Background: Macro histone variant expression is induced during cell differentiation.
Results: Human pluripotent cells knocked down for macro histone variants show improper in vitro differentiation.
Conclusion: Macro histone variants are critical for the proper silencing of genes during in vitro differentiation.
Significance: Proper macro histone deposition is critical for the in vitro generation of good quality differentiated cell types.
Keywords: Chromatin, Differentiation, Induced Pluripotent Stem (iPS) Cell, Stem Cells, Macro Histone Variants, Pluripotency
Abstract
We have previously shown that macro histone variants (macroH2A) are expressed at low levels in stem cells and are up-regulated during differentiation. Here we show that the knockdown of macro histone variants impaired the in vitro and in vivo differentiation of human pluripotent cells, likely through defects in the silencing of pluripotency-related genes. ChIP experiments showed that during differentiation macro histone variants are recruited to the regulatory regions of pluripotency and developmental genes marked with H3K27me3 contributing to the silencing of these genes.
Introduction
Chromatin structure plays fundamental roles in the regulation of gene expression during development and differentiation. The nucleosome is composed of two molecules of each of the core histone proteins, H2A, H2B, H3, and H4, wrapped by DNA. Histone and DNA modifications have been proposed to play essential roles in the regulation of gene expression. Additionally, the incorporation of certain histone variants to chromatin can provide additional regulation. In higher organisms each histone subtype is represented by a family of genes encoding multiple variants that, in most cases, are highly similar and likely redundant. However, some histone variants, known as replacement variants, show unique features and play very precise roles in critical aspects of chromatin structure regulation. Most characterized replacement variants are the H3 variants H3.3 (1) and CENPA (2), and the H2A variants H2A.Z (3), H2A.X (4), and macroH2A4 (5).
The macroH2A variant contains an amino-terminal histone-like region that is similar to histone H2A and a carboxyl-terminal globular domain, called the macro domain (5), that protrudes out of the nucleosome. Although generally referred to as macroH2A, two different genes, H2AFY and H2AFY2, encode for two macroH2A isoforms called macroH2A.1 and macroH2A.2, respectively (6). MacroH2A.1 is characterized by two splicing variants, macroH2A.1.1 and macroH2A.1.2. Of these two splicing variants macroH2A.1.1 is capable of binding NAD+ (7) metabolites through the macro domain and likely plays additional NAD+-dependent roles.
MacroH2A has been suggested to be involved in transcriptional repression through two different mechanisms. In a passive way, the macro domain physically impedes the access of transcription factors and ATP-dependent chromatin remodelers to chromatin (8). More actively, the macro domain can interact with histone deacetylases (9) and certain subunits of the Polycomb complex (10) and thus, might participate in the recruitment or stabilization of repressor complexes at defined genomic locations.
Accordingly, macroH2A was originally described to be involved in X inactivation (11), but other reports have challenged that view (12). Moreover, recent reports have located macroH2A in several autosomal genes (10, 13, 14). Despite the fact that macroH2A occupies mostly transcriptionally inactive genes, its knockdown has been reported to block the induction of genes by serum starvation (13). Therefore, the potential involvement of macro histone variants in transcriptional activation remains controversial.
Several recent reports (14–16) have linked the expression of macro histone variants to differentiation. MacroH2A.1 and macroH2A.2 are expressed at low levels in human embryonic (ES), induced pluripotent (iPS) and adult stem cells but induced during their in vitro and in vivo differentiation. Also, adult somatic cells express high levels of both variants compared with pluripotent cells (14). Therefore, it is likely that these histone variants are involved in the process of differentiation.
The epigenetic mechanisms that contribute to establishing cell identity during differentiation are not completely known. Several studies (16, 17), including ours (14), have previously shown that macroH2A expression protects cells against reprogramming to pluripotency, and its expression is needed for the proper differentiation of mouse cells (15). However, mechanistic roles in the differentiation of human embryonic stem cells (ESCs) and induced pluripotent cells (iPSCs) remain unaddressed. Our results show that macro histone variants play essential roles in the in vitro differentiation of human pluripotent cells, likely through the coordinated repression of pluripotency genes and bivalent germ layer-specific genes.
EXPERIMENTAL PROCEDURES
Cell Culture
The human embryonic stem cell lines ES[4] (18) and the KiPSCs (14) lines were grown in Matrigel-coated plates, in the presence of irradiated mouse embryonic fibroblast-conditioned HES medium (knock-out DMEM supplemented with 20% KO serum replacement, 1× MEM nonessential amino acids, 2 mm l-glutamine, and 50 μm β-mercaptoethanol) supplemented with 10 ng/ml FGF and subcultured using trypsin.
In Vitro Differentiation of Pluripotent Cells
Cells were trypsinized into a single cell suspension and resuspended in mouse embryonic fibroblast-conditioned HES medium. Embryoid body (EB) formation was induced by seeding 100,000 cells in each well of 96-well V-bottom, low attachment plates and centrifuging the plates at 950 × g for 5 min to aggregate the cells. After 3 days the EBs were transferred to 0.1% gelatin-coated dishes and cultured in differentiation medium (KO DMEM supplemented with 20% fetal bovine serum, 1× MEM nonessential amino acids, 2 mm l-glutamine, and 50 μm β-mercaptoethanol) up to 20 days. The medium was changed every 4 days. For Activin A treatment, cells grown in monolayer were treated with 100 ng/ml Activin A for 1 day in DMEM/F12 supplemented with 1× MEM nonessential amino acids, 2 mm l-glutamine, and 0.5 mg/ml human albumin. During the next 2 days cells were incubated in the same medium in the presence of 1% fetal bovine serum. At day 3 cells were trypsinized and stained with antibodies against CXCR4 for FACS analysis.
Real Time PCR
Total mRNA was isolated using TRIzol, and 1 μg was used to synthesize cDNA using the Cloned AMV First-strand cDNA synthesis kit (Invitrogen). One μl of the reaction was used to quantify gene expression by quantitative PCR (qPCR). Oligonucleotides for amplification of differentiation genes and human macro histone variants were described previously (14, 19).
Cell Staining and Flow Cytometry
Cells were trypsinized, fixed, permeabilized using the FIX & PERM Cell Permeabilization Kit (Invitrogen), and incubated with anti-OCT4 antibody (PE Mouse anti-OCT3/4; BD Biosciences) or anti-CXCR4 antibody (555976; BD Biosciences). The analysis was performed on a Beckman Coulter Gallios Flow Cytometer using the Kaluza Flow Cytometry Analysis software.
Immunofluorescence
Cells were grown on plastic coverslide chambers and fixed with 4% paraformaldehyde at room temperature for 20 min. The immunodetection was performed with TBS/0.2% Triton X-100 for permeabilization, and primary antibodies were incubated at 4 °C overnight and secondary antibodies at 37 °C for 2 h. The following antibodies were used: OCT4 (sc-5279 from Santa Cruz), FOXA2 (AF2400 from R&D Systems), α1-fetoprotein (A0008 from Dako). Secondary antibodies used were all cyanine-conjugated from Jackson (all 1:400). Images were taken using Leica SP5 AOBS confocal microscope.
Teratoma Formation and Analysis
Severe combined immunodeficient beige mice (Charles River Laboratories) were anesthetized, and approximately 1 million KiPSCs were injected into the testis. Two clones each of control KiPSCs (shRD) and KiPSCs knocked down for macroH2A.1 (shM1) or macroH2A.2 (shM2) were injected in duplicate. Mice were euthanized 6 weeks after cell injection, and tumors were fixed with 4% paraformaldehyde overnight at 4 °C. Samples were embedded in paraffin and sectioned completely in a sequential way. To quantitatively analyze the OCT4 content, anti-OCT4 (sc-5279 from Santa Cruz Biotechnology) and the envision kit with DAB (K4061 from Dako) were used. Counting was done with the MetaMorph software (from Molecular Devices), comparing the DAB signal versus the hematoxylin staining of all nuclei. The percentage of OCT4-positive cells was determined using at least 10 random pictures per slide. For precursors versus mature neurons distribution analysis, the number of rosettes analyzed was 10 for shRD, 20 for shM1, and 18 for shM2, using anti-TUJ1 (MMS-435P from Covance) and anti-Sox9 (AF3075 from R&D Systems) antibodies.
Chromatin Immunoprecipitation
The chromatin immunoprecipitation (ChIP) assays were performed according to the Millipore protocol. Briefly, 1 million human ESCs were used for each immunoprecipitation. Cells were fixed using 1% formaldehyde, harvested, resuspended in ChIP lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris-HCl, pH 8.1), and sonicated using the Branson Digital Sonifier to generate fragments of 150–500 bp. Soluble chromatin was diluted 8-fold in ChIP radioimmuneprecipitation assay buffer (10 mm Tris-HCl, pH 7.5, 140 mm NaCl, 1 mm EDTA, 0.5 mm EGTA, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate) and incubated with Dynabeads protein A (Invitrogen) coupled to normal rabbit IgG or specific antibodies against macroH2A.1 (07-219 from Millipore), macroH2A.2 (10), or H3K27me3 (07-449 from Millipore). After incubation, the immunocomplexes were washed sequentially with low salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 150 mm NaCl), high salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, 500 mm NaCl), LiCl wash buffer (0.25 m LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mm EDTA, 10 mm Tris-HCl, pH 8.1), and TE. Immunocomplexes were eluted in ChIP elution buffer (1% SDS, 0.1 m NaHCO3), and the cross-linking was reverted overnight at 65 °C. Samples were treated with proteinase K and RNase A, and DNA was extracted using the Qiagen PCR purification kit. Purified chromatin was quantified using qPCR and the following oligonucleotides: OCT4D forward, CTTGGCAGACAGCAGAGAGATG and reverse, ATCTCAATCCCCAGGACAGAAC; OCT4P forward, CAGTTGTGTCTCCCGGTTTTC and reverse, CGAAGGATGTTTGCCTAATGG; NanogD forward, GACAGGGTTTCACCATGTTGGT and reverse, CCGAGCCAGGTGCATCAT; NanogP forward, CGGTTTTCTAGTTCCCCACCTA and reverse, CCAAGGCCATTGTAATGCAA; Sox17 forward, TGAAGGCCAGGAGTTTGCA and reverse, CCGTCTGTCCAGTCTTGCTTATT; ACTB forward, GCCCACCCGGTCTTGTG and reverse, CCTGTCCTTGTCACCCTTTCTT; FOXA2 forward, CAGCAGCGGGCGAGTTA and reverse, TGCAAAGCGCTGTCCTATTTAG; p21 forward, CGAGCGGTTTTGTTTTCGTT and reverse, AAAGCACGGGATGAGTCAGATC; GAPDH forward, GGTGCGTGCCCAGTTGA and reverse, TACTTTCTCCCCGCTTTTT.
RESULTS
The Knockdown of Macro Histone Variants Impairs the in Vitro Differentiation of Human Pluripotent Cell Lines
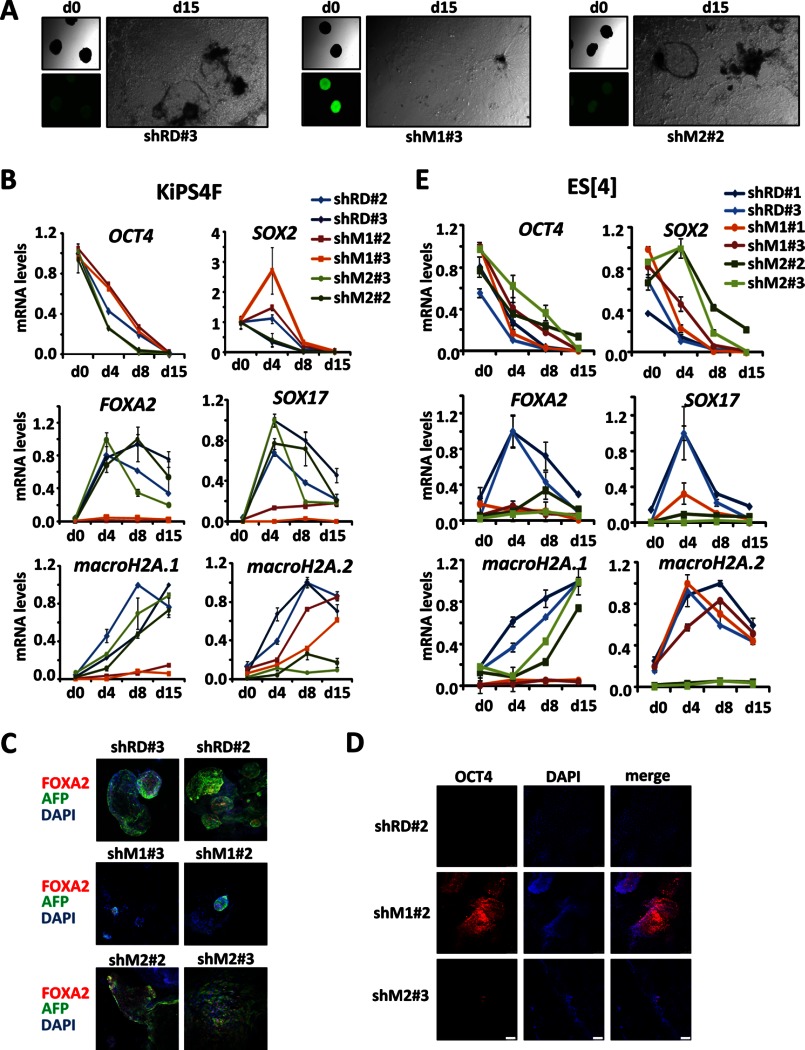
We have previously shown that human KiPSCs derived from keratinocytes knocked down for macroH2A.1 or macroH2A.2 show effective down-regulation of both variants. These lines can be expanded normally and, compared with control KiPSCs, do not show differences in self-renewal properties (14). However, the differentiation capabilities of such cells remain unaddressed. To answer this question we performed in vitro differentiation experiments in the form of EBs and monitored the formation of endoderm, an embryonic layer that could be readily detected after several days in culture with differentiation medium containing 20% fetal bovine serum and absence of FGF. All the tested KiPSC clones were able to initially produce EBs of similar size and shape, but displayed morphological differences after several days in differentiation medium, with clones knocked down for macroH2A.1 showing fewer cystic structures compared with control and macroH2A.2 knockdown clones (Fig. 1A). Next, we monitored the expression of differentiation and pluripotency genes during differentiation. Knockdown of macroH2A.1 in KiPSCs caused a delay in the silencing of the pluripotency genes OCT4 and SOX2, whereas differentiation genes, such as FOXA2 and SOX17, were practically not induced compared with control or macroH2A.2 knockdown KiPSC clones (Fig. 1B). Immunohistochemistry confirmed that endodermal areas marked by the co-presence of FOXA2 and AFP were almost absent in the EBs from the macroH2A.1 knockdown (Fig. 1C) and that these EBs still retained certain expression of OCT4 after 15 days of differentiation (Fig. 1D). We next produced two lines each of human ES control or knockdown cells for each variant and tested their differentiation abilities. Interestingly, human ESCs knocked down for macroH2A.2 also showed impaired differentiation, which correlated with a stronger depletion of macroH2A.2 in the ESC clones compared with the KiPSC lines (Fig. 1E).
FIGURE 1.
Effects of macroH2A.1 and macroH2A.2 knockdown in the differentiation of human pluripotent cell lines. A, phase contrast and fluorescence images of EBs obtained from KiPSCs control (shRD), knockdown for macroH2A.1 (shM1), and macroH2A.2 (shM2) at days 0 and 15 of differentiation. B, mRNA levels determined by qPCR of pluripotency and differentiation genes during the differentiation of two clones each of control KiPS (shRD) and KiPSCs knocked down for macroH2A.1 (shM1) or macroH2A.2 (shM2). mRNA levels were normalized to GAPDH and plotted relatively to day 0 (d0) for pluripotency markers and relatively to the maximum expression level for differentiation genes. Each clone was tested in two independent differentiation experiments with similar results. Graphs show the mean ± S.D. (error bars) of triplicates of one representative and independent differentiation experiment per clone. C, immunocytochemical analysis of the expression of the endodermal genes FOXA2 (red) and AFP (green) in two clones each of control KiPSCs (shRD) and KiPSCs knocked down for macroH2A.1 (shM1) or macroH2A.2 (shM2) at day 20 of EB differentiation. D, OCT4 immunodetection in EBs obtained from one clone each of control KiPSCs (shRD), knocked down for macroH2A.1 (shM1) or macroH2A.2 (shM2) at 15 days of differentiation. E, mRNA levels determined by qPCR of pluripotency and differentiation genes during the differentiation of two clones each of ES[4] control (shRD) ES[4], knockdown for macroH2A.1 (shM1) or macroH2A.2 (shM2). mRNA levels were normalized to GAPDH and plotted relative to the maximum expression level. Graphs show the mean ± S.D. (error bars) of triplicates.
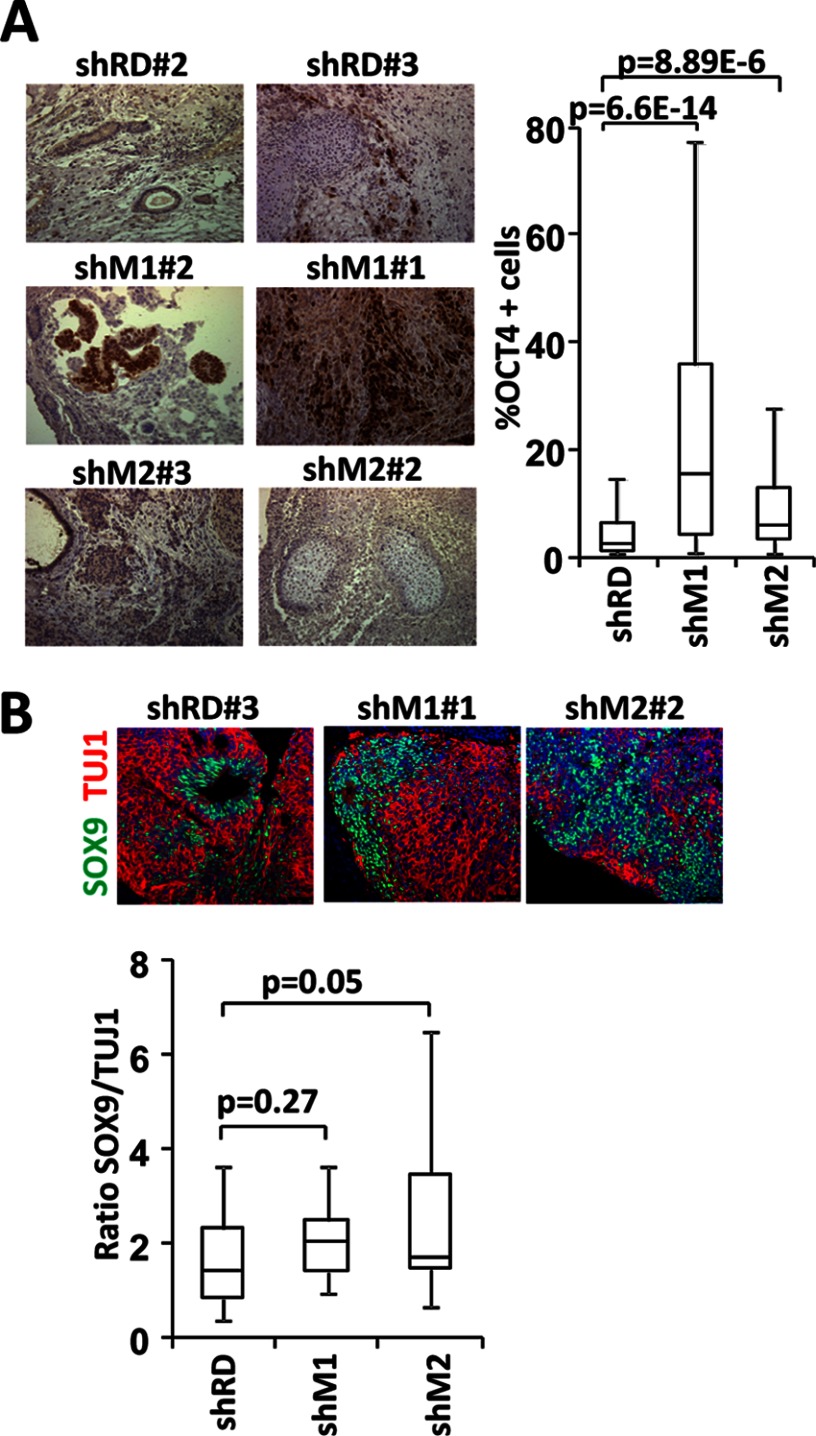
Teratomas from MacroH2A Knockdown KiPS4F Show Residual OCT4 Expression and Large Areas of Precursor Cells
We have previously reported that KiPSC lines knocked down for macro variants are able to produce teratomas positive for markers of the three embryonic layers (14). To evaluate further potential defects in these teratomas we checked the potential retention of OCT4 expression. Similar to in vitro differentiated lines, a higher number of OCT4-positive cells were found in the teratomas produced by the macroH2A.1 knockdown KiPSCs compared with control and macroH2A.2 knockdown cell lines (Fig. 2A). We have also reported that macroH2A.2 is expressed at low levels in neural precursors and induced during their differentiation (14). Therefore, we asked whether the depletion of macro histone variants might have consequences for the balance between differentiated and precursor neural cells. The analysis of the relative content of neural precursors (SOX9-positive cells) versus mature neurons (TUJ1-positive cells) suggests that the teratomas produced by the macroH2A.2 knockdown KiPSC clones had a tendency to accumulate precursors versus mature neurons. Moreover, whereas control rosettes were characterized by the presence of precursor cells in the center and more mature cells on the periphery, 78% of shM2 rosettes showed a disorganized distribution of the SOX9- and TUJ1-positive areas (Fig. 2B).
FIGURE 2.
Characterization of teratomas. A, levels of OCT4 expression were determined by immunohistochemistry in teratomas from two lines each of control KiPSCs (shRD) and KiPSCs knocked down for macroH2A.1 (shM1) or macroH2A.2 (shM2). The left panel shows one representative picture of OCT4 immunostaining per line, visualized using DAB (brown) over hematoxylin (blue). The right panel shows a box plot representation of the percentage of OCT4-positive cells found in teratomas from both lines of shRD, shM1 and shM2. B, upper panel shows a representative picture of neural rosettes in teratomas from control and macroH2A.1 or macroH2A.2 knocked down KiPSCs, showing SOX9 (green) and TUJ1 (red) immunolocalization. Lower panel shows a box plot representation of the relative ratio of Sox9- to Tuj1-expressing cells in neuronal rosettes detected by immunohistochemistry.
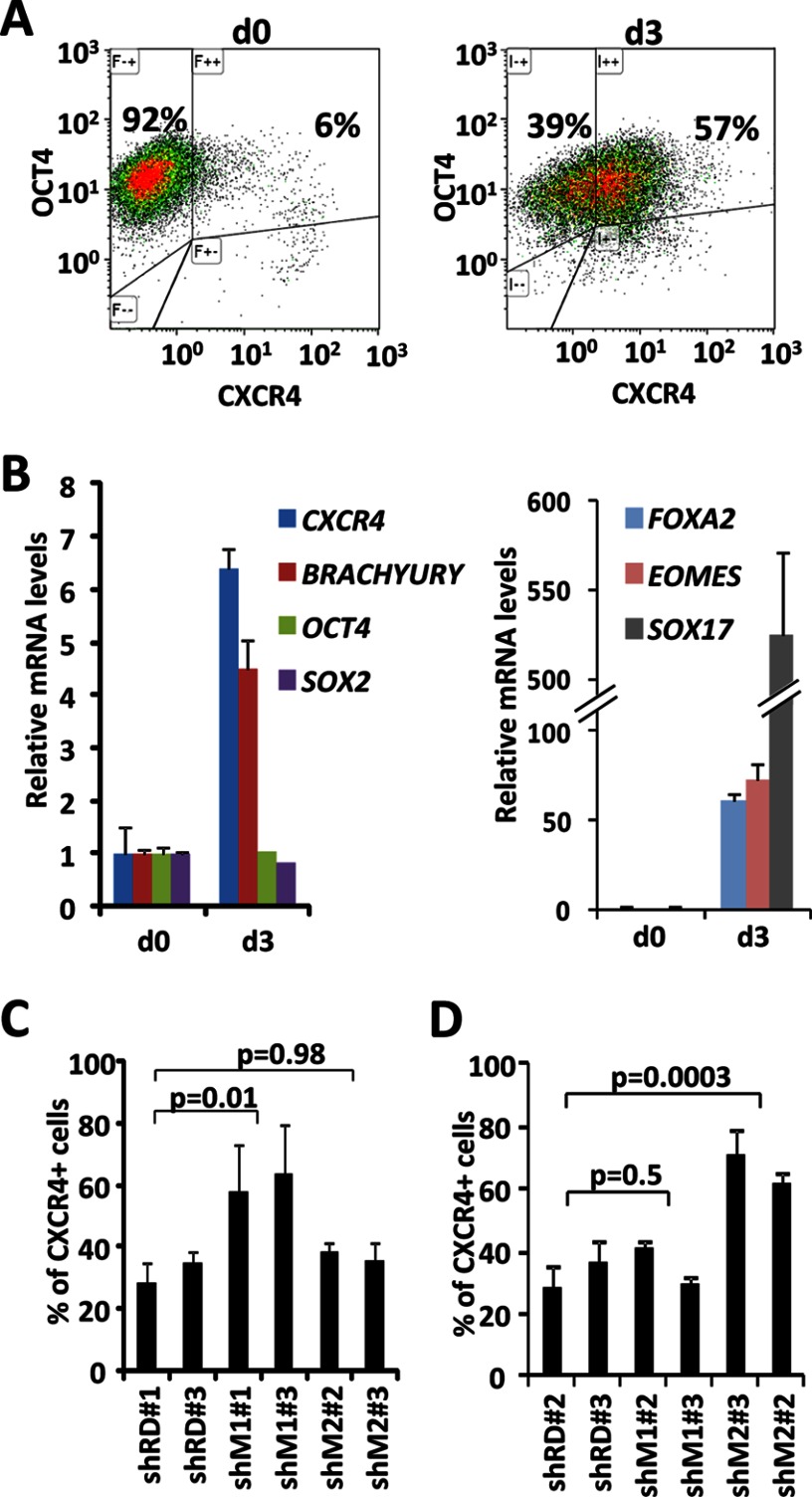
Human Pluripotent Lines Knocked Down for Macro Histone Variants Show Induction of Activin A Target Genes
The differentiation of pluripotent lines into EBs is a highly coordinated process in which silencing of pluripotency genes and the induction of developmental genes take place in a coordinated fashion. Thus, the defects in induction of differentiation genes observed in vitro could be an indirect consequence of the defective silencing of the pluripotency network. Indeed, the residual expression of OCT4 in teratomas and the fact that macroH2A.1 occupies repressed genes marked with H3K27me3 in human keratinocytes (14) suggest that the effects on the silencing of the pluripotency network might be the direct cause of impaired differentiation. To further test this we tested the ability of the KiPS cell lines to activate genes in response to high concentrations of Activin A, using a protocol adapted from D'Amour et al. (20) and evaluating the percentage of cells expressing the Activin A target gene CXCR4 by FACS after 3 days of treatment. Fig. 3A shows that human ESCs increase the percentage of CXCR4-expressing cells after 3 days of treatment. This induction is consistent with the up-regulation of other endodermal genes as judged by qPCR (Fig. 3B). Importantly, the levels of the pluripotency genes OCT4 and SOX2 remain unaffected, suggesting that the induction of Activin A target genes can take place independently of the down-regulation of pluripotency genes. After 3 days of treatment with high concentrations of Activin A all of the tested ESC lines (Fig. 3C) and KiPSCs (Fig. 3D) showed induction of CXCR4 expression and equal or higher percentages of CXCR4-positive cells as control cell lines, indicating that the knockdown of macro histone variants does not affect the ability of genes to respond to Activin A. These data suggest that macro histone variants are more likely to affect differentiation through the impairment of the silencing of pluripotency genes than directly affecting the activation of differentiation genes.
FIGURE 3.
Activin A activates target genes in pluripotent lines knocked down for macro histone variants. A, FACS analysis of expression of OCT4 and CXCR4 in ESCs before (day 0) and after 3 days (day 3) of treatment with Activin A. B, mRNA levels determined by qPCR of pluripotency and differentiation genes in ESCs before (d0) and after 3 days (d3) of Activin A treatment. mRNA levels were normalized to GAPDH. Graphs show the mean ± S.D. (error bars) of triplicates. C, FACS analysis of the percentage of cells that express CXCR4 after 3 days of Activin A treatment in two lines of ES[4] control (shRD), knocked down for macroH2A.1 (shM1) or for macroH2A.2 (shM2). Graphs show the mean ± S.D. of at least four independent treatments. D, FACS analysis of the percentage of cells that express CXCR4 after 3 days of Activin A treatment in two lines of KiPS control (shRD), knocked down for macroH2A.1 (shM1) or for macroH2A.2 (shM2). Graphs show the mean ± S.D. of at least four independent treatments.
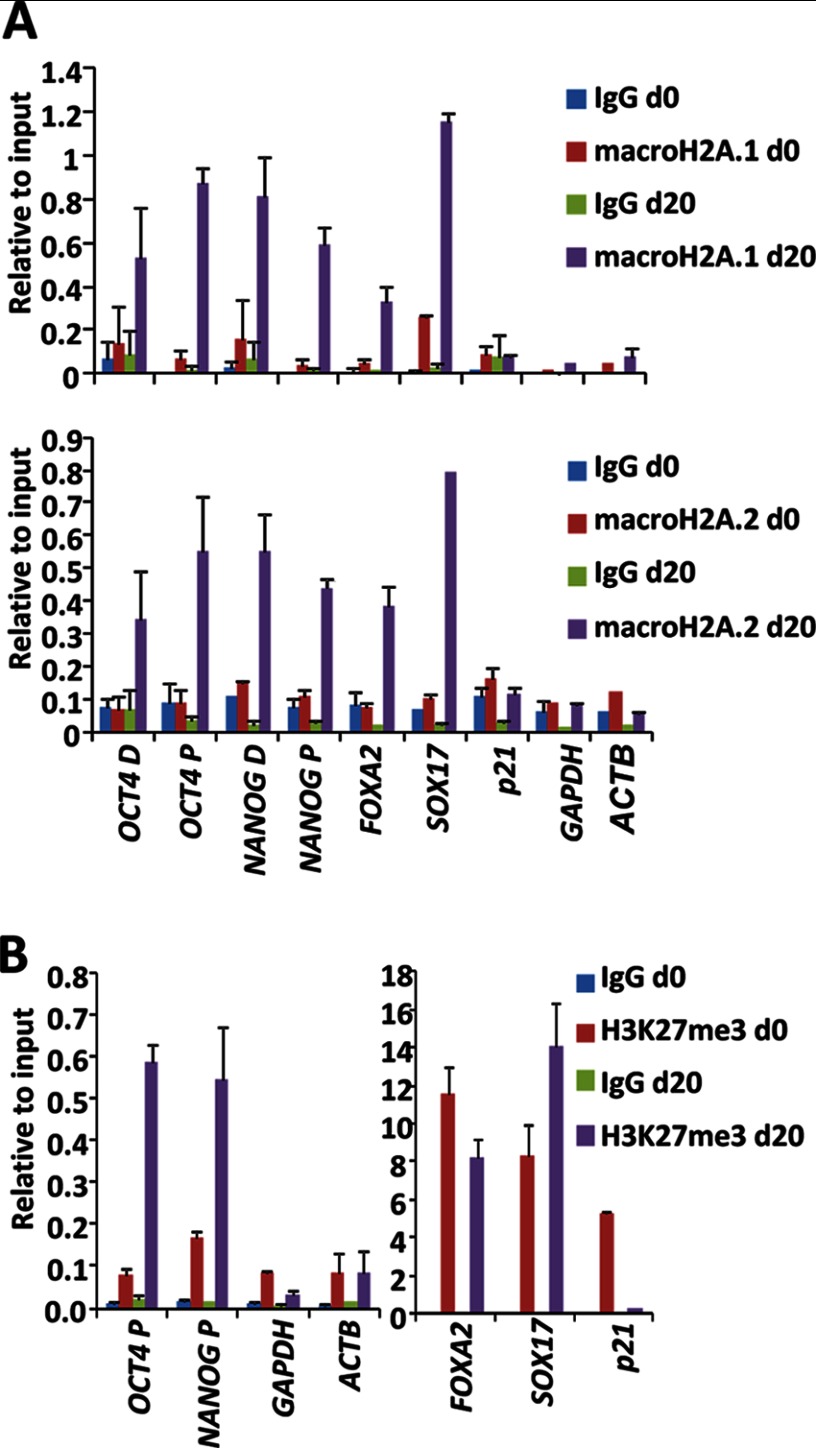
Macro Histone Variants Accumulate at the Promoters of Pluripotency and Differentiation Genes during Differentiation
We have previously shown (14) that macroH2A.1 occupies the regulatory regions of genes marked with H3K27me3 in human keratinocytes. These genes include bivalent developmental genes and genes of the pluripotency network that are expressed at low levels in human keratinocytes, including epigenetic regulators (LIN28a, CBX2, DNMT3B, and RCOR2), genes involved in signaling pathways (NODAL, LEFTY1, and LEFTY2) and transcription factors (NANOG, PRDM14, and ZSCAN10). These data point to a potential role of macroH2A.1 in the silencing of developmental and pluripotency genes during differentiation. Important pluripotency genes such as OCT4 and NANOG have been reported to acquire H3K27me3 as they become repressed during differentiation (21). To test the possibility that pluripotency and differentiation genes are direct targets of macroH2A during differentiation, we performed chromatin immunoprecipitation assays in undifferentiated human ESCs and EBs at day 20 of differentiation. Both macroH2A.1 and macroH2A.2 accumulated at two tested locations of the regulatory regions of the pluripotency genes OCT4 and NANOG in differentiated human ESCs and at the master endodermal-differentiation genes FOXA2 and SOX17 (Fig. 4A). However, we could not detect the presence of these histone variants at the regulatory regions of highly expressed genes such as GAPDH and ACTB. The presence of macro variants correlated with the presence of H3K27me3 (Fig. 4B), both at genes that gained H3K27me3 during differentiation (OCT4 and NANOG) and bivalent genes (FOXA2 and SOX17) that preserve H3K27me3 in most cells, because only about 30% of the cells become positive for these markers in our in vitro differentiation conditions (data not shown). As expected, macro histone variants were not incorporated into the bivalent gene p21, which loses H3K27me3 during differentiation as it becomes induced in most cells.
FIGURE 4.
MacroH2A.1 and macroH2A.2 are recruited to the regulatory regions of pluripotency and differentiation genes during human ESC differentiation. A, chromatin immunoprecipitation assays in undifferentiated ES[4] cells (d0) and after 20 days of differentiation (d20) using control IgGs and specific antibodies against macroH2A.1 (upper panel) and macroH2A.2 (lower panel). Levels relative to 1% of the input are shown as mean ± S.D. (error bars) of triplicates. B, chromatin immunoprecipitation assays in undifferentiated ES[4] cells (d0) and after 20 days of differentiation (d20) using specific antibodies against H3K27me3. Levels relative to 1% of the input are shown as mean ± S.D. of triplicates.
DISCUSSION
Our work shows that the expression of macrohistone variants is critical for the in vitro differentiation of human pluripotent cells. We performed in vitro differentiation experiments in the form of embryoid bodies and presence of fetal bovine serum. In this context, differentiation is driven by the physiological combination of signaling molecules present in the serum and the tridimensional structure of the embryoid body, which contributes to recapitulate early embryonic development (23). During embryoid body differentiation, similar to embryonic development, the coordinated repression of the pluripotency network and the induction of developmental genes are essential for proper differentiation. This type of differentiation is particularly relevant for studying human cells in which in vivo developmental experiments cannot be performed. Although several protocols have been developed to direct the differentiation of embryonic stem cells into certain cell types relevant for the use in the clinic, they often entail the use of nonphysiological concentrations of signaling molecules that overcome the natural sequence of events that takes place during development.
In our study we found that the depletion of macroH2A.1 had consistent effects in the differentiation of both KiPS and human ES cells. However, depletion of macroH2A.2 affected the differentiation of human ES cells but not the differentiation of KiPSCs. Although differences between the differentiation potential of ES and iPS cells have been previously described (24, 25), we believe that the most likely explanation for the differences that we observe is that the knockdown of macroH2A.2 in KiPSCs is not as efficient as in ES[4]. Both histone variants have been reported likely to be recruited to the same set of target genes (13), suggesting that their functions might be redundant. However, these variants can be differentially regulated at the level of gene expression (14), making the relative content of macroH2A.1 and macroH2A.2 different in each particular cell type. Therefore, the knockdown effects might finely depend on how much the depletion of each variant contributes to reduce the total macroH2A pool.
Similarly, we found variability in the percentage of cells expressing CXCR4 after Activin A treatment of KiPS and human ES cells depleted of macrohistone variants. Whereas all cell lines were able to induce the expression of CXCR4 in response to Activin A, KiPSCs depleted of macroH2A.2 showed a significant higher number of CXCR4-positive cells. Further analysis will be needed to understand the nature of this observation.
Defects in differentiation after depletion of macroH2A.1 have been recently reported in mouse ESC (15), but whether macro histone variants could be directly involved in the induction of differentiation genes remains uncertain (15, 26). Our data show that Activin A target genes can be efficiently induced in the absence of macroH2A.1 or macroH2A.2 whereas differentiation in the presence of fetal bovine serum, which requires proper silencing of the pluripotency network for the induction of developmental genes, is impaired. Despite the fact that all tested KiPSC lines can form teratomas, those originated from cells knocked down for macro histone variants appear more immature, as judged by the larger number of cells expressing OCT4 in the macroH2A.1 knockdown and a larger number of neural precursors in the macroH2A.2 knockdown. We also found that macro histone variants are recruited to the regulatory regions of genes that retain or gain H3K27me3 during differentiation, but not to those that lose H3K27me3. Moreover, further evidence suggests the involvement of these variants in gene silencing during differentiation. First, the patterns of macroH2A recruitment to target genes during differentiation resemble those of the repressive linker histone H1 (27). Second, ES cells depleted from the repressive Polycomb subunit Suz12 or histone H1.0 show similar deregulated patterns of gene expression during differentiation to cells knocked down for macro histone variants (27, 28). Therefore, our data point to a critical role of macro histone variants in repressing gene expression during differentiation. Interestingly, proper differentiation might not only require the repression of pluripotency-related genes but also the silencing of bivalent developmental genes, previously reported to be occupied by macroH2A.1 in human somatic cells (14), in a germ layer-specific manner.
In our in vitro differentiation experiments in the form of embryoid bodies, we could clearly detect the formation of nice endodermal structures, and therefore our study has focused on the formation of this particular germ layer. Because the depletion of macrohistone variants impairs the down-regulation of pluripotency-related genes during differentiation, it is likely that the formation of proper mesoderm and ectoderm is also compromised. Although macrohistone variants were previously reported to be critical for X chromosome inactivation in mouse female cells (11, 17), the effects reported here in human cells are independent of X chromosome inactivation because all the cell lines analyzed in this study were male cells.
The possibility of generating iPS cells has open up unprecedented opportunities for cell therapy in regenerative medicine. However, the clinical use of in vitro differentiated cells derived from pluripotent cells has still one major drawback, which is the risk of tumor formation (29). Moreover, estimation of the quality of human iPS lines is limited by the impossibility to test their contribution to quimera formation or tetraploid complementation (30). According to our study, the induction and recruitment of macro histone variants to target genes appear essential for the proper in vitro differentiation of human pluripotent cells. Relevant to this topic, macroH2A.1 reduced expression has been correlated with higher risk of cancer recurrence (31) and with increased melanoma malignancy in humans (32). Therefore, proper recruitment of macro histone variants to targets genes during iPS differentiation appears critical to guarantee the quality and safety of transplantable differentiated cell types.
Acknowledgments
We thank J. Castaño and B. Kuebler for technical assistance, J. M. A. Vaquero for FACS analysis, and the histology and embryo micromanipulation platforms at the Center for Regenerative Medicine.
This work was supported by Ministerio de Ciencia e Innovación of Spain (MICINN) Grants RYC-2007-01510 and SAF2009-08588 (to M. J. B.).
- macroH2A
- macro histone H2A
- EB
- embryoid body
- ESC
- embryonic stem cell
- iPS
- induced pluripotent
- iPSC
- iPS cell
- qPCR
- quantitative PCR.
REFERENCES
- 1. Ahmad K., Henikoff S. (2002) The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol. Cell 9, 1191–1200 [DOI] [PubMed] [Google Scholar]
- 2. Sullivan K. F., Hechenberger M., Masri K. (1994) Human CENP-A contains a histone H3-related histone fold domain that is required for targeting to the centromere. J. Cell Biol. 127, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faast R., Thonglairoam V., Schulz T. C., Beall J., Wells J. R., Taylor H., Matthaei K., Rathjen P. D., Tremethick D. J., Lyons I. (2001) Histone variant H2A.Z is required for early mammalian development. Curr. Biol. 11, 1183–1187 [DOI] [PubMed] [Google Scholar]
- 4. Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 [DOI] [PubMed] [Google Scholar]
- 5. Pehrson J. R., Fried V. A. (1992) MacroH2A, a core histone containing a large nonhistone region. Science 257, 1398–1400 [DOI] [PubMed] [Google Scholar]
- 6. Chadwick B. P., Willard H. F. (2001) Histone H2A variants and the inactive X chromosome: identification of a second macroH2A variant. Hum. Mol. Genet. 10, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 7. Karras G. I., Kustatscher G., Buhecha H. R., Allen M. D., Pugieux C., Sait F., Bycroft M., Ladurner A. G. (2005) The macro domain is an ADP-ribose binding module. EMBO J. 24, 1911–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angelov D., Molla A., Perche P. Y., Hans F., Côté J., Khochbin S., Bouvet P., Dimitrov S. (2003) The histone variant macroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol. Cell 11, 1033–1041 [DOI] [PubMed] [Google Scholar]
- 9. Chakravarthy S., Gundimella S. K., Caron C., Perche P. Y., Pehrson J. R., Khochbin S., Luger K. (2005) Structural characterization of the histone variant macroH2A. Mol. Cell. Biol. 25, 7616–7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buschbeck M., Uribesalgo I., Wibowo I., Rué P., Martin D., Gutierrez A., Morey L., Guigó R., López-Schier H., Di Croce L. (2009) The histone variant macroH2A is an epigenetic regulator of key developmental genes. Nat. Struct. Mol. Biol. 16, 1074–1079 [DOI] [PubMed] [Google Scholar]
- 11. Costanzi C., Pehrson J. R. (1998) Histone macroH2A1 is concentrated in the inactive X chromosome of female mammals. Nature 393, 599–601 [DOI] [PubMed] [Google Scholar]
- 12. Csankovszki G., Panning B., Bates B., Pehrson J. R., Jaenisch R. (1999) Conditional deletion of Xist disrupts histone macroH2A localization but not maintenance of X inactivation. Nat. Genet. 22, 323–324 [DOI] [PubMed] [Google Scholar]
- 13. Gamble M. J., Frizzell K. M., Yang C., Krishnakumar R., Kraus W. L. (2010) The histone variant macroH2A1 marks repressed autosomal chromatin, but protects a subset of its target genes from silencing. Genes Dev. 24, 21–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrero M. J., Sesé B., Kuebler B., Bilic J., Boue S., Martí M., Izpisua Belomonte J. C. (2013) Macrohistone variants preserve cell identity by preventing the gain of H3K4me2 during reprogramming to pluripotency. Cell Rep. S2211–1247, 00105–00108 [DOI] [PubMed] [Google Scholar]
- 15. Creppe C., Janich P., Cantariño N., Noguera M., Valero V., Musulén E., Douet J., Posavec M., Martín-Caballero J., Sumoy L., Di Croce L., Benitah S. A., Buschbeck M. (2012) MacroH2A1 regulates the balance between self-renewal and differentiation commitment in embryonic and adult stem cells. Mol. Cell. Biol. 32, 1442–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasque V., Radzisheuskaya A., Gillich A., Halley-Stott R. P., Panamarova M., Zernicka-Goetz M., Surani M. A., Silva J. C. (2012) Histone variant macroH2A marks embryonic differentiation in vivo and acts as an epigenetic barrier to induced pluripotency. J. Cell Sci. 125, 6094–6104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasque V., Gillich A., Garrett N., Gurdon J. B. (2011) Histone variant macroH2A confers resistance to nuclear reprogramming. EMBO J. 30, 2373–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raya A., Rodríguez-Pizà I., Arán B., Consiglio A., Barri P. N., Veiga A., Izpisúa Belmonte J. C. (2008) Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harbor Symp. Quant. Biol. 73, 127–135 [DOI] [PubMed] [Google Scholar]
- 19. Aasen T., Raya A., Barrero M. J., Garreta E., Consiglio A., Gonzalez F., Vassena R., Bilic J., Pekarik V., Tiscornia G., Edel M., Boue S., Belmonte J. C. (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 26, 1276–1284 [DOI] [PubMed] [Google Scholar]
- 20. D'Amour K. A., Agulnick A. D., Eliazer S., Kelly O. G., Kroon E., Baetge E. E. (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 [DOI] [PubMed] [Google Scholar]
- 21. Pan G., Tian S., Nie J., Yang C., Ruotti V., Wei H., Jonsdottir G. A., Stewart R., Thomson J. A. (2007) Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1, 299–312 [DOI] [PubMed] [Google Scholar]
- 22.Deleted in proof
- 23. Keller G. (2005) Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 19, 1129–1155 [DOI] [PubMed] [Google Scholar]
- 24. Kim K., Doi A., Wen B., Ng K., Zhao R., Cahan P., Kim J., Aryee M. J., Ji H., Ehrlich L. I., Yabuuchi A., Takeuchi A., Cunniff K. C., Hongguang H., McKinney-Freeman S., Naveiras O., Yoon T. J., Irizarry R. A., Jung N., Seita J., Hanna J., Murakami P., Jaenisch R., Weissleder R., Orkin S. H., Weissman I. L., Feinberg A. P., Daley G. Q. (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Polo J. M., Liu S., Figueroa M. E., Kulalert W., Eminli S., Tan K. Y., Apostolou E., Stadtfeld M., Li Y., Shioda T., Natesan S., Wagers A. J., Melnick A., Evans T., Hochedlinger K. (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 28, 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamble M. J., Kraus W. L. (2010) Multiple facets of the unique histone variant macroH2A: from genomics to cell biology. Cell Cycle 9, 2568–2574 [DOI] [PubMed] [Google Scholar]
- 27. Terme J. M., Sesé B., Millán-Ariño L., Mayor R., Izpisúa Belmonte J. C., Barrero M. J., Jordan A. (2011) Histone H1 variants are differentially expressed and incorporated into chromatin during differentiation and reprogramming to pluripotency. J. Biol. Chem. 286, 35347–35357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pasini D., Bracken A. P., Hansen J. B., Capillo M., Helin K. (2007) The Polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol. Cell. Biol. 27, 3769–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barrero M. J. (2012) The stability of the induced epigenetic programs. Comp. Funct. Genomics 2012, 434529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boué S., Paramonov I., Barrero M. J., Izpisúa Belmonte J. C. (2010) Analysis of human and mouse reprogramming of somatic cells to induced pluripotent stem cells: what is in the plate? PloS One 5, e12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sporn J. C., Kustatscher G., Hothorn T., Collado M., Serrano M., Muley T., Schnabel P., Ladurner A. G. (2009) Histone macroH2A isoforms predict the risk of lung cancer recurrence. Oncogene 28, 3423–3428 [DOI] [PubMed] [Google Scholar]
- 32. Kapoor A., Goldberg M. S., Cumberland L. K., Ratnakumar K., Segura M. F., Emanuel P. O., Menendez S., Vardabasso C., Leroy G., Vidal C. I., Polsky D., Osman I., Garcia B. A., Hernando E., Bernstein E. (2010) The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 468, 1105–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]