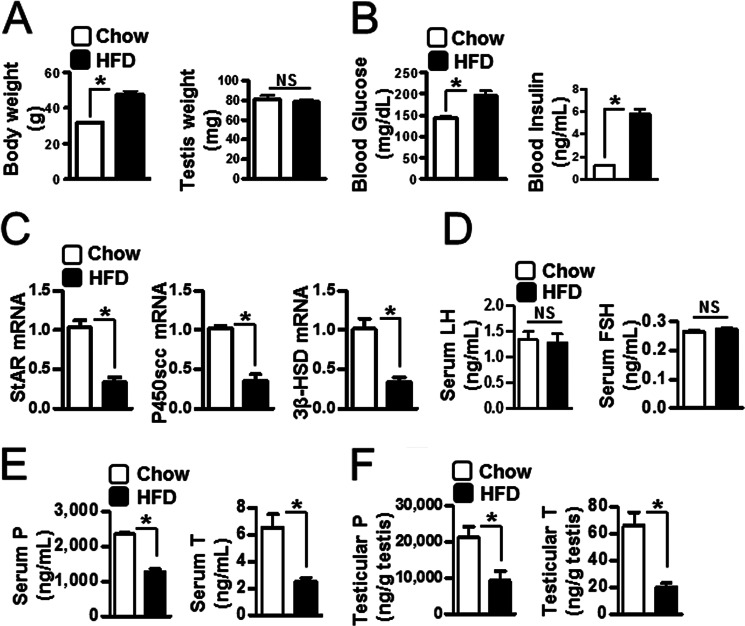
Regulation of Testicular Steroidogenesis in HFD-fed Mice
Obese humans and those with T2D show low testosterone level and decreased insulin sensitivity (2–4). We measured testicular steroidogenic enzyme gene expression and steroidogenesis in HFD-fed mice as a model of T2D. Body weight was increased significantly in HFD-fed mice when compared with control chow diet (left panel), but testis weight was unchanged (right panel) (Fig. 5A). As expected, blood glucose (left panel) and insulin (right panel) levels were high in HFD-fed mice (Fig. 5B). We assessed the steroidogenic enzyme mRNA level to determine whether steroidogenic enzyme gene expression was affected in testes of HFD-fed mice. Expression of StAR, P450scc, and 3β-HSD was decreased in HFD-fed mice when compared with control chow diet (Fig. 5C). Because testosterone concentration is affected by LH and FSH levels via the hypothalamic-pituitary-gonadal axis (1, 2), we measured LH and FSH. Both were unchanged in HFD-fed mice (Fig. 5D). However, both progesterone and testosterone were significantly decreased in serum and testes of HFD-fed mice (Fig. 5, E and F). These results indicate that testicular steroidogenesis is negatively regulated in the HFD-fed condition.
FIGURE 5.
The regulation of testicular steroidogenesis in HFD-fed mice. A, C57BL/6J mice were used to measure body weight (left) and testis weight (right) in chow diet-fed (12 weeks) and HFD-fed (12 weeks) mice (n = 4). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. NS, not significant. B, blood glucose (left) and insulin (right) were measured in HFD-fed mice (12 weeks) (n = 4). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. C, total RNA was isolated for real-time PCR from the testes of chow diet-fed and HFD-fed mice (n = 4). StAR, P450scc, and 3β-HSD expression was measured in the testes of chow diet-fed (12 weeks) and HFD-fed (12 weeks) mice (n = 4). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. D, serum LH and FSH levels were measured in HFD-fed mice (n = 4). NS, not significant. E, serum from panel C was collected for progesterone (P, left) and testosterone (T, right) measurement by radioimmunoassay (n = 4). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. F, testes from panel C were collected for progesterone (left) and testosterone (right) measurement by radioimmunoassay (n = 4). Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test.
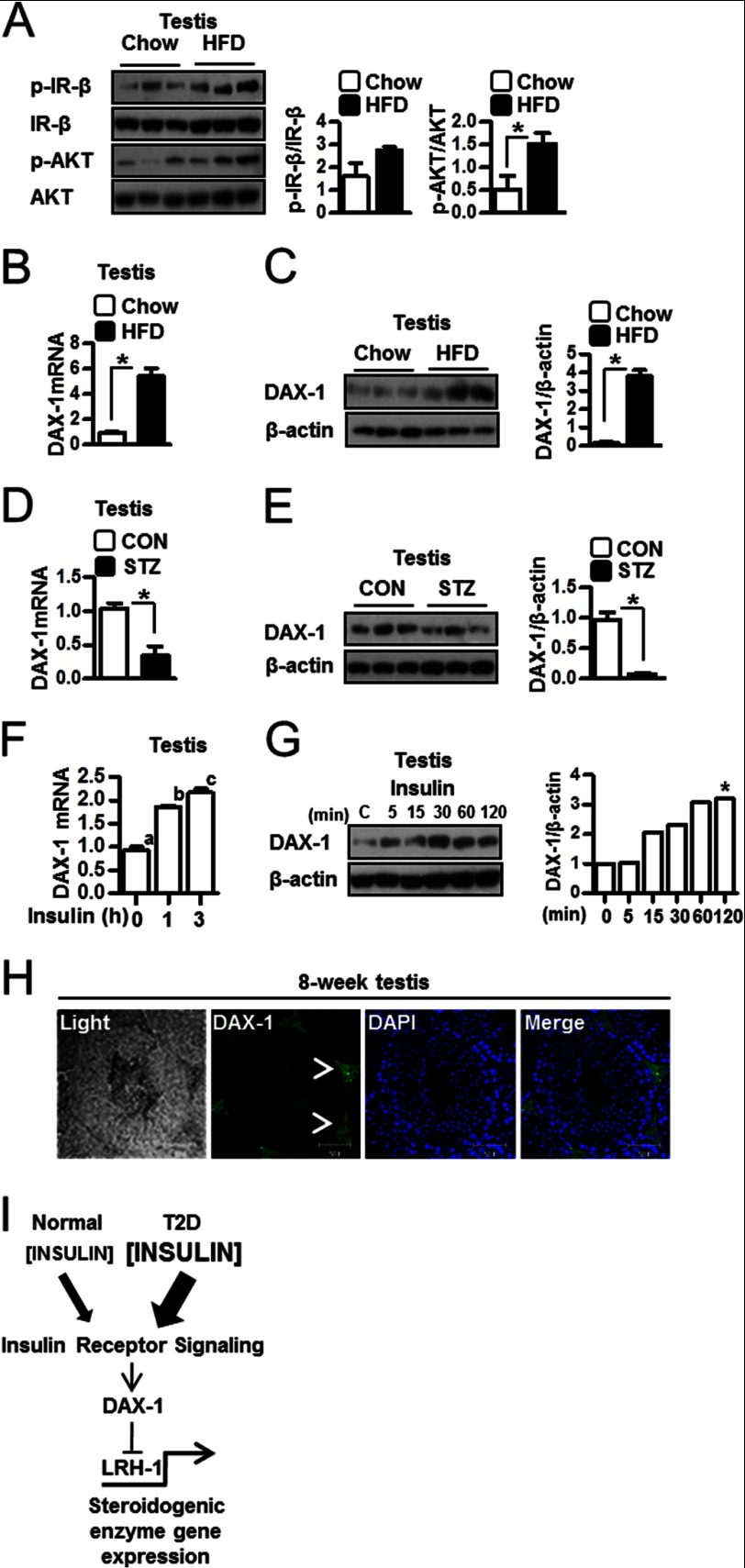
Increased Induction of DAX-1 in Testes of HFD-fed Mice
Western blot analysis was performed to determine the effect of insulin in HFD-fed mouse testis. Phospho-IR-β and phospho-AKT were low in liver of HFD-fed mice (data not shown), but both signals were significantly higher than those in testes from HFD-fed mice (Fig. 6A). Previously, we have reported that insulin inhibits gluconeogenesis via induction of DAX-1 in liver cells (16) and that LH promotes steroidogenesis via down-regulation of DAX-1 in Leydig cells (17). In the present study, DAX-1 mRNA and protein levels were increased in testes of HFD-fed mice when compared with testes of chow diet-fed mice (Fig. 6, B and C). In contrast, DAX-1 mRNA and protein levels were low in testes of streptozotocin mice when compared with control (Fig. 6, D and E). Furthermore, insulin injection increased DAX-1 gene expression in testis (Fig. 6F) and DAX-1 protein levels in chow diet-fed mice (Fig. 6G). Moreover, immunohistochemistry showed that DAX-1 is expressed primarily if not exclusively in the Leydig cells of the testes (Fig. 6F, arrowheads). Overall, these findings suggest a role for insulin in regulation of testicular steroidogenesis via the induction of DAX-1.
FIGURE 6.
Induction of DAX-1 in testis. A, testes of HFD-fed mice were prepared for Western blot assay. Samples were extracted, and Western blot analyses were performed using the phospho-IR-β (p-IR-β), IR-β, phospho-AKT (p-AKT), and AKT antibodies. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. B, total RNA was isolated for real-time PCR from chow diet-fed and HFD-fed mice testes (n = 3). DAX-1 mRNA level is measured in testis. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. C, protein samples were prepared for Western blot assay from chow diet-fed and HFD-fed mice testes (n = 3). DAX-1 protein level was measured in testis. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from chow group by two-tailed t test. D, total RNA was isolated for real-time PCR from chow and streptozotocin (STZ) mice testes (n = 3). DAX-1 mRNA level was measured in testis. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from control (CON) group by two-tailed t test. E, protein samples were prepared for Western blot assay from control and streptozotocin mice testes (n = 3). DAX-1 protein level was measured in testis. Each data point represents the mean ± S.E. *, p < 0.05, significantly different from control group by two-tailed t test. F, total RNA was isolated for real-time PCR from insulin-injected 8-week-old mouse testes (n = 5). DAX-1 expression was measured at the indicated time. Each data point represents the mean ± S.E. Bars with different letters in this panel indicate statistically significant differences in mean values from each other (p < 0.05), as determined by ANOVA and Student-Newman-Keuls test. G, 8-week-old C57BL/6J mice were injected insulin (1 unit/kg). Testes of C57BL/6J mice were prepared for Western blot assay (n = 3). Samples were extracted, and Western blot analyses were performed using the DAX-1 and β-actin antibodies. * indicates the highest DAX-1 protein level. H, testes were collected from 8-week-old C57BL/6J mice for immunohistochemistry. Expression of DAX-1 was only observed in the Leydig cells of the testis. The arrowheads show the DAX-1 stain in 8-week testis. I, proposed mechanism for the importance of regulation of steroidogenesis by insulin. Insulin induces DAX-1 gene expression, and DAX-1 regulates LRH-1-mediated steroidogenic enzyme gene expression and steroidogenesis in testis. The low level of insulin in individuals with normal insulin sensitivity mildly opposes the stimulation of steroidogenesis by LH. However, greater up-regulation of DAX-1 by insulin is observed in individual with insulin resistance, and therefore, high insulin level inhibits testicular steroidogenesis in the T2D diabetes model.
DISCUSSION
In this study, we demonstrated that insulin-treated MA-10 Leydig cells activated insulin receptor signal pathway and repressed cAMP-mediated steroidogenesis via induction of DAX-1. Moreover, the high insulin level in HFD-fed mice activated IR signaling in testis that correlated with reduced steroid level in serum and the testis. These results indicate that insulin directly regulated testicular steroidogenesis via induction of DAX-1. Moreover, insulin injection significantly induced testicular DAX-1 in vivo. Furthermore, insulin-induced DAX-1 gene expression decreased testicular steroidogenesis mediated by the nuclear receptor LRH-1 in vitro. However, the effects of insulin on testicular steroidogenesis were abolished by DAX-1 knockdown in Leydig cells. Based on these results, we propose that insulin inhibits the LH/cAMP-mediated steroidogenesis through induction of DAX-1.
The role of insulin in testis has been controversial. Schoeller et al. (28) reported that Akita diabetic mice, which express a mutant form of insulin 2 (Ins2) in testes and pancreas, show infertility, whereas exogenous insulin treatment rescues fertility through the hypothalamic-pituitary-gonadal axis. However, other studies showed that overexpression of insulin in Leydig cells decreases germ cells and causes infertility in transgenic mice (14). Intriguingly, it was reported that type 1 diabetes (T1D) does not affect testosterone level (29) but decreases sex hormone-binding globulin in human subjects (30, 31). Moreover, other previous studies have shown positive effect of insulin on Leydig cells (32) and have shown that tungstate, an insulin signaling mimic, restores Leydig cell function in type 1 diabetes (33). It has been reported that insulin receptor signal transducers are expressed in rat testis (27), and we found in this study that IR-β, IRS1, and AKT were activated by insulin both in vivo and in vitro. Our study strongly suggests that insulin activates the insulin receptor signaling pathway, leading to decreased steroidogenic gene expression and steroid production in testicular Leydig cells without any change in serum LH or FSH levels. From previously published studies, it can be suggested that normal insulin level is necessary for Leydig cell function (28). However, the present study suggests that high levels of insulin, such as those obtained in obesity and T2D, may induce negative effect on steroidogenesis in Leydig cells. However, further studies are required to fully elucidate the precise biological correlation between insulin and Leydig cells.
It is known that tissue levels of insulin are invariably higher than serum insulin levels and also do not correlate with serum insulin levels (34–37). This has been shown for brain, heart, liver, cultured lymphocytes, cultured fibroblasts, and testes. Although this phenomenon may not be fully understood, it has been hypothesized that insulin is synthesized by many cells other than pancreatic beta cells (38). Our finding that the insulin receptor signaling pathway is activated more in the testes of HFD-fed mice than in WT mice (Fig. 6) strongly indicates that the higher serum level of insulin produces a greater effect in the HFD-fed mice than in the WT mice. Moreover, we found that DAX-1 gene expression was high in HFD-fed and insulin-injected mice testes (Fig. 6, B–E). Indeed, induction of DAX-1 by insulin suppressed basal level and LH/cAMP-induced progesterone and testosterone levels (Fig. 6G). It has been reported that insulin sensitivity is preserved in the kidney, sympathetic nervous system, and blood vessels of human subjects and animal models that have insulin resistance (39). Moreover, because insulin sensitivity is preserved in these tissues of insulin-resistant individuals and/or serum insulin levels are greatly elevated in these individuals, insulin effects on these tissues may be larger than normal in these individuals, which likely contributes to the development of hypertension in patients with the metabolic syndrome. Based on the results of the current study, we speculate that steroidogenesis is reduced because of the action of insulin in HFD-fed and insulin-injected mice, that insulin sensitivity of the testes is preserved in insulin-resistant mice, and that elevated serum insulin level produces a negative effect in testis that is larger than normal. Moreover, the high amount of insulin of HFD-fed mice may increase DAX-1 gene expression and subsequently down-regulate testicular steroidogenesis. Overall, it seems likely that the molecular mechanism by which insulin inhibits testicular steroidogenesis is mediated by activation of insulin receptor signal pathway, leading to the induction of the transcriptional corepressor DAX-1.
We have shown previously that gluconeogenic enzyme genes expressed in Leydig cells are required for testicular steroidogenesis (21). Insulin-induced DAX-1 decreases gluconeogenic enzyme genes expression in the liver (16). Moreover, we previously reported that DAX-1 represses steroidogenesis by decreasing Nur77 transcriptional activity in Leydig cells (17). We examined whether insulin inhibits gluconeogenic enzyme gene expression or whether DAX-1 mediates insulin-induced suppression of gluconeogenic enzyme gene expression. PEPCK and G6Pase were inhibited by insulin treatment, and these effects were reversed by knockdown of DAX-1 regulated by IR signaling pathway in MA-10 cells (data not shown). These results indicate that insulin-induced DAX-1 directly repressed gluconeogenic and steroidogenic enzyme gene expression in Leydig cells. Taken together, we suggest that insulin-induced DAX-1 down-regulated steroidogenesis in Leydig cells and testes (Figs. 1D and 4, E and F).
In conclusion, our results suggest that induction of DAX-1 by insulin represents a novel regulatory mechanism of steroidogenesis in Leydig cells and that insulin-mediated induction of DAX-1 in Leydig cells of testis may be a key regulatory step of serum sex hormone level in states where insulin is elevated. Inhibition of steroidogenesis by the insulin-mediated induction of DAX-1 provides new insights into differential regulation of testicular steroidogenesis between normal individuals and T2D patients (Fig. 6G).
Acknowledgments
We thank our laboratory members for helpful suggestions.
This work was supported by the National Creative Research Initiatives Center for Nuclear Receptor Signals from the Korean Ministry of Education, Science, and Technology (Grant 20110018305) and the Future-based Technology Development Program (BIO Fields) through the National Research Foundation of Korea (NRF), by the Ministry of Education, Science, and Technology (Grant 20100019512) (to H. S. C.), and by the Korea Research Institute of Bioscience & Biotechnology (KRIBB) Research Initiative Program of Korea, Republic of Korea (to C. H. L.).
- T2D
- type 2 diabetes
- LH
- luteinizing hormone
- LH-R
- luteinizing hormone receptor
- IR-β
- insulin receptor-β
- DAX-1
- dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1
- LRH-1
- liver receptor homolog-1
- HFD
- high fat diet
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- StAR
- steroidogenic acute regulatory protein
- 8-Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- DMSO
- dimethyl sulfoxide
- MOI
- multiplicity of infection
- ANOVA
- analysis of variance
- IRS
- insulin receptor substrate(s)
- Ad
- adenovirus.
REFERENCES
- 1. Dhindsa S., Prabhakar S., Sethi M., Bandyopadhyay A., Chaudhuri A., Dandona P. (2004) Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 5462–5468 [DOI] [PubMed] [Google Scholar]
- 2. Pitteloud N., Hardin M., Dwyer A. A., Valassi E., Yialamas M., Elahi D., Hayes F. J. (2005) Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 90, 2636–2641 [DOI] [PubMed] [Google Scholar]
- 3. Phillips G. B. (1977) Relationship between serum sex hormones and glucose, insulin, and lipid abnormalities in men with myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 74, 1729–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haffner S. M., Karhapää P., Mykkänen L., Laakso M. (1994) Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 43, 212–219 [DOI] [PubMed] [Google Scholar]
- 5. Haffner S. M., Valdez R. A., Stern M. P., Katz M. (1993) Obesity, body fat distribution, and sex hormones in men. Int. J. Obes. Relat. Metab. Disord. 17, 643–649 [PubMed] [Google Scholar]
- 6. Mårin P., Holmäng S., Gustafsson C., Jönsson L., Kvist H., Elander A., Eldh J., Sjöström L., Holm G., Björntorp P. (1993) Androgen treatment of abdominally obese men. Obes. Res. 1, 245–251 [DOI] [PubMed] [Google Scholar]
- 7. Simon D., Charles M. A., Lahlou N., Nahoul K., Oppert J. M., Gouault-Heilmann M., Lemort N., Thibult N., Joubert E., Balkau B., Eschwege E. (2001) Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care 24, 2149–2151 [DOI] [PubMed] [Google Scholar]
- 8. Liu S., Navarro G., Mauvais-Jarvis F. (2010) Androgen excess produces systemic oxidative stress and predisposes to β-cell failure in female mice. PLoS One 5, e11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Isidori A. M., Caprio M., Strollo F., Moretti C., Frajese G., Isidori A., Fabbri A. (1999) Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 84, 3673–3680 [DOI] [PubMed] [Google Scholar]
- 10. Tena-Sempere M., Pinilla L., González L. C., Diéguez C., Casanueva F. F., Aguilar E. (1999) Leptin inhibits testosterone secretion from adult rat testis in vitro. J. Endocrinol. 161, 211–218 [DOI] [PubMed] [Google Scholar]
- 11. Khaw K. T., Barrett-Connor E. (1992) Lower endogenous androgens predict central adiposity in men. Ann. Epidemiol. 2, 675–682 [DOI] [PubMed] [Google Scholar]
- 12. Pasquali R., Casimirri F., De Iasio R., Mesini P., Boschi S., Chierici R., Flamia R., Biscotti M., Vicennati V. (1995) Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J. Clin. Endocrinol. Metab. 80, 654–658 [DOI] [PubMed] [Google Scholar]
- 13. Le T. N., Nestler J. E., Strauss J. F., 3rd, Wickham E. P., 3rd (2012) Sex hormone-binding globulin and type 2 diabetes mellitus. Trends Endocrinol. Metab. 23, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirneshan K., Binder S., Böhm D., Wolf S., Sancken U., Meinhardt A., Schmid M., Engel W., Adham I. M. (2008) Directed overexpression of insulin in Leydig cells causes a progressive loss of germ cells. Mol. Cell Endocrinol. 295, 79–86 [DOI] [PubMed] [Google Scholar]
- 15. Miller W. L. (1988) Molecular biology of steroid hormone synthesis. Endocr. Rev. 9, 295–318 [DOI] [PubMed] [Google Scholar]
- 16. Nedumaran B., Hong S., Xie Y. B., Kim Y. H., Seo W. Y., Lee M. W., Lee C. H., Koo S. H., Choi H. S. (2009) DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4α and negatively regulates gluconeogenic enzyme gene expression. J. Biol. Chem. 284, 27511–27523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Song K. H., Park Y. Y., Park K. C., Hong C. Y., Park J. H., Shong M., Lee K., Choi H. S. (2004) The atypical orphan nuclear receptor DAX-1 interacts with orphan nuclear receptor Nur77 and represses its transactivation. Mol. Endocrinol. 18, 1929–1940 [DOI] [PubMed] [Google Scholar]
- 18. Hong C. Y., Park J. H., Ahn R. S., Im S. Y., Choi H. S., Soh J., Mellon S. H., Lee K. (2004) Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor α. Mol. Cell Biol. 24, 2593–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim Y. D., Park K. G., Lee Y. S., Park Y. Y., Kim D. K., Nedumaran B., Jang W. G., Cho W. J., Ha J., Lee I. K., Lee C. H., Choi H. S. (2008) Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57, 306–314 [DOI] [PubMed] [Google Scholar]
- 20. Ascoli M. (1981) Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108, 88–95 [DOI] [PubMed] [Google Scholar]
- 21. Ahn S. W., Gang G. T., Tadi S., Nedumaran B., Kim Y. D., Park J. H., Kweon G. R., Koo S. H., Lee K., Ahn R. S., Yim Y. H., Lee C. H., Harris R. A., Choi H. S. (2012) Phosphoenolpyruvate carboxykinase and glucose-6-phosphatase are required for steroidogenesis in testicular Leydig cells. J. Biol. Chem. 287, 41875–41887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chanda D., Xie Y. B., Choi H. S. (2010) Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation. Nucleic Acids Res. 38, 4607–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kokk K., Veräjänkorva E., Laato M., Wu X. K., Tapfer H., Pöllänen P. (2005) Expression of insulin receptor substrates 1–3, glucose transporters GLUT-1–4, signal regulatory protein 1α, phosphatidylinositol 3-kinase, and protein kinase B at the protein level in the human testis. Anat. Sci. Int. 80, 91–96 [DOI] [PubMed] [Google Scholar]
- 24. Kokk K., Veräjänkorva E., Wu X. K., Tapfer H., Põldoja E., Simovart H. E., Pöllänen P. (2007) Expression of insulin signaling transmitters and glucose transporters at the protein level in the rat testis. Ann. N.Y. Acad. Sci. 1095, 262–273 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki T., Kasahara M., Yoshioka H., Morohashi K., Umesono K. (2003) LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol. Cell Biol. 23, 238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fayard E., Auwerx J., Schoonjans K. (2004) LRH-1: an orphan nuclear receptor involved in development, metabolism, and steroidogenesis. Trends Cell Biol. 14, 250–260 [DOI] [PubMed] [Google Scholar]
- 27. Lubik A. A., Gunter J. H., Hendy S. C., Locke J. A., Adomat H. H., Thompson V., Herington A., Gleave M. E., Pollak M., Nelson C. C. (2011) Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 71, 5754–5764 [DOI] [PubMed] [Google Scholar]
- 28. Schoeller E. L., Albanna G., Frolova A. I., Moley K. H. (2012) Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 61, 1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ebeling P., Stenman U. H., Seppälä M., Koivisto V. A. (1995) Androgens and insulin resistance in type 1 diabetic men. Clin. Endocrinol. (Oxf.) 43, 601–607 [DOI] [PubMed] [Google Scholar]
- 30. Pitteloud N., Mootha V. K., Dwyer A. A., Hardin M., Lee H., Eriksson K. F., Tripathy D., Yialamas M., Groop L., Elahi D., Hayes F. J. (2005) Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care 28, 1636–1642 [DOI] [PubMed] [Google Scholar]
- 31. Christensen L., Hagen C., Henriksen J. E., Haug E. (1997) Elevated levels of sex hormones and sex hormone binding globulin in male patients with insulin dependent diabetes mellitus. Effect of improved blood glucose regulation. Dan. Med. Bull. 44, 547–550 [PubMed] [Google Scholar]
- 32. Lin T., Haskell J., Vinson N., Terracio L. (1986) Characterization of insulin and insulin-like growth factor I receptors of purified Leydig cells and their role in steroidogenesis in primary culture: a comparative study. Endocrinology 119, 1641–1647 [DOI] [PubMed] [Google Scholar]
- 33. Ballester J., Domínguez J., Muñoz M. C., Sensat M., Rigau T., Guinovart J. J., Rodríguez-Gil J. E. (2005) Tungstate treatment improves Leydig cell function in streptozotocin-diabetic rats. J. Androl. 26, 706–715 [DOI] [PubMed] [Google Scholar]
- 34. Rosenzweig J. L., Havrankova J., Lesniak M. A., Brownstein M., Roth J. (1980) Insulin is ubiquitous in extrapancreatic tissues of rats and humans. Proc. Natl. Acad. Sci. U.S.A. 77, 572–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agardh C. D., Lesniak M. A., Gerritsen G. C., Roth J. (1986) The influence of plasma insulin concentrations on tissue insulin levels in rodents: a study of the diabetic Chinese hamster and the ob/ob mouse. Metabolism. 35, 244–249 [DOI] [PubMed] [Google Scholar]
- 36. Havrankova J., Roth J., Brownstein M. J. (1979) Concentrations of insulin and insulin receptors in the brain are independent of peripheral insulin levels. Studies of obese and streptozotocin-treated rodents. J. Clin. Invest. 64, 636–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grizard G., Fournet M., Rigaudière N., Lombard-Vignon N., Grizard J. (1991) Insulin binding to Leydig cells and insulin levels in testicular interstitial fluid at different stages of development in the rat. J. Endocrinol. 128, 375–381 [DOI] [PubMed] [Google Scholar]
- 38. Kojima H., Fujimiya M., Matsumura K., Nakahara T., Hara M., Chan L. (2004) Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc. Natl. Acad. Sci. U.S.A. 101, 2458–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sechi L. A. (1999) Mechanisms of insulin resistance in rat models of hypertension and their relationships with salt sensitivity. J. Hypertens. 17, 1229–1237 [DOI] [PubMed] [Google Scholar]