Background: Gastric parietal cell atrophy causes metaplasia, reactive stem cell proliferation, and increased risk for cancer.
Results: Atrophy induces proliferation of CD44-positive epithelial cells that requires ERK → CD44 → STAT3 signaling.
Conclusion: CD44 is a putative gastric stem cell marker that regulates normal and metaplasia-associated proliferation.
Significance: Targeted pharmacological inhibition of ERK/CD44/STAT3 signaling may help block or reverse proliferation in precancerous atrophic/metaplastic lesions.
Keywords: Cd44, ERK, Gastric Cancer, Signaling, Stem Cells, Hyaluronan, PEP-1, Tamoxifen, U0126, WP1066
Abstract
The stem cell in the isthmus of gastric units continually replenishes the epithelium. Atrophy of acid-secreting parietal cells (PCs) frequently occurs during infection with Helicobacter pylori, predisposing patients to cancer. Atrophy causes increased proliferation of stem cells, yet little is known about how this process is regulated. Here we show that CD44 labels a population of small, undifferentiated cells in the gastric unit isthmus where stem cells are known to reside. Loss of CD44 in vivo results in decreased proliferation of the gastric epithelium. When we induce PC atrophy by Helicobacter infection or tamoxifen treatment, this CD44+ population expands from the isthmus toward the base of the unit. CD44 blockade during PC atrophy abrogates the expansion. We find that CD44 binds STAT3, and inhibition of either CD44 or STAT3 signaling causes decreased proliferation. Atrophy-induced CD44 expansion depends on pERK, which labels isthmal cells in mice and humans. Our studies delineate an in vivo signaling pathway, ERK → CD44 → STAT3, that regulates normal and atrophy-induced gastric stem/progenitor-cell proliferation. We further show that we can intervene pharmacologically at each signaling step in vivo to modulate proliferation.
Introduction
Tumors of the stomach are the second leading cause of cancer-related death worldwide (1, 2). Most of these tumors occur in the setting of chronic infection with the bacterium Helicobacter pylori, which causes atrophy (death) of the acid-secreting parietal cells (PC).4 PC atrophy in turn causes precancerous, metaplastic changes in other epithelial cells (3–6). In normal corpus gastric units, PCs concentrate in the middle (neck) portion among mucous neck cells (7) and below the isthmus that houses the stem cell. Classical 32P-radiolabeling studies indicate that one or a few cells in the isthmus constantly regenerate cells that undergo bidirectional migration, up to the mucosal surface and down to the gland base, as they differentiate into mature cells of the gastric unit (4, 8). Neck cells migrate slowly from their birth into the base, where they rapidly transition into digestive enzyme-secreting zymogenic cells.
PC atrophy in humans, mice, and other model animals causes existing zymogenic cells to re-express neck cell markers (6, 9–11). This aberrant zymogenic cell differentiation pattern is known as spasmolytic polypeptide expressing metaplasia (SPEM) due to greatly increased expression of the neck cell marker spasmolytic polypeptide (TFF2). PC atrophy also causes increased proliferation of normal stem/progenitor cells in the isthmus (6, 7). The pattern of chronic PC atrophy and SPEM has been associated with 90% of resected gastric cancers and is thought to be a key predisposing factor, but the molecular mechanisms causing SPEM as well as progenitor expansion have not been elucidated (12–14). Given that eradication of H. pylori seems to cause only partial reversion of metaplasia and risk for cancer (15–18), developing additional treatment strategies that would encourage reversion of these lesions can potentially greatly decrease the risk for gastric cancers worldwide.
Our understanding of the molecular regulation of gastric corpus isthmal stem cell proliferation, even under normal homeostasis, is still rudimentary despite considerable recent work having elucidated gene products marking stem cells in the intestines (e.g. LGR5 (19), LRIG1 (20), BMI (21)) and even in the more distal gastric antrum (19, 22). A handful of molecular pathways and markers (23–25) have been proposed for the gastric epithelium, but no mechanistic studies revealing molecules that regulate proliferation of the canonical isthmal stem cell either under normal conditions or in response to injury have been reported (4). Furthermore, the mechanisms underlying altered patterns of stem cell behavior during precancerous conditions in any tissue are only beginning to be explored.
We have recently shown that a ≥3 mg/20-g body weight dose of tamoxifen is toxic specifically to PCs, in an estrogen receptor independent manner, within the mouse stomach (26). Nearly all PCs atrophy by 3 days after a single intraperitoneal injection of tamoxifen, and death begins within hours, leading to SPEM (26) that eventually reverses several weeks later if no more tamoxifen is injected. PC death is accompanied by rapid activation of stem and progenitor cells in the isthmus region (26). Thus, tamoxifen causes PC atrophy and isthmal stem cell activation that is rapid, synchronous, and robust, affording us a novel tool to study the induction of stem cell activity in response to PC atrophy within an animal model. Here, we report the signaling mechanisms by which gastric corpus epithelial stem cells maintain homeostasis. We find that CD44 labels undifferentiated, proliferating cells within the isthmus, that expand dramatically during atrophy induced by Helicobacter infection and tamoxifen. Base-line isthmal progenitor proliferation is reduced in Cd44−/− mice. Moreover, wild-type (WT) mice treated with PEP-1, a peptide that blocks the interaction between hyaluronic acid (HA) and CD44, also show both inhibited normal proliferation as well as blocked expansion during atrophy. We next show that, along with CD44, STAT3 phosphorylation is critical for isthmal cell proliferation in response to injury and that STAT3 activation depends on CD44. We find ERK signaling is activated almost immediately after PC damage and acts as the upstream modulator of Cd44 and the atrophy induced proliferative response, as determined by a kinase activation screen. Finally, we show that cells expressing pERK in their nuclei expand in the isthmus of mice during PC atrophy and in atrophic and metaplastic lesions in human patients. Our results identify for the first time an in vivo signaling pathway that mediates the response of the normal stem/progenitor cell compartment to a metaplasia-inducing injury.
EXPERIMENTAL PROCEDURES
Animals and Injections
All experiments involving animals were performed according to protocols approved by the Washington University School of Medicine Animal Studies Committee. Mice were maintained in a specified-pathogen-free barrier facility under a 12-h light cycle. Wild-type C57BL/6 and Cd44−/− mice were purchased from The Jackson Laboratory. Mice from all treatment groups were given an intraperitoneal injection of a mixture of 5-bromo-2′-deoxyuridine (BrdU, 120 mg/kg) and 5-fluoro-2′-deoxyuridine (12 mg/kg) 90 min before sacrifice to label S-phase cells. Vehicles used for all injections were sterile water, sterile saline, ethanol in sunflower seed oil, or DMSO in sunflower seed oil; no phenotypes were induced by injection of any of the vehicles alone. For detailed concentration, dosage, injection and H. pylori infection schemes, please see supplemental Materials.
Human Tissues
Examination of human gastric pathological tissue specimens was approved by the Institutional Review Board of Washington University School of Medicine, the Comité de Bioetica of Nicaragua for Universidad Nacional Autonoma De Nicaragua-Facultad De Ceincias Medicas Managua, and the Research Ethics Board Manager for Health Sciences at the University of Toronto. Serial sections (4–6 μm thick) obtained from paraffin-embedded tissue samples (hematoxylin and eosin and Alcian blue-periodic acid-Schiff stains) were reviewed by two pathologists in Italy with specific expertise in gastrointestinal diseases, and a consensus on the score for each pertinent histologic variable was reached. Diagnoses and selection of specific regions of transitions among normal stomach, atrophic stomach, and intestinal metaplasia was performed by a third pathologist in the United States.
Immunofluorescence and Immunohistochemistry
Stomachs were prepared, stained, and imaged using methods modified from Ramsey et al. (27).
Western Blotting
Western blot analysis was performed as described (26). Antibodies used for blotting are listed under supplemental Materials. Immobilon Western Chemiluminescent HRP substrate (Millipore) was used for detection.
Immunoprecipitation
Immunoprecipitation was performed using the Pierce Crosslink IP kit (Thermo Scientific, Rockford, IL) using the manufacturer's instructions. Rabbit anti-Stat3 (1:200, Cell Signaling Technology) was used for pulldown, and Western blots analysis was done as described.
Microscopy
Light and epifluorescence micrographs were taken as described (7).
Graphing and Statistics
All graphs and statistics were performed in GraphPad Prism using Student's t test (one-tailed or two-tailed as appropriate) for comparison of two groups of data and one-way analysis of variance with either Dunnett's or Tukey's test for multiple comparison tests.
RESULTS
CD44 Is Expressed in Isthmal Cells and Regulates Normal Base-line Proliferation
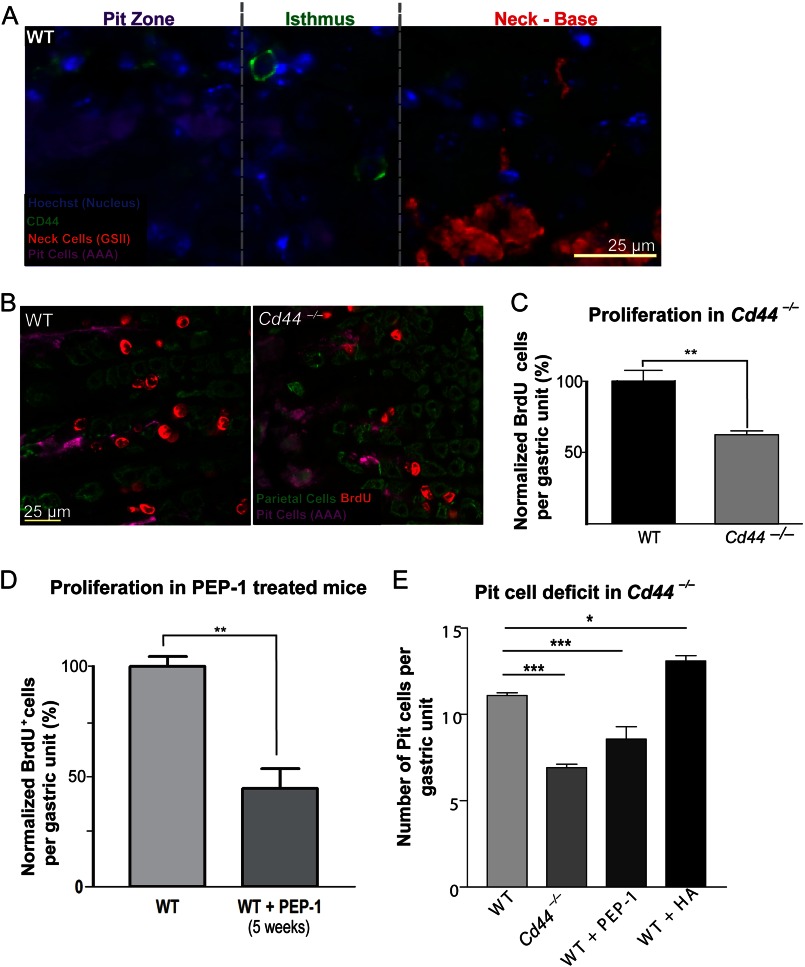
CD44 is a cell-surface adhesion molecule widely described as a marker of cells with highest proliferative capacity in cancers of the breast (28, 29), colon (30, 31), and stomach (32). CD44 is highly expressed in gastric cancer cell lines (32), H. pylori-infected human patient epithelia (32, 33), gastric carcinomas (34, 35), intestinal metaplasia (35, 36), and dysplasia (37). Although CD44 is expressed in gastric tumors (32, 33, 38), its expression has not been characterized in normal mouse corpus gastric epithelial tissue (39), but it has been observed in the antral epithelium (32) and at the squamous-corpus junction (40). We found that CD44 was expressed throughout the scant interglandular mesenchymal cells (supplemental Fig. 1A), but CD44+ epithelial cells could also be found in epithelial cells within the isthmus (Fig. 1A) and in the foveolar/pit region of wild-type mice (supplemental Fig. 1A, white bracket). CD44+ isthmal epithelial cells were small and undifferentiated, as they did not co-stain with markers of differentiated cells, such as AAA and GSII (Fig. 1A). Because CD44 is known to affect proliferation (41), we next investigated the requirement for CD44 signaling in gastric epithelial stem cell proliferation. In mice lacking Cd44, the basal rate of proliferation was half that of the WT controls (Fig. 1, B and C; n = 10), suggesting a role for CD44 in normal stem cell homeostasis.
FIGURE 1.
CD44 labels undifferentiated cells in the normal stem cell zone, i.e. the isthmus, of the gastric unit, and its loss stunts basal rates of proliferation. In the normal mouse gastric unit, CD44 labeled small, distinct cells in the isthmus region (A). Mice lacking the Cd44 gene have about half the number of proliferating cells per gastric unit compared with WT controls (B and C). PEP-1 is a peptide inhibitor that blocks the interaction between hyaluronic acid and CD44. Treatment with PEP-1 for 5 weeks reduced basal rates of proliferation (D) and resulted in lower numbers of pit cells (E), similar to the Cd44−/− animals (E). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
CD44 can interact with multiple ligands in the extracellular matrix such as osteopontin, collagen, fibronectin, laminin, and chondroitin sulfate, but its principal ligand is HA (42). HA activates CD44 by binding to its N-terminal functional domain (43). To determine whether direct CD44 activation was required for isthmal cell proliferation, we next treated adult mice with PEP-1 twice a week for 5 weeks. PEP-1 inhibits CD44-mediated signaling by blocking the binding of its ligand, HA. Blocking the CD44-HA interaction with PEP-1 caused a statistically significant decrease in proliferation of normal stem cells to levels phenocopying Cd44−/− mice (Fig. 1D, n = 12 mice total, 3 mice per experiment, 50 gastric units analyzed per mouse). Cd44−/− gastric units also showed stunting of the gastric unit zone between the gastric lumen and the isthmus, the pit/foveolar zone (supplemental Fig. 2, A and B) and overall decreased census of pit cells (Fig. 1E), a phenotype that was recapitulated by five-week treatment of WT mice with PEP-1 (supplemental Fig. 2C). Pit cells slough rapidly after emergence from the isthmal stem cell zone (half-life of ∼3 days (44)) and would be expected to be most affected by decreased stem cell proliferation due to their high turnover rate. We also treated mice for 5 weeks with HA, which caused statistically significant increased isthmal cell proliferation (supplemental Fig. 2E , n=7) and pit/foveolar zones relative to wild-type (Fig. 1E), showing that injection of the CD44 activating ligand was sufficient to induce increased proliferation and further confirming a direct role of CD44 signaling in regulating isthmal stem cell proliferation. PCs and other non-pit cell epithelial lineages did not differ in their base-line census whether CD44 was activated, inhibited, or deleted (supplemental Fig. 2F). Taken together, our data indicate that CD44 is expressed in undifferentiated epithelial cells within the isthmus and regulates normal rates of gastric epithelial stem cell proliferation.
Infection with H. pylori Causes PC Atrophy and Expansion of CD44 into the Base of Gastric Units
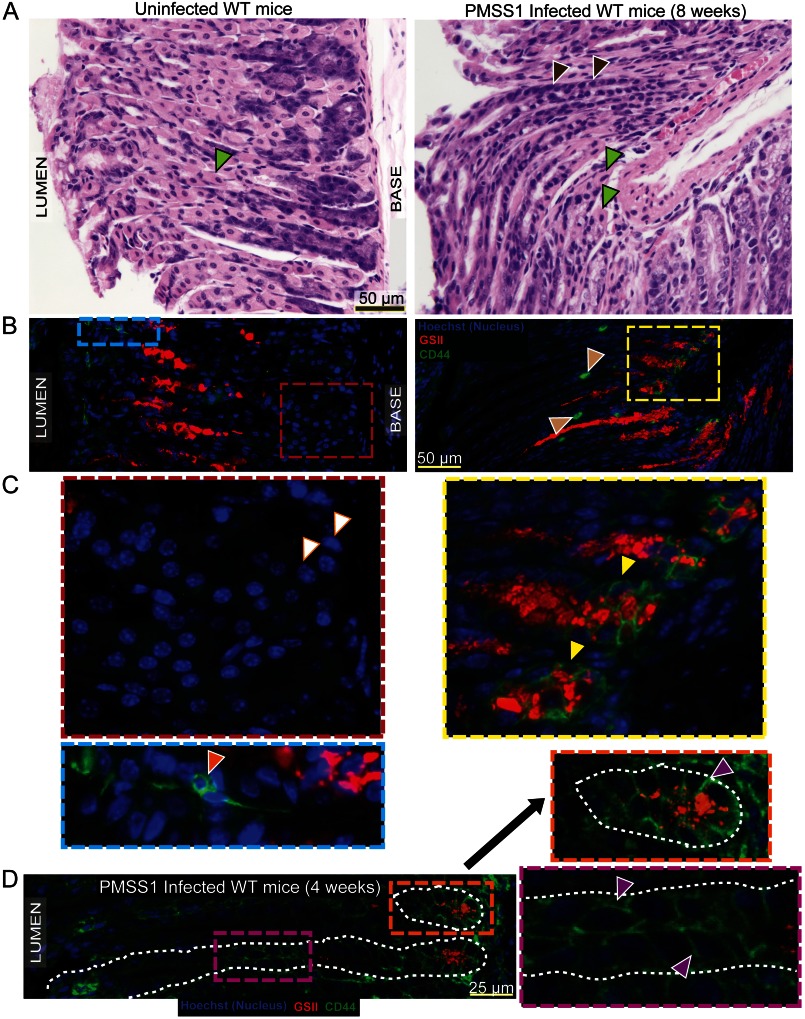
We sought to determine whether CD44 expression in gastric epithelial cells was affected by PC atrophy, which induces proliferation in mice and humans. Infection of humans with CagA+ strains of H. pylori is a major predisposing factor for the development of gastric adenocarcinoma (45). We infected WT mice with a CagA+ strain of H. pylori, PMSS1, for 8 weeks (n = 5 mice). As expected, in uninfected mice, there was no parietal cell death (Fig. 2A, left, the green arrowhead indicates a PC), and CD44 was expressed in the epithelium in the isthmus (Fig. 2, B and C, left; orange arrowhead). In contrast, 8 weeks after H. pylori infection, most PCs were atrophic (Fig. 2A, right, note only rare residual PCs in a section of the gastric corpus; green arrowheads), and CD44 expression was found diffusely in the base of gastric units (Fig. 2, B and C) in zymogenic cells, which co-expressed the neck cell marker, GSII (yellow arrowheads), indicating they were metaplastic.
FIGURE 2.
H. pylori infection causes parietal cell atrophy and expansion of CD44 expression. Hematoxylin- and eosin-stained sections of wild-type, uninfected mice show healthy parietal cells (A, left, green arrowhead), whereas those infected for 8 weeks with the cag+ PMSS1 strain of H. pylori showed diffuse loss of parietal cells (A, right). The gastric unit is largely replaced with metaplastic cells (A, right; brown arrowheads), and only a few parietal cells remained (A, right; green arrowheads). CD44 also labeled occasional immune cells infiltrating interglandular regions (B, right; beige arrowheads). CD44 is expressed in the uninfected gastric epithelium in the isthmus (B, blue box, and C, orange arrowheads) but expands to the base of the unit upon infection with H. pylori (B, right, yellow box). In uninfected mice, the zymogenic cells did not express CD44 (C, left, white arrowheads); however, upon infection, they became metaplastic and expressed neck cell markers such as GSII (red) as well as CD44 (C, right, yellow arrowheads). In mice infected with H. pylori for a shorter time period of 4 weeks (D), CD44 expansion can be seen to extend from the isthmus (purple box) and into the base (orange box) in some gastric units. Exemplar CD44+ cells are marked with purple arrowheads (D, insets); gastric units are outlined by dashed white line.
Tamoxifen Induced Parietal Cell Atrophy Causes a Burst of CD44+ Progenitor Cell Proliferation
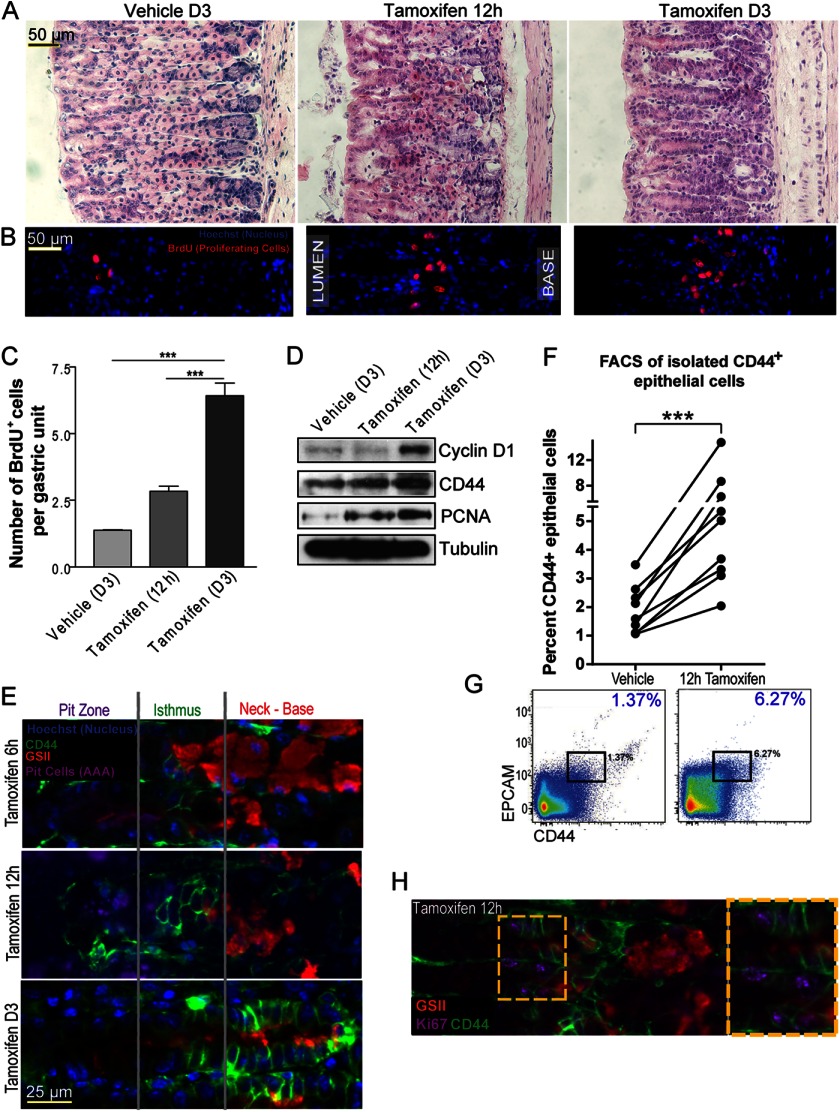
H. pylori infection in mice and humans is chronic and often focal and asynchronous across the stomach. Elucidation of the molecular mechanisms underlying atrophy-induced proliferation in the stomach requires a system for inducing atrophy that is synchronous, rapid, and global throughout the whole stomach. We have shown that a single injection of 5 mg/20-g body weight of tamoxifen causes dramatic rearrangement of the gastric mucosa, an effect that does not depend on the estrogenic or anti-estrogenic effects of tamoxifen but instead causes direct PC toxicity (26). Fig. 3 shows how within 3 days, >90% of PCs atrophied, yet complete recovery of PC census occurred by 21 days (Fig. 3A and Ref. 26). As PCs atrophied, proliferation accelerated in the normal stem cell region, the isthmus, reaching 6-fold base-line levels by day 3 (Fig. 3, B and C, and Ref. 26). Even by 12 h, almost half of the PCs had atrophied, and isthmal progenitor cells could be seen expanding toward the base of the unit, the region vacated by dying PCs (Fig. 3, B and C). Western blots showed that CD44 and the proliferation markers, PCNA and cyclin D1, increased throughout the gastric corpus (Fig. 3D).
FIGURE 3.
CD44 expands and labels proliferating cells upon parietal cell atrophy and is required for this injury induced expansion of progenitor cells. Hematoxylin- and eosin-stained sections of wild-type mice at 3 days (A, left) after intraperitoneal injection of vehicle and at 12 h (A, middle) and 3 days (A, right) after intraperitoneal injection of 5 mg/20-g body weight of tamoxifen. Wild-type mouse stomach treated with vehicle showed normal stomach epithelium, whereas those injected with tamoxifen showed a progressive loss of PCs. PC loss is coupled with an expansion in proliferation, measured by BrdU incorporation (stained in red) at 12 h (B, middle; C) and 3 days (B, right, C) after tamoxifen treatment compared with vehicle controls (B, left, C). Cyclin D1 and PCNA, which are markers of proliferation, were also increased on PC atrophy at 12 h and day 3 by Western blot of whole corpus stomach regions (D). The blot also shows that CD44 expression increases upon atrophy. A CD44+ epithelial population started expanding from the time point (6 h) when PCs first began to die (E) and reached the base of the unit by day 3 (E). The number of CD44+ epithelial cells expanded ∼3–5-fold during this time, as shown by multiple FACS experiments (F). One of the FACS plots graphed in F is shown in G. Many CD44-expressing cells co-stain with Ki67 after treatment with tamoxifen (H, the yellow box is magnified in the inset at the right).
In short, tamoxifen causes PC atrophy, increased CD44 expression, and proliferation, similar to infection with Helicobacter but has a rapid and synchronous timeframe for atrophy and injury response across the whole stomach allowing for biochemical analysis of the process. Furthermore, we have shown previously that tamoxifen treatment does not cause substantial inflammatory cell infiltrate (26). Unlike infection with CagA+ Helicobacter; the changes are almost wholly confined to the mucosal cells already present at time of treatment, reducing confounding variables in analyzing differences in global analysis of changes in signaling pathways and gene expression.
We next set out to use tamoxifen-induced atrophy as a tool to determine the origin of CD44-positive cells after PC atrophy. CD44+ isthmal cells began to expand as early as 6 h after tamoxifen injection (Fig. 3E). The initial increase in CD44-positive epithelial cells occurred in the isthmal progenitor zone, from which they expanded into the base until there were CD44+, E-cadherin double-positive epithelial cells from isthmus to base by D3 (Fig. 3E, supplemental Fig. 1, B and C). This pattern was similar to the chronic CD44 labeling that occurred in the base of Helicobacter-infected corpus units. By day 3, many of the CD44+ cells in the base labeled SPEM-type metaplastic cells, co-labeling with GSII (Fig. 3E). We next decided to look at an earlier time point in our Helicobacter-infected mice (4 weeks post infection) to determine whether, in certain units, CD44+ cells could also be identified expanding from the isthmus as occurred shortly after tamoxifen-induced atrophy. Fig. 2D shows that whereas many units already show full metaplasia with GS-II+/CD44+ cells only at the base, as in the 8-week post-infection animals, some units showed CD44 extending from isthmus to the base.
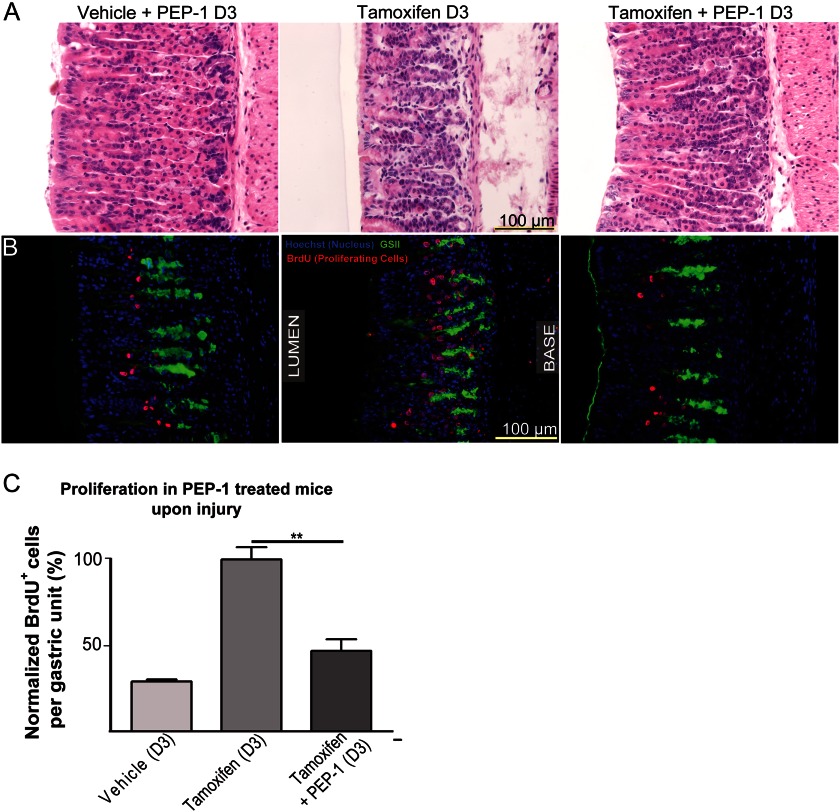
We next quantified the expansion of CD44+ epithelial cells. Their census increased ∼3–5-fold over the course of the 3-day tamoxifen treatment, as determined by flow cytometry (n = 18 mice across 9 experiments) (Fig. 3, F and G, supplemental Fig. 3). Furthermore, there was extensive co-labeling of the CD44+ population (at 12 h) with the proliferation marker Ki67 (Fig. 3H). CD44 activating ligand, HA, was found in the mesenchyme between gastric glands (supplemental Fig. 4A) and increased after atrophy, as did the enzymes that synthesize it, HAS1 and HAS2 (supplemental Fig. 4B). Thus, both CD44 and HA were increased in expression in tandem after atrophy, with HA present in the region of the basement membrane of the epithelial cells expressing CD44. During response to PC atrophy, Cd44−/− mice showed a statistically significant reduction in isthmal proliferation compared with controls (n = six mice, two experiments) (supplemental Fig. 5A), and a short, 3-day pretreatment with PEP-1 was sufficient to nearly completely abrogate the proliferative response induced by PC atrophy (Fig. 4; n = 5, two experiments). Thus, both stem cell normal homeostasis and response to PC atrophy are mediated by HA-CD44 interactions, although in mice null for Cd44 from conception, compensatory mechanisms allow for some degree of non-CD44-mediated proliferation increase (note in supplemental Fig. 5B that atrophy still caused an increase in cyclin D1 expression in Cd44−/−mice).
FIGURE 4.
CD44 is necessary for elevating the rate of progenitor cell proliferation upon induction of atrophy. PEP-1 blockade of CD44 activation halved atrophy-induced proliferation (B and C), measured by BrdU incorporation (red, B), whereas parietal cells died upon PEP-1 inhibition of CD44 (A).
Therefore, our data show that CD44 (a) is expressed by cells in the normal stem cell compartment, (b) marks proliferating progenitor cells during injury, and (c) is necessary for maintaining the normal and injury-responsive proliferative capacity of the gastric units. We next sought to determine the mechanism by which CD44 regulates progenitor cell proliferation.
CD44 Regulates Gastric Progenitor Cell Proliferation through STAT3
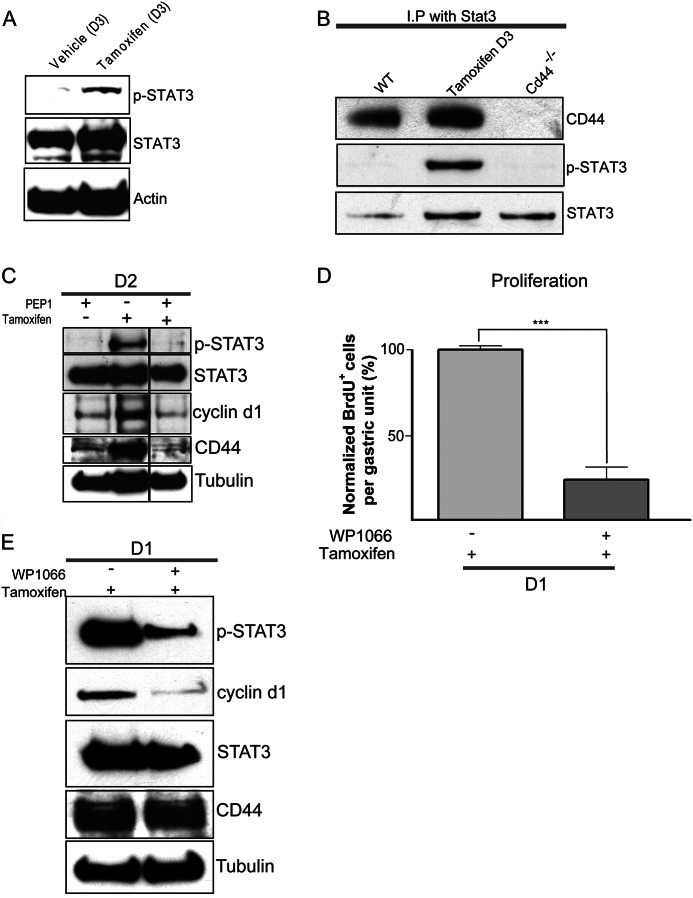
Signal transducer and activator of transcription 3 (STAT3) controls diverse cellular functions, such as growth, differentiation, and apoptosis (46). When activated by cytokines and growth factors, STAT3 localizes to the nucleus and regulates transcription of target genes that control proliferation and apoptosis (32, 46, 47). STAT3 increases normal and cancer stem cell proliferation (48) by inducing its targets survivin and cyclin D1 (49, 50) and is activated by cagA+ strains of H. pylori in host cells, in vitro and in vivo (51). CD44 increases cyclin D1 expression by directly interacting with active STAT3 (52). As we observed increased proliferation and cyclin D1 expression in the CD44-dependent response of the gastric unit to PC atrophy, we hypothesized that the mechanism of CD44 action might be via STAT3-cyclin D1. Consistent with our hypothesis, we found that although activated STAT3 (STAT3 phosphorylated on Tyr 705) was at low levels in normal mucosa, there was abundant p-STAT3 during atrophy (Fig. 5A). We then checked whether STAT3 bound CD44 by co-immunoprecipitation and found indeed that there was more CD44 associated with STAT3 in response to atrophy (Fig. 5B) when compared with controls. Immunoprecipitated STAT3 was phosphorylated only in tamoxifen-treated samples and not in control or Cd44−/− stomachs (Fig. 5B). STAT3 phosphorylation was also decreased in the absence of CD44 or when CD44-HA interactions were blocked by PEP-1 (Fig. 5C; supplemental Fig. 5B). CD44 expression was also reduced by PEP-1 blockade of its HA ligand (Fig. 5C). When we blocked STAT3 activation by injecting its specific inhibitor, WP1066 (53) with and without tamoxifen, we found that STAT3 inhibition greatly reduced atrophy-induced cyclin D1 expression and stem cell proliferation (Fig. 5, D and E) without affecting CD44 expression (Fig. 5E). Hence, we conclude that STAT3 is activated upon injury in the gastric epithelium, and CD44 binds to STAT3 to regulate the injury-induced burst of proliferation of progenitor cells.
FIGURE 5.
CD44 regulates gastric progenitor cell proliferation through STAT3. STAT3 was activated by tyrosine phosphorylation after atrophy induction with tamoxifen (A). Immunoprecipitation (IP) with antibody against STAT3 followed by Western blot for CD44 showed increased association between CD44 and STAT3 during tamoxifen-induced atrophy compared with controls; also, phosphorylated STAT3 was pulled down only during tamoxifen-induced atrophy (B). Inhibition of STAT3 and CD44 functions (using WP1066 and PEP-1, respectively) caused decreased cyclin D1 expression (C and E) and proliferation as quantified in tissue using BrdU (D); D1, 1 day post tamoxifen; D2, 2 days. CD44 levels were not affected by STAT3 inhibition, whereas pSTAT3 was significantly reduced upon CD44 inhibition with PEP-1 (C and E). CD44 expression was reduced upon blocking interaction with its HA ligand by PEP-1 (C).
ERK Signaling Regulates Progenitor Cell Proliferation through CD44
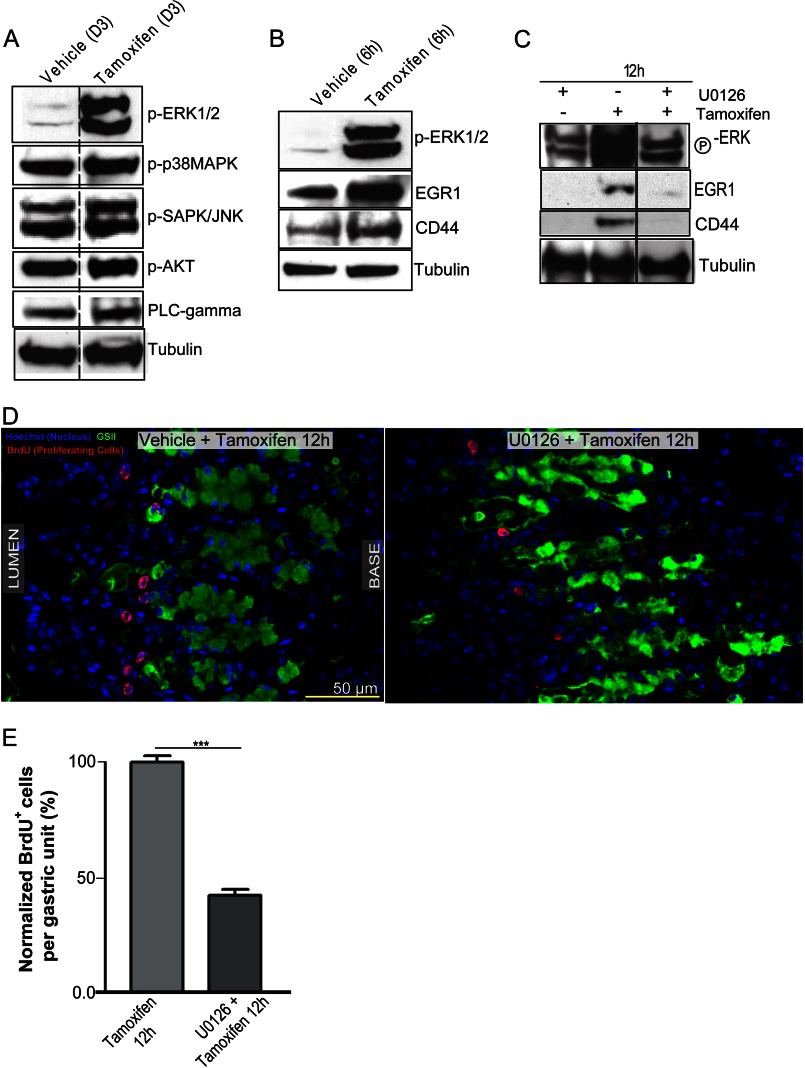
To determine the upstream signal causing CD44 expansion and increased proliferation, we surveyed multiple signaling pathways known to affect proliferation, such as the MAP kinases (p38MAPK, ERK, and JNK), AKT, and phospholipase Cγ in tamoxifen-induced atrophy. Whereas most proliferation mediators were either not substantially or only marginally increased, there was a dramatic increase in active ERK at the time of peak atrophy (Fig. 6A). ERK is known to increase proliferation in normal (54) as well as neoplastic cells (54, 55), although its role in gastric stem cell homeostasis has not previously been assessed. Accordingly, ERK was strongly activated as early as 6 h after treatment with tamoxifen (Fig. 6B), which was further confirmed by the concomitant increase in expression of its downstream target, EGR1, as well as CD44 (Fig. 6B) (56). If ERK signaling was indeed involved in regulating proliferation in the gastric epithelium after PC atrophy, blocking it should block atrophy-induced proliferation. ERK phosphorylation can be blocked with the kinase-specific inhibitor of MEK, U-0126 (57). We co-injected mice intraperitoneally with U-0126 along with tamoxifen. Fig. 6C shows that U-0126 successfully blocked ERK phosphorylation in the stomach during tamoxifen-induced atrophy and prevented a downstream increase in ERK transcriptional target EGR1 (58, 59). Confirming our hypothesis that pERK mediates PC-atrophy induced CD44, U-0126 injection into mice blocked the increase in CD44 (n = 10 mice across 3 experiments) (Fig. 6C). We observed similar results with another inhibitor of ERK activation, PD 98059 (60) (unpublished data; n = three mice, two experiments). As expected, ERK inhibition also blocked the proliferative response to atrophy (Fig. 6, D and E), much like loss or inhibition of CD44. BrdU incorporation per gastric unit at 12 h post tamoxifen treatment was reduced by 56 ± 5% (n = 3 mice; 50 gastric units counted per mouse) in mice treated with U-0126 compared with those receiving tamoxifen and vehicle.
FIGURE 6.
ERK signaling is activated early upon induction of injury and is required to induce CD44. Shown are Western blots of candidate signaling pathways that might be involved in increasing the proliferative response after PC atrophy by tamoxifen (A). PLC, phospholipase C. Of the pathways analyzed, only ERK signaling showed a dramatic increase in activation after tamoxifen compared with vehicle controls. ERK1/2 were tyrosine-phosphorylated soon after PC damage ensued (6 h after tamoxifen treatment); EGR1 and CD44, downstream targets of ERK signaling, were already increased in expression at this early time point (B). Co-injection of tamoxifen with U-0126, a specific inhibitor of ERK phosphorylation, muted the metaplastic induction of pERK along with downstream targets, EGR1 and CD44, by Western blot (C) and muted the proliferative response shown by BrdU immunostaining in red (D), quantified in E.
ERK Signaling Is Increased in Multiple Models of Gastric Metaplasia and Labels Isthmal Cells
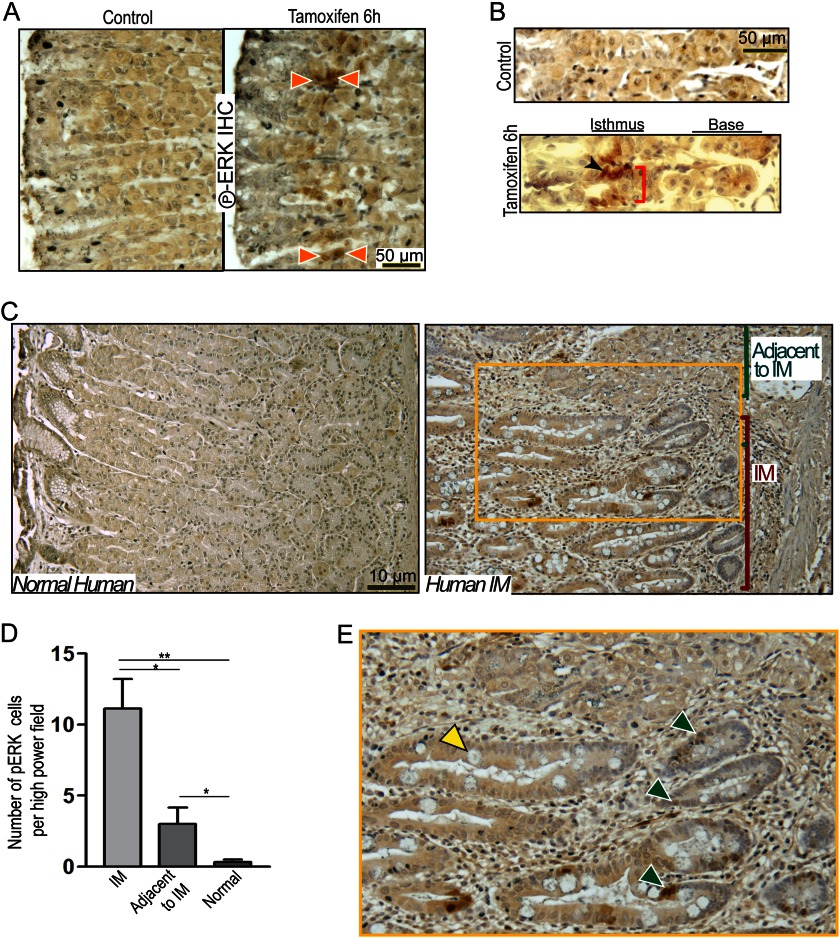
If ERK activation mediates the expansion of stem/progenitor cells, then phosphorylated ERK should be identifiable within those cells. In agreement, Fig. 7, A and B, show that whereas control mice showed no detectable pERK 6 h after induction of atrophy, we identified 2–3 undifferentiated cells per unit with nuclear pERK within the canonical isthmal stem cell zone (Fig. 7, A and B). pERK could also be detected in multiple cell nuclei per unit in tox176 mice (61) that show constitutive PC atrophy due to PC-specific expression of attenuated diphtheria toxin5 and in mice 8 weeks after infection with Helicobacter (supplemental Fig. 7A, right panels), whereas pERK was completely absent in uninfected controls (supplemental Fig. 7A, left panels). Serial histological sections immunostained with anti-pERK and anti-CD44 from mice injected with tamoxifen for 12 h showed a population of positive cells in the same location in the isthmus (supplemental Fig. 6). Finally, we examined a small cohort (n = 3) of human gastric resection specimens for pERK staining (10). pERK+ cells were almost never observed in normal control stomach specimens (Fig. 7C) or in regions without atrophy. However, pERK+ epithelial cells could be found in regions of gastric differentiation neighboring or contiguous with intestinal metaplasia (Fig. 7C, magnified in E). pERK+ cells were also particularly prominent in intestinal metaplasia lesions that neighbored regions of gastric differentiation. To test this pattern of pERK staining in regions of transition between normal and metaplastic differentiation in a broader cohort of samples, we extended our study to include 10 more gastric specimens with chronic parietal cell atrophy and inflammation caused by infection with H. pylori. The specimens were acquired in collaboration with an international consortium studying gastric carcinogenesis in Nicaragua. of these specimens showed the same pattern of pERK staining, with high pERK staining in transition regions adjacent to metaplastic tissue. One specimen did not display metaplasia nor did it stain with pERK. The remaining sample did not stain with pERK even though it displayed intestinal metaplasia. We took three random pERK-stained patient slides and quantified regions showing transitions between gastric and intestinal differentiation. In regions of intestinal metaplasia with residual gastric epithelium, there were 11 ± 3.46 pERK+ cells per high power field of 60× magnification (quantification from n = 3 different patient specimens, representative of staining patterns observed in 9/11 specimens; p < 0.01). In the gastric epithelium adjacent to intestinal metaplasia, pERK-positive cells were also increased significantly (3.67 ± 1.15 per high power field, p < 0.05) compared with regions in the same specimens that had residual normal differentiation patterns with preserved parietal cells (∼0.33 pERK+ cells per high power field) (Fig. 7D; supplemental Fig. 7, B and C). Given the data in humans and mice, we posit that activation of ERK signaling plays a key role in the cell proliferative response to PC atrophy and is the upstream regulator of the CD44-STAT3 proliferation axis.
FIGURE 7.
pERK labels metaplasia-associated cells in mice and humans. pERK labeled nuclei of isthmal progenitor cells (orange arrowheads) at 6 h of tamoxifen treatment (A and B), whereas there was no pERK signal in vehicle-treated controls. PCs did not stain positive for pERK in either vehicle- or tamoxifen-treated mice. Gastric units of human patients showed focal intestinal metaplasia (C, right) in a region with mixed gastric and intestinal differentiation (magnified in E), indicating transitional/early metaplasia (goblet cell is marked with a yellow arrowhead) stain positive for pERK (green arrowheads). Shown is quantification of pERK expression in human IM patients (n = 3) displaying such a transitional epithelium by counting the number of pERK+ cells in the intestinal metaplasia (IM) regions and in residual gastric-differentiation regions “Adjacent to IM” epithelium versus those in normal humans showed a dramatic increase in pERK+ cells in IM regions, with a significant increase in pERK+ cells even in gastric regions adjacent to IM when compared with normal human patients (D). IHC, immunohistochemistry.
DISCUSSION
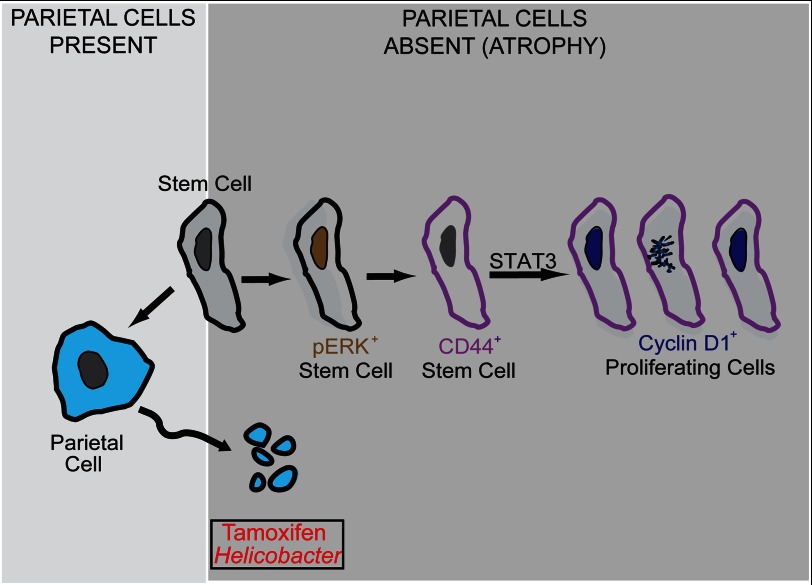
Here we demonstrate that parietal cell atrophy, which increases the risk for gastric cancer (62), causes increased isthmal stem/progenitor cell proliferation, which depends on activation of ERK. ERK induces CD44, which is critical for both normal proliferation and the atrophy-induced expansion. CD44 signaling maintains normal proliferation and increases proliferation after PC atrophy via interaction with its ligand HA. STAT3 binds CD44 and is phosphorylated only when CD44 is present and can interact with its ligand HA. Inhibition of STAT3 phosphorylation inhibits atrophy-induced stem cell proliferation but does not affect increased expression of CD44. Thus, we conclude that atrophy-induced proliferation depends on an ERK → CD44 → STAT3 axis (Fig. 8).
FIGURE 8.
Model for stem/progenitor cell renewal during normal and atrophic injury conditions. The isthmal stem cell (SC) is self-renewing and also gives rise to acid-secreting PCs among other cell-types within the stomach. Upon atrophy of PCs by toxins or H. pylori infection, the stem cells activate ERK to ramp up proliferation. ERK activation is required for expanded CD44 expression and the association between CD44 and pSTAT3, which in turn up-regulates cyclin D1 to drive proliferation.
To our knowledge, this is the first report delineating an in vivo mechanism for isthmal cell expansion almost immediately after PC damage and atrophy. We utilize tamoxifen treatment as a rapid, synchronous, and inducible model for PC atrophy that recapitulates what we observe in mice in chronic H. pylori infection. Soon after PC atrophy begins, there is a dramatic increase in activation of ERK in isthmal cells, possibly due to release of a damage-induced pro-proliferative signal or loss of a proliferation-inhibiting signal from the surrounding PCs. Our finding that ERK is critical for inducing stem cell proliferation is consistent with other reports. In the juvenile rat, premature weaning also induces isthmal proliferation that depends on ERK signaling (63). In patients infected with H. pylori, it has been proposed that bacterial CagA activates the ERK cascade in gastric epithelial cells, which initiates the development of gastric cancer (64). It has also been shown that systemic constitutive activation of the K-ras oncogene, which is upstream of ERK in the ERK-MAPK pathway, causes gastric hyperplasia and increased proliferation (65). It appears that ERK is a consistent injury-induced proliferative signal in precancerous lesions of the gastrointestinal tract, such as esophageal dysplasia (66), Barrett's adenocarcinoma cells (67), and pancreatic ductal adenocarcinoma (68).
Once active, ERK increases the expression of its target CD44. CD44 expression expands from the isthmus soon after PC damage. CD44 is a well known cancer stem cell marker and co-labels proliferating cells in the gastric epithelium upon injury. CD44 labels a population of cells that includes the LGR5+ population in the bases of the pyloric glands (Ref. 19 and data not shown)5; however, we show here it also labels occasional, small epithelial cells in the corpus gastric unit isthmus. Lack of CD44 signaling in Cd44−/− mice and in mice treated with PEP-1 stunts stem cell proliferation. Cd44−/− mice display a defect in the numbers of pit cells possibly due to a faster turnover rate of these cells compared with parietal and other epithelial cells, which leads to an accumulation of the deficit over time. Another explanation for this observation could be that CD44 might regulate pit cell progenitor proliferation selectively, as we do observe mesenchymal CD44 accumulation adjacent to pit cells. When subjected to PC injury by tamoxifen, the PEP-1 mice are unable to elicit the same proliferative response as control mice, whereas Cd44 nulls are better at coping with PC atrophy, probably due to compensatory mechanisms established during a lifelong lack of CD44. This is confirmed by the fact that although PEP-1-treated mice do not show an expansion in cyclin D1 expression after injury, the Cd44−/− mice are still able to elevate cyclin D1 to control levels, suggesting they have developed other ways of responding to injury that are independent of the normal CD44-dependent mechanism. Both PEP-1-treated and Cd44−/− mice, when injured with tamoxifen, undergo PC atrophy, establishing that the proliferative response is downstream of the initial attack of tamoxifen, i.e. the ablation of PCs (note in Fig. 4, PEP-1-treated mice have lower proliferation, even though there are almost no PCs remaining). It will be interesting to see whether loss of CD44 in Cd44−/− or PEP-1-inhibited mice affects the course of H. pylori infection. In addition to its role in proliferation, CD44 has recently been shown to impart cells with resistance against reactive oxygen species (69). As most gastric pathology involves inflammatory responses and inflammation induces the release of reactive oxygen species, CD44 might play a dual role in overcoming such injurious stimuli by increasing proliferation and protecting the dividing cells from the surrounding toxicity, thereby increasing their lifespan.
CD44 induces cyclin D1 by associating with active STAT3 (52). Abolishing STAT3 activity decreases proliferation despite PC atrophy without affecting Cd44 expression. Tamoxifen-treated Cd44−/− and PEP-1-injected mice show reduced pSTAT3, establishing a role for CD44 in STAT3 activation in a feed-forward proliferation circuit. Interestingly, Cd44−/− mice are able to increase cyclin D1 without the presence of active STAT3, confirming that the CD44-independent mechanism regulating proliferation in cases of acute PC injury is also STAT3-independent. As PEP-1 has a more dramatic effect on atrophy-induced proliferation than loss of CD44, it is possible that HA signaling through its other receptors, such as TLR4 (70), which would be blocked by PEP-1 but would still function in Cd44−/− mice, might compensate loss of CD44. However, treating Cd44−/− mice with PEP-1 does not further reduce the rate of proliferation in uninjured mice,5 suggesting that HA receptors other than CD44 might not play key roles in maintaining normal stem cell turnover. Taken together, it appears that the ERK → CD44 → STAT3 axis is involved in expansion of proliferating isthmal stem/progenitor cells under conditions of acute injury.
There is only scant literature on the intracellular signaling pathways that regulate non-neoplastic turnover of progenitor cells in the corpus (4). On the other hand, some of the signals regulating stem cell response extrinsically have been identified. For example, Sonic hedgehog and BMP2/4/7 are critical for regulation of normal stem cell turnover because deficiencies in those factors cause increased isthmal proliferation (71, 72), although those effects might be indirect via loss of PCs causing decreased acid and increased gastrin, which is a known corpus stem cell mitogen (73, 74). Gastrin works in part by stimulating histamine release by enterochromaffin cells, which also regulates proliferation of isthmal stem cells (75–77). EGFR stimulating factors like EGF, TGFα, and amphiregulin work through ERK and other downstream signaling pathways; all cause increased proliferation (78, 79).
It is unclear which ligand stimulates activation of ERK in the responsive isthmal cells. It might be derived from neighboring dying PCs. We do not believe the early stem cell response to atrophy we observe depends on gastrin, as gastrin transcript levels are unchanged until at least 3 days after tamoxifen treatment (26). Signaling through the EGFR receptor tends to cause pit/foveolar cell-specific proliferation as opposed to stimulation of the multipotent, isthmal stem cell (80). Thus, EGF family ligands are less likely candidates.
Inhibition of the ERK pathway decreases proliferation and CD44 expression. Although regulation of biological processes like proliferative response to injury in vivo is undoubtedly complex, we posit three possible, non-mutually exclusive mechanisms by which ERK could mediate increase in CD44. First, ERK activation could increase CD44 transcriptionally leading to an increase in CD44+ cell proliferation. Second, ERK could increase proliferation of CD44+ isthmal cells without increasing CD44 expression directly. Third, ERK signaling could increase HA in the basement membrane/mesenchyme by directly activating HAS, which in turn would increase CD44-dependent proliferation. Our data support the first interpretation. The second mechanism does not seem plausible because if it were true, then blocking CD44 action would not affect proliferation, yet our data show that CD44 is necessary for atrophy-induced increase in proliferation. The third interpretation seems less likely because we observe pERK in isthmal cells in the epithelium as early as 6 h after atrophy induction, just before CD44+ cells start expanding from the same region. We do not observe pERK in the mesenchyme, so there are likely to be other mechanisms leading to up-regulation of HAS.
It is intriguing to speculate that the resident CD44+ epithelial population in wild-type mice might mark a normal corpus stem cell, as these cells are isthmal, occasional, have high N:C ratios under normal conditions, and expand greatly upon injury. CD44+ cells also co-label with LGR5+ cells in the antrum/pyloric region of the stomach, which can regenerate the entire pyloric unit, consistent with multipotency.
One seeming paradox in our CD44 data is that loss of CD44 under homeostatic conditions decreases census of surface/pit cells, whereas during response to parietal cell atrophy it affects proliferation of cells expanding away from the pit zone and into the base. One explanation is that in homeostasis, pit cells turnover far more rapidly than any other cells in the gastric unit, so defective CD44 signaling manifests as decreased census of those cells. During parietal cell atrophy, there is rapid turnover of parietal cells, deeper in the unit, so CD44 mediates expansion of cells toward the base of the unit to replace the lost parietal cells.
Lifetime risk for development of gastric cancer has been reported to be decreased only partially by eradication of H. pylori (15–17). It is likely that aberrant epithelial differentiation patterns, such as atrophy, metaplasia, and increased proliferation, persist after treatment and must also be treated to reduce cancer risk substantially. The experiments in the current study identify both the novel role of a specific signaling pathway involved in the proliferative response to PC atrophy and show, as proof of principle, that those pathways can be pharmacologically inhibited at multiple steps. Ultimately, the identification of clear pharmaceutical targets in the metaplasia/atrophy sequence might be critical for reversing the risk for cancer progression from precursor lesions.
Supplementary Material
Acknowledgments
We thank the Washington University Digestive Diseases Research Core Center (DDRCC), DDRCC Biobank, and Morphology Core, Developmental Biology Histology and Morphology Core (Washington University), the Digestive Diseases Center of Texas Medical Center, and the Vanderbilt University Digestive Disease Research Center. We also thank Vincenza Guzzardo (University of Padua) for technical support, Drs. Reyna Victoria Palacios Gonzalez (Managua, Nicaragua) and Hala El-Zimaity (University Health Network, Toronto) for pathological diagnoses, and Dr. Lawrence Paszat for funding and coordinating acquisition of Nicaraguan samples.
This work was supported, in whole or in part, by National Institutes of Health Grants DK079798-3 and -4 and 2P30 DK052574–12 (to J. C. M.), DK33165 and DK55753 (to W. F. S.), R01 DK58587, R01 CA 77955, and P01 116037 (to R. M. P.), F32 CA 153539 (to J. N.), and P30 DK 508404 (to Vanderbilt Digestive Diseases Research Core Center).

This article contains supplemental Materials and Figs. S1–S7.
S. S. Khurana, T. E. Riehl, B. D. Moore, M. Fassan, M. Rugge, J. Romero-Gallo, J. Noto, R. M. Peek, Jr., W. F. Stenson, and J. C. Mills, unpublished data.
- PC
- parietal cell
- SPEM
- spasmolytic polypeptide expressing metaplasia
- HA
- hyaluronic acid.
REFERENCES
- 1. Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010) Estimates of worldwide burden of cancer in 2008. GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 2. Roder D. M. (2002) The epidemiology of gastric cancer. Gastric Cancer 5, 5–11 [DOI] [PubMed] [Google Scholar]
- 3. Correa P., Houghton J. (2007) Carcinogenesis of Helicobacter pylori. Gastroenterology 133, 659–672 [DOI] [PubMed] [Google Scholar]
- 4. Mills J. C., Shivdasani R. A. (2011) Gastric epithelial stem cells. Gastroenterology 140, 412–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nomura S., Baxter T., Yamaguchi H., Leys C., Vartapetian A. B., Fox J. G., Lee J. R., Wang T. C., Goldenring J. R. (2004) Spasmolytic polypeptide expressing metaplasia to preneoplasia in H. felis-infected mice. Gastroenterology 127, 582–594 [DOI] [PubMed] [Google Scholar]
- 6. Nozaki K., Ogawa M., Williams J. A., Lafleur B. J., Ng V., Drapkin R. I., Mills J. C., Konieczny S. F., Nomura S., Goldenring J. R. (2008) A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 134, 511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bredemeyer A. J., Geahlen J. H., Weis V. G., Huh W. J., Zinselmeyer B. H., Srivatsan S., Miller M. J., Shaw A. S., Mills J. C. (2009) The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev. Biol. 325, 211–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karam S. M., Leblond C. P. (1993) Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec 236, 259–279 [DOI] [PubMed] [Google Scholar]
- 9. El-Zimaity H. M., Ota H., Graham D. Y., Akamatsu T., Katsuyama T. (2002) Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer 94, 1428–1436 [DOI] [PubMed] [Google Scholar]
- 10. Lennerz J. K., Kim S. H., Oates E. L., Huh W. J., Doherty J. M., Tian X., Bredemeyer A. J., Goldenring J. R., Lauwers G. Y., Shin Y. K., Mills J. C. (2010) The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma. Am. J. Pathol. 177, 1514–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nam K. T., Lee H. J., Sousa J. F., Weis V. G., O'Neal R. L., Finke P. E., Romero-Gallo J., Shi G., Mills J. C., Peek R. M., Jr., Konieczny S. F., Goldenring J. R. (2010) Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology 139, 2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldenring J. R., Nam K. T. (2010) Oxyntic atrophy, metaplasia, and gastric cancer. Prog Mol Biol Transl Sci 96, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee H. J., Nam K. T., Park H. S., Kim M. A., Lafleur B. J., Aburatani H., Yang H. K., Kim W. H., Goldenring J. R. (2010) Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology 139, 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldenring J. R., Nam K. T., Wang T. C., Mills J. C., Wright N. A. (2010) Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia. Time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology 138, 2207–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romero-Gallo J., Harris E. J., Krishna U., Washington M. K., Perez-Perez G. I., Peek R. M., Jr. (2008) Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab. Invest. 88, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Vries A. C., Kuipers E. J., Rauws E. A. (2009) Helicobacter pylori eradication and gastric cancer. When is the horse out of the barn? Am. J. Gastroenterol. 104, 1342–1345 [DOI] [PubMed] [Google Scholar]
- 17. Graham D. Y., Uemura N. (2006) Natural history of gastric cancer after Helicobacter pylori eradication in Japan. After endoscopic resection, after treatment of the general population, and naturally. Helicobacter 11, 139–143 [DOI] [PubMed] [Google Scholar]
- 18. Niv Y., Hazazi R. (2008) Helicobacter pylori recurrence in developed and developing countries. Meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter 13, 56–61 [DOI] [PubMed] [Google Scholar]
- 19. Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M., Danenberg E., van den Brink S., Korving J., Abo A., Peters P. J., Wright N., Poulsom R., Clevers H. (2010) Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 [DOI] [PubMed] [Google Scholar]
- 20. Powell A. E., Wang Y., Li Y., Poulin E. J., Means A. L., Washington M. K., Higginbotham J. N., Juchheim A., Prasad N., Levy S. E., Guo Y., Shyr Y., Aronow B. J., Haigis K. M., Franklin J. L., Coffey R. J. (2012) The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sangiorgi E., Capecchi M. R. (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat. Genet. 40, 915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiao X. T., Gumucio D. L. (2011) Current molecular markers for gastric progenitor cells and gastric cancer stem cells. J Gastroenterol 46, 855–865 [DOI] [PubMed] [Google Scholar]
- 23. Mills J. C., Andersson N., Hong C. V., Stappenbeck T. S., Gordon J. I. (2002) Molecular characterization of mouse gastric epithelial progenitor cells. Proc. Natl. Acad. Sci. U.S.A. 99, 14819–14824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kikuchi M., Nagata H., Watanabe N., Watanabe H., Tatemichi M., Hibi T. (2010) Altered expression of a putative progenitor cell marker DCAMKL1 in the rat gastric mucosa in regeneration, metaplasia, and dysplasia. BMC Gastroenterol 10, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Modlin I. M., Kidd M., Lye K. D., Wright N. A. (2003) Gastric stem cells. An update. Keio. J. Med. 52, 134–137 [DOI] [PubMed] [Google Scholar]
- 26. Huh W. J., Khurana S. S., Geahlen J. H., Kohli K., Waller R. A., Mills J. C. (2012) Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 142, 21–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramsey V. G., Doherty J. M., Chen C. C., Stappenbeck T. S., Konieczny S. F., Mills J. C. (2007) The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development 134, 211–222 [DOI] [PubMed] [Google Scholar]
- 28. Sheridan C., Kishimoto H., Fuchs R. K., Mehrotra S., Bhat-Nakshatri P., Turner C. H., Goulet R., Jr., Badve S., Nakshatri H. (2006) CD44+/CD24− breast cancer cells exhibit enhanced invasive properties. An early step necessary for metastasis. Breast Cancer Res. 8, R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ricardo S., Vieira A. F., Gerhard R., Leitão D., Pinto R., Cameselle-Teijeiro J. F., Milanezi F., Schmitt F., Paredes J. (2011) Breast cancer stem cell markers CD44, CD24, and ALDH1. Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 64, 937–946 [DOI] [PubMed] [Google Scholar]
- 30. Du L., Wang H., He L., Zhang J., Ni B., Wang X., Jin H., Cahuzac N., Mehrpour M., Lu Y., Chen Q. (2008) CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res. 14, 6751–6760 [DOI] [PubMed] [Google Scholar]
- 31. Horst D., Kriegl L., Engel J., Kirchner T., Jung A. (2009) Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 27, 844–850 [DOI] [PubMed] [Google Scholar]
- 32. Takaishi S., Okumura T., Tu S., Wang S. S., Shibata W., Vigneshwaran R., Gordon S. A., Shimada Y., Wang T. C. (2009) Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27, 1006–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fan X., Long A., Goggins M., Fan X., Keeling P. W., Kelleher D. (1996) Expression of CD44 and its variants on gastric epithelial cells of patients with Helicobacter pylori colonisation. Gut 38, 507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chong J. M., Fukayama M., Hayashi Y., Funata N., Takizawa T., Koike M., Muraoka M., Kikuchi-Yanoshita R., Miyaki M., Mizuno S. (1997) Expression of CD44 variants in gastric carcinoma with or without Epstein-Barr virus. Int. J. Cancer 74, 450–454 [DOI] [PubMed] [Google Scholar]
- 35. Higashikawa K., Yokozaki H., Ue T., Taniyama K., Ishikawa T., Tarin D., Tahara E. (1996) Evaluation of CD44 transcription variants in human digestive tract carcinomas and normal tissues. Int. J. Cancer 66, 11–17 [DOI] [PubMed] [Google Scholar]
- 36. Dhingra S., Feng W., Brown R. E., Zhou Z., Khoury T., Zhang R., Tan D. (2011) Clinicopathologic significance of putative stem cell markers, CD44 and nestin, in gastric adenocarcinoma. Int. J. Clin. Exp. Pathol. 4, 733–741 [PMC free article] [PubMed] [Google Scholar]
- 37. Washington K., Gottfried M. R., Telen M. J. (1994) Expression of the cell adhesion molecule CD44 in gastric adenocarcinomas. Hum. Pathol. 25, 1043–1049 [DOI] [PubMed] [Google Scholar]
- 38. Mayer B., Jauch K. W., Günthert U., Figdor C. G., Schildberg F. W., Funke I., Johnson J. P. (1993) De novo expression of CD44 and survival in gastric cancer. Lancet 342, 1019–1022 [DOI] [PubMed] [Google Scholar]
- 39. Ghaffarzadehgan K., Jafarzadeh M., Raziee H. R., Sima H. R., Esmaili-Shandiz E., Hosseinnezhad H., Taghizadeh-Kermani A., Moaven O., Bahrani M. (2008) Expression of cell adhesion molecule CD44 in gastric adenocarcinoma and its prognostic importance. World J. Gastroenterol. 14, 6376–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishimoto T., Oshima H., Oshima M., Kai K., Torii R., Masuko T., Baba H., Saya H., Nagano O. (2010) CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci. 101, 673–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Götte M., Yip G. W. (2006) Heparanase, hyaluronan, and CD44 in cancers. A breast carcinoma perspective. Cancer Res. 66, 10233–10237 [DOI] [PubMed] [Google Scholar]
- 42. Naor D., Sionov R. V., Ish-Shalom D. (1997) CD44. Structure, function, and association with the malignant process. Adv. Cancer Res. 71, 241–319 [DOI] [PubMed] [Google Scholar]
- 43. Lesley J., Hyman R. (1992) CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur. J. Immunol. 22, 2719–2723 [DOI] [PubMed] [Google Scholar]
- 44. Karam S. M., Leblond C. P. (1993) Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat. Rec. 236, 280–296 [DOI] [PubMed] [Google Scholar]
- 45. Herrera V., Parsonnet J. (2009) Helicobacter pylori and gastric adenocarcinoma. Clin. Microbiol. Infect. 15, 971–976 [DOI] [PubMed] [Google Scholar]
- 46. Sherry M. M., Reeves A., Wu J. K., Cochran B. H. (2009) STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells 27, 2383–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G. H., Wei H., Lv S. Q., Ji H., Wang D. L. (2010) Knockdown of STAT3 expression by RNAi suppresses growth and induces apoptosis and differentiation in glioblastoma stem cells. Int. J. Oncol. 37, 103–110 [PubMed] [Google Scholar]
- 48. Ho P. L., Lay E. J., Jian W., Parra D., Chan K. S. (2012) Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer Res. 72, 3135–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanda N., Seno H., Konda Y., Marusawa H., Kanai M., Nakajima T., Kawashima T., Nanakin A., Sawabu T., Uenoyama Y., Sekikawa A., Kawada M., Suzuki K., Kayahara T., Fukui H., Sawada M., Chiba T. (2004) STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene 23, 4921–4929 [DOI] [PubMed] [Google Scholar]
- 50. Leslie K., Lang C., Devgan G., Azare J., Berishaj M., Gerald W., Kim Y. B., Paz K., Darnell J. E., Albanese C., Sakamaki T., Pestell R., Bromberg J. (2006) Cyclin D1 is transcriptionally regulated by and required for transformation by activated signal transducer and activator of transcription 3. Cancer Res. 66, 2544–2552 [DOI] [PubMed] [Google Scholar]
- 51. Bronte-Tinkew D. M., Terebiznik M., Franco A., Ang M., Ahn D., Mimuro H., Sasakawa C., Ropeleski M. J., Peek R. M., Jr., Jones N. L. (2009) Helicobacter pylori cytotoxin-associated gene A activates the signal transducer and activator of transcription 3 pathway in vitro and in vivo. Cancer Res. 69, 632–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee J. L., Wang M. J., Chen J. Y. (2009) Acetylation and activation of STAT3 mediated by nuclear translocation of CD44. J. Cell Biol. 185, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iwamaru A., Szymanski S., Iwado E., Aoki H., Yokoyama T., Fokt I., Hess K., Conrad C., Madden T., Sawaya R., Kondo S., Priebe W., Kondo Y. (2007) A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26, 2435–2444 [DOI] [PubMed] [Google Scholar]
- 54. Khavari T. A., Rinn J. (2007) Ras/Erk MAPK signaling in epidermal homeostasis and neoplasia. Cell Cycle 6, 2928–2931 [DOI] [PubMed] [Google Scholar]
- 55. Fritz J. M., Dwyer-Nield L. D., Malkinson A. M. (2011) Stimulation of neoplastic mouse lung cell proliferation by alveolar macrophage-derived, insulin-like growth factor-1 can be blocked by inhibiting MEK and PI3K activation. Mol. Cancer 10, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maegawa M., Arao T., Yokote H., Matsumoto K., Kudo K., Tanaka K., Kaneda H., Fujita Y., Ito F., Nishio K. (2009) EGFR mutation up-regulates EGR1 expression through the ERK pathway. Anticancer Res. 29, 1111–1117 [PubMed] [Google Scholar]
- 57. Marampon F., Bossi G., Ciccarelli C., Di Rocco A., Sacchi A., Pestell R. G., Zani B. M. (2009) MEK/ERK inhibitor U0126 affects in vitro and in vivo growth of embryonal rhabdomyosarcoma. Mol. Cancer Ther. 8, 543–551 [DOI] [PubMed] [Google Scholar]
- 58. Murai T., Miyauchi T., Yanagida T., Sako Y. (2006) Epidermal growth factor-regulated activation of Rac GTPase enhances CD44 cleavage by metalloproteinase disintegrin ADAM10. Biochem. J. 395, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bourguignon L. Y., Gilad E., Peyrollier K. (2007) Heregulin-mediated ErbB2-ERK signaling activates hyaluronan synthases leading to CD44-dependent ovarian tumor cell growth and migration. J. Biol. Chem. 282, 19426–19441 [DOI] [PubMed] [Google Scholar]
- 60. Baines P., Fisher J., Truran L., Davies E., Hallett M., Hoy T., Burnett A. K. (2000) The MEK inhibitor, PD98059, reduces survival but does not block acute myeloid leukemia blast maturation in vitro. Eur. J. Haematol. 64, 211–218 [DOI] [PubMed] [Google Scholar]
- 61. Syder A. J., Guruge J. L., Li Q., Hu Y., Oleksiewicz C. M., Lorenz R. G., Karam S. M., Falk P. G., Gordon J. I. (1999) Helicobacter pylori attaches to NeuAc α2,3Gal-β1,4-glycoconjugates produced in the stomach of transgenic mice lacking parietal cells. Mol. Cell 3, 263–274 [DOI] [PubMed] [Google Scholar]
- 62. Fox J. G., Wang T. C. (2007) Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 117, 60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Osaki L. H., Figueiredo P. M., Alvares E. P., Gama P. (2011) EGFR is involved in control of gastric cell proliferation through activation of MAPK and Src signalling pathways in early-weaned rats. Cell Prolif. 44, 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yang J. J., Cho L. Y., Ma S. H., Ko K. P., Shin A., Choi B. Y., Han D. S., Song K. S., Kim Y. S., Chang S. H., Shin H. R., Kang D., Yoo K. Y., Park S. K. (2011) Oncogenic CagA promotes gastric cancer risk via activating ERK signaling pathways. A nested case-control study. PLoS One 6, e21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matkar S. S., Durham A., Brice A., Wang T. C., Rustgi A. K., Hua X. (2011) Systemic activation of K-ras rapidly induces gastric hyperplasia and metaplasia in mice. Am. J. Cancer Res. 1, 432–445 [PMC free article] [PubMed] [Google Scholar]
- 66. Quante M., Bhagat G., Abrams J. A., Marache F., Good P., Lee M. D., Lee Y., Friedman R., Asfaha S., Dubeykovskaya Z., Mahmood U., Figueiredo J. L., Kitajewski J., Shawber C., Lightdale C. J., Rustgi A. K., Wang T. C. (2012) Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Souza R. F., Shewmake K., Pearson S., Sarosi G. A., Jr., Feagins L. A., Ramirez R. D., Terada L. S., Spechler S. J. (2004) Acid increases proliferation via ERK and p38 MAPK-mediated increases in cyclooxygenase-2 in Barrett's adenocarcinoma cells. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G743–G748 [DOI] [PubMed] [Google Scholar]
- 68. Collisson E. A., Trejo C. L., Silva J. M., Gu S., Korkola J. E., Heiser L. M., Charles R. P., Rabinovich B. A., Hann B., Dankort D., Spellman P. T., Phillips W. A., Gray J. W., McMahon M. (2012) A central role for RAF→MEK→ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2, 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., Masuko T., Shimizu T., Ishikawa T., Kai K., Takahashi E., Imamura Y., Baba Y., Ohmura M., Suematsu M., Baba H., Saya H. (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19, 387–400 [DOI] [PubMed] [Google Scholar]
- 70. Riehl T. E., Ee X., Stenson W. F. (2012) Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G377–D388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xiao C., Ogle S. A., Schumacher M. A., Orr-Asman M. A., Miller M. L., Lertkowit N., Varro A., Hollande F., Zavros Y. (2010) Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology 138, 550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shinohara M., Mao M., Keeley T. M., El-Zaatari M., Lee H. J., Eaton K. A., Samuelson L. C., Merchant J. L., Goldenring J. R., Todisco A. (2010) Bone morphogenetic protein signaling regulates gastric epithelial cell development and proliferation in mice. Gastroenterology 139, 2050–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jain R. N., Samuelson L. C. (2006) Differentiation of the gastric mucosa. II. Role of gastrin in gastric epithelial cell proliferation and maturation. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G762–G765 [DOI] [PubMed] [Google Scholar]
- 74. Wang T. C., Dangler C. A., Chen D., Goldenring J. R., Koh T., Raychowdhury R., Coffey R. J., Ito S., Varro A., Dockray G. J., Fox J. G. (2000) Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology 118, 36–47 [DOI] [PubMed] [Google Scholar]
- 75. Grandi D., Schunack W., Morini G. (2006) Epithelial cell proliferation is promoted by the histamine H(3) receptor agonist (R)-α-methylhistamine throughout the rat gastrointestinal tract. Eur. J. Pharmacol. 538, 141–147 [DOI] [PubMed] [Google Scholar]
- 76. Nakamura E., Kataoka T., Furutani K., Jimbo K., Aihara T., Tanaka S., Ichikawa A., Ohtsu H., Okabe S. (2004) Lack of histamine alters gastric mucosal morphology. Comparison of histidine decarboxylase-deficient and mast cell-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1053–G1061 [DOI] [PubMed] [Google Scholar]
- 77. Kobayashi T., Tonai S., Ishihara Y., Koga R., Okabe S., Watanabe T. (2000) Abnormal functional and morphological regulation of the gastric mucosa in histamine H2 receptor-deficient mice. J. Clin. Invest. 105, 1741–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tremblay E., Monfils S., Ménard D. (1997) Epidermal growth factor influences cell proliferation, glycoproteins, and lipase activity in human fetal stomach. Gastroenterology 112, 1188–1196 [DOI] [PubMed] [Google Scholar]
- 79. Goldenring J. R., Ray G. S., Soroka C. J., Smith J., Modlin I. M., Meise K. S., Coffey R. J., Jr. (1996) Overexpression of transforming growth factor-α alters differentiation of gastric cell lineages. Dig. Dis. Sci. 41, 773–784 [DOI] [PubMed] [Google Scholar]
- 80. Coffey R. J., Washington M. K., Corless C. L., Heinrich M. C. (2007) Menetrier disease and gastrointestinal stromal tumors. Hyperproliferative disorders of the stomach. J. Clin. Invest. 117, 70–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.