Background: Dectin-1 is able to recognize and phagocytose the fungal carbohydrate, β-1,3-glucan, but its contribution to phagosomal maturation has not been explored.
Results: Dectin-1-dependent Syk activation promotes phagolysosomal fusion and acidification.
Conclusion: Dectin-1-dependent Syk-activation permits egress of early phagosomes to mature phagolysosomes.
Significance: The surface recognition receptor, Dectin-1 shapes anti-fungal responses by controlling fungal phagosome maturation.
Keywords: Lysosomes; Macrophages; Pathogen-associated Molecular Pattern (PAMP); Pattern Recognition Receptor; Phagocytosis; β-1,3-Glucan; Dectin-1; Syk; Acidification; Phagosome
Abstract
Elimination of fungal pathogens by phagocytes requires phagosome maturation, a process that involves the recruitment and fusion of intracellular proteins. The role of Dectin-1, a β-1,3-glucan receptor, critical for fungal recognition and triggering of Th17 responses, to phagosomal maturation has not been defined. We show that GFP-Dectin-1 translocates to the fungal phagosome, but its signal decays after 2 h. Inhibition of acidification results in retention of GFP-Dectin-1 to phagosome membranes highlighting the requirement for an acidic pH. Following β-1,3-glucan recognition, GFP-Dectin-1 undergoes tyrosine phosphorylation by Src kinases with subsequent Syk activation. Our results demonstrate that Syk is activated independently of intraphagosomal pH. Inhibition of Src or Syk results in prolonged retention of GFP-Dectin-1 to the phagosome signifying a link between Syk and intraphagosomal pH. β-1,3-glucan phagosomes expressing a signaling incompetent Dectin-1 failed to mature as demonstrated by prolonged Dectin-1 retention, presence of Rab5B, failure to acquire LAMP-1 and inability to acidify. Phagosomes containing Candida albicans also require Dectin-1-dependent Syk activation for phagosomal maturation. Taken together, these results support a model where Dectin-1 not only controls internalization of β-1,3-glucan containing cargo and triggers proinflammatory cytokines, but also acts as a master regulator for subsequent phagolysosomal maturation through Syk activation.
Introduction
There has been an unprecedented increase in the number of invasive fungal infections owing to the availability of more potent chemotherapeutic and biologic agents (1–4). Additionally, specific genetic polymorphisms result in increased susceptibility to fungal infections (5). Our ability to define the critical molecular events required for innate immune cells to recognize, phagocytose, and elicit an adaptive immune response is essential to develop strategies for induction of sterilizing immunity (6, 7).
β-1,3-Glucan is a dominant fungal cell wall component of multiple fungal species including the major fungal pathogens Candida albicans, Aspergillus fumigatus, and Pneumocystis jirovecii (8, 9) and is detected by the type II lectin receptor, Dectin-1 (8, 10), on phagocytic innate immune cells. Dectin-1 recognition of β-1,3-glucan is critical in mounting anti-fungal immunity as patients with Dectin-1 deficiencies suffer from recurrent mucocutaneous candidiasis (11, 12). Mice lacking Dectin-1 also show defective ability to clear candidal infections (13, 14).
Upon ligation, the intracellular domain of Dectin-1 is phosphorylated by Src kinases resulting in activation of spleen tyrosine kinase (Syk)3 and an intracellular signaling cascade resulting in proinflammatory cytokine production capable of polarizing Th17 cells, essential for anti-fungal immunity (15–22). Dectin-1 also triggers phagocytosis, and in innate immune cells places cargo in membrane-delineated compartments termed phagosomes. Maturation of these compartments occurs as a result of intracellular protein recruitment and vesicular fusion through protein chaperones such as Rab GTPase family members, or direct fusion with lysosomes. Recent observations from bacterial and fungal model systems demonstrate that specific signaling cascades as well as cross-presentation are influenced by phagosome maturation (23, 24). Intraphagosomal sampling by pattern-recognition receptors (PRR) can result in unique signaling cascades (23, 25, 26). To date, it is not known whether Dectin-1 plays a role in β-1,3-glucan or fungal phagosome maturation.
In this study, we explore the contribution of Dectin-1 and subsequent Syk signaling to phagosomal maturation. To reduce the complexity of fungal cell wall ligands, we used a fungal-like platform that displays monodispersed, purified β-1,3-glucan on a polystyrene platform (β-1,3-glucan beads) (27). Our results show that Dectin-1 translocates to β-1,3-glucan-containing phagosomes and is retained to the phagosomal membrane in a pH-dependent process. Through the use of chemical inhibitors or a signaling incompetent mutant of Dectin-1 incapable of activating Syk, we demonstrate that Dectin-1-dependent Syk activation is critical for acidification of β-1,3-glucan-containing phagosomes. Furthermore, we show that Dectin-1-dependent Syk signaling is required not only for phagosomal pH control but also for egress of phagosomes from an early phagosomal stage into mature phagolysosomes. These data indicate that in addition to triggering phagocytosis and the elaboration of pro-inflammatory cytokines, Dectin-1 controls the transition of nascent phagosomes to mature phagolysosomes through a Syk-dependent mechanism.
EXPERIMENTAL PROCEDURES
Reagents
All products purchased from Invitrogen (Carlsbad, CA) or Sigma unless otherwise indicated. Piceatannol (Syk inhibitor) and PP2 (Src inhibitor) purchased from EMD-Calbiochem (Billerica, MA). Bafilomycin A1 (BafA1) from A.G. Scientific, Inc. (San Diego, CA). PP2, piceatannol and BafA1 used at 50 μm, 100 μm and 100 nm, respectively. Antibodies for LAMP-1, Rab5B, and isotype controls (Santa Cruz Biotechnologies, Santa Cruz, CA), Dectin-1 (R&D systems, Minneapolis, MN), anti-phospho SYK antibody (Cell Signaling, Danvers, MA) and anti-β-actin antibody (Abcam, Cambridge, MA).
Cell Lines and Culture
RAW 264.7 macrophage (RAW) cells purchased from ATCC (Manassas, VA). RAW cells cultured in Dulbecco's-modified Eagle's medium (DMEM) containing 10% FBS (ThermoScientific, Logan, UT), 1% penicillin/streptomycin, and 1% l-glutamine (complete media). Cells were grown at 37 °C in a humidified incubator supplemented with 5% CO2. For cell lysates and phagosome isolations, RAW cells were plated into 6-well plates (Corning, Tewksbury, MA). Puromycin at 5 μg/ml used for selection of transduced cells. C. albicans, SC5314, a wild-type strain (gift from Dr. Eleftherios Mylonakis) grown from −80 °C stock overnight in YPD (Beckton-Dickinson, Sparks, MD and Sigma) with 100 μg/ml ampicillin (Sigma) in an orbital shaker at 30 °C. Yeast were heat-killed at 95 °C for 10 min in 300 μl of PBS, and counted by hemacytometer.
Cell Lysates
RAW cells grown in 6-well plates were stimulated with ligand, washed (PBS), and lysed in 0.5 ml 1% nonidet P40 (American Bioanalytical, Natick, MA) containing protease inhibitors (Roche, Indianapolis, IN) for 1 h nutating at 4 °C. Following microcentrifugation, samples were prepared for SDS-PAGE. For anti-phospho-SYK Western blots, 1 mm sodium orthovanadate (New England Biolabs, Ipswich, MA) was added to cell lysis solution for phosphatase inhibition.
Generation of GFP-Dectin-1ΔY15 Mutant
Using wild-type Dectin-1 gene as a template, a tyrosine to phenylalanine substitution at amino acid position 15 within the intracytoplasmic domain (GFP-Dectin-1ΔY15) in pMAX vector with GFP fused at the N terminus (gift from Dr. David Underhill). The intracytoplasmic substitution was performed using QuikChangeXL site-directed mutagenesis (Agilent Technologies, Santa Clara, CA) with the following primers: 5′-GAG AAT CTG GAT GAA GAT GGA TTT ACT CAA TTA GAC TTC AGC AC-3′ (forward) and 5′-GTG CTG AAG TCT AAT TGA GTA AAT CCA TCT TCA TCC AGA TTC TC-3′ (reverse). The final construct was confirmed by sequencing.
Lentiviral Transduction
HEK293T cells were used to generate lentivirus as described (28). GFP-Dectin-1 or GFP-Dectin-1ΔY15 was subcloned into pHAGE II, a fourth-generation lentiviral self-inactivating nonreplicative vector (28) (29). 50 μl of concentrated viral supernatant was placed onto RAW cells at 37 °C overnight. Transduced cells were selected using 5 μg/ml puromycin. Expression of both receptor fusions, GFP-Dectin-1 and GFP-Dectin-1ΔY15, were comparable as measured by flow cytometry (data not shown).
Generation of β-1,3-Glucan Beads
β-1,3-Glucan beads were produced as described (27). Briefly, 3 μm amine-terminated polystyrene beads (Polysciences, Warrington, PA) were washed in DMSO (Sigma) using centrifugal 0.45 μm PTFE membrane filters (Ultrafree brand, Millipore, Billerica, MA). Amino groups were activated with 0.5 m 1,1′-carbonyldiimidazole (CDI, Sigma) at room temperature for 1 h. After rinsing, beads were resuspended in 10 mm β-1,3-glucan (Wellmune, Biothera, Eagan, MN) dissolved in DMSO for 1 h. Beads were stored at 4 °C in PBS. All batches were tested for conjugation β-1,3-glucan as described (27).
Surface Fluorescence Labeling of β-1,3-Glucan Beads
5 × 106 β-1,3-glucan beads were fluorescently labeled for imaging by mixing with 30 μg of N-hydroxysuccinimidyl AF dye (Invitrogen) in PBS for 1 h at room temperature. For the described experiments, AF647 (ex: 650/em: 655) was used.
Immunofluorescence Staining of Phagosomes
For phagoFACS of purified phagosomes, 1 × 105 phagosomes were immunostained for 30 min at room temperature using 0.1 μg anti-LAMP-1 antibody or isotype control and 0.4 μg adsorbed anti-rabbit IgG AF647 antibody (Jackson Immunoresearch, West Grove, PA). After washing, phagosomes were assessed using FACS Calibur (BD Biosciences, San Jose, CA). Flow analysis performed using FlowJo software (Tree Star Inc., Ashland, OR). Phagosome index was calculated by determining the percent positive phagosome compared with beads alone multiplied by the geometric mean fluorescence.
Confocal Microscopy
For visualization of subcellular compartments, RAW cells were plated onto 8-chambered slides (LabTek, ThermoScientific, Rochester, NY). Cells were incubated with AF647-β-1,3-glucan beads for 30 min at 37 °C. For visualizing acidic compartments, cells were pre-loaded with lysotracker red (Invitrogen) at 100 nm for 20 min. After washing, cells were mounted on a Nikon Ti-E inverted microscope equipped with a CSU-X1 confocal spinning-disk head (Yokogawa, Sugar Land, TX). A Coherent, 4 Watt laser (Coherent, Santa Clara, CA) was used as an excitation light source to produce wavelengths of 488, 568, and 647 nm. To acquire high-quality fluorescence images, a high-magnification, high-numerical aperture objective was used (Nikon, 1003, 1.49 numerical aperture, oil immersion). A piezo stage (Prior Instruments, Rockland, MA) capable of X, Y, Z movement was used for z-stack acquisition. A halogen light source and an air condenser (0.52 numerical aperture) were used for bright field illumination. A polarizer (Nikon, MEN51941) and Wollaston prisms (Nikon, MBH76190) were used to acquire differential interference contrast (DIC) images. Emission light from the sample was collected after passage through the appropriate emission filters (Semrock, Rochester, NY). Images were acquired using an EM-CCD camera (Hamamatsu C9100-13, Bridgewater, NJ). Image acquisition was performed using MetaMorph software (Molecular Devices, Downingtown, PA). Images were then cropped using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
Phagosome Isolation
Phagosome isolation was adopted from previous protocols (30). RAW cells were incubated with 1–4 × 106 β-1,3-glucan beads for the indicated time, and 0.5 ml of hypotonic lysis buffer (2 mm MgCl2, 6 mm β-mercaptoethanol, 10 mm HEPES) with protease inhibitor mixture (Roche) added. Cells were mechanically sheared by aspirating and ejecting cells through a 1cc syringe with ½ inch 26G needle (Fisher, Agawam, MA) for 15 cycles. Hypotonic buffer was adjusted to isotonicity with 62% (w/v) sucrose solution to 40%. A discontinuous gradient was then constructed in ultracentrifugation tubes (Beckman Polyallomer, Brea, CA) overlayering 2 ml of 62%, 40% (sample), 30%, 25%, and 10%. Sucrose gradients centrifuged at 24,000 rpm (80,000 × g) for 1 h at 4 °C (Beckman, SW-28 rotor, L8-M ultracentrifuge, Brea, CA). Following centrifugation, phagosomes were isolated at the interface of the 25%/10% sucrose layers. Phagosomes were counted using a hemacytometer in preparation for phagoFACS, Western blot analysis or sorting (BD FACSAria, BD Biosciences).
Western Blot
0.5–1 × 106 phagosomes or cell lysate in 1X loading buffer/reducing agent (Invitrogen) were heated to 95 °C for 5 min and resolved using 12% SDS-PAGE. Following electrophoresis, gels were removed and methanol-activated PVDF membrane (PerkinElmer, Waltham MA) applied to the gel in transfer buffer (0.025 m Tris, 0.192 m glycine, 20% methanol). All buffer components from National Diagnostics (Atlanta, GA) or Sigma. The gel and PVDF membrane were sandwiched between transfer sponge/blotting paper and subjected to electrophoretic transfer at 100 volts for 1 h at 4 °C.
For detection of specific proteins, PVDF-immobilized gel transfers were blocked with 5% milk in PBS-0.02% Tween-20 (PBS-T, Sigma). Blots were incubated with primary antibody in 1% milk in PBS-T for 1 h at room temperature unless noted otherwise. Following washes in PBS-T, rat anti-mouse HRP secondary (Cell Signaling Technology, Danvers, MA) was added at 1:20,000 in 1% milk in PBS-T for 1 h at room temperature. Following washes in PBS, the blot was visualized using Western Lighting Plus ECL chemiluminescent substrate (PerkinElmer, Waltham, MA) on Kodak BioMax XAR Film (Sigma). Films were then scanned, cropped and contrast adjusted evenly to entire image using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA).
Statistical Significance
Student's t test with a value of p < 0.05 was used as a measure of statistical significance. Representative data are expressed as means + S.E.
RESULTS
GFP-Dectin-1 Retention to β-Glucan-containing Phagosomes Is Dependent on Phagosomal pH
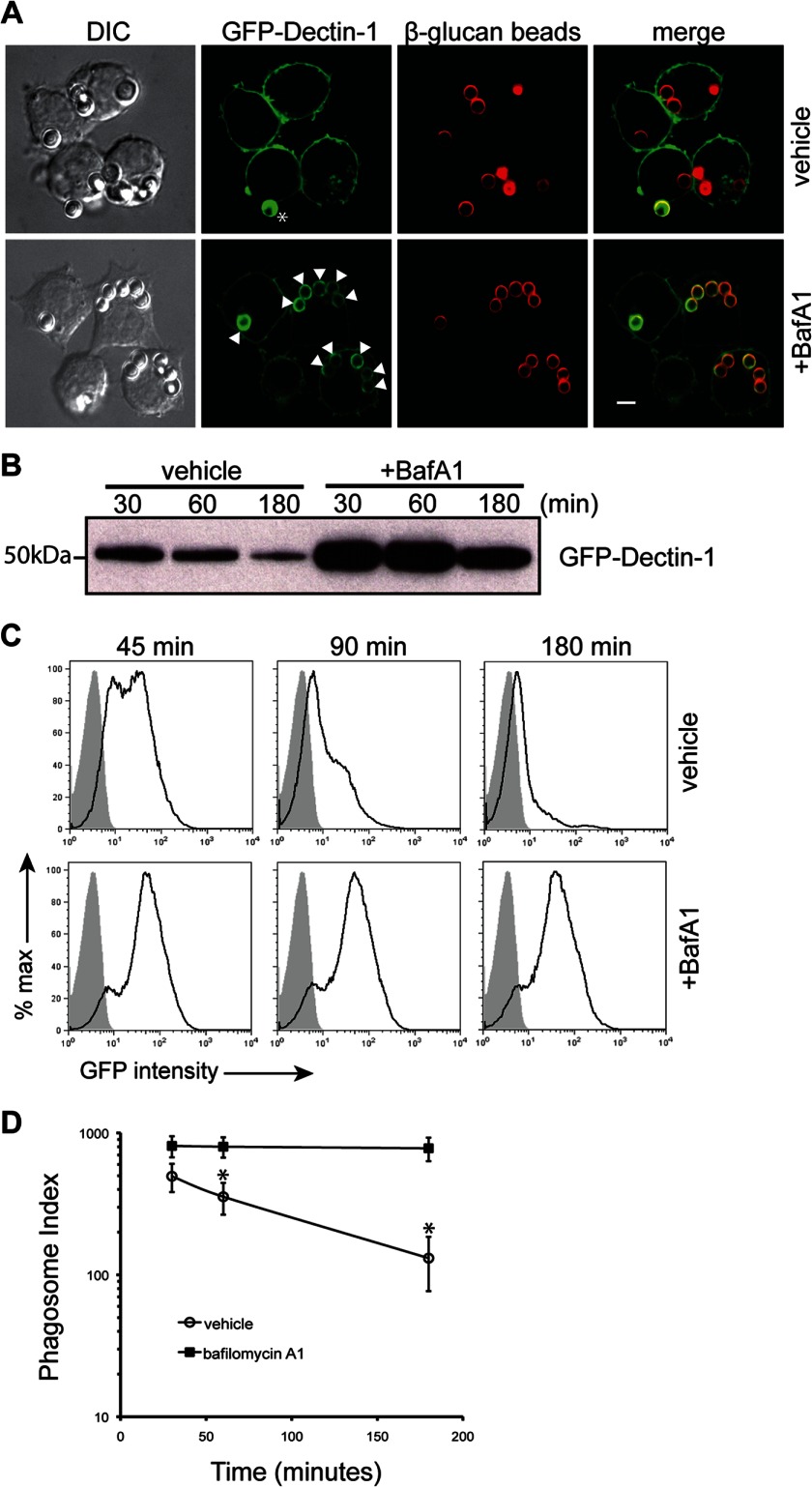
We first explored the kinetics of normal Dectin-1 translocation following capture and phagocytosis of β-1,3-glucan beads. RAW macrophage cells expressing GFP-Dectin-1 (RAW GFP-Dectin-1) cells show a loss of phagosomal-associated GFP-Dectin-1 within 90 min (Fig. 1, panel A). Occasionally, a phagocytic cup was visualized marking the onset of capture and initiation of phagocytosis (Fig. 1, panel A, asterisk). Since most macrophage phagosomes acidify within this time, we hypothesized that loss of Dectin-1 from the phagosome was related to intraphagosomal pH. To explore pH effects, RAW GFP-Dectin-1 were treated with the vacuolar-type H+-ATPase inhibitor, bafilomycin A1 (BafA1). BafA1 treatment had no effect on phagocytosis (data not shown). BafA1 treated RAW GFP-Dectin-1 had intense and prolonged retention of GFP-Dectin-1 to β-1,3-glucan-containing phagosomes (Fig. 1, panel A, arrowheads). To quantify GFP-Dectin-1 to the phagosomal membrane, RAW GFP-Dectin-1 cells were incubated with β-1,3-glucan beads followed by phagosome isolation. Cytospin and flow analysis of purified phagosomes revealed minimal debris to suggest isolation of highly purified phagosomes (data not shown). Phagosomes from BafA1 treated RAW GFP-Dectin-1 cells had intense retention of GFP-Dectin-1 for up to 3 h by Western blot analysis (Fig. 1, panel B). Similarly, surface GFP-Dectin-1 recruited to the phagosomal membrane assessed by flow cytometry (phagoFACS, Fig. 1, panel C) demonstrates acidification-dependent loss of GFP-Dectin-1 signal. The calculated phagosomal indices from compiled phagoFACS data revealed a significantly impaired decay rate of GFP-Dectin-1 from BafA1 treated RAW GFP-Dectin-1 cells (Fig. 1, panel D). Having confirmed a direct correlation between GFP-Dectin-1 phagosomal retention and pH, we next sought to understand the contribution of Dectin-1 signaling to intraphagosomal acidification.
FIGURE 1.
Dectin-1 retention to β-1,3-glucan containing phagosomes is pH-dependent. Panel A, RAW GFP-Dectin-1 cells were pre-incubated BafA1 or vehicle control for 30 min. β-1,3-glucan particles labeled with AF647 (red) were added at a bead to cell ratio of 5:1 for 90 min. Cells were then imaged using confocal imaging. Arrowheads denote presence of recruited GFP-Dectin-1 around β-1,3-glucan-containing phagosomes. Asterisk marks the initial formation of a phagocytic cup. Bar indicates 5 μm. Panel B, β-1,3-glucan particles were incubated with RAW GFP-Dectin-1 for the times indicated in the presence of BafA1 or vehicle control. 1 × 106 purified phagosomes were resolved by SDS-PAGE and blotted for GFP-Dectin-1. Panel C, purified β-1–3-glucan phagosomes (5 × 104) from BafA1 or vehicle pre-treated RAW GFP-Dectin-1 were assessed for surface recruitment of GFP-Dectin-1 (black line, open histogram) by phagoFACS and compared with bead-only control (shaded gray histogram). Panel D, phagosomal indices were determined from purified β-1–3-glucan phagosome phagoFACS over time comparing BafA1 versus vehicle treated RAW GFP-Dectin-1 cells. Data are representative of five independent experiments. * denotes p < 0.05.
Inhibition of Syk-dependent Signaling Results in GFP-Dectin-1 Retention to β-1,3-Glucan-containing Phagosomes
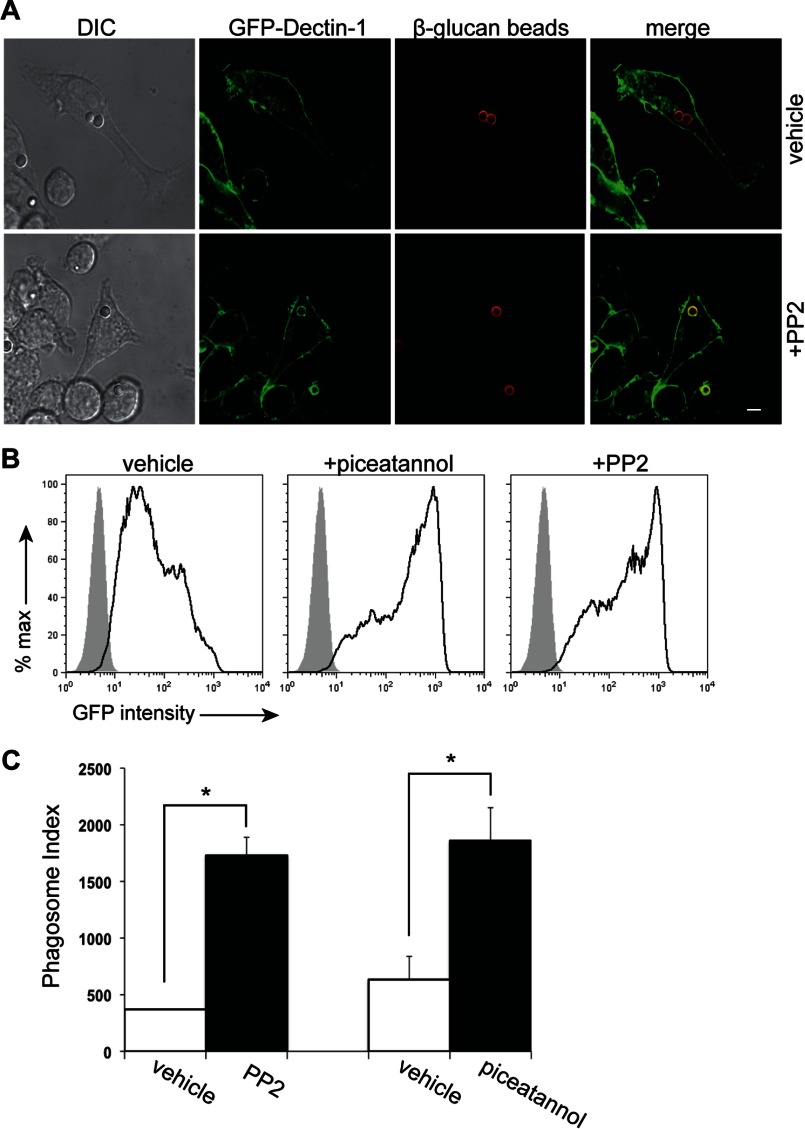
Since the dominant intracellular signaling pathway resulting from Dectin-1 ligation is through Src/Syk activation, we sought to determine the potential contribution of Syk to Dectin-1 phagosomal retention. RAW GFP-Dectin-1 cells were pre-treated with PP2 (Src inhibitor), or vehicle and exposed to β-1,3-glucan beads. Phagocytosis occurred with normal kinetics in both conditions when examined by light and confocal microscopy (data not shown). More intense GFP-Dectin-1 retention was visualized to the β-1,3-glucan-containing phagosomal surface in the PP2 treated cells as compared with vehicle (Fig. 2, panel A). To quantify GFP-Dectin-1 retention to the β-1,3-glucan-containing phagosomes, RAW GFP-Dectin-1 cells were pre-treated with PP2, piceatannol (Syk inhibitor), or vehicle. Purified β-1,3-glucan-containing phagosomes were isolated from treated cells and GFP-Dectin-1 assessed by phagoFACS (Fig. 2, panel B). As compared with vehicle, the phagosome index shows a significant increase in surface recruitment of GFP-Dectin-1 in the PP2 and piceatannol group (Fig. 2, panel C). These data suggest a direct role for Syk in the control of intraphagosomal pH and subsequent Dectin-1 retention.
FIGURE 2.
Dectin-1 retention to β-1,3-glucan containing phagosomes is enhanced by Src and Syk kinase inhibition. Panel A, RAW GFP-Dectin-1 cells were pre-treated with PP2, or vehicle control for 30 min. AF647-labeled β-1,3-glucan beads (red) were added at a bead to cell ratio of 5:1 for 30 min and imaged using confocal microscopy. Bar indicates 5 μm. Panel B, RAW GFP Dectin-1 cells pre-treated with piceatannol, PP2, or vehicle control for 30 min then incubated with β-1,3-glucan particles for 1 h. Following phagosome isolation, recruitment of GFP-Dectin-1 (black line, open histogram) to the phagosome surface was assessed by phagoFACS and compared with bead-only control (gray shaded histogram). Panel C, phagosomal indices were determined from purified β-1,3-glucan phagosome phagoFACS over time comparing PP2, piceatannol, and vehicle-treated RAW GFP-Dectin-1 cells. Data are representative of three independent experiments. * denotes p < 0.05.
Syk Signaling-incompetent GFP-Dectin-1 Demonstrates Prolonged Retention to β-1,3-Glucan-containing Phagosomes
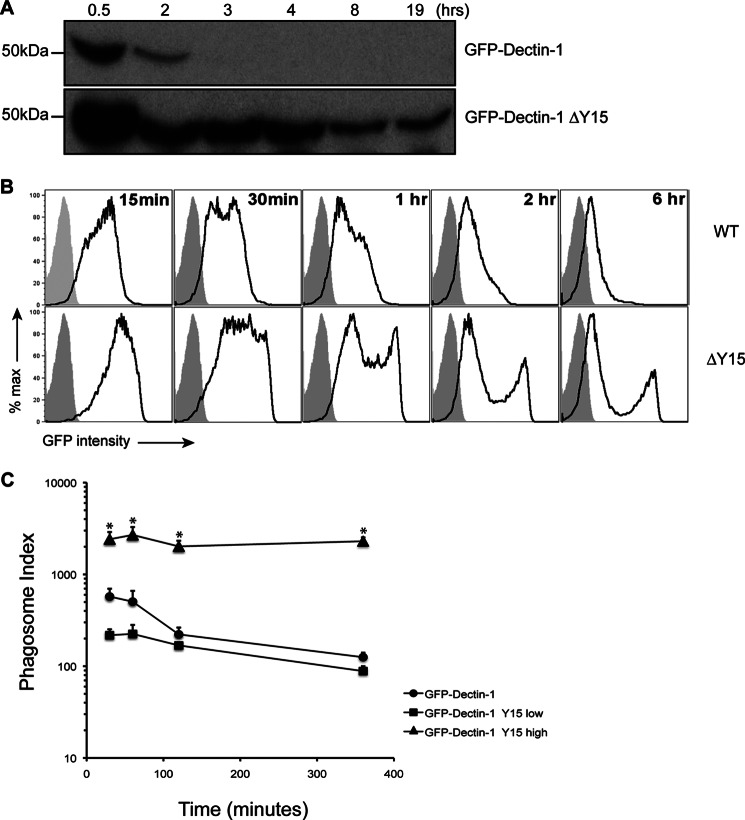
Given the potential for off-target effects of chemical kinase inhibitors, we sought to validate the effect of Syk activation to retention using a molecular approach. A tyrosine to phenylalanine substitution at amino acid position 15 within the intracellular domain of GFP-Dectin-1 (ΔY15) was introduced disrupting Dectin-1-dependent Syk activation (15). RAW GFP-Dectin-1ΔY15 cells were capable of capturing and completing phagocytosis of β-1,3-glucan beads and heat-killed C. albicans though the rate of phagocytosis was marginally slower as compared with wild-type RAW GFP-Dectin-1 (data not shown). Moreover, non-phagocytic HEK 293 cells transduced with GFP-Dectin-1ΔY15 became phagocytic for β-1,3-glucan beads (data not shown). Using purified phagosomes, the kinetics of GFP-Dectin-1ΔY15 at the phagosomal surface revealed retention of up to 19 h as compared with 2 h with wild-type GFP-Dectin-1 by Western (Fig. 3, panel A). Interestingly, Western analysis shows a “step-down” pattern of GFP-Dectin-1 content from 0.5 to 2 h and another from 4 to 8 h (Fig. 3, panel A). When examined by phagoFACS, the distribution of GFP-Dectin-1ΔY15 positive phagosomes was not uniform but exhibited a bimodal distribution with a composition of 30% GFP-Dectin-1ΔY15high and 70% GFP-Dectin-1ΔY15low population (Fig. 3, panel B). This ratio was unchanged even when lower β-1,3-glucan bead to cell ratios were used (data not shown) suggesting a receptor “sink” effect is unlikely. Phagosome index analysis illustrates a marginal decay rate of the GFP-Dectin-1ΔY15high decorated phagosomes as compared with a similar decay rate with GFP-Dectin-1 wild type or GFP-Dectin-1ΔY15low (Fig. 3, panel C). After confirming that Dectin-1-dependent Syk-activation is critical for GFP-Dectin-1 loss from β-1,3-glucan-containing phagosomes, we next sought to explore the role of pH on Syk activation.
FIGURE 3.
Signaling-incompetent GFP-Dectin-1 shows enhanced retention to β-1,3-glucan-containing phagosomes. Panel A, RAW GFP-Dectin-1 (wild-type, WT) or GFP-Dectin-1ΔY15F (ΔY15) cells were incubated with β-1,3-glucan beads for the indicated time points. Following phagosome purification, recruitment of GFP-Dectin-1 WT or GFP-Dectin-1 ΔY15 was assessed by Western blot for Dectin-1 following SDS-PAGE. Panel B, GFP-Dectin-1 or GFP-Dectin-1ΔY15F recruited to purified phagosomes at various time points was determined by phagoFACS (black line, open histogram) compared with bead-only control (gray-shaded histogram). Panel C, phagosomal indices were determined from the GFP-Dectin-1 ΔY15Fhigh, GFP-Dectin-1 ΔY15Flow, and GFP-Dectin-1 WT over time. Data are representative of three independent experiments. * denotes p < 0.05.
Dectin-1-dependent Syk Phosphorylation Is Independent of Phagosomal Acidification
Given the dependence of Syk activity for Dectin-1 retention to β-1,3-glucan-containing phagosomes, we next investigated the role of phagosomal acidification on Syk phosphorylation. RAW GFP-Dectin-1 and RAW GFP-Dectin-1ΔY15 cells were incubated with β-1,3-glucan beads for various time points followed by whole cell lysis and probed for Syk phosphorylation. Syk was rapidly phosphorylated by GFP-Dectin-1 cells within 30 min of stimulation, which returned to baseline by 5 h (Fig. 4, panel A, arrowhead). In contrast, as expected, GFP-Dectin-1ΔY15 cells were unable to phosphorylate Syk (Fig. 4, panel A). RAW GFP-Dectin-1 cells pre-incubated with BafA1 and subsequently stimulated with β-1,3-glucan beads or heat-killed C. albicans revealed no difference in phosphorylated Syk content compared with vehicle (Fig. 4, panel B). In contrast, PP2-treated cells resulted in significantly reduced phosphorylated Syk species (Fig. 4, panel B). Despite heat-killed C. albicans possessing a more rich complement of pattern-associated molecular patterns (PAMPs), the degree of Syk phosphorylation was significantly reduced with PP2 pre-treatment. These data demonstrate that an acidic intraphagosomal pH environment is not required for Dectin-1-specific Syk activation.
FIGURE 4.
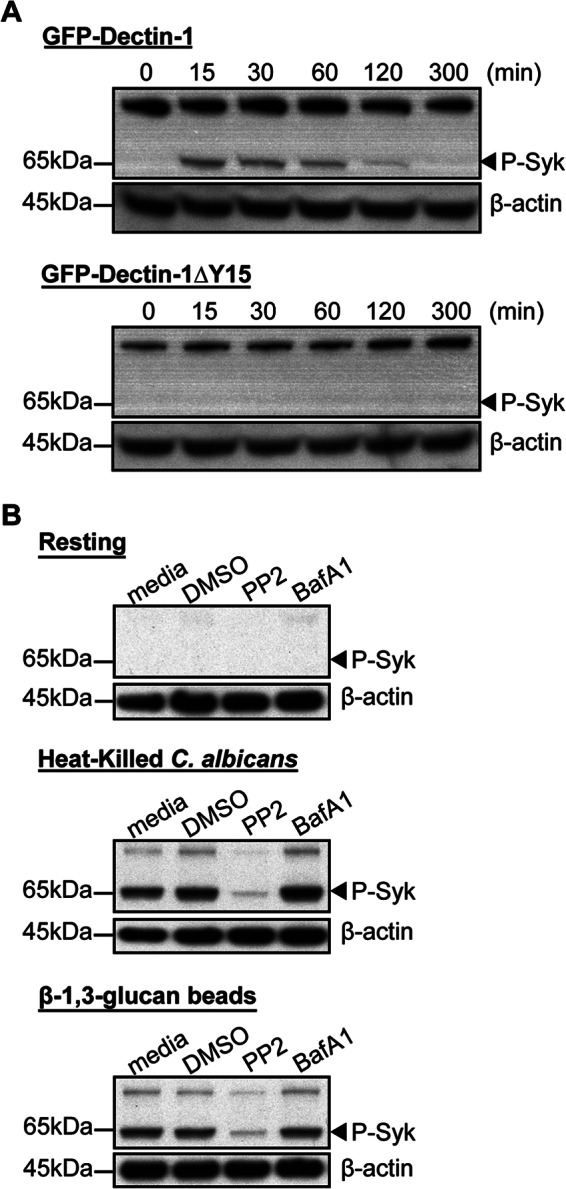
Dectin-1-dependent Syk activation is pH-independent. Panel A, RAW GFP-Dectin-1 or GFP-Dectin-1ΔY15F cells were incubated with β-1,3-glucan particles for the times indicated. Cells were lysed using Nonidet P-40 in the presence of protease and phosphatase inhibitors. Total cell lysates were resolved on SDS-PAGE and membranes blotted for phosphorylated Syk (P-Syk, arrowhead). Panel B, RAW GFP-Dectin-1 cells were pre-incubated with PP2, BafA1 or vehicle control for 30 min. Stimulation was performed using heat-killed C. albicans (yeast to macrophage ratio of 1:1), β-1,3-glucan beads (bead to cell ratio of 5:1) or resting conditions for 20 min. Following lysis, total cell lysate was resolved by SDS-PAGE and blotted for phosphorylated Syk (P-Syk, arrowhead). Representative β-actin blots are shown as loading controls.
Phagosomal Maturation of β-1,3-Glucan-containing Phagosomes Is Arrested with Inhibition of Syk or Src Kinases
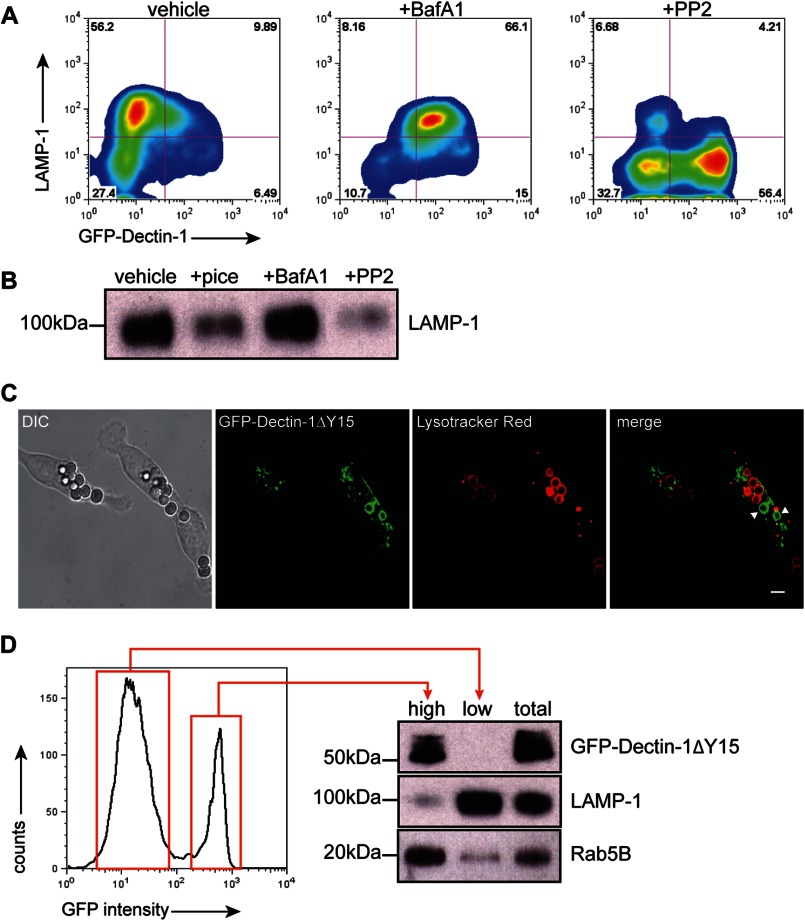
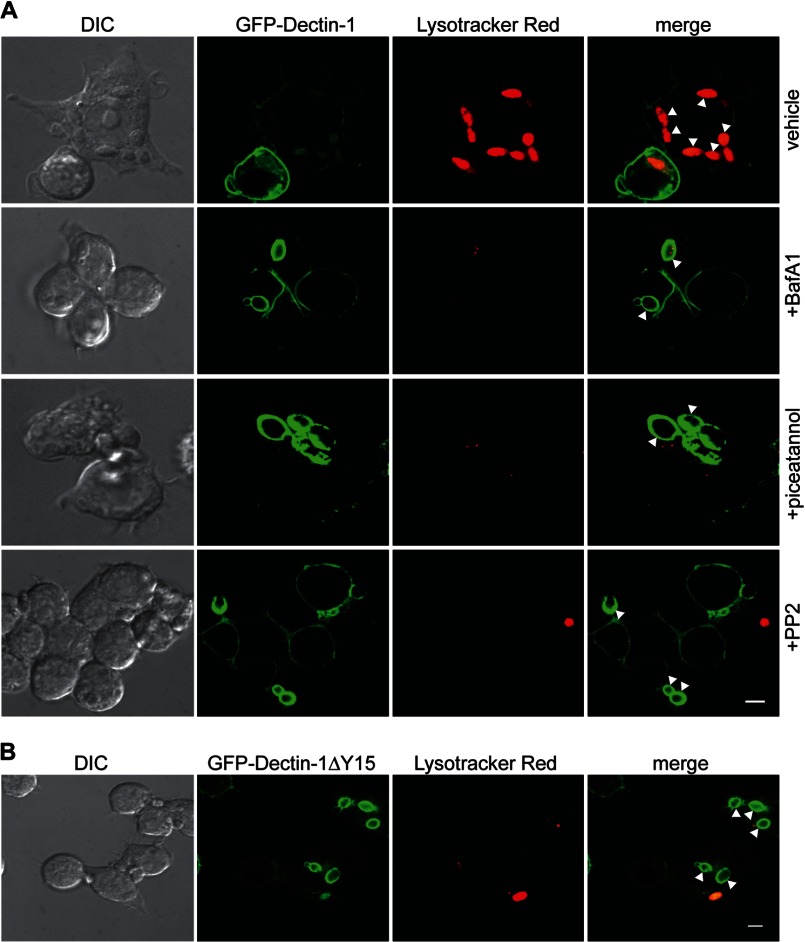
We next explored the role of Dectin-1-dependent Syk activation to downstream phagosomal maturation including recruitment of Rab GTPase chaperones and lysosomal fusion. Phagosomes undergo a series of surface and biochemical changes following pathogen capture that mark early phagosomal stages such as recruiting surface Rab5B, followed by late stage markers, such as recruitment of lysosome-associated membrane protein-1 (LAMP-1) and the progressive development of intraphagosomal acidic environment. We first examined the effect of Src kinase inhibition on the ability to recruit LAMP-1. RAW GFP-Dectin-1 cells were pre-treated with PP2, BafA1, or vehicle. Purified β-1,3-glucan-containing phagosomes were immunostained with antibodies to LAMP-1 and assessed by two-color phagoFACS. β-1,3-Glucan-containing phagosomes from vehicle-treated RAW GFP-Dectin-1 cells are GFP-Dectin-1dim and LAMP-1high marking maturation into a late phagolysosomal phenotype. BafA1-treated β-1,3-glucan-containing phagosomes were GFP-Dectin-1high, and LAMP-1high signifying that lysosomal fusion occurred independent of phagosomal acidification. In contrast, PP2-treated cells are unable to recruit LAMP-1 remaining LAMP-1dim and strongly GFP-Dectin-1high (Fig. 5, panel A). To confirm lysosomal fusion, purified phagosomes were resolved by SDS-PAGE and probed for LAMP-1. As compared with vehicle, there was no difference with BafA1 treatment, which had an equivalent amount of LAMP-1. On the other hand, PP2 and piceatannol treatment both illustrate a diminished amount of LAMP-1 (Fig. 5, panel B). These results indicate that Src and Syk activation is required for egress from an early to late phagolysosomal stage.
FIGURE 5.
Blockade of Syk activation arrests β-1,3-glucan-containing phagosomes at an early endosomal stage. Panel A, RAW GFP-Dectin-1 cells were pre-treated with piceatannol, PP2, BafA1, or vehicle for 30 min. β-1,3-Glucan beads were added for 1 h. Purified phagosomes were immunostained with anti-LAMP-1. Two-color phagoFACS was performed to determine surface recruitment of GFP-Dectin-1 (x axis) and LAMP-1 (y axis). Panel B,1 × 106 purified phagosomes from panel A were resolved by SDS-PAGE blotted for LAMP-1. Panel C, RAW GFP-Dectin-1ΔY15F cells were incubated with β-1,3-glucan beads (unlabeled) for 1 h in the presence of lysotracker (red) to visualize intracellular acidified compartments. Arrowheads delineate phagosomes positive for GFP-Dectin-1ΔY15F. Bar indicates 5 μm. Panel D, RAW GFP-Dectin-1ΔY15F cells were incubated with β-1,3-glucan particles for 1 h. Recruitment of GFP-Dectin-1ΔY15F to purified phagosomes was determined by phagoFACS. Phagosomes with bright (high) or dim (low) GFP-Dectin-1ΔY15F fluorescence were sort purified. Pre-sorted phagosomes (total) and post-sort phagosomes were counted and 0.5 × 106 per lane resolved by SDS-PAGE. Western blotting performed for GFP-Dectin-1ΔY15F, LAMP-1, and Rab5B.
RAW GFP-Dectin-1ΔY15 cells were incubated with β-1,3-glucan beads and the acidotropic dye, lysotracker, and imaged by confocal microscopy. All β-1,3-glucan-containing phagosomes that were GFP-Dectin-1ΔY15high had no lysotracker accumulation suggesting a non-acidic phagosomal pH (Fig. 5, panel C, arrowhead). In sharp contrast, GFP-Dectin-1ΔY15low phagosomes were brightly lysotracker positive indicating GFP-Dectin-1 ΔY15 retention inversely correlates to the pH of an individual phagosome within the same macrophage. Despite the suggestion that GFP-Dectin-1ΔY15high phagosomes were unable to acidify, we pursued a more rigorous approach to define the maturity stage of the phagosomes. Following incubation with β-1,3-glucan beads, RAW GFP-Dectin-1ΔY15 cells were lysed, phagosomes isolated and then sorted into GFP-Dectin-1ΔY15high and GFP-Dectin-1ΔY15low populations. Phagosomal proteins were resolved by SDS-PAGE and probed for Dectin-1, LAMP-1, and Rab5B. GFP-Dectin-1ΔY15high phagosomes were positive for GFP-Dectin-1 ΔY15 and the early phagosome marker, Rab5B, but not LAMP-1. In contrast, GFP-Dectin-1ΔY15low phagosomes were negative for GFP-Dectin-1ΔY15, and Rab5B, but highly enriched for LAMP-1 suggesting GFP-Dectin-1ΔY15high were unable to proceed with lysosomal fusion. RAW Dectin-1ΔY15 confirms that the inability to activate Syk results in the arrest of β-1,3-glucan-containing phagosome maturation at an early phagosomal stage even within the same macrophage.
C. albicans-containing Phagosomes Remain at an Early Endosomal Stage with Syk Inhibition
Since β-1,3-glucan beads do not contain the full complement of cell wall-associated PAMPs found on the surface of C. albicans, we next determined the effect of Syk activation on maturation of C. albicans-containing phagosomes. RAW GFP-Dectin-1 cells were incubated with heat-killed C. albicans (unlabeled, Fig. 6, panel A, white arrowheads) and pre-treated with piceatannol, BafA1, PP2, or vehicle. Lysotracker was used to demarcate acidified phagosomes under live cell confocal microscopy. Vehicle-treated cells show a striking accumulation of lysotracker within C. albicans-containing phagosomes indicating the presence of an acidic pH (Fig. 6, panel A, top row). In addition, C. albicans-containing phagosomes in the vehicle-treated group showed the normal decay of GFP-Dectin-1 signal from the phagosomal membrane. In contrast, RAW GFP-Dectin-1 cells pre-treated with BafA1 showed no phagosomal accumulation of lysotracker within C. albicans-containing phagosomes, but instead GFP-Dectin-1 retention to the phagosome membrane remained intense. Similar to β-1,3-glucan-containing phagosomes, C. albicans-containing phagosomes in Src or Syk inhibitor pre-treated cells were devoid of lysotracker, and retained GFP-Dectin-1 to the phagosomal membrane (Fig. 6, panel A) suggesting blockade of acidification. To confirm these findings using a molecular approach, we next explored the ability of RAW GFP-Dectin-1ΔY15 cells to undergo phagosome maturation with C. albicans-containing phagosomes. Heat-killed C. albicans were incubated with RAW GFP-Dectin-1ΔY15 in the presence of lysotracker and imaged by confocal microscopy. After 20 min, C. albicans-containing phagosomes that were GFP-Dectin-1ΔY15high had no accumulation of lysotracker, in contrast to GFP-Dectin-1ΔY15low, which were strongly lysotracker positive (Fig. 6, panel B). Despite using heat-killed C. albicans, which displays a more complete array of fungal ligands that trigger TLRs, these data suggest that Syk activation is a dominant signal for phagosomal transition from early to late phagolysosomes.
FIGURE 6.
C. albicans-containing phagosomes are incapable of acidifying following Src or Syk blockade. Panel A, RAW GFP-Dectin-1 cells were pre-incubated with BafA1, piceatannol, PP2, or vehicle for 30 min. Heat-killed C. albicans (unlabeled) were incubated with RAW GFP-Dectin-1 cells (yeast to macrophage ratio of 1:1) for 20 min in the presence of lysotracker to demarcate acidified compartments. RAW GFP-Dectin-1 cells were then imaged using spinning-disc live cell confocal imaging. Panel B, RAW GFP-Dectin-1ΔY15 cells were incubated with heat-killed C. albicans (yeast to macrophage ratio of 1:1) for 20 min in the presence of lysotracker and imaged using confocal microscopy. Arrowheads denote intracellular C. albicans. Bar indicates 5 μm.
DISCUSSION
Fungal pathogens are recognized by their complex carbohydrate cell walls at the cell surface of innate immune cells by PRR. Surface membrane PRRs including Toll-like receptor (TLR)-2 (31, 32) and C-type lectins such as Dectin-1 are crucial for the elaboration of proinflammatory cytokines. However, there is mounting evidence that a well-coordinated immune response requires continued intraphagosomal cargo-specific sampling (23–26). While Dectin-1 is essential to the recognition of pathogens, its contribution to intracellular phagosome maturation is not clear. In this study, we assessed the contribution of GFP-Dectin-1 to β-1,3-glucan-containing phagosomal maturation in macrophages.
Our experiments demonstrate rapid GFP-Dectin-1 translocation to the phagosome with an off-rate of two hours in contrast to previous publications with off-rate of 20 min (33). Several unique differences including the nature of the cargo may account for this observation; our studies utilize highly purified, homogenous β-1,3-glucan beads whereas many studies rely on whole yeast or the crude cell wall extract, zymosan. Interpretation of data from both yeast and/or zymosan poses considerable challenges in that there are several receptor/ligands occurring simultaneously. The loss of phagosomal GFP-Dectin-1 may be a result of loss of binding as the grooves on the extracellular domains are predicted to interact with glucan through hydrophobic and hydrogen bonding (34). These may be disrupted in a mature phagolysosome. Indeed, our data using BafA1 demonstrates that retention to the phagosome is pH-dependent. Yet, further work will be required to address if Dectin-1 loss is a result of receptor/ligand disruption and/or receptor degradation in the presence of lysosomal enzymes. Recent work shows that Dectin-1 does not recycle following translocation from the plasma surface suggesting the presence of an intracellular sink (35). Another limitation of our study includes the use of macrophage cell lines that may not faithfully reproduce primary macrophage biology. The generation of a GFP-Dectin-1 knock-in mouse may represent the best future tool to study phagosome biology in the native state.
Our results establish Syk-dependent control of fungal phagosomal pH including lysosomal fusion. The dominant signaling cascades downstream of Dectin-1-dependent Syk activation is a complex composed of MALT-1/Bcl-10/Card9 (reviewed in Refs. 36, 37), and which of these is responsible for lysosomal fusion, VTPase recruitment and the phagosomal egress from early to mature phagolysosomes remains unclear. A number of alternate signaling candidates could be directed by the Dectin-1-Syk axis including Raf-1 (38), NFAT (39), protein kinase C-δ (40), and the NLRP3 inflammasome (14). Recently, Dectin-1 has been shown to activate reactive oxygen species and the autophagic machinery including light chain 3, which correlate with enhanced MHC II presentation (41). Interestingly, 30% of GFP-Dectin-1ΔY15 phagosomes undergo phagosomal arrest suggesting that other Src- or Syk-dependent processes may contribute to the 70% remaining phagosomes, which mature but remain susceptible to chemical inhibition of Src or Syk kinases. Complement receptor 3, and CD36 are potential Src/Syk-dependent receptors shown to bind glucan and may contribute to phagosomal maturation (8, 42–44). The difference in the GFP-Dectin-1ΔY15 bimodal phagosome distribution awaits further proteosome-based experiments.
Through the use of a uniform monodisperse polystyrene β-1,3-glucan bead, we have eliminated the potential contribution of other fungal cell wall ligands. Strikingly, as fungi are phagocytosed there is considerable change in the gene and protein expression highlighting the impressive and dynamic rearrangement within phagosomes (45, 46). Given the limitation imposed by antigenic heterogeneity, it will be vital to investigate the individual contribution(s) and cross talk of other major fungal cell wall components to phagosomal maturation. Interestingly, our experiments using heat-killed C. albicans-containing phagosomes continue to show limited egress from early endosomal stages suggesting that the control exerted by the β-1,3-glucan-Dectin-1-Syk axis is likely dominant.
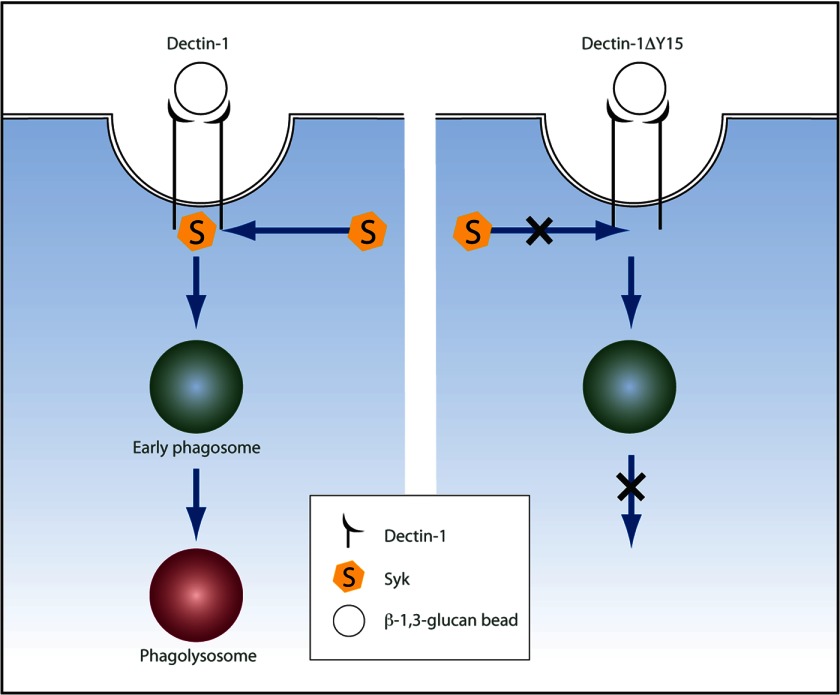
In this study, we sought to determine the role of Dectin-1 in the fate of phagocytosed β-1,3-glucan cargo. The model we propose suggests that Dectin-1-dependent Syk activation is required for subsequent lysosomal fusion and acidification of the phagosomal compartment (Fig. 7) and without this checkpoint phagosomal maturation is arrested at an early stage. Taken together, our results indicate that Dectin-1 not only captures and triggers phagocytosis, but also acts as a regulator for β-1,3-glucan phagosome maturation through a Dectin-1-dependent Syk mechanism.
FIGURE 7.
Schematic representation of phagosomal maturation for β-1,3-glucan-containing phagosomes. Initial recognition and ligation of GFP-Dectin-1 with β-1,3-glucan cargo result in phagocytosis and Syk activation. β-1,3-glucan-containing early phagosomes mature and undergo lysosomal fusion resulting in phagolysosome formation. Signaling-incompetent GFP-Dectin-1ΔY15 forms early phagosomes, but is incapable of activating Syk and does not permit phagolysosomal maturation.
Acknowledgment
We thank Arch MacInnes for assistance with artwork.
This work was supported, in whole or in part, by National Institutes of Health Grants NIAID T32-AI007061-35 (to M. K. M. and J. L. R.), NIAID R01AI079198 (to L. M. S.), and NIAID 1R01AI092084 (to J. M. V.).
- Syk
- spleen tyrosine kinase
- PRR
- pattern-recognition receptor
- CDI
- 1,1′-carbonyldiimidazole
- LAMP
- lysosome-associated membrane protein.
REFERENCES
- 1. Pappas P. G., Alexander B. D., Andes D. R., Hadley S., Kauffman C. A., Freifeld A., Anaissie E. J., Brumble L. M., Herwaldt L., Ito J., Kontoyiannis D. P., Lyon G. M., Marr K. A., Morrison V. A., Park B. J., Patterson T. F., Perl T. M., Oster R. A., Schuster M. G., Walker R., Walsh T. J., Wannemuehler K. A., Chiller T. M. (2010) Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50, 1101–1111 [DOI] [PubMed] [Google Scholar]
- 2. Arendrup M. C. (2010) Epidemiology of invasive candidiasis. Curr. Opin. Crit. Care 16, 445–452 [DOI] [PubMed] [Google Scholar]
- 3. Neofytos D., Fishman J. A., Horn D., Anaissie E., Chang C.-H., Olyaei A., Pfaller M., Steinbach W. J., Webster K. M., Marr K. A. (2010) Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect. Dis. 12, 220–229 [DOI] [PubMed] [Google Scholar]
- 4. Brown G. D., Denning D. W., Gow N. A. R., Levitz S. M., Netea M. G., White T. C. (2012) Hidden killers: human fungal infections. Science Translational Medicine 4, 165rv113. [DOI] [PubMed] [Google Scholar]
- 5. Carvalho A., Cunha C., Bozza S., Moretti S., Massi-Benedetti C., Bistoni F., Aversa F., Romani L. (2012) Immunity and tolerance to fungi in hematopoietic transplantation: principles and perspectives. Front. Immun. 3, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Segal B. H. (2009) Aspergillosis. N. Engl. J. Med. 360, 1870–1884 [DOI] [PubMed] [Google Scholar]
- 7. LeibundGut-Landmann S., Wüthrich M., Hohl T. M. (2012) Immunity to fungi. Curr. Opin. Immunol. 24, 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodridge H. S., Wolf A. J., Underhill D. M. (2009) Beta-glucan recognition by the innate immune system. Immunol. Rev. 230, 38–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saijo S., Fujikado N., Furuta T., Chung S.-H., Kotaki H., Seki K., Sudo K., Akira S., Adachi Y., Ohno N., Kinjo T., Nakamura K., Kawakami K., Iwakura Y. (2007) Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8, 39–46 [DOI] [PubMed] [Google Scholar]
- 10. Brown G. D., Gordon S. (2001) Immune recognition. A new receptor for beta-glucans. Nature 413, 36–37 [DOI] [PubMed] [Google Scholar]
- 11. Chai L. Y., de Boer M. G., van der Velden W. J., Plantinga T. S., van Spriel A. B., Jacobs C., Halkes C. J., Vonk A. G., Blijlevens N. M., van Dissel J. T., Donnelly P. J., Kullberg B.-J., Maertens J., Netea M. G. (2011) The Y238X stop codon polymorphism in the human β-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J.Infect. Dis. 203, 736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferwerda B., Ferwerda G., Plantinga T. S., Willment J. A., van Spriel A. B., Venselaar H., Elbers C. C., Johnson M. D., Cambi A., Huysamen C., Jacobs L., Jansen T., Verheijen K., Masthoff L., Morré S. A., Vriend G., Williams D. L., Perfect J. R., Joosten L. A., Wijmenga C., van der Meer J. W., Adema G. J., Kullberg B. J., Brown G. D., Netea M. G. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N. Engl. J. Med. 361, 1760–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor P. R., Tsoni S. V., Willment J. A., Dennehy K. M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G. D. (2007) Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat. Immunol. 8, 31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hise A. G., Tomalka J., Ganesan S., Patel K., Hall B. A., Brown G. D., Fitzgerald K. A. (2009) An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 [DOI] [PubMed] [Google Scholar]
- 17. Gross O., Gewies A., Finger K., Schäfer M., Sparwasser T., Peschel C., Förster I., Ruland J. (2006) Card9 controls a non-TLR signaling pathway for innate anti-fungal immunity. Nature 442, 651–656 [DOI] [PubMed] [Google Scholar]
- 18. Kerrigan A. M., Brown G. D. (2010) Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol. Rev. 234, 335–352 [DOI] [PubMed] [Google Scholar]
- 19. Carvalho A., Giovannini G., De Luca A., D'Angelo C., Casagrande A., Iannitti R. G., Ricci G., Cunha C., Romani L. (2012) Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol. Immunol. 9, 276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skrzypek F., Cenci E., Pietrella D., Rachini A., Bistoni F., Vecchiarelli A. (2009) Dectin-1 is required for human dendritic cells to initiate immune response to Candida albicans through Syk activation. Microbes Infect. 11, 661–670 [DOI] [PubMed] [Google Scholar]
- 21. Osorio F., LeibundGut-Landmann S., Lochner M., Lahl K., Sparwasser T., Eberl G., Reis e Sousa C. (2008) DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 38, 3274–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dennehy K. M., Willment J. A., Williams D. L., Brown G. D. (2009) Reciprocal regulation of IL-23 and IL-12 following co-activation of Dectin-1 and TLR signaling pathways. Eur. J. Immunol. 39, 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Underhill D. M., Goodridge H. S. (2012) Information processing during phagocytosis. Nature Rev. Immunol. 12, 492–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Luca A., Iannitti R. G., Bozza S., Beau R., Casagrande A., D'Angelo C., Moretti S., Cunha C., Giovannini G., Massi-Benedetti C., Carvalho A., Boon L., Latgé J.-P., Romani L. (2012) CD4+ T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease. J. Clin. Invest. 122, 1816–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Husebye H., Aune M. H., Stenvik J., Samstad E., Skjeldal F., Halaas O., Nilsen N. J., Stenmark H., Latz E., Lien E., Mollnes T. E., Bakke O., Espevik T. (2010) The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33, 583–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zanoni I., Ostuni R., Marek L. R., Barresi S., Barbalat R., Barton G. M., Granucci F., Kagan J. C. (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147, 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tam J. M., Mansour M. K., Khan N. S., Yoder N. C., Vyas J. M. (2012) Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr. Biol. 4, 220–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Artavanis-Tsakonas K., Love J. C., Ploegh H. L., Vyas J. M. (2006) Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc. Natl. Acad. Sci. U.S.A. 103, 15945–15950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kasperkovitz P. V., Cardenas M. L., Vyas J. M. (2010) TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J. Immunol. 185, 7614–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stuart L. M., Boulais J., Charriere G. M., Hennessy E. J., Brunet S., Jutras I., Goyette G., Rondeau C., Letarte S., Huang H., Ye P., Morales F., Kocks C., Bader J. S., Desjardins M., Ezekowitz R. A. (2007) A systems biology analysis of the Drosophila phagosome. Nature 445, 95–101 [DOI] [PubMed] [Google Scholar]
- 31. West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., Walsh M. C., Choi Y., Shadel G. S., Ghosh S. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472, 476–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dennehy K. M., Ferwerda G., Faro-Trindade I., Pyz E., Willment J. A., Taylor P. R., Kerrigan A., Tsoni S. V., Gordon S., Meyer-Wentrup F., Adema G. J., Kullberg B.-J., Schweighoffer E., Tybulewicz V., Mora-Montes H. M., Gow N. A., Williams D. L., Netea M. G., Brown G. D. (2008) Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur. J. Immunol. 38, 500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Heinsbroek S. E., Taylor P. R., Martinez F. O., Martinez-Pomares L., Brown G. D., Gordon S. (2008) Stage-specific sampling by pattern recognition receptors during Candida albicans phagocytosis. PLoS Pathog. 4, e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brown J., O'callaghan C. A., Marshall A. S. J., Gilbert R. J. C., Siebold C., Gordon S., Brown G. D., Jones E. Y. (2007) Structure of the fungal beta-glucan-binding immune receptor dectin-1: implications for function. Protein Sci. 16, 1042–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Esteban A., Popp M. W., Vyas V. K., Strijbis K., Ploegh H. L., Fink G. R. (2011) Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. U.S.A. 108, 14270–14275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reid D. M., Gow N. A., Brown G. D. (2009) Pattern recognition: recent insights from Dectin-1. Curr. Opin. Immunol. 21, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goodridge H. S., Shimada T., Wolf A. J., Hsu Y.-M., Becker C. A., Lin X., Underhill D. M. (2009) Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 182, 1146–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gringhuis S. I., den Dunnen J., Litjens M., van der Vlist M., Wevers B., Bruijns S. C., Geijtenbeek T. B. (2009) Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 10, 203–213 [DOI] [PubMed] [Google Scholar]
- 39. Goodridge H. S., Simmons R. M., Underhill D. M. (2007) Dectin-1 stimulation by Candida albicans yeast or zymosan triggers NFAT activation in macrophages and dendritic cells. J. Immunol. 178, 3107–3115 [DOI] [PubMed] [Google Scholar]
- 40. Strasser D., Neumann K., Bergmann H., Marakalala M. J., Guler R., Rojowska A., Hopfner K.-P., Brombacher F., Urlaub H., Baier G., Brown G. D., Leitges M., Ruland J. (2012) Syk Kinase-Coupled C-type Lectin Receptors Engage Protein Kinase C-δ to Elicit Card9 Adaptor-Mediated Innate Immunity. Immunity 36, 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma J., Becker C., Lowell C. A., Underhill D. M. (2012) Dectin-1-triggered recruitment of light chain 3 protein to phagosomes facilitates major histocompatibility complex class II presentation of fungal-derived antigens. J. Biol. Chem. 287, 34149–34156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levitz S. M. (2010) Innate recognition of fungal cell walls. PLoS Pathog. 6, e1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang H., Ostroff G. R., Lee C. K., Agarwal S., Ram S., Rice P. A., Specht C. A., Levitz S. M. (2012) Relative Contributions of Dectin-1 and Complement to Immune Responses to Particulate β-Glucans. J. Immunol. 189, 312–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilkinson B., Koenigsknecht-Talboo J., Grommes C., Lee C. Y., Landreth G. (2006) Fibrillar beta-amyloid-stimulated intracellular signaling cascades require Vav for induction of respiratory burst and phagocytosis in monocytes and microglia. J. Biol. Chem. 281, 20842–20850 [DOI] [PubMed] [Google Scholar]
- 45. Fernández-Arenas E., Cabezón V., Bermejo C., Arroyo J., Nombela C., Diez-Orejas R., Gil C. (2007) Integrated proteomics and genomics strategies bring new insight into Candida albicans response upon macrophage interaction. Mol. Cell Proteomics 6, 460–478 [DOI] [PubMed] [Google Scholar]
- 46. Rai M. N., Balusu S., Gorityala N., Dandu L., Kaur R. (2012) Functional genomic analysis of Candida glabrata-macrophage interaction: role of chromatin remodeling in virulence. PLoS Pathog. 8, e1002863. [DOI] [PMC free article] [PubMed] [Google Scholar]