Background: CRTC2 translocates to the nucleus upon glucagon stimulation, yet its role in regulating blood glucose remains controversial.
Results: CRTC2 promotes expression of enzymes that direct amino acids toward gluconeogenesis and is a key regulator of glucagon clearance.
Conclusion: CRTC2 has antagonistic cell-autonomous and endocrine effects on glucose homeostasis.
Significance: We present new insights into glucagon/CRTC2 physiology.
Keywords: Amino Acid, Diabetes, Gluconeogenesis, Liver, Physiology, CRTC2, Glucagon
Abstract
cAMP-responsive element-binding protein (CREB)-regulated transcription coactivator 2 (CRTC2) regulates transcription of gluconeogenic genes by specifying targets for the transcription factor CREB in response to glucagon. We used an antisense oligonucleotide directed against CRTC2 in both normal rodents and in rodent models of increased gluconeogenesis to better understand the role of CRTC2 in metabolic disease. In the context of severe hyperglycemia and elevated hepatic glucose production, CTRC2 knockdown (KD) improved glucose homeostasis by reducing endogenous glucose production. Interestingly, despite the known role of CRTC2 in coordinating gluconeogenic gene expression, CRTC2 KD in a rodent model of type 2 diabetes resulted in surprisingly little alteration of glucose production. However, CRTC2 KD animals had elevated circulating concentrations of glucagon and a ∼80% reduction in glucagon clearance. When this phenomenon was prevented with somatostatin or a glucagon-neutralizing antibody, endogenous glucose production was reduced by CRTC2 KD. Additionally, CRTC2 inhibition resulted in reduced expression of several glucagon-induced pyridoxal 5′-phosphate-dependent enzymes that convert amino acids to gluconeogenic intermediates, suggesting that it may control substrate availability as well as gluconeogenic gene expression. CRTC2 is an important regulator of gluconeogenesis with tremendous impact in models of elevated hepatic glucose production. Surprisingly, it is also part of a previously unidentified negative feedback loop that degrades glucagon and regulates amino acid metabolism to coordinately control glucose homeostasis in vivo.
Introduction
CRTC23 is a coactivator of CREB that enhances CREB binding to target cAMP-responsive elements (1), as well as specifying cAMP response genes (2). In response to glucagon, CRTC2 is dephosphorylated (1) and CREB-phosphorylated (3), allowing these factors to translocate to the nucleus to orchestrate the gluconeogenic transcriptional response. Conversely, by activating Akt2 and its downstream kinase Sik-2, insulin stimulates the phosphorylation of CRTC2 at Ser-171, leading to its exclusion from the nucleus (4). AMP kinase is similarly reported to phosphorylate CRTC2 at Ser-171 to prevent its nuclear translocation (5, 6). In type 2 diabetes mellitus (T2DM), however, insulin receptor desensitization precludes the effective activation of Akt2 and Sik-2, resulting in inappropriate nuclear activity of CRTC2 (4, 7). Furthermore, in states of chronic hyperglycemia, O-glycosylation of Ser-171 further prevents its inhibitory phosphorylation by Sik-2 (7).
In addition to phosphorylation and dephosphorylation at Ser-171, other post-translational modifications have also been proposed to influence the activity of CRTC2 (1, 8–10). For example, SirT1 is reported to deacetylate CRTC2 during prolonged periods of fasting, leading to its ubiquitination and degradation (6). However, the role of CRTC2 in glucose homeostasis remains controversial. For instance, a recently generated liver-specific CRTC2 knock-out exhibited normal glucose tolerance (8).
In healthy animals, induction of hepatic glucose production during fasting is regulated largely through transcription of prototypical gluconeogenic genes such as glucose-6-phosphatase (G6pase) and phosphoenolpyruvate carboxykinase (PEPCK) under the control of the transcription factors CREB and FoxO1. Similarly, in T2DM, fasting hyperglycemia is attributable to increased endogenous glucose production (11, 12), much of which results from hepatic gluconeogenesis (13). Surprisingly, however, expression of G6Pase and PEPCK correlates poorly with rates of HGP in diabetic human and rodent livers (14), even though inhibition of CREB and FoxO1 can correct this defect (15, 16). These results suggest that pathologic induction of gluconeogenesis during T2D is attributable to mechanisms other than just transcription of these prototypical, fasting-induced gene products.
Given the important role of CRTC2 in modulating CREB gene targeting, combined with evidence that gene targets beside G6pase and PEPCK control pathologic HGP during diabetes, we hypothesized that altered CRTC2 activity may be responsible for the inappropriate induction of hepatic glucose production through previously underappreciated gene targets in T2D. Here we report that CRTC2, in addition to its known role in regulating gluconeogenic gene transcription, controls both glucagon clearance and hepatic amino acid catabolism to regulate glucose metabolism.
EXPERIMENTAL PROCEDURES
Animals
The Institutional Animal Care and Use Committee (IACUC) of Yale University approved all procedures. The T2DM rat model was induced as previously reported (17). Rats were individually housed and were on a 12:12-h light/dark cycle. CRTC2 and control ASO solutions were prepared in normal saline and injected intraperitoneally twice a week at a dose of 37.5 mg/kg body weight for 4 weeks to achieve maximal knockdown. Delivery of ASO by this method has been shown to result in target knockdown in liver, white adipose tissue, kidney, and macrophages (15, 16). The T1D model was induced with a 65 mg/kg intraperitoneal streptozotocin injection into SD rats fed normal chow following 4 weeks of ASO injections. Rats were studied 3 days after streptozotocin injection. The T2DM model was created by administering 175 mg/kg nicotinamide in combination with 65 mg/kg streptozotocin followed by high fat feeding (55% kcal from fat; Harlan Teklad 93075) for 4 weeks to obtain insulin resistance with mild β-cell dysfunction, as described in (17, 18). Rats were assigned to CRTC2 versus control ASO groups following streptozotocin/nicotinamide treatment by matching semi-fed glucose levels as described previously (17). For mouse studies, C57BL/6 mice (6–8 weeks old, Jackson Laboratories) were injected with 40 μg glucagon/mouse and sacrificed 2 h later for hepatic harvest and subsequent quantitative PCR analysis. Glucagon bioactivity was confirmed by verifying hyperglycemia 20 min after injection.
Selection of CRTC2 ASO
To identify rat CRTC2 antisense inhibitors, rapid-throughput screens were performed in primary rat hepatocytes. In brief, ∼80 ASOs were designed to target a binding site against the CRTC2 mRNA sequence. The reduction of target gene expression was analyzed with real time quantitative RT-PCR after transfection of the cells with 165 nm ASOs for 24 h. Based on target reduction, 8 ASOs were selected and further characterized in a dose-response screen. The two most potent ASOs from the screen were chosen, and their in vivo activity was confirmed in lean Sprague-Dawley rats. The most potent ASO, ISIS 384680, 5′-GCAGTAAGGTCCCCTCACTG-3′, was chosen as the CRTC2 ASO for subsequent studies. All of the ASOs screened have a uniform phosphorothioate backbone and a 20-base chimeric design with 2′-O-(methoxy)-ethyl (2′-MOE) modification on the first five and last five bases. This modification enhances their binding affinity to complementary sequences and their resistance to the action of nucleases. A negative control ASO (ISIS 141923), which has the same chemical composition as the CRCT2 ASO but no complementarity to any known gene sequence, was also included in the studies.
Glucose Metabolism Studies
Prior to the clamp studies, catheters were inserted into the right internal jugular vein to the right atrium and left carotid artery extending into the aortic arch on week 3 of the ASO injections. Rats were given 1 week to recover from the surgery. All infusions were administered via the arterial cannula, and all sampling was obtained via the jugular cannula. For the glucose metabolism studies, at 6 p.m., rats were fasted overnight, and in the morning (6 a.m.), rats were infused with 99% [6,6-2H]glucose (1.1 mg/kg prime, 0.1 mg/kg) to assess the basal glucose turnover. After the basal period, the hyperinsulinemic-euglycemic clamp was conducted until euglycemia (∼100 mg/dl) was reached infusing 20% dextrose spiked with 2.5% [6,6-2H]glucose with a primed/continuous infusion of insulin into the carotid artery (400 milliunits/kg primed over 5 min, 4 microunits/kg per min of constant infusion) to reach a hyperinsulinemic state (100 microunits/ ml). The somatostatin studies were performed as described above for the clamp studies except somatostatin-14 was infused following the basal period (20 μg/kg prime for 5 min, 2 μg/kg per min of infusion) for a second basal period, and during the hyperinsulinemic-euglycemic clamp. Once rats maintained euglycemia for 30 min, plasma samples were taken for clamp calculations. The hepatic glucose production was calculated using the rate of infusion of [6,6-2H]glucose over the atom percent excess in the plasma minus the rate of glucose being infused. The insulin-stimulated whole body glucose uptake was calculated by adding the total glucose infusion rate plus the hepatic glucose production. For the glucose tolerance tests, rats were fasted the previous night at 6 p.m. The following morning rats were injected with 0.75 g/kg glucose and 1 ml of normal saline in to the jugular vein. Blood samples were taken at 1, 2, 5, 10, 20, 30, and 45 min for glucose, insulin, and glucagon. After the completion of the clamp or glucose tolerance test, sodium pentobarbital was injected via the venous catheter administered at 150 mg/kg. After rats were completely anesthetized, tissues were extracted and frozen with the use of liquid cooled N2 tongs. The samples were stored at −80 °C until further analysis. Plasma was obtained for glucagon or glucagon-like peptide-1 measurements using tubes containing 500 kallikrein-inactivating units of aprotinin per ml of blood.
Glucagon Antibody
T2D rats were fasted overnight, and the following morning basal blood glucose was extracted from the jugular vein catheter. Subsequently, 4 mg/kg glucagon antibody (NovoNordisk) was injected into the jugular vein. After 2 h blood glucose values were measured.
Glucagon Clearance
Somatostatin-14 (2 μg/kg per min) was infused through an arterial placed catheter for 2 h. Subsequently glucaGen (Bedford Laboratories) dissolved in 1% BSA/0.9% saline, was coinfused with somatostatin at 2 ng/kg per min. Blood samples were drawn prior and throughout the 2-h glucagon infusion. The final plasma glucagon value minus the initial plasma glucagon value after the somatostatin infusion was considered the change in plasma glucagon from base line.
Biochemical Analysis and Calculations
Plasma glucose values were determined using a Beckman Glucose Analyzer II (Beckman Coulter) by a glucose oxidase method, YSI 2700 Select (YSI), or an AlphaTRAK blood glucose monitoring system (Abbott). Plasma insulin, glucagon, and corticosterone concentrations were determined by a radioimmune system kit (Linco). Plasma total glucagon-like peptide-1 (7–36 and 7–37) concentrations were measured using a commercially available kit (Meso Scale Diagnostics) for a Sector Imager 2400A, model 1250 (Meso Scale Diagnostics). The enrichment of plasma glucose was determined as described previously (17).
Liver Triglycerides
Triglycerides were extracted from the method of Bligh and Dyer (19) and measured with the use of a commercially available triglyceride kit (DCL Triglyceride Reagent; Diagnostic Chemicals Ltd.).
Total RNA Preparation, Real Time Quantitative RT-PCR, and Western Blot Analysis
RNA was extracted with a Qiagen RNeasy kit (Qiagen). The mRNA was transcribed to cDNA using MuLV reverse transcriptase (New England Biolabs) following treatment with DNase I to eliminate genomic DNA. The abundance of transcripts was assessed by real time PCR on a 7500 Fast Real-Time PCR System (Applied Biosystems). Each run was evaluated in duplicate for both the gene of interest and 18S or actin as a control. The expression data for the gene of interest and 18S or actin were normalized for the efficiency of amplification determined by the standard curve included for each data acquisition. Primer sequences are as following: 18S forward, 5′-TCCGATAACGAACGAGACTC-3′ and reverse, 5′-TGGCTGAACGCCACTTGTC-3′; actin forward, 5′-CCAGATCATGTTTGAGACCTTC-3′ and reverse, 5′-CATGAGGTAGTCTGTCAGGTCC-3′; CRTC2 forward, 5′-GAGTACCTGGCTTTGAGGTGTCA-3′ and reverse, 5′-CATGCGCAACTCATCTTCCA-3′; G6Pase catalytic forward, 5′-GAAGGCCAAGAGATGGTGTGA-3′ and reverse, 5′-TGCAGCTCTTGCGGTACATG-3′; G6Pase translocase forward, 5′-GGCCAGTTCTTCCTTATCCA-3′ and reverse, 5′-CCTACAAGGCCTCCAACCT-3′; FBPase forward, 5′-AATGAGCCTTCGGAGAAAGA-3 and reverse, 5′-CAGTTGACGCCACAATTCAT-3′; PEPCK forward, 5′-TCGCCCCTTCCCGCT-3′ and reverse, 5′-CAGCATGCTTGCTGGTTT-3′; PGC-1α forward, 5′-TCTGGAACTGCAGGCCTAACTC-3′, and reverse, 5′-GCAAGAGGGCTTCAGCTTTG-3′; FGF21 forward, 5′-GCAAAGGCTCTACCATGCTC-3′ and reverse, 5′-AGGCCTGCAGTTTCAGAGAG-3′; SDS forward, 5′-CACTGGCCTCGCTGGTTGTCATT-3′ and reverse, 5′-GTGGCCAGGGCAGCAGCAGAT-3′; GPT1 forward, 5′-GTGCGGGTTTCGTGGTGGCTATGT-3′ and reverse, 5′-GGACGGCTCGGAGGGTGTTGG-3′, PC forward, 5′-ATCCAGCGGCGGCACCAGA-3′ and reverse, 5′-GCGGGAATTGACCTCGATGAAGTA-3′, CTH forward, 5′-CAGAGCCGGAGCAATGGAGTT-3′ and reverse, 5′-AAGGCCCCGAGCGAAGGTCA-3′; GOT1 forward, 5′-CCTGGGCCTGGCGGAGTT-3′ and reverse, 5′-ACGGGCGTGTTCTTGTTGTCTGTG-3′; TBP forward, 5′-CTTACGGCACAGGGCTTACT-3′ and reverse, 5′-GTGTGGGCTGCTGAGATGTTG-3′; HPRT forward, 5′-GCCAAGTACAAAGCCTAAAAGACA-3′ and reverse, 5′-AAAAGGGACGCAGCAACAGACATT-3′; HSP90 forward, 5′-TTGCCCAGTTAATGTCCTTGAT-3′ and reverse, 5′-TCCTTCCCCGAGTCCAGTT-3′; GUSB forward, 5′-GGGCCCCTGACCACCTTC and reverse, 5′-GCAGCCCCGCATAGTTGA-3′; RPL13a forward, 5′-CTGGGCCGAAAGGTGGTGGTTGT-3′ and reverse, 5′-TAGGGGCCTCGAGACGGGTTGG-3′.
Western Blotting
Liver tissue was lysed in radioimmuneprecipitation assay buffer supplemented with EDTA-mini protease inhibitor mixture tablets (Roche Applied Science), sodium orthovanadate, sodium fluoride, EDTA, EGTA, and DTT and diluted to a protein concentration of 1 mg/ml in Laemmli buffer. 15 μg of total protein was run on SDS-PAGE, transferred to nitrocellulose membranes, and probed for CRTC2 (Calbiochem/Millipore), GPT1 (Novus), and actin (Cell Signaling).
RESULTS
To evaluate the role of CRTC2 in glycemic control, we administered an ASO by intraperitoneal injection to reduce CRTC2 expression in rodent liver, white adipose tissue, and kidneys. To validate the CRTC2 ASO, we first examined the effect of CRTC2 knockdown (KD) on gluconeogenesis in a hyperglycemic T1D rat model created by treating rats with the β-cell toxin streptozotocin (STZ). As expected, streptozotocin treatment led to a dramatic reduction in plasma insulin concentrations (Fig. 1B). This reduction in insulin was associated with a large increase in plasma glucose concentrations (Fig. 1A), resulting from a 150% increase in endogenous glucose production (Fig. 1D). These data were consistent with the well described function of insulin in suppressing gluconeogenesis.
FIGURE 1.
CRTC2 ASO reduces endogenous glucose production and hyperglycemia in a T1D rat model. A–C, fasting plasma glucose (A), insulin (B), and glucagon (C). D, fasting endogenous glucose production rates calculated using stable isotopes. E–G, hepatic expression of key metabolic genes as assessed by quantitative RT-PCR (n = 6–8/group). *, p < 0.05; **p < 0.005, comparing Control ASO STZ versus Control ASO saline. $, p < 0.05; $$, p < 0.005, comparing CRTC2 ASO STZ versus Control ASO Saline. #, p < 0.05; ##, p < 0.005, comparing CRTC2 ASO STZ versus Control ASO STZ. One-way ANOVA and Tukey's Multiple Comparison Test were used. Error bars, S.E.
Treating these T1D rats with CRTC2 ASO led to an 89% decrease in hepatic CRTC2 expression compared with control ASO (Table 1) and a complete reversal of the STZ-dependent induction of the hepatic gluconeogenic genes catalytic G6Pase, FBpase, and PGC-1α (Fig. 1, E–G). These transcriptional changes lead to a 30% decrease in HGP (Fig. 1D) and similar reduction in plasma glucose concentration (Fig. 1A). Interestingly, we found that plasma glucagon concentrations tended to be higher in CRTC2 ASO-treated rats despite the persistent hyperglycemia (Fig. 1C). ASO treatment was not associated with any toxicity as shown by similar plasma alanine aminotransferase concentrations compared with control rats (Table 1).
TABLE 1.
Profiles of control ASO and CRTC2 ASO T1D rats
Data are presented as mean ± S.E. (n = 5–8 rats per group). *, p < 0.05; **, p < 0.005, comparing control ASO STZ versus control ASO saline. $, p < 0.05; $$, p < 0.005, comparing CRTC2 ASO STZ versus control ASO saline. #, p < 0.05; ##, p < 0.005, comparing CRTC2 ASO STZ versus control ASO STZ.
| Parameters | Control ASO saline | Control ASO STZ | CRTC2 ASO STZ |
|---|---|---|---|
| CRTC2 mRNA liver (relative) | 1.00 ± 0.14 | 2.10 ± 0.53* | 0.23 ± 0.06$## |
| CRTC2 mRNA white adipose tissue (relative) | 1.00 ± 0.08 | 0.92 ± 0.11 | 0.32 ± 0.05$$## |
| CRTC2 mRNA muscle (relative) | 1.00 ± 0.06 | 1.11 ± 0.04 | 1.34 ± 0.09 |
| Liver triglycerides (mg/g of tissue) | 8.8 ± 1.8 | 7.0 ± 1.1 | 6.5 ± 1.3 |
| Plasma corticosterone (ng/ml) | 219 ± 44 | 312 ± 69 | 341 ± 67 |
| Plasma triglycerides (mg/dl) | 26.1 ± 5.5 | 95.4 ± 25.4* | 30.3 ± 6.4# |
| Plasma free fatty acids (meq/liter) | 0.73 ± 0.08 | 1.68 ± 0.27* | 0.79 ± 0.12# |
| Ketone bodies (mmol/liter) | 0.89 ± 0.07 | 3.06 ± 0.50** | 1.90 ± 0.43 |
| Total cholesterol (mg/dl) | 53.1 ± 4.3 | 29.4 ± 4.6* | 42.1 ± 7.5 |
| HDL cholesterol (mg/dl) | 11.0 ± 0.7 | 8.3 ± 1.3 | 11.3 ± 1.1 |
| Plasma alanine aminotransferase (units/liter) | 20.3 ± 3.3 | 25.9 ± 1.1 | 18.8 ± 3.0 |
Similar results were obtained using CRTC2 KD in hyperglycemic Zucker diabetic fatty rats. In this model, CRTC2 ASO treatment also decreased plasma glucose concentrations, endogenous glucose production, and gluconeogenic gene transcription (data not shown). As in the T1D model, Zucker diabetic fatty rats in which CRTC2 was knocked down exhibited increased plasma glucagon concentrations.
We also tested CRTC2 ASO in a T2D rat model created by administering nicotinamide in combination with streptozotocin and high fat feeding for 4 weeks (17, 18). This model shows mild fasting hyperglycemia due to insulin resistance combined with a relative (but not absolute) insulin deficiency, but lacks the confounding effects of hypoinsulinemia or hypercorticosteronemia, which are present in leptin-deficient and leptin receptor-deficient rodents (14). In our view, this model provides the most faithful representation of human T2D.
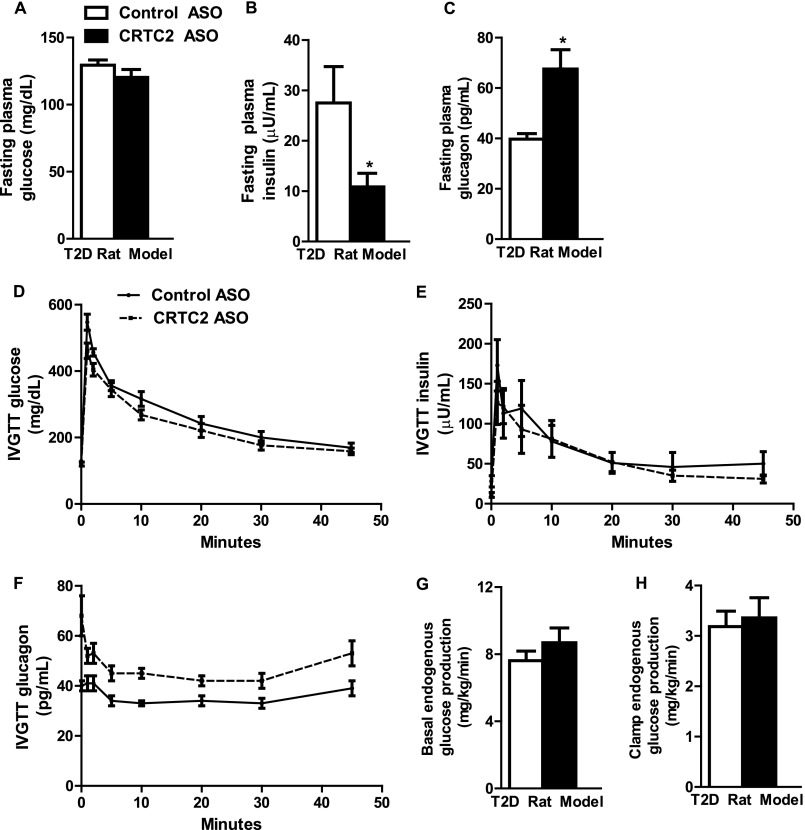
ASO treatment reduced hepatic CRTC2 expression by 94% in this model (Table 2). Surprisingly, fasting plasma glucose concentrations were unchanged by CRTC2 KD (Fig. 2A). However, plasma insulin concentrations were decreased 61% (Fig. 2B), and plasma glucagon concentrations were increased 70% (Fig. 2C). Nevertheless, we observed no improvement in glucose tolerance or reduction in endogenous glucose production in CRTC2 ASO-treated T2D rats (Fig. 2, D and E, G and H). Based on the trends we had observed in previous models toward increased plasma glucagon, we hypothesized that the lack of difference in the GTT and hyperinsulinemic-euglycemic clamp might be attributable to increased plasma glucagon concentrations induced by CRTC2 KD. Consistent with this hypothesis, we found that plasma glucagon concentrations were significantly increased throughout the glucose tolerance test (Fig. 2F; control ASO area under the curve 1597 versus CRTC2 ASO area under the curve 2019; p < 0.05).
TABLE 2.
Profiles of control ASO and CRTC2 ASO T2D rats
Data are presented as mean ± S.E. (n = 6–10 rats per group). *, p < 0.05; **, p < 0.005, comparing CRTC2 ASO versus control ASO.
| Parameters | Control ASO | CRTC2 ASO |
|---|---|---|
| CRTC2 mRNA liver (relative) | 1.00 ± 0.53 | 0.06 ± 0.14** |
| PEPCK mRNA liver (relative) | 1.00 ± 0.19 | 0.31 ± 0.09* |
| FBPase mRNA liver (relative) | 1.00 ± 0.06 | 0.61 ± 0.05** |
| G6Pase mRNA liver (relative) | 1.00 ± 0.16 | 0.60 ± 0.14 |
| Liver triglycerides (mg/g of tissue) | 11.3 ± 2.0 | 9.9 ± 0.8 |
| Plasma GLP-1 (pg/ml) | 30.3 ± 2.3 | 28.1 ± 1.1 |
| Plasma triglycerides (mg/dl) | 24.7 ± 4.0 | 19.4 ± 2.0 |
| Plasma free fatty acids (meq/liter) | 0.77 ± 0.10 | 0.69 ± 0.08 |
| Total cholesterol (mg/dl) | 48.3 ± 4.5 | 54.9 ± 7.4 |
| HDL cholesterol (mg/dl) | 18.4 ± 1.3 | 24.7 ± 3.4 |
FIGURE 2.
CRTC2 ASO treatment yields elevated plasma glucagon in a model of T2D. A–C, fasting plasma glucose (A), insulin (B), and glucagon (C) between control and CRTC2 ASO-treated T2D rats. D–F, glucose (D), insulin (E), and glucagon (F) during an intravenous glucose tolerance test. G and H, basal (G) and clamp (H) glucose production during a hyperinsulinemic-euglycemic clamp (n = 6–8/group). *, p < 0.05 Control ASO versus CRTC2 ASO; Student's t test. Error bars, S.E.
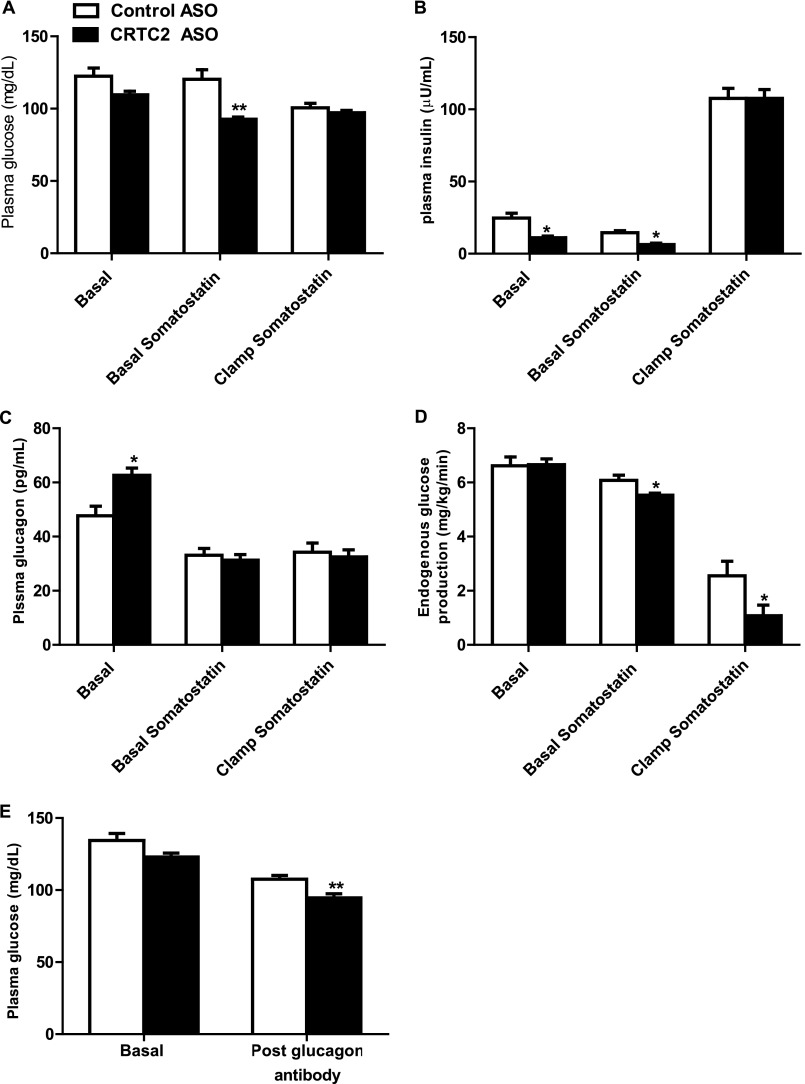
To explore the hypothesis that increased glucagon may be blunting the expected improvements in endogenous glucose production in CRTC2 ASO-treated rats, we suppressed pancreatic insulin and glucagon secretion during a hyperinsulinemic-euglycemic clamp by simultaneously infusing somatostatin in the T2D rat model. The somatostatin infusion effectively normalized the increased plasma glucagon concentrations induced by CRTC2 ASO treatment (Fig. 3C) and reduced insulin concentrations in the basal period (Fig. 3B). Accordingly, infusion of somatostatin during the basal period decreased plasma glucose concentrations 16% in the CRTC2 ASO-treated animals, but only 1% in the control ASO-treated animals (Fig. 3A), thereby supporting the hypothesis that the increased glucagon concentrations seen in CRTC2 ASO-treated rats blunted the expected reductions in glucose concentration. Compared with the surprising results seen without somatostatin infusion (Figs. 2, G and H, and 3D, basal) coinfusion of somatostatin unmasked a decrease in endogenous glucose production during the basal period and the hyperinsulinemic-euglycemic clamp in the CRTC2 ASO-treated rats compared with control-treated rats (Fig. 3D).
FIGURE 3.
Glucagon blockade unmasks metabolic improvements in a T2D model. A–D, plasma glucose (A), insulin (B), and glucagon (C) and endogenous glucose production rate (D) during the three infusion periods of hyperinsulinemic-euglycemic clamp with somatostatin coinfusion. E, plasma glucose concentrations before and after treatment with glucagon-blocking antibody (n = 4–8/group). *, p < 0.05; **, p < 0.01 Control ASO versus CRTC2 ASO; Student's t test. Error bars, S.E.
Because somatostatin affects the regulation of other glucoregulatory hormones in addition to glucagon (20) and reduces the splanchnic blood flow (21), the aforementioned results were confirmed by using an antibody specific for glucagon (22–24). After the injection of the glucagon-neutralizing antibody, fasting blood glucose concentrations were lower in the CRTC2 ASO group relative to the control ASO-treated rats (Fig. 3E). Taken together, these studies suggest that plasma glucagon was blunting the expected effects of CRTC2 KD on hepatic glucose metabolism.
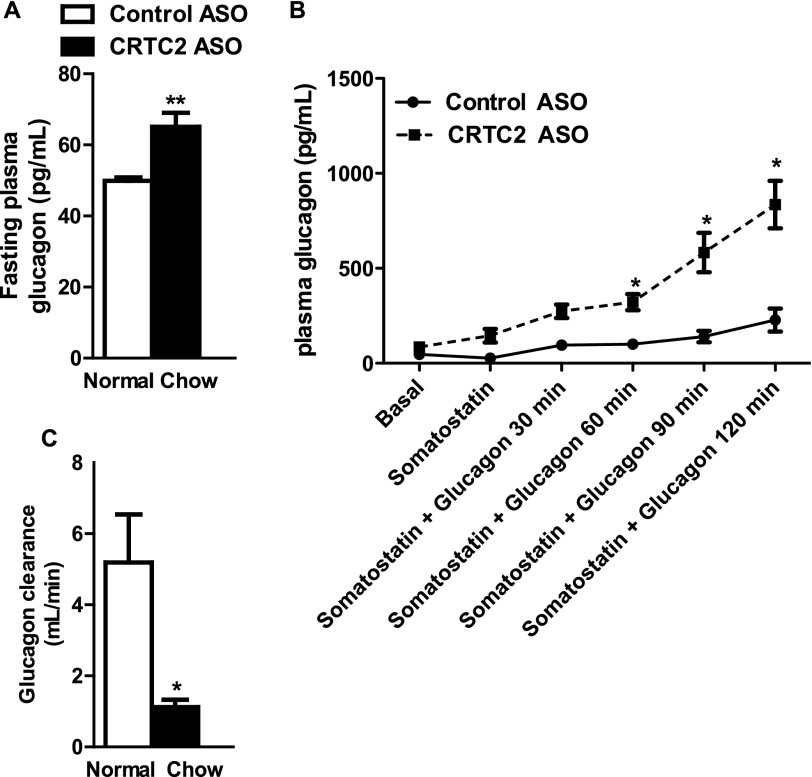
The elevated plasma glucagon observed in CRTC2 ASO-treated rats could be due to increased production or reduced clearance. We considered whether glucagon secretion could be increased, either because of unintended knockdown of CRTC2 in the pancreas, or indirectly through a mediator such as Fgf21. However, neither pancreatic CRTC2 expression nor hepatic Fgf21 expression was altered in CRTC2 ASO-treated nondiabetic (chow-fed) rats (data not shown). In normal rats, as in diabetic rats, fasting plasma glucagon was significantly increased by CRTC2 ASO treatment (Fig. 4A). When we infused glucagon in combination with somatostatin to test glucagon clearance in the absence of endogenous glucagon production, we found that the infused glucagon rapidly accumulated in the plasma of CRTC2 ASO-treated animals due to reduced clearance (Fig. 4, B and C). Poor kidney function is unlikely to have caused the reduced glucagon clearance, as blood urea nitrogen did not differ between animals treated with control ASO and CRTC2 ASO (data not shown).
FIGURE 4.
CRTC2 ASO slows glucagon clearance. A, elevated fasting plasma glucagon in normal, nondiabetic rats. B, accumulation of infused plasma glucagon in CRTC2 ASO-treated normal rats. C, glucagon clearance (n = 4–8/group). *, p < 0.05; **, p < 0.01 Control ASO versus CRTC2 ASO; Student's t test, Two-way ANOVA. Error bars, S.E.
To refine our understanding of how CRTC2 might transcriptionally regulate gluconeogenesis, we performed mRNA profiling of hepatic transcriptomes from both groups of ASO-treated normal rats. Interestingly, we found that CRTC2 ASO-treated rats showed substantially reduced expression of several enzymes involved in the conversion of amino acids to gluconeogenic intermediates. These include glutamic-oxaloacetic transaminase 1 (GOT1), pyruvate carboxylase (PC), glutamic-pyruvate transaminase (GPT1), and serine dehydratase (SDS) (Fig. 5A). These genes convert alanine, cysteine, serine, and aspartate to pyruvate and oxaloacetate (Fig. 5D). To demonstrate that these gene expression changes translated into protein expression, we examined GPT1 protein and found that it was, indeed, strongly suppressed in CRTC2 ASO-treated animals (Fig. 5B). Interestingly, no differences were observed in expression profiles of the canonical gluconeogenic genes G6Pase or PEPCK (Fig. 5A), perhaps due to redundant or alternative transcriptional control (such as by FoxO1). To further explore the regulation of these genes, we treated mice with either glucagon or vehicle. As expected, we found that many of the genes that were reduced by CRTC2 KD were induced by glucagon stimulation (Fig. 5C). This suggests that upon activation by glucagon, CRTC2 enhances the availability of amino acid-derived gluconeogenic substrates to augment hepatic glucose output. Such a hypothesis could corroborate published data showing that gluconeogenic flux is well correlated with TCA cycle activity (25). Plasma amino acids have been shown to stimulate glucagon release in the pancreatic α-cell (26) and therefore could theoretically contribute to the altered plasma glucagon concentrations observed in CRTC2 ASO-treated rats. However, we did not observe significant differences in the concentrations of key amino acid regulators of glucagon: alanine, glutamine, glycine, and phenylalanine (data not shown) between the CRTC2 ASO and control ASO.
FIGURE 5.

CRTC2 promotes amino acid entry into gluconeogenesis. A, hepatic gene expression with CRTC2 knockdown in chow-fed, ASO-treated rats. (n = 4/group). *, p < 0.05; **, p < 0.005; Student's t test. B, hepatic protein expression of GPT1 and CRTC2 in the same group of rats. C, hepatic gene expression after injection of glucagon or vehicle in wild type mice (n = 3–4/group). *, p < 0.05; Student's t test. D, schematic illustration of how these enzymes impinge on amino acid traffic to promote gluconeogenesis.
DISCUSSION
CRTC2 is a coactivator of CREB that is activated by glucagon and assists in orchestrating hepatic gluconeogenesis during fasting. Accordingly, we found that in models of extreme hyperglycemia, where gluconeogenesis is significantly elevated, CRTC2 knockdown reduced endogenous glucose production and fasting glucose concentrations. In a model of T2D, however, the improvement in glucose tolerance and glucose production was not as profound. Our investigations into this initially confusing result demonstrated that knockdown of CRTC2 raised circulating plasma glucagon concentrations, thereby blunting the expected changes in endogenous glucose production. This increase in plasma glucagon was likely attributable to decreased glucagon clearance, although increased glucagon secretion could also contribute. Although the molecular mechanism of CRTC2-dependent control of glucagon clearance remains unclear, these data may indicate that CRTC2 serves as a feedback mechanism to prevent detrimental increases in plasma glucagon concentrations during fasting. This could explain the increased plasma glucagon concentrations observed with glucagon receptor knockdown (27), because CRTC2 is downstream of the glucagon receptor. When the effects of glucagon were suppressed, either with somatostatin or a glucagon-neutralizing antibody, the expected improvement in glucose production was unmasked.
The competing effects of CRTC2 on gluconeogenic gene transcription and glucagon clearance could underlie the model put forth by a recent publication showing that CRTC2 is important for transcriptional modification of gluconeogenic genes but is not absolutely necessary for normal glucose homeostasis in vivo (8). Although Le Lay et al. did not observe differences in plasma glucagon concentrations, such studies are technically challenging because of the rapid degradation by plasma proteases and large amount of plasma volume required for accurate measurement. Moreover, it is possible that this differing result is due to ASO-mediated knockdown of CRTC2 in the kidney, which is the primary site of glucagon clearance.
The result of CRTC2 knockdown in our three distinct diabetic animal models elucidates an important potential source of confusion in this field. In extreme diabetic models, such as our T1D and Zucker diabetic fatty rats, gluconeogenic rates are dramatically increased, resulting in profound fasting hyperglycemia. In this setting, differences in plasma glucagon are relatively unimportant compared with the absence of insulin (in the T1D model) or abundance of corticosterone (in the Zucker diabetic fatty model), and the effects of CRTC2 knockdown on gluconeogenesis are as expected. Comparatively, when such extreme induction of gluconeogenesis is not present, this effect becomes masked by opposing effects on glucagon clearance and amino acid catabolism. Moreover, although CRTC2 is an important contributor to the transcription of gene products that aid in gluconeogenesis, it is not the only controller; for example, FoxO1 and several nuclear transcription factors are important regulators of gluconeogenic transcription. Thus, the degree of hyperglycemia and hormonal milieu are critical to the physiologic outcome of CRTC2 knockdown. This may explain why other models of CRTC2 knockdown have shown more impressive reductions in plasma glucose and glucose production (9, 10). Alternatively, such differences may be due to the degree to which CRTC2 expression was reduced or the species used.
In addition to regulating gluconeogenic gene transcription by controlling the half-life of glucagon, CRTC2 strongly regulates the expression of genes that facilitate the shuttling of substrates such as alanine, cysteine, serine, and aspartate into gluconeogenesis. This finding overlaps with recent reports demonstrating that knock-out of the glucagon pro-peptide leads to reduced expression of hepatic amino acid-catabolizing genes and suggests that CRTC2 is the intracellular transducer of this effect (28–30). Through this mechanism, CRTC2 may provide additional feedback to regulate glucagon secretion; by shuttling amino acids toward gluconeogenesis, CRTC2 will reduce amino acid-induced stimulation of glucagon secretion by the pancreas and thereby suppress glucagon-induced CRTC2 activation. Although it is somewhat puzzling that we did not observe differences in plasma amino acids in our model, it is still possible that amino acid flux, if not concentration, may be altered. Moreover, it is possible that the kidney, which also participates in gluconeogenesis and in which CRTC2 is reduced in our model, may concurrently participate in amino acid metabolism and could contribute to stabilizing plasma amino acid concentrations. From an entirely different perspective, it is interesting to note that GPT1 (also known as alanine aminotransferase), and GOT1 (aspartate aminotransferase) are proteins whose expression appears highly dependent on CRTC2 activity. As testing of release of these proteins into plasma is commonly performed clinically to assess liver injury, it is worth considering whether such tests may require a different interpretation in diabetic patients, in whom CRTC2 activity may be altered.
Our work and that of others have demonstrated that CRTC2 is an important regulator of gluconeogenic gene expression. Yet some investigators have argued that CRTC2 does not seem to be as important as expected for in vivo glucose homeostasis. We believe these discordant findings are attributable to competing roles played by CRTC2. We found that in extreme models of diabetes, knockdown of CRTC2 led to dramatic metabolic improvement. Although these models represent a small percentage of the diabetic patient population, CRTC2 ASO could be of therapeutic utility in patients with uncontrollable fasting hyperglycemia. In a model of T2D that may better approximate many human patients, metabolic improvement in response to CRTC2 ASO was only clearly revealed with administration of somatostatin or glucagon-blocking antibody. Moreover, regulation of amino acid catabolism by CRTC2 may account for differences in gluconeogenic flux that cannot be entirely attributed to expression of prototypical genes. These results argue that inhibition of CRTC2 could be a beneficial treatment for controlling hyperglycemia in diverse diabetic phenotypes if combined with multimodal therapies aimed at glucagon metabolism.
Acknowledgments
We thank Jianying Dong, Yanna Kosover, and Todd May for expert technical assistance with the studies; Novo Nordisk for proving the glucagon antibody; Aida Groszmann for performing the hormone assays; and Thanh Erion for insightful commentary.
This work was supported by United States Public Health Service Grants R01 DK-40936 and P30 DK-45735 (to G. I. S.) and the University of Iowa. S. F. M. and S. B. own stock and/or hold stock options in Isis Pharmaceuticals.
- CRTC2
- CREB-regulated transcription coactivator 2
- ASO
- antisense oligonucleotide
- CREB
- cAMP-responsive element-binding protein
- STZ
- streptozotocin
- T1D
- type 1 diabetes
- T2DM
- type 2 diabetes mellitus.
REFERENCES
- 1. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 2. Ravnskjaer K., Kester H., Liu Y., Zhang X., Lee D., Yates J. R., 3rd, Montminy M. (2007) Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J. 26, 2880–2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mayr B., Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat. Rev. Mol. Cell Biol. 2, 599–609 [DOI] [PubMed] [Google Scholar]
- 4. Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J., 3rd, Montminy M. (2007) Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 [DOI] [PubMed] [Google Scholar]
- 5. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, Olefsky J., Guarente L., Montminy M. (2008) A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dentin R., Hedrick S., Xie J., Yates J., 3rd, Montminy M. (2008) Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 8. Le Lay J., Tuteja G., White P., Dhir R., Ahima R., Kaestner K. H. (2009) CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 10, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saberi M., Bjelica D., Schenk S., Imamura T., Bandyopadhyay G., Li P., Jadhar V., Vargeese C., Wang W., Bowman K., Zhang Y., Polisky B., Olefsky J. M. (2009) Novel liver-specific TORC2 siRNA corrects hyperglycemia in rodent models of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 297, E1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y., Inoue H., Ravnskjaer K., Viste K., Miller N., Liu Y., Hedrick S., Vera L., Montminy M. (2010) Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc. Natl. Acad. Sci. U.S.A. 107, 3087–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reaven G. M. (1988) Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 37, 1595–1607 [DOI] [PubMed] [Google Scholar]
- 12. DeFronzo R. A., Bonadonna R. C., Ferrannini E. (1992) Pathogenesis of NIDDM: a balanced overview. Diabetes Care 15, 318–368 [DOI] [PubMed] [Google Scholar]
- 13. Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. (1992) Increased rate of gluconeogenesis in type II diabetes mellitus: a 13C nuclear magnetic resonance study. J. Clin. Invest. 90, 1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samuel V. T., Beddow S. A., Iwasaki T., Zhang X. M., Chu X., Still C. D., Gerhard G. S., Shulman G. I. (2009) Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl. Acad. Sci. U.S.A. 106, 12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samuel V. T., Choi C. S., Phillips T. G., Romanelli A. J., Geisler J. G., Bhanot S., McKay R., Monia B., Shutter J. R., Lindberg R. A., Shulman G. I., Veniant M. M. (2006) Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55, 2042–2050 [DOI] [PubMed] [Google Scholar]
- 16. Erion D. M., Ignatova I. D., Yonemitsu S., Nagai Y., Chatterjee P., Weismann D., Hsiao J. J., Zhang D., Iwasaki T., Stark R., Flannery C., Kahn M., Carmean C. M., Yu X. X., Murray S. F., Bhanot S., Monia B. P., Cline G. W., Samuel V. T., Shulman G. I. (2009) Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 10, 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erion D. M., Yonemitsu S., Nie Y., Nagai Y., Gillum M. P., Hsiao J. J., Iwasaki T., Stark R., Weismann D., Yu X. X., Murray S. F., Bhanot S., Monia B. P., Horvath T. L., Gao Q., Samuel V. T., Shulman G. I. (2009) SirT1 knockdown in liver decreases basal hepatic glucose production and increases hepatic insulin responsiveness in diabetic rats. Proc. Natl. Acad. Sci. U.S.A. 106, 11288–11293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masiello P., Broca C., Gross R., Roye M., Manteghetti M., Hillaire-Buys D., Novelli M., Ribes G. (1998) Experimental NIDDM: development of a new model in adult rats administered streptozotocin and nicotinamide. Diabetes 47, 224–229 [DOI] [PubMed] [Google Scholar]
- 19. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 20. Koerker D. J., Ruch W., Chideckel E., Palmer J., Goodner C. J., Ensinck J., Gale C. C. (1974) Somatostatin: hypothalamic inhibitor of the endocrine pancreas. Science 184, 482–484 [DOI] [PubMed] [Google Scholar]
- 21. Wahren J. (1976) Influence of somatostatin on carbohydrate disposal and absorption in diabetes mellitus. Lancet 2, 1213–1216 [DOI] [PubMed] [Google Scholar]
- 22. Brand C. L., Rolin B., Jørgensen P. N., Svendsen I., Kristensen J. S., Holst J. J. (1994) Immunoneutralization of endogenous glucagon with monoclonal glucagon antibody normalizes hyperglycaemia in moderately streptozotocin-diabetic rats. Diabetologia 37, 985–993 [DOI] [PubMed] [Google Scholar]
- 23. Brand C. L., Jørgensen P. N., Knigge U., Warberg J., Svendsen I., Kristensen J. S., Holst J. J. (1995) Role of glucagon in maintenance of euglycemia in fed and fasted rats. Am. J. Physiol. 269, E469–477 [DOI] [PubMed] [Google Scholar]
- 24. Sørensen H., Brand C. L., Neschen S., Holst J. J., Fosgerau K., Nishimura E., Shulman G. I. (2006) Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 55, 2843–2848 [DOI] [PubMed] [Google Scholar]
- 25. Burgess S. C., He T., Yan Z., Lindner J., Sherry A. D., Malloy C. R., Browning J. D., Magnuson M. A. (2007) Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 5, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocha D. M., Faloona G. R., Unger R. H. (1972) Glucagon-stimulating activity of 20 amino acids in dogs. J. Clin. Invest. 51, 2346–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sloop K. W., Cao J. X., Siesky A. M., Zhang H. Y., Bodenmiller D. M., Cox A. L., Jacobs S. J., Moyers J. S., Owens R. A., Showalter A. D., Brenner M. B., Raap A., Gromada J., Berridge B. R., Monteith D. K., Porksen N., McKay R. A., Monia B. P., Bhanot S., Watts L. M., Michael M. D. (2004) Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J. Clin. Invest. 113, 1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe C., Seino Y., Miyahira H., Yamamoto M., Fukami A., Ozaki N., Takagishi Y., Sato J., Fukuwatari T., Shibata K., Oiso Y., Murata Y., Hayashi Y. (2012) Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61, 74–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yang J., MacDougall M. L., McDowell M. T., Xi L., Wei R., Zavadoski W. J., Molloy M. P., Baker J. D., Kuhn M., Cabrera O., Treadway J. L. (2011) Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor (GCGR) knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genomics 12, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mu J., Qureshi S. A., Brady E. J., Muise E. S., Candelore M. R., Jiang G., Li Z., Wu M. S., Yang X., Dallas-Yang Q., Miller C., Xiong Y., Langdon R. B., Parmee E. R., Zhang B. B. (2012) Anti-diabetic efficacy and impact on amino acid metabolism of GRA1, a novel small-molecule glucagon receptor antagonist. PloS One 7, e49572. [DOI] [PMC free article] [PubMed] [Google Scholar]