Background: One goal of diabetic regenerative medicine is to convert mature pancreatic acinar cells into insulin-producing cells.
Results: Ligand-bound thyroid hormone receptor α (TRα), which interacts with p85α, induces phosphatidylinositol 3-kinase (PI3K) signaling and insulin expression.
Conclusion: PI3K signaling must be activated for TRα-induced reprogramming of pancreatic acinar cells.
Significance: TRα is critical for postnatal expansion of the β-cell mass.
Keywords: Diabetes, Insulin, Pancreas, Phosphatidylinositol 3-Kinase, Thyroid Hormone
Abstract
One goal of diabetic regenerative medicine is to instructively convert mature pancreatic exocrine cells into insulin-producing cells. We recently reported that ligand-bound thyroid hormone receptor α (TRα) plays a critical role in expansion of the β-cell mass during postnatal development. Here, we used an adenovirus vector that expresses TRα driven by the amylase 2 promoter (AdAmy2TRα) to induce the reprogramming of pancreatic acinar cells into insulin-producing cells. Treatment with l-3,5,3-triiodothyronine increases the association of TRα with the p85α subunit of phosphatidylinositol 3-kinase (PI3K), leading to the phosphorylation and activation of Akt and the expression of Pdx1, Ngn3, and MafA in purified acinar cells. Analyses performed with the lectin-associated cell lineage tracing system and the Cre/loxP-based direct cell lineage tracing system indicate that newly synthesized insulin-producing cells originate from elastase-expressing pancreatic acinar cells. Insulin-containing secretory granules were identified in these cells by electron microscopy. The inhibition of p85α expression by siRNA or the inhibition of PI3K by LY294002 prevents the expression of Pdx1, Ngn3, and MafA and the reprogramming to insulin-producing cells. In immunodeficient mice with streptozotocin-induced hyperglycemia, treatment with AdAmy2TRα leads to the reprogramming of pancreatic acinar cells to insulin-producing cells in vivo. Our findings suggest that ligand-bound TRα plays a critical role in β-cell regeneration during postnatal development via activation of PI3K signaling.
Introduction
The formation of the pancreas and its subsequent differentiation into various types of exocrine and endocrine cells during development are controlled by the activation or repression of a large number of genes (1). The expression of these genes is regulated by a well organized cascade of transcription factors. Pancreatic and duodenal homeobox 1 (Pdx1), basic helix-loop-helix factor neurogenin 3 (Ngn3), and MafA are transcription factors that are essential for the transdifferentiation of endocrine cells (1, 2). Pdx1 controls the growth and development of the pancreatic bud; Ngn3 is required for formation of endocrine progenitors; and MafA and Pdx1 are required for the maturation of β-cells (3).
Cell differentiation type can be reprogrammed by overexpression of selected transcription factors, usually a subset of the transcription factors required for formation of the relevant cell type during normal development. Zhou et al. reported a reprogramming of pancreatic exocrine cells to β-like cells in vivo by introduction of genes for the three transcription factors, Pdx1, Ngn3, and MafA (4). Other studies have revealed that mature cells have high plasticity in their differentiation capacity. Pancreatic acinar cells can transdifferentiate into endocrine cells. Indeed, under appropriate culture conditions, dedifferentiated acinar cells can be induced to become insulin-expressing cells via Ngn3 expression (5). Cell lineage studies have also indicated that pancreatic acinar cells possess sufficient plasticity to transdifferentiate into endocrine cells.
Thyroid hormone influences various physiological processes, including cell cycle progression and cell differentiation/development in the vertebrate nervous system. The actions of triiodothyronine (T3)2 are mediated through specific thyroid hormone nuclear receptors (TR)s that function as ligand-dependent transcription factors that increase or decrease the expression of target genes (6, 7). Two TR genes located on different chromosomes encode four TR isoforms, designated as α1, β1, β2, and β3, which all bind to T3. These TRs regulate target gene transcription by binding to specific DNA sequences (thyroid hormone response elements on promoters. TR-mediated transcription is regulated at multiple levels. In addition to these genomic or thyroid hormone response element-mediated effects of T3, nonnuclear or thyroid hormone response element-independent actions of ligand-bound TR have recently been described (8–11). These results indicate that T3 rapidly modulates membrane potential, cellular depolarization, and contractile activity by regulating ion flux across plasma membrane ion channels.
Regarding the mechanism of transdifferentiation of pancreatic acinar cells, PI3K, Notch, and/or leukocyte inhibitory factor/signal transducers and activators of transcription (LIF/STAT) signals are thought to be involved in the process, based mainly on studies with signaling inhibitor compounds (5, 12, 13). However, the precise roles of these signals in the transdifferentiation are not clear. Members of the steroid hormone receptor superfamily, such as estrogen, vitamin D, and TRs, cross-couple to the PI3K/Akt pathway, leading to the downstream activation of the PI3K signaling (14). Indeed, thyroid hormone modulates the interaction of TR with the p85α subunit of PI3K, leading to the activation of Akt and endothelial NOS in vascular endothelial cells (11).
We have reported that intrapancreatic injection of adenovirus vector that expresses TRα leads to the restoration of islet function and an increase in the β-cell mass in immunodeficient mice with streptozotocin (STZ)-induced diabetes (15). These results suggest that ligand-bound TRα plays a critical role in β-cell replication and expansion of the β-cell mass during postnatal development. In the present study, we investigated the physiological importance of the activation of PI3K by TRα and the influence of TRα on the reprogramming of pancreatic exocrine cells to insulin-producing cells.
EXPERIMENTAL PROCEDURES
Primary Cell Culture
Immunodeficient, 4-week-old nude mice (BALB/cAJc1-nu/nu) that were treated with 200 mg/kg STZ (Sigma) were sacrificed, and their pancreases were removed and digested with 1 mg/ml collagenase (Sigma). By Ficoll gradient centrifugation, the exocrine fraction was prepared as a pellet (5). Subsequently, the cells were cultured for 6 h on 35-mm culture dishes (Thermo Fisher Scientific). Floating cells were collected and replated on 2-methacryloxyethyl phosphorylcholine-coated plates (Cosmo Bio). The purified cells were cultured in RPMI 1640 Gluta MAX-I medium supplemented with 10% resin-stripped FBS (16) at 37 °C under 5% CO2 atmosphere.
Construction of Recombinant Adenoviral Vectors
The murine amylase2 promoter was PCR-amplified from mouse liver genomic DNA. The PCR primers were: Amy2-KpnI-5′ (AAGGTACCGCAGGATGGCCTCAGAAGTAAGAT) and Amy2-3′-XhoI (AACTCGAGAGTTGTCAGTGTTCTCTGTAGCAC) (17). The enzyme-digested promoter fragment was ligated into the KpnI and XhoI sites of pGL3 basic vector (Promega). Pancreatic exocrine cell-specific activation of these promoters has been established (17, 18) and confirmed by reporter assay in AR42J cells, a rat pancreatic exocrine cell line (data not shown). The FLAG-TRα1 plasmid (15) was used as the template for cloning human TRα1 into pENTR-1A Dual Selection (Invitrogen) by using PCR. The PCR primers were: kozak-SalI 5′ (GGGGTCGACCACCATGGACTACAAAGACGATGACGACAAG) and SpeI 3′ (GGGCATCTCAGGATGTTAGACTTCCTGATCCTCAAAGAC). Then, amylase2 promoter-driven adenovirus vector (AdAmy2TRα) was constructed by using the pAd/PL-DEST Gateway vector kit (Invitrogen) according to the manufacturer's protocol. Cre-recombinase-expressing adenovirus under control of the amylase2 promoter (AdAmy2Cre) was assembled from synthetic oligonucleotides or PCR products. The fragments were cloned into attL1 and attL2 sites by using the pAd/PL-DEST Gateway vector kit.
Lineage Tracing Study
Wheat germ agglutinin (WGA) is a lectin that binds to N-acetylglucosamine (19). Because of the higher expression of this sugar on exocrine cells, WGA labels only the acinar cells (20). For the specific labeling of acinar cells, freshly isolated exocrine cells were incubated with 4 nm Qdot-conjugated WGA (Invitrogen) in RPMI 1640 Gluta MAX-I medium (Invitrogen) without serum for 10 min and washed three times. To analyze the reprogramming of exocrine cells, culture medium was supplemented with adenovirus at a multiplicity of infection (m.o.i.) of 30 and 100 nm T3 with or without 10 μm LY294002 (Cell Signaling Technology), a PI3K signal-specific inhibitor. The culture medium was not changed throughout the experiment. For lineage tracing with the Cre/loxP-based system, ROSA26-lacZ mice (B6.129S4-Gt (ROSA) 26Sortm1Sor) were purchased from the Jackson Laboratory. Purified pancreatic acinar cells from ROSA26-lacZ mice were coinfected with 30 m.o.i. of AdAmy2TRα and AdAmy2Cre with T3 treatment. After 24, 48, or 72 h of incubation, cells were fixed in 10% buffered formalin and subsequently embedded in paraffin. Then, 3-μm sections were permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 10 min at room temperature and blocked with 10% BSA and 0.2% Tween 20.
Real-time RT-PCR
RNA was extracted from tissues or cells by using an RNeasy mini kit (Qiagen) according to the manufacturer's instructions. cDNA synthesis and real-time RT-PCR were performed as described (15). TaqMan probes for glucokinase, amylase2, insulin1, insulin2, Hnf6, Foxa2, Neurod1, Nkx2.2, Ngn3, Mafa, Pdx1, and 18S were purchased from Applied Biosystems.
Western Blot Analysis
Protein lysate was prepared by using cell lysis buffer (Cell Signaling Technology) according to the manufacturer's instructions. Determination of the protein abundance by Western blot analysis was performed as described (15) with the following primary antibodies: anti-Ngn3 antibody (BD Biosciences); anti-MafA, anti-Akt, anti-elastase, anti-GLUT2, anti-GCK, anti-receptor-type tyrosine-protein phosphatase-like N, and anti-carboxypeptidase E antibody (Santa Cruz Biotechnology); and anti-prohormone convertase 1/3, anti-p85α, anti-Pdx1, anti-phosphorylated Akt (Ser-473), and anti-phosphorylated Akt (Thr-308) antibody (Cell Signaling Technology).
Negative control siRNA (Stealth RNAi) and siRNA against p85α (Stealth RNAi) were purchased from Invitrogen. Pancreatic acinar cells (1.0–1.3 × 105) infected with adenovirus (m.o.i., 30) were incubated with or without T3 for 12 h. Fifty nanomoles of control siRNA or a mixture of two anti-p85α siRNAs were then transfected into the cells by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. After 48 h, the cells were washed with phosphate-buffered saline (Ca2+/Mg2+-free), and Western blot analysis was performed.
Immunochemistry
Purified acinar cells that were infected with 30 m.o.i. of AdAmy2TRα were incubated with 100 nm T3 for 12 h. Fifty nanomoles of control siRNA or anti-p85α siRNAs was transfected. After 24, 48, or 72 h of incubation, the cells were fixed in 10% buffered formalin and subsequently embedded in paraffin.
Coimmunoprecipitation Analysis
To analyze the protein expression of endogenous TRα in the cells, 500 μg of protein lysate from purified β-cells or acinar cells was immunoprecipitated with 5 μg of the C3 mouse monoclonal antibody against the C terminus of TRα (Santa Cruz Biotechnology) or with 5 μg of mouse IgG by using the Dynabeads protein G immunoprecipitation kit (Invitrogen) according to the manufacturer's protocol. Western blot analysis was performed with anti-TRα antibody (Santa Cruz Biotechnology) against an N-terminal TRα peptide.
The association of p85α and TRα in purified acinar cells was determined by coimmunoprecipitation. To determine the interaction of endogenous p85α and transfected TRα, acinar cells infected with AdAmy2TRα at an m.o.i. of 30 were cultured with or without 100 nm T3. Cells were harvested after 24 h, and cell lysates were immunoprecipitated with 5 μg of anti-FLAG antibody by using the Dynabeads protein G immunoprecipitation kit, followed by Western blot analysis with anti-p85α antibody.
Electron Microscopy
Purified acinar cells that were infected with AdAmy2TRα were incubated with 100 nm T3 for 12 h. Fifty nanomoles of control siRNA or anti-p85α siRNAs was transfected. After 48 h, the cells were pre-fixed with 2% glutaraldehyde in phosphate buffer (pH 7.4) at 4 °C. After fixation the specimen was post-fixed with 2% osmium tetroxide in phosphate buffer (pH 7.4) for 45 min. Then, the specimens were dehydrated in a graded series of ethanol replaced with propylene oxide and embedded in epoxy resin (Quetol 812). Ultrathin sections (90–100 nm) were cut by using an Ultracut-UCT (Leica) with a diamond knife and stained with 2% uranyl acetate in distilled water for 15 min followed by a lead staining solution (21) for 5 min. Sections were examined with a JEM-1200EX (JEOL) electron microscope at 80 kV.
For immunoelectron microscopy, samples were fixed with 0.5% glutaraldehyde and 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) at 4 °C. Afterward, these were dehydrated in a graded series of ethanol and embedded in acrylate resin (LR-White). The polymerization was performed using an ultraviolet lamp equipped machine at −20 °C. Ultrathin sections (100–120 nm) were cut using a Ultracut-UCT (Leica) and pre-treated with 2% BSA. They were subsequently incubated with anti-insulin antibody (diluted 1:100 with 2% BSA in PBS) at 4 °C overnight and then with colloidal gold-conjugated (15 nm) anti-guinea pig IgG (BB International) at a dilution of 1:100 with 2% BSA. The sections were washed with distilled water prior to staining with 2% uranyl acetate for 5 min following by a lead staining solution (21) for 1 min.
Animal Experiments and Vector Injection
Immunodeficient, 4-week-old nude mice (BALB/cAJc1-nu/nu) were purchased from Clea Japan. A single dose of 200 mg/kg STZ was injected intraperitoneally (15, 22). Depletion of β-cells was confirmed by immunohistochemical staining of the pancreas as well as by detection of severe hyperglycemia. After 7 days, mice that showed hyperglycemia (almost 70% of STZ-injected mice) were randomly divided into experimental groups (n = 6) that received 6 × 1010 plaque-forming units/mouse of AdAmy2TRα or AdAmy2LacZ, which are both controlled by the amylase2 promoter. Mice were anesthetized, and adenovirus was injected through the ampulla of Vater, also known as the hepatopancreatic ampulla, into the pancreatic duct. The abdomen was closed by using 2 layers of 4-0 vicryl sutures. T3 (0.25 μg/g body weight) was intraperitoneally injected once a day for 3 days. At day 4 following pancreatic duct injection, blood glucose levels or plasma insulin concentrations were analyzed as described (15), and organs were removed from mice, fixed in 10% buffered formalin, and subsequently embedded in paraffin. Intraperitoneal glucose tolerance tests in STZ-treated mice were analyzed as described previously (15). At day 4 following adenovirus injection, mice were intravenously injected with a 5-bromo-2′-deoxyuridine (BrdU) labeling reagent, and BrdU incorporation was analyzed according to the manufacturer's protocol (Roche Applied Science).
Statistics
Data are expressed as means ± S.D. Statistical analysis was performed by using one-way ANOVA or the unpaired two-tailed Student's t test. Probability (23) values <0.05 were considered to be significant.
RESULTS
Interaction of TRα with PI3K
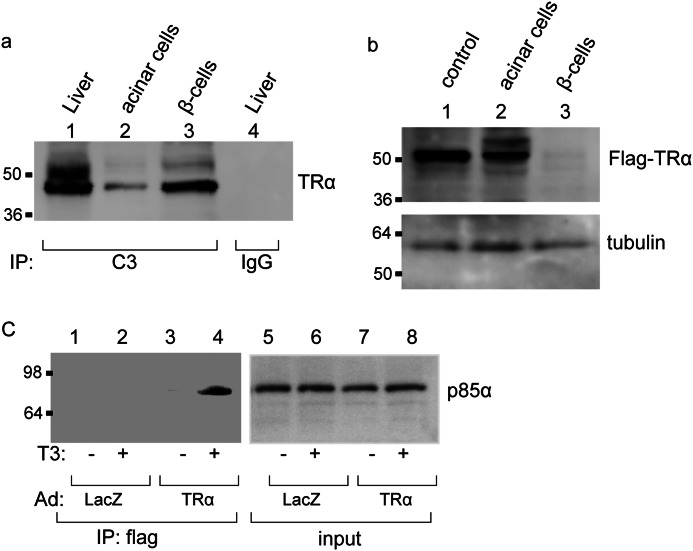
We first analyzed the expression of endogenous TRα in the pancreatic acinar or β-cells by coimmunoprecipitation of the whole cell lysate with a monoclonal antibody that recognizes the TR C-terminal region or with control mouse IgG. The immunoprecipitation was followed by Western blot analysis with antibody against an N-terminal TRα peptide. As shown in Fig. 1a, TRα protein expression was clearly observed in islets, but the TRα protein level was diminished in acinar cells. There was no signal for TRα in mouse IgG-precipitated samples of liver cells. These findings indicate that the expression levels of TRα are different between pancreatic acinar cells and β-cells and that large amounts of TRα are present in insulin-producing cells. In contrast, little TRα was expressed in pancreatic acinar cells.
FIGURE 1.
Ligand-dependent interaction of TRα with PI3K. a, cell lysates (500 μg) prepared from acinar cells or β-cells were immunoprecipitated (IP) with 5 μg of mouse monoclonal anti-TRα antibody, which recognizes the TRα C terminus (C3) or 5 μg of normal mouse IgG. Then, Western blot analysis of the precipitates was performed by using an antibody against an N-terminal TRα peptide. The same amount of protein extract prepared from the liver was used as a positive control. b, to analyze the specific expression of AdAmy2TRα, 10 μg of cell lysates prepared from AdAmy2TRα-infected acinar cells was immunoblotted by anti-FLAG antibody. The same amount of protein lysates from acinar cells infected with cytomegalovirus promoter-driven flag-tagged TRα-expressing adenovirus (15) was used as a positive control. c, AdAmy2TRα- or control virus-infected acinar cells were treated with or without 100 nm T3 for 24 h. Cell lysates (500 μg) were immunoprecipitated with anti-FLAG antibody or normal IgG, and then the immunoprecipitate or 10 μg of lysate was immunoblotted by anti-p85α antibody.
To explore the effects of TRα on the reprogramming of pancreatic acinar cells, we constructed adenovirus vector that expresses AdAmy2TRα. Ficoll-purified acinar cells or β-cells were infected with AdAmy2TRα at an m.o.i. of 30. After 48 h of incubation, the specific expression of FLAG-tagged TRα in purified acinar cells but not in AdAmy2TRα-infected β-cells was shown by Western blot analysis (Fig. 1b).
Several reports suggested that activation of EGF signaling induces transdifferentiation of pancreatic exocrine cells into endocrine cells (5, 20). To understand the functional consequences of ligand-bound TRα with downstream signaling molecules of the EGF receptor, we focused on PI3K. Previous reports indicated that TRα binds to the regulatory subunit of PI3K, p85α, and activates the PI3K/Akt pathway in vascular endothelial cells (11). To determine whether ligand-bound TRα can interact with p85α in pancreatic exocrine cells, we performed coimmunoprecipitation studies with anti-FLAG and anti-p85α antibodies (Fig. 1c). The expression levels of endogenous p85α are no different in the lysates of purified acinar cells infected with AdAmy2TRα with or without 100 nm T3 treatment. By using the coimmunoprecipitation assay, we found that TRα and endogenous p85α are associated in a T3-dependent manner in pancreatic acinar cells.
Ligand-bound TRα Enhances the Expression of Transcription Factors Critical in Pancreatic Development
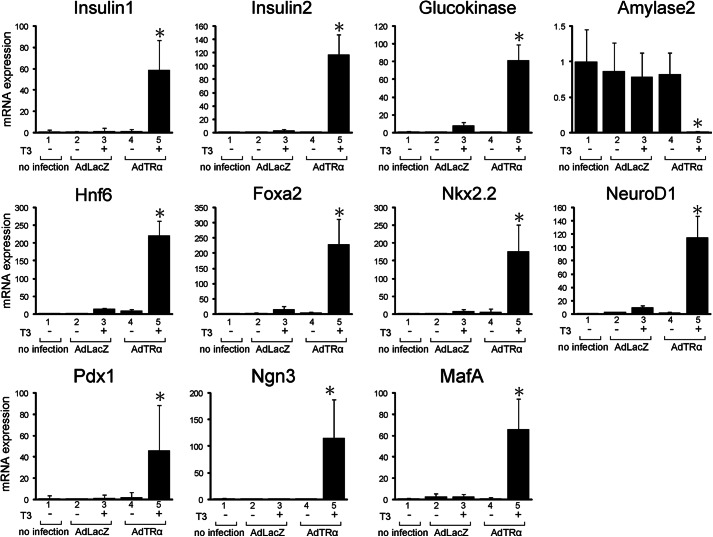
To identify the role of TRα in the reprogramming of pancreatic β-cells, purified pancreatic acinar cells were infected with Ad Amy2TRα and treated with or without T3. The expression of transcription factors that are involved in the differentiation of pancreatic endocrine cells was then analyzed by quantitative RT-PCR (Fig. 2 and Table 1). Infection with Ad Amy2TRα and incubation with T3 for 72 h significantly enhanced the mRNA expression of insulin1, insulin2, and glucokinase and induced the expression of transcription factors expressed at high levels in the early developing pancreas, such as Hnf6, Foxa2, Nkx2.2, NeuroD1, Pdx1, Ngn3, and MafA. In contrast, the expression level of amylase2 was decreased in the AdAmy2TRα-infected exocrine cells treated with T3. These results indicate that ligand-bound TRα specifically induced the genes characteristic of pancreatic β-cells and suppressed the expression of amylase, which is specifically expressed in pancreatic exocrine cells.
FIGURE 2.
Reprogramming of pancreatic acinar cells induced by AdAmy2TRα. mRNA levels for 11 genes associated with the differentiation of β-cells were assayed in AdAmy2TRα-infected acinar cells treated with 100 nm T3. From four independent samples, total RNA was isolated. The amounts of insulin1, insulin2, glucokinase, amylase2, Hnf6, Foxa2, Nkx2.2, NeuroD1, Pdx1, Ngn3, and Mafa mRNA were determined by quantitative real-time RT-PCR with 100 ng of cDNA in triplicate. Relative quantification of target cDNA was determined by arbitrarily setting the control value from untreated samples to 1. All data are expressed as the mean ± S.D. (error bars). *, p < 0.05 compared with untreated cells.
TABLE 1.
mRNA expression levels induced by ligand-bound TRα
mRNA for 11 genes associated with the differentiation of β-cells was measured in AdAmy2TRα-infected acinar cells treated with T3. From four independent samples, total RNA was isolated. Amounts of mRNA were determined by quantitative real-time RT-PCR with 100 ng of cDNA in triplicate. Relative quantification of target cDNA was determined by arbitrarily setting the control value from untreated samples to 1. All data are expressed as the mean ± S.D.
| Time | Insulin1 | Insulin2 | Glucokinase | Amylase | Hnf6 | Foxa2 | Nkx2.2 | NeuroD1 | Pdx1 | Ngn3 | MafA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1.0 ± 1.5 | 1.0 ± 0.7 | 1.0 ± 0.5 | 1.0 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.5 | 1.0 ± 0.9 | 1.0 ± 0.3 | 1.0 ± 2.5 | 1.0 ± 1.4 | 1.0 ± 0.4 |
| 24 h | 12 ± 10.7 | 8 ± 3.5 | 44 ± 32.8 | 0.3 ± 0.3 | 48 ± 18.0 | 16 ± 25.2 | 502 ± 23.0 | 170 ± 21.0 | 68 ± 14.5 | 478 ± 21.1 | 588 ± 21.1 |
| 48 h | 25 ± 24.1 | 45 ± 36.4 | 48 ± 42.1 | 0.1 ± 0.1 | 48 ± 13.2 | 43 ± 33.7 | 286 ± 89.0 | 121 ± 53.3 | 98 ± 24.6 | 195 ± 15.6 | 189 ± 43.2 |
| 72 h | 59 ± 27.9 | 117 ± 29.9 | 81 ± 18.3 | 0.0 ± 0.0 | 222 ± 40.1 | 230 ± 82.6 | 177 ± 74.2 | 115 ± 32.5 | 46 ± 13.2 | 116 ± 24.5 | 66 ± 28.1 |
Activation of PI3K Signals Required for Reprogramming of Acinar Cells
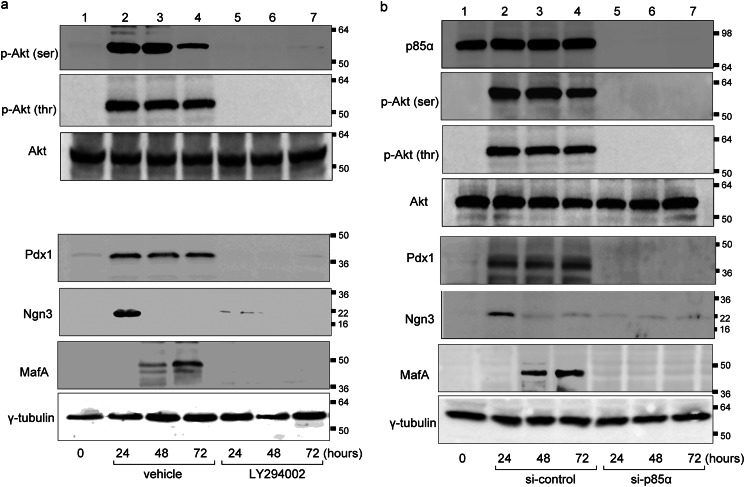
We then investigated cellular signaling of PI3K in the ligand-bound TRα-associated reprogramming of purified pancreatic acinar cells. Ligand-bound TRα-activated exocrine cells were treated with or without LY294002, a specific inhibitor of PI3K. AdAmy2TRα-infected acinar cells (30 m.o.i.) were treated with 100 nm T3 in the presence or absence of LY294002. After 24, 48, or 72 h, cells were collected, and the expression of phosphorylated Akt, total Akt, Pdx1, Ngn3, and MafA was examined by Western blot analysis. Phosphorylated Akt was markedly expressed in pancreatic acinar cells after 24 and 48 h of incubation with ligand-bound TRα (Fig. 3a). Akt was not activated in LY294002-treated cells. There was no difference in the abundance of total Akt protein between cells with or without LY294002 treatment. The expression of Pdx1, Ngn3, and MafA was induced after 24 h of incubation with ligand-bound TRα. Pdx1, Ngn3, and MafA were not induced in LY294002-treated cells.
FIGURE 3.
Activation of PI3K pathway in AdAmy2TRα-infected exocrine cells. a, expression levels of phosphorylated Akt, total Akt, Pdx1, Ngn3, and MafA protein in AdAmy2TRα-infected cells exposed to 100 nm T3 for 24, 48 or 72 h with or without LY294002 treatment. b, effects of siNRA knockdown of p85α (or control siRNA) on AdAmy2TRα-infected cells incubated with 100 nm T3 for 24, 48, or 72 h and analyzed by Western blotting of cell extracts. Loading controls for γ-tubulin are shown in the bottom panel. Western blot analysis was performed with 20 μg of protein.
To examine the effect of p85α protein depletion in AdAmy2TRα-induced reprogramming of acinar cells, we performed siRNA-mediated p85α knockdown. The protein abundance of p85α was completely diminished in acinar cells transfected with p85α-targeted siRNAs compared with control siRNA-transfected cells (Fig. 3b). In control siRNA-transfected acinar cells, AdAmy2TRα induced the expression of phosphorylated Akt, Pdx1, MafA, and Ngn3 by Western blot analysis. The levels of phosphorylated Akt, Pdx1, MafA, and Ngn3 were significantly reduced in AdAmy2TRα-infected acinar cells transfected with p85α-targeted siRNAs.
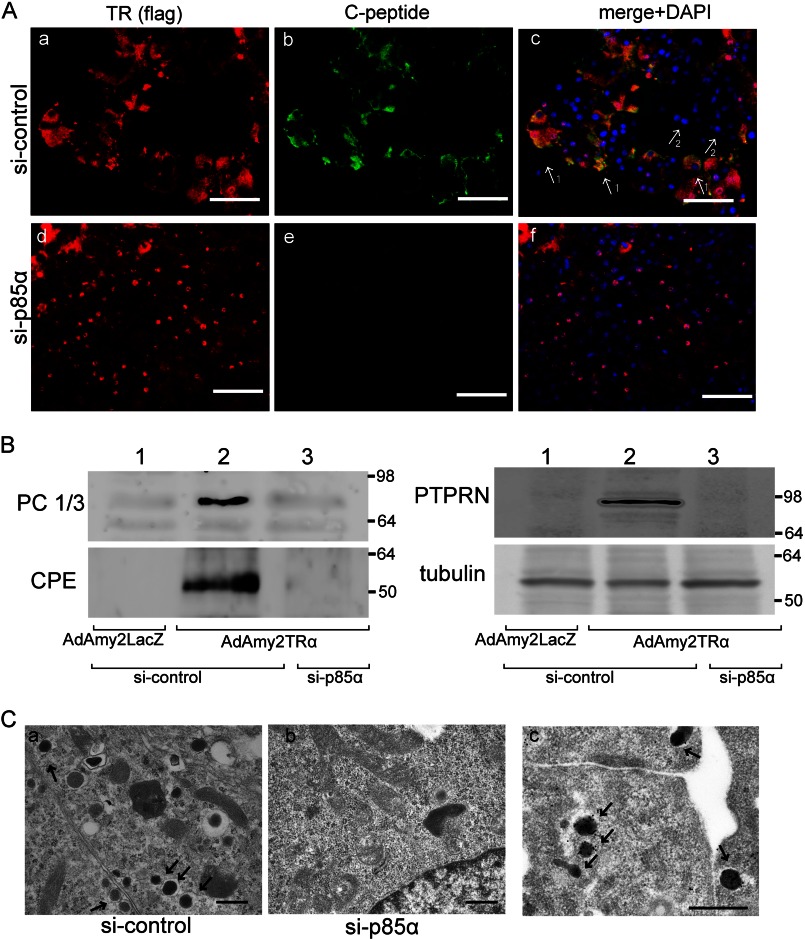
Immunochemical analysis indicated that the efficiency of viral infection was 74.2% ± 12.4% after 72 h of incubation. Ligand-bound TRα induced the expression of C-peptide in the cytoplasm (Fig. 4Ac), whereas C-peptide expression was not induced in the cells with no TRα signal (Fig. 4Ag, arrow 1 versus 2). The TRα-induced reprogramming of acinar cells did not occur in cells that were transfected with p85α-targeted siRNAs (Fig. 4Ad).
FIGURE 4.
Reprogramming of AdAmy2TRα-infected acinar cells. A, AdAmy2TRα-infected cells transfected with control siRNA (a–c) or p85α siRNA (d–f) and incubated with 100 nm T3 for 48 h were analyzed by immunostaining. The FLAG-tagged TRα-expressing cells were visualized by using anti-FLAG antibody and Alexa Fluor 555-conjugated secondary antibody (red). C-peptide-positive mature acinar cells were visualized with Alexa Fluor 488 (green). The 1 arrows identify the FLAG-TR TRα-expressing cells, which express insulin. The 2 arrows identify insulin-negative cells, which do not express FLAG-tagged TRα. B, the expression of prohormone convertase 1/3 (PC 1/3), carboxypeptidase E (CPE), or receptor-type tyrosine-protein phosphatase-like N (PTPRN) in AdAmy2TRα-infected cells transfected with control siRNA or p85α siRNA was analyzed by Western blotting. Loading controls for γ-tubulin are shown in the lower panel. Western blot analysis was performed with 20 μg of protein. C, electron microscopic analysis of AdAmy2TRα-infected cells transfected with control siRNA (a) or p85α siRNA (b) was performed. Scale bars, 500 nm. Insulin immunoreactivities were detected in the secretory granules in AdAmy2TRα-infected cells transfected with control siRNA (c). Scale bar, 500 nm. The arrows identify gold particles.
Western blot analysis showed that AdAmy2TRα infection in control siRNA-transfected acinar cells induced the expression of prohormone convertase 1/3, carboxypeptidase E, and receptor-type tyrosine-protein phosphatase-like N, which are critical for the secretion of insulin-containing secretory granules (Fig. 4B). The expression of these proteins was significantly diminished in AdAmy2TRα-infected acinar cells transfected with p85α-targeted siRNAs. Furthermore, at the ultrastructural level, the reprogrammed cells possess the small dense secretory granules characteristic of insulin granules (Fig. 4Ca, arrow), and insulin immunoreactivity was localized in the granules. In contrast, insulin granules were not observed in AdAmy2TRα-infected acinar cells transfected with p85α-targeted siRNAs (Fig. 4Cb), nor do the reprogrammed cells express any other pancreatic hormones such as glucagon, somatostatin, pancreatic polypeptide, or ghrelin (data not shown). These results indicate that the interaction between TRα and p85α plays a critical role in the activation of PI3K signaling and induces insulin biosynthesis in mature pancreatic acinar cells.
Reprogramming of Acinar Cells to Insulin-producing Cells
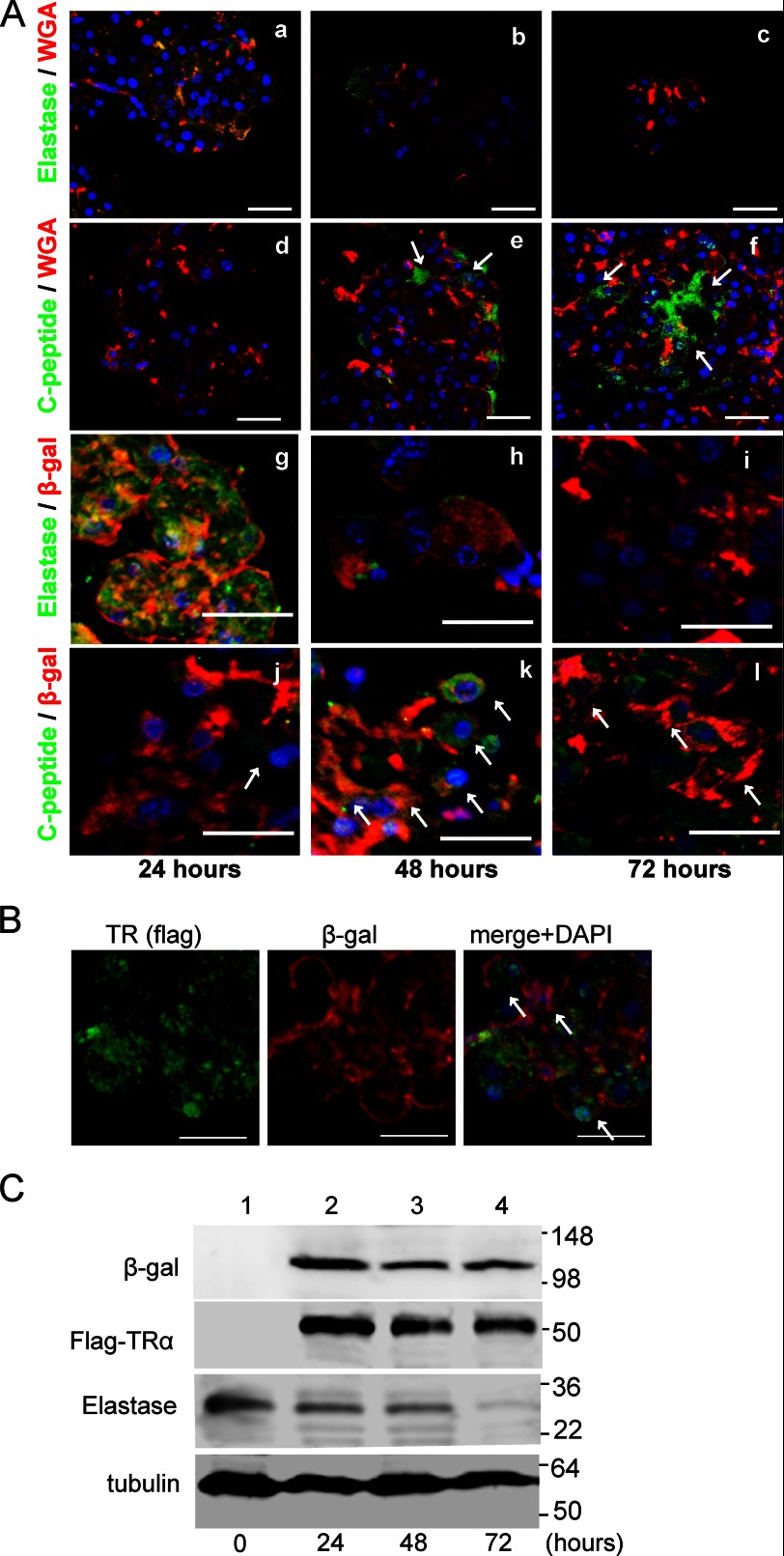
The lectin WGA binds to N-acetylglucosamine and remains in the cytoplasm of pancreatic acinar cells (20). To visualize acinar cells, the purified exocrine fraction was incubated with fluorescein-conjugated lectin and infected with adenovirus. After 24 h of incubation, WGA was localized in acinar cells, with 62.5% ± 4.8% of elastase-positive acinar cells being labeled (Fig. 5Aa). In contrast, WGA staining was <0.1% in endocrine cells that were stained with C-peptide-specific antibodies (Fig. 5Ad). There were no WGA signals in duct cells stained with cytokeratin-19 antibody, vascular cells stained with PECAM, antibodies or mesenchymal cells stained with nestin after 24 h of treatment with lectin (data not shown). These results suggest that WGA is a highly selective and efficient marker for acinar cells. After 48 and 72 h of incubation with ligand-bound TRα, the number of elastase-expressing cells was decreased (4.6% ± 1.1% and 0.3% ± 0.2%, respectively) in WGA-labeled acinar cells (Fig. 5A, b and c). Otherwise, the number of C-peptide-expressing cells was significantly enhanced in WGA-labeled exocrine cells infected with AdAmy2TRα and treated with T3 for 48 and 72 h (35.2% ± 5.5% and 78.6% ± 13.2%, respectively) (Fig. 5A, e and f).
FIGURE 5.
A, cell lineage tracing by a lectin-associated system or Cre/loxP-based system. AdAmy2TRα-infected pancreatic acinar cells (m.o.i. 30) were stained for elastase (a marker of mature exocrine cells) and C-peptide and were analyzed by fluorescence microscopy. After 24, 48, or 72 h of incubation with 100 nm T3, Qdot 655-conjugated WGA-labeled cells (red) expressed elastase (a–c) (green) or C-peptide (d–f) (green). Pancreatic acinar cells from ROSA26-lacZ mice were labeled by infection with AdAmy2Cre. AdAmy2Cre and AdAmy2TRα-coinfected acinar cells, elastase (g–i) and C-peptide (j–l) expression was detected by using anti-elastase or anti-C-peptide antibody, respectively. Blue represents nuclear staining with DAPI. The arrows identify the insulin-producing cells, which are WGA- or β-gal-positive and express C-peptide. Scale bars, 20 μm. B, expression of FLAG-TRα and β-gal in AdAmy2TRα (30 m.o.i.)- and AdAmy2Cre (30 m.o.i.)-infected acinar cells of ROSA26-lacZ mice. After 48 h of incubation with 100 nm T3, the FLAG-tagged TRα-expressing cells were visualized by using anti-FLAG antibody and Alexa Fluor 488-conjugated secondary antibody (green). β-Gal-positive acinar cells were visualized with Alexa Fluor 555-conjugated secondary antibody (red). The arrows identify both FLAG-TRα and β-gal-positive acinar cells. C, expression of β-gal, FLAG-TRα, or elastase in AdAmy2TRα (30 m.o.i.)- and AdAmy2Cre (30 m.o.i.)-infected acinar cells of ROSA26-lacZ mice. The cells were incubated with 100 nm T3 for 24, 48, or 72 h and analyzed by Western blotting. Loading controls for γ-tubulin are shown in the bottom panel.
To confirm this observation, we generated adenovirus vector, AdAmy2Cre, in which the amylase2 promoter drives the expression of Cre recombinase, for the genetic tracing of pancreatic acinar cells. In purified acinar cells derived from ROSA26-lacZ mice, the expression of β-gal was activated by the removal of a transcriptional stop sequence by Cre recombinase. When groups of 100 AdAmy2TRα- and AdAmy2Cre-infected acinar cells were examined, 72.3% ± 4.6% (n = 3) of the acinar cells were labeled after 24 h (Fig. 5B). The expression levels of elastase were significantly decreased after 48 or 72 h of incubation (Fig. 5A, h and I, and C). In contrast, β-gal signals were observed after 72 h by immunochemistry (Fig. 5A, g–l) or Western blot analysis (Fig. 5C). After 72 h of infection with AdAmy2TRα, FLAG signals were still observed by Western blotting analysis (Fig. 5C). In immunofluorescent analysis of acinar cells from ROSA26-lacZ mice, AdAmy2TRα induced the expression of C-peptide after 24 h of incubation (Fig. 5Aj). When groups of 100 β-gal-expressing acinar cells were analyzed, 75.3% ± 11.1% (n = 3) of cells expressed C-peptide after 48 h of coinfection with AdAmy2Cre and AdAmy2TRα (Fig. 5Ak). These results demonstrate that the insulin-producing cells induced by ligand-bound TRα originated from mature acinar cells.
Adenovirus Vector-mediated TRα Gene Transfer Induces the Reprogramming of Pancreatic Exocrine Cells into Insulin-producing Cells in Vivo
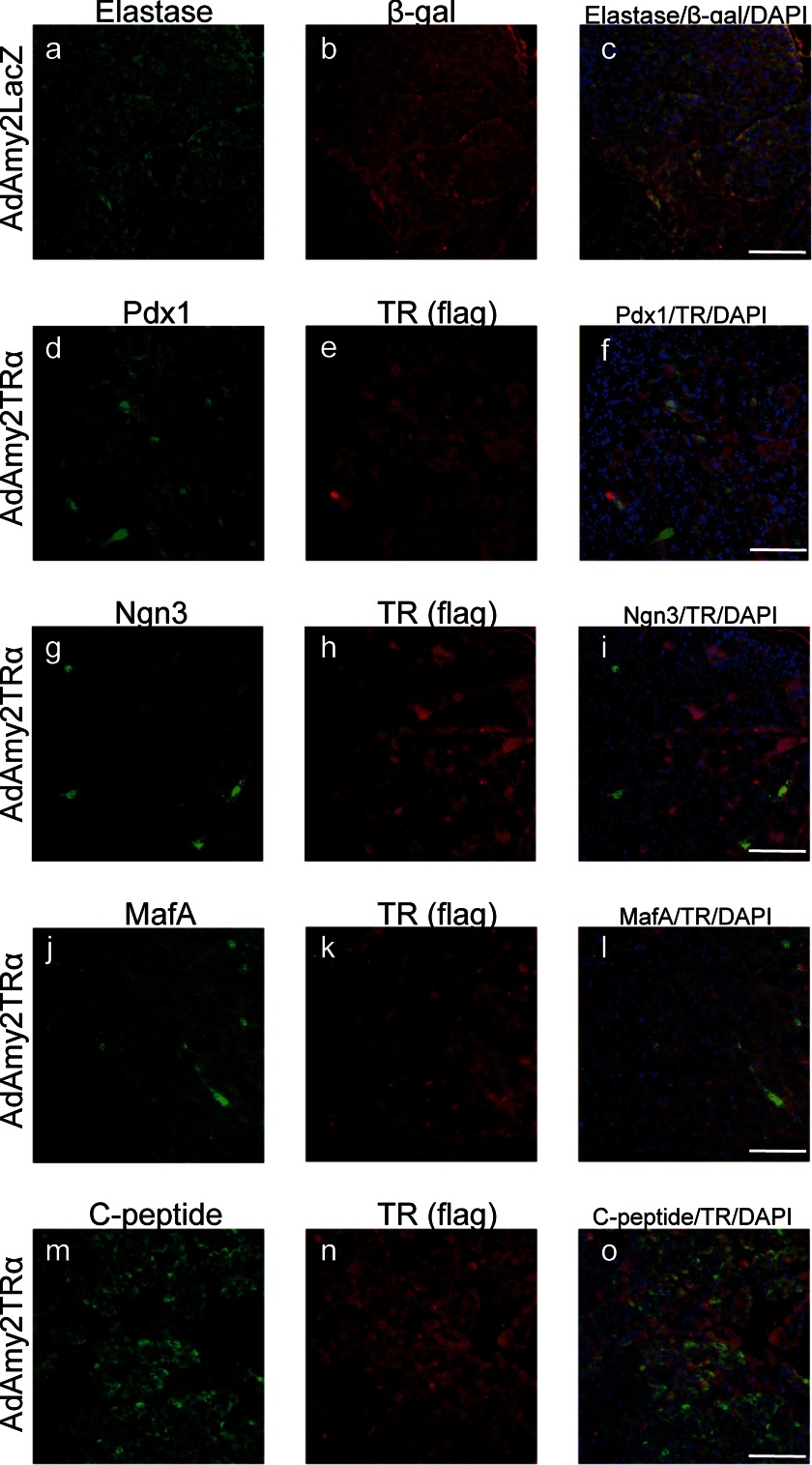
To analyze the effect of TRα on postnatal pancreas regeneration, adenovirus that expresses TRα1 or LacZ under an amylase2 promoter (AdAmy2TRα or AdAmy2LacZ, respectively) was injected into the pancreatic duct of immunodeficient mice treated with STZ. In the AdAmy2LacZ-infected pancreas, acinar cells expressed β-galactosidase and elastase (Fig. 6, a–c). These results indicate that the Amy2 promoter has specific activation in pancreatic acinar cells. The expression of Pdx1, Ngn3, MafA, and C-peptide was not observed in AdAmy2LacZ-infected acinar cells (data not shown). In AdAmy2TRα-infected pancreas, transfected FLAG-tagged TRα was localized in the nucleus and cytoplasm. Expression of Pdx-1 (d–f), Ngn3 (g–i), and MafA (j–l) protein occurred in 73.4% ± 6.3%, 68.8% ± 4.3%, and 52.6% ± 8.3% of AdAmy2TRα-infected acinar cells, respectively. Furthermore, histological analysis of AdAmy2TRα-infected pancreas demonstrated insulin production in 55.4% ± 3.3% of AdAmy2TRα-infected acinar cells that were costained with anti-FLAG antibodies and anti-C-peptide antibodies. The BrdU incorporation assay indicated that AdAmy2TRα treatment had no effect on the proliferation of pancreatic acinar cells (data not shown).
FIGURE 6.
Reprogramming of pancreatic acinar cells induced by AdAmy2TRα. Immunodeficient mice were injected with AdAmy2LacZ or AdAmy2TRα into the pancreatic duct. The marker of pancreatic exocrine cells, elastase, was visualized with anti-elastase antibody and Alexa Fluor 488-conjugated secondary antibody (green); transfected-TRα was visualized with an anti-FLAG antibody and Alexa Fluor 555-conjugated secondary antibody (red). Transcription factors that are essential for the differentiation of pancreatic endocrine cells were visualized with Alexa Fluor 488 (green). Insulin-producing cells were stained with anti-C-peptide antibody. Scale bars, 100 μm.
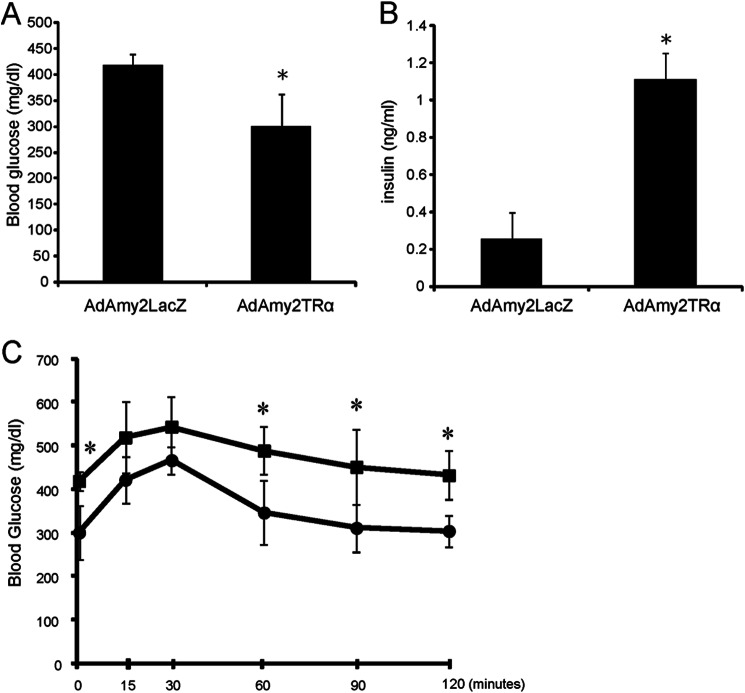
To explore the function of insulin-producing acinar cells, we analyzed the blood glucose levels and insulin levels of AdAmy2LacZ- or AdAmy2TRα-infected mice that were pretreated with STZ (Fig. 7). When subsequently injected with AdAmy2TRα, fasting blood glucose levels that showed a significant improvement compared with animals injected with control virus (n = 6) had increased insulin levels in the serum. In addition, the AdAmy2TRα-injected animals had increased glucose tolerance (Fig. 7). These results are consistent with immunochemical results in Fig. 6 that demonstrated that AdAmy2TRα-infected mice possess large numbers of insulin-producing cells. These results suggest that TRα-induced β-cells might release insulin in vivo.
FIGURE 7.
Insulin secretory properties of pancreatic exocrine-derived cells. A and B, morning postprandial levels of blood glucose (A) and plasma insulin (B) in adenovirus-injected STZ-treated mice (n = 6). All data are mean ± S.D. (error bars). *, p < 0.05 compared with AdAmy2LacZ-treated mice. C, glucose tolerance test. Glucose tolerance improved in diabetic mice after injection with AdAmy2TRα (circles) compared with control virus (squares). n = 6 animals. *, p < 0.05. Data are means ±S .D. (error bars).
DISCUSSION
We have shown that thyroid hormone can nontranscriptionally activate the PI3K/Akt pathway in pancreatic exocrine cells. In a ligand-dependent manner, TRα was shown to interact with PI3K in purified acinar cells by coimmunoprecipitation. The activation of the PI3K/Akt pathway by ligand-bound TRα led to increased expression of Pdx1, Ngn3, and MafA and reprogramming into insulin-producing cells. These results indicate that some of the transdifferentiation effects of the ligand-bound TRα are attributed to the expression of islet-specific transcription factors and insulin and suggest that the activation of PI3K by ligand-bound TRα may be therapeutically beneficial in diabetics.
We previously developed and validated an in vitro model for β-cell regeneration by stimulation of replicated β-cells with overexpressed ligand-bound TRα (15). Upon treatment with TRα-expressing adenovirus vector and T3, a large increase in the absolute number of β-cells was observed (15). The size of the β-cell mass is governed by the balance between the growth (differentiation and replication) and death (apoptosis) of these cells, but the mechanisms that sense the β-cell mass and maintain its homeostasis are largely unknown (24). It has also been reported that new β-cells arise from transdifferentiation of acinar cells, based on a genetic lineage tracing study (5, 25). Several mechanisms have been proposed to explain the process of adult β-cell mass expansion, including neogenesis from pancreatic duct cells or hematopoietic tissues, replication of highly active β-cell progenitors within the islets, and simple β-cell proliferation. In the present study, we have expanded on our previous study by showing that overexpression of ligand-bound TRα induces insulin expression in pancreatic acinar cells in accordance with the activation of PI3K signaling. These results suggest the possibility that regenerated β-cells can be derived from several pathways, including by dedifferentiation from exocrine cells.
A role for ligand-bound TRα in pancreas remodeling is supported by previous studies. Exocrine pancreas remodeling occurs at metamorphosis in the Xenopus laevis tadpole (26). Metamorphosis in X. laevis involves the remodeling of organs including the skin, brain, intestine, liver, and pancreas. The addition of thyroid hormone to the rearing water induces organ remodeling, and anti-thyroid chemicals prevent remodeling. Thyroid hormone induces dedifferentiation of the entire exocrine pancreas to a progenitor state at the climax of metamorphosis (27). These cells then redifferentiate in the frog to form a typical vertebrate pancreas. Additionally, a role for TR in diverse metamorphic programs was demonstrated based on the inhibitory properties of a dominant negative form of TR on the metamorphosis of various cell types and organs (28–30). These results support the hypothesis that ligand-bound TR has an important role in the process of reprogramming pancreatic exocrine cells into insulin-producing cells.
Previous results suggested that three transcription factors, Pdx1, Ngn3, and MafA, are important for the embryonic development of the pancreas and β-cells (3, 31). Studies on the embryonic development of the endocrine pancreas have shown that the transient expression of the basic helix-loop-helix transcription factor Ngn3 drives a proendocrine fate (32, 33). Ectopic expression of Ngn3 drives the development of proexocrine ductal cells and leads to a partial recapitulation of endocrine differentiation (34). In the developing pancreas, Ngn3 is a marker for cells that are in transit from undifferentiated epithelial progenitor cells to mature endocrine cells. Our results indicate that Pdx1, Ngn3, and MafA expression is crucial for the reprogramming of pancreatic exocrine cells induced by ligand-bound TRα. These findings suggest that AdAmy2TRα-infected acinar cells regain the properties of immature pancreatic cells.
Our present results demonstrate that the PI3K signaling pathway plays a critical role in the transdifferentiation of pancreatic exocrine cells into insulin-producing cells. In the embryonic pancreas, EGF, which is upstream of the PI3K signaling pathway, increases the number of undifferentiated endocrine precursor cells; upon removal of EGF, a large number of β-cells are differentiated (35). Therefore, PI3K signaling might be important in the proliferation of endocrine precursors and endow the cells with a commitment to the endocrine lineage. Several reports indicated that thyroid hormone activates EGF- or TGF-induced PI3K activation (36, 37). Indeed, our results suggest that overexpression of ligand-bound TRα induced the activation of the PI3K signaling pathway and transdifferentiation of exocrine cells. This conversion is demonstrated with the lectin cell tracing method.
The pancreas is composed of 2% endocrine cells and >90% exocrine cells. Both the endocrine and exocrine cells originate from a common pancreas progenitor, but the lineages diverge around the onset of the secondary transition of pancreas development. It has remained uncertain whether neogenesis of islets, by budding of new islets or islet cells from progenitor cells in the ducts, contributes to the postnatal β-cell mass. Recently, lineage tracing studies have demonstrated that during normal pancreas homeostasis in mice, new β-cells arise exclusively by proliferation of preexisting β-cells (38). We previously reported that it is possible to stimulate rodent β-cell replication, but it remains unclear whether a significant β-cell mass expansion can result from the replication. β-Cell regeneration can be studied in models where the β-cells are selectively destroyed by the injection of STZ, a DNA-alkylating agent. After infusion of β-cellulin to STZ-diabetic animals, β-cell neoformation occurs from somatostatin-positive islet cells (39). Minami et al. reported that activation of the EGF pathway induced pancreatic acinar cell transdifferentiation into insulin-secreting cells in vitro (5). Thus, transdifferentiation of pancreatic acinar cells into ductal cells (12) and insulin-producing cells (5) has been proven under certain conditions without gene transfer in vitro. However, direct evidence in vivo has been provided only for acinar-to-ductal transdifferentiation in transgenic mice having sole expression of Pdx1 in the pancreatic acinar cells (40). Herein, overexpression of ligand-bound TRα by adenovirus vector effectively induced the expression of transcription factors that are key molecular factors involved in the development of islets in pancreatic exocrine cells of diabetic mice. These results indicate that gene transfer can induce the reprogramming of pancreatic exocrine cells into insulin-producing cells in vivo.
Improved understanding of the signals regulating the growth and survival of adult β-cells remains one of the main challenges in diabetes research. The mechanisms regulating pancreatic β-cell mass are poorly understood. One goal of regenerative medicine is to instructively convert adult cells into other cell types for tissue repair and regeneration. In this study, by using a strategy of reexpressing key developmental regulators, we identified a specific combination of nuclear hormone-dependent transcription factors that reprogram pancreatic exocrine cells into cells that closely resemble β-cells. Although many additional factors are also required for β-cell development, further studies are necessary to understand the mechanism of β-cell regeneration.
Acknowledgments
We thank H. Takase (Hanaichi Ultra Structure Research Institute, Aichi, Japan) for expert assistance with electron microscopy and the staff of Life Technology Japan (Tokyo, Japan) for preparing the adenoviruses.
This work was supported by The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 24591359.
- T3
- triiodothyronine
- AdAmy2Cre
- Cre-recombinase-expressing adenovirus under control of the amylase2 promoter
- AdAmy2TRα
- amylase2 promoter-driven adenovirus vector
- m.o.i.
- multiplicity of infection
- STZ
- streptozotocin
- TR
- thyroid hormone nuclear receptor
- WGA
- wheat germ agglutinin.
REFERENCES
- 1. Edlund H. (1998) Transcribing pancreas. Diabetes 47, 1817–1823 [DOI] [PubMed] [Google Scholar]
- 2. Schwitzgebel V. M., Scheel D. W., Conners J. R., Kalamaras J., Lee J. E., Anderson D. J., Sussel L., Johnson J. D., German M. S. (2000) Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127, 3533–3542 [DOI] [PubMed] [Google Scholar]
- 3. Murtaugh L. C., Melton D. A. (2003) Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol. 19, 71–89 [DOI] [PubMed] [Google Scholar]
- 4. Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D. A. (2008) In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455, 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minami K., Okuno M., Miyawaki K., Okumachi A., Ishizaki K., Oyama K., Kawaguchi M., Ishizuka N., Iwanaga T., Seino S. (2005) Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc. Natl. Acad. Sci. U.S.A. 102, 15116–15121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lazar M. A. (1993) Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 14, 184–193 [DOI] [PubMed] [Google Scholar]
- 7. Chin W. W. (1994) Molecular mechanisms of thyroid hormone action. Thyroid 4, 389–393 [DOI] [PubMed] [Google Scholar]
- 8. Sakaguchi Y., Cui G., Sen L. (1996) Acute effects of thyroid hormone on inward rectifier potassium channel currents in guinea pig ventricular myocytes. Endocrinology 137, 4744–4751 [DOI] [PubMed] [Google Scholar]
- 9. Sun Z. Q., Ojamaa K., Coetzee W. A., Artman M., Klein I. (2000) Effects of thyroid hormone on action potential and repolarizing currents in rat ventricular myocytes. Am. J. Physiol. Endocrinol. Metab. 278, E302–307 [DOI] [PubMed] [Google Scholar]
- 10. Sen L., Sakaguchi Y., Cui G. (2002) G protein modulates thyroid hormone-induced Na+ channel activation in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 283, H2119–2129 [DOI] [PubMed] [Google Scholar]
- 11. Hiroi Y., Kim H. H., Ying H., Furuya F., Huang Z., Simoncini T., Noma K., Ueki K., Nguyen N. H., Scanlan T. S., Moskowitz M. A., Cheng S. Y., Liao J. K. (2006) Rapid nongenomic actions of thyroid hormone. Proc. Natl. Acad. Sci. U.S.A. 103, 14104–14109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Means A. L., Meszoely I. M., Suzuki K., Miyamoto Y., Rustgi A. K., Coffey R. J., Jr., Wright C. V., Stoffers D. A., Leach S. D. (2005) Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 132, 3767–3776 [DOI] [PubMed] [Google Scholar]
- 13. Minami K., Okano H., Okumachi A., Seino S. (2008) Role of cadherin-mediated cell-cell adhesion in pancreatic exocrine-to-endocrine transdifferentiation. J. Biol. Chem. 283, 13753–13761 [DOI] [PubMed] [Google Scholar]
- 14. Hafezi-Moghadam A., Simoncini T., Yang Z., Limbourg F. P., Plumier J. C., Rebsamen M. C., Hsieh C. M., Chui D. S., Thomas K. L., Prorock A. J., Laubach V. E., Moskowitz M. A., French B. A., Ley K., Liao J. K. (2002) Acute cardiovascular protective effects of corticosteroids are mediated by nontranscriptional activation of endothelial nitric-oxide synthase. Nat. Med. 8, 473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furuya F., Shimura H., Yamashita S., Endo T., Kobayashi T. (2010) Liganded thyroid hormone receptor-α enhances proliferation of pancreatic beta-cells. J. Biol. Chem. 285, 24477–24486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Samuels H. H., Stanley F., Casanova J. (1979) Depletion of l-3,5,3′-triiodothyronine and l-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105, 80–85 [DOI] [PubMed] [Google Scholar]
- 17. Dematteo R. P., McClane S. J., Fisher K., Yeh H., Chu G., Burke C., Raper S. E. (1997) Engineering tissue-specific expression of a recombinant adenovirus: selective transgene transcription in the pancreas using the amylase promoter. J. Surg. Res. 72, 155–161 [DOI] [PubMed] [Google Scholar]
- 18. Gumucio D. L., Wiebauer K., Dranginis A., Samuelson L. C., Treisman L. O., Caldwell R. M., Antonucci T. K., Meisler M. H. (1985) Evolution of the amylase multigene family: YBR/Ki mice express a pancreatic amylase gene which is silent in other strains. J. Biol. Chem. 260, 13483–13489 [PubMed] [Google Scholar]
- 19. Maylié-Pfenninger M. F., Jamieson J. D. (1979) Distribution of cell surface saccharides on pancreatic cells. II. Lectin-labeling patterns on mature guinea pig and rat pancreatic cells. J. Cell Biol. 80, 77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baeyens L., Bonné S., Bos T., Rooman I., Peleman C., Lahoutte T., German M., Heimberg H., Bouwens L. (2009) Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 136, 1750–1760 [DOI] [PubMed] [Google Scholar]
- 21. Hanaichi T., Sato T., Iwamoto T., Malavasi-Yamashiro J., Hoshino M., Mizuno N. (1986) A stable lead by modification of Sato's method. J. Electron Microsc. 35, 304–306 [PubMed] [Google Scholar]
- 22. Shifrin A. L., Auricchio A., Yu Q. C., Wilson J., Raper S. E. (2001) Adenoviral vector-mediated insulin gene transfer in the mouse pancreas corrects streptozotocin-induced hyperglycemia. Gene Ther. 8, 1480–1489 [DOI] [PubMed] [Google Scholar]
- 23. Furuya F., Hanover J. A., Cheng S. Y. (2006) Activation of phosphatidylinositol 3-kinase signaling by a mutant thyroid hormone β receptor. Proc. Natl. Acad. Sci. U.S.A. 103, 1780–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heit J. J., Karnik S. K., Kim S. K. (2006) Intrinsic regulators of pancreatic beta cell proliferation. Annu. Rev. Cell Dev. Biol. 22, 311–338 [DOI] [PubMed] [Google Scholar]
- 25. Baeyens L., De Breuck S., Lardon J., Mfopou J. K., Rooman I., Bouwens L. (2005) In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 48, 49–57 [DOI] [PubMed] [Google Scholar]
- 26. Bollin E., Jr., Carlson C. A., Kim K. H. (1973) Anuran pancreas development during thyroxine-induced metamorphosis of Rana catesbeiana: RNA metabolism during the regressive phase of pancreas development. Dev. Biol. 31, 185–191 [DOI] [PubMed] [Google Scholar]
- 27. Mukhi S., Mao J., Brown D. D. (2008) Remodeling the exocrine pancreas at metamorphosis in Xenopus laevis. Proc. Natl. Acad. Sci. U.S.A. 105, 8962–8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das B., Schreiber A. M., Huang H., Brown D. D. (2002) Multiple thyroid hormone-induced muscle growth and death programs during metamorphosis in Xenopus laevis. Proc. Natl. Acad. Sci. U.S.A. 99, 12230–12235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marsh-Armstrong N., Cai L., Brown D. D. (2004) Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 101, 165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown D. D., Cai L., Das B., Marsh-Armstrong N., Schreiber A. M., Juste R. (2005) Thyroid hormone controls multiple independent programs required for limb development in Xenopus laevis metamorphosis. Proc. Natl. Acad. Sci. U.S.A. 102, 12455–12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jensen J. (2004) Gene regulatory factors in pancreatic development. Dev. Dyn. 229, 176–200 [DOI] [PubMed] [Google Scholar]
- 32. Gu G., Dubauskaite J., Melton D. A. (2002) Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 [DOI] [PubMed] [Google Scholar]
- 33. Gradwohl G., Dierich A., LeMeur M., Guillemot F. (2000) Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. U.S.A. 97, 1607–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heremans Y., Van De Casteele M., in't Veld P., Gradwohl G., Serup P., Madsen O., Pipeleers D., Heimberg H. (2002) Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J. Cell Biol. 159, 303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cras-Méneur C., Elghazi L., Czernichow P., Scharfmann R. (2001) Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro: a balance between proliferation and differentiation. Diabetes 50, 1571–1579 [DOI] [PubMed] [Google Scholar]
- 36. Stern P. H., Krieger N. S., Nissenson R. A., Williams R. D., Winkler M. E., Derynck R., Strewler G. J. (1985) Human transforming growth factor-α stimulates bone resorption in vitro. J. Clin. Invest. 76, 2016-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shih A., Zhang S., Cao H. J., Tang H. Y., Davis F. B., Davis P. J., Lin H. Y. (2004) Disparate effects of thyroid hormone on actions of epidermal growth factor and transforming growth factor-α are mediated by 3′,5′-cyclic adenosine 5′-monophosphate-dependent protein kinase II. Endocrinology 145, 1708–1717 [DOI] [PubMed] [Google Scholar]
- 38. Dor Y., Brown J., Martinez O. I., Melton D. A. (2004) Adult pancreatic beta cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- 39. Li L., Seno M., Yamada H., Kojima I. (2003) Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to beta cells in streptozotocin-treated mice. Am. J. Physiol. Endocrinol. Metab. 285, E577–583 [DOI] [PubMed] [Google Scholar]
- 40. Miyatsuka T., Kaneto H., Shiraiwa T., Matsuoka T. A., Yamamoto K., Kato K., Nakamura Y., Akira S., Takeda K., Kajimoto Y., Yamasaki Y., Sandgren E. P., Kawaguchi Y., Wright C. V., Fujitani Y. (2006) Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through STAT3 activation. Genes Dev. 20, 1435–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]