Background: New crystallographic data for HIV-1 RT containing an RNA/DNA hybrid show important interactions involving the p51 C terminus.
Results: Altered hydrogen bonding at the p51 C terminus affects both NNRTI sensitivity and enzyme activity.
Conclusion: An intact p51 subunit is necessary to faithfully recapitulate activities of the RT heterodimer.
Significance: The extreme C terminus of p51 plays an important role in RNA/DNA hybrid positioning and hydrolysis.
Keywords: Enzyme Mechanisms, Enzyme Structure, HIV, Protein-Nucleic Acid Interaction, Reverse Transcription
Abstract
Recent crystallographic analysis of p66/p51 human immunodeficiency virus (HIV) type 1 reverse transcriptase (RT) complexed with a non-polypurine tract RNA/DNA hybrid has illuminated novel and important contacts between structural elements at the C terminus of the noncatalytic p51 subunit and the nucleic acid duplex in the vicinity of the ribonuclease H (RNase H) active site. In particular, a short peptide spanning residues Phe-416–Pro-421 was shown to interact with the DNA strand, cross the minor groove of the helix, and then form Van der Waals contacts with the RNA strand adjacent to the scissile phosphate. At the base of the adjoining α-helix M′, Tyr-427 forms a hydrogen bond with Asn-348, the latter of which, when mutated to Ile, is implicated in resistance to both nucleoside and non-nucleoside RT inhibitors. Based on our structural data, we analyzed the role of the p51 C terminus by evaluating selectively mutated p66/p51 heterodimers carrying (i) p51 truncations that encroach on α-M′, (ii) alterations that interrupt the Asn-348-Tyr-427 interaction, and (iii) alanine substitutions throughout the region Phe-416–Pro-421. Collectively, our data support the notion that the p51 C terminus makes an important contribution toward hybrid binding and orienting the RNA strand for catalysis at the RNase H active site.
Introduction
An asymmetric subunit organization of the human immunodeficiency virus type 1 reverse transcriptase (HIV-1 RT) p66/p51 heterodimer has been documented for more than 25 years (1). Despite this, the precise contribution from the p51 subunit, derived from p66 via cleavage by the viral protease (2), remains to be clarified. Because the absence of a C-terminal ribonuclease H (RNase H) domain and alternative configuration of its subdomains render p51 subunit enzymatically inactive (3), its potential functions have included providing a structural support and facilitating p66 loading onto nucleic acid (4). Multiple structures of the RT apo enzyme and cocrystals containing nucleic acid support the notion that although p66, which contains both the DNA polymerase and the RNase H catalytic centers, undergoes large scale motions, the p51 subunit is essentially rigid (3–12). However, observations that (a) the p51 mutation N348I confers resistance to nucleoside and non-nucleoside RT inhibitors (NRTIs2 and NNRTIs, respectively (13–16)), (b) p51 residues Lys-395 and Glu-396 contribute to the architecture of the RNase H primer grip/phosphate binding pocket (9, 17–20), and (c) p51 C-terminal deletions induce alterations in both RNase H activity (21) and RNase H inhibitor sensitivity (22) convincingly demonstrate that p51 of the p66/p51 RT heterodimer is not inert.
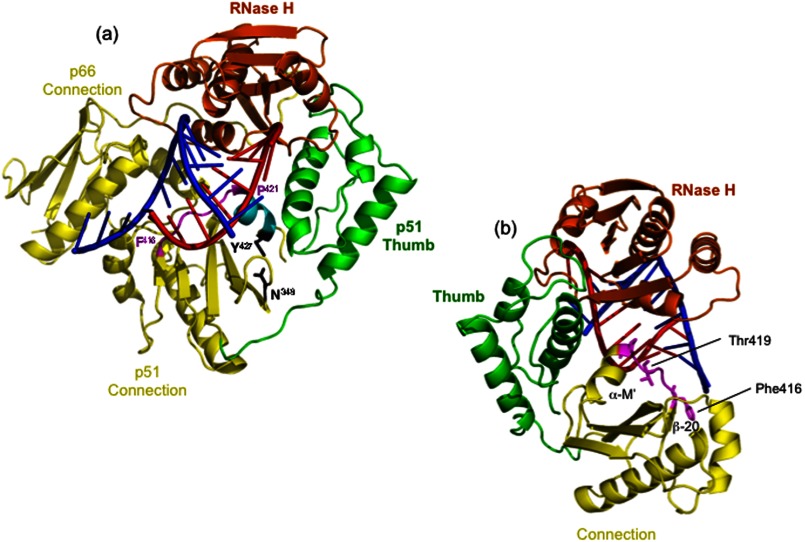
In this study, we have taken advantage of our recently solved structure of HIV-1 RT containing an NNRTI and a non-polypurine tract RNA/DNA hybrid (PDB ID 4B3O) that differs structurally from all previously reported RT-nucleic acid complexes and is compatible with RNA degradation (23) to evaluate the effect of point mutations, deletions, and alanine scanning mutagenesis on positioning of α-helix M′ (Pro-421–Tyr-427), which constitutes the last structural element of the p51 subunit visible from crystallography. Our studies were prompted by observations that the p51 and p66 are further separated ∼2.0 Å and that p51 helix α-helix M′ and surrounding residues are in the core of such movement (23). The RT-RNA/DNA cocrystal structure also demonstrated hydrogen bonding between p51 residues Asn-348 and Tyr-427, in contrast to previous structures where Asn-348 was surrounded by hydrophobic side chains (23). Lastly, the peptide comprising Phe-416–Pro-421 preceding α-M′ was shown to interact with the DNA strand and cross the shallow minor groove to form Van der Waals contacts with the RNA strand adjacent to the scissile phosphate (Fig. 1). Because 13- and 19-residue C-terminal p51 deletions of the 440-residue p51 subunit were shown to inhibit polymerization-independent RNase H activity (21) and DNA polymerase activity, respectively (24), our recent crystallographic data predicted that altering α-M′ architecture could affect both subunit dynamics and correct positioning of nucleic acid at the RNase H catalytic center. In this study, we have tested these predictions by combining site-directed and alanine scanning mutagenesis with additional studies of the selectively deleted RT mutant p66/p51Δ13 (1–427 amino acids), whose C terminus is Tyr-427. Collectively, our data highlight a hitherto unappreciated importance of p51 C-terminal architecture in activity of the parental heterodimer. Our data also provide a cautionary note for crystal engineering of HIV-1 RT when attempting to define contacts with RNA/DNA hybrids.
FIGURE 1.
Structural elements of the HIV-1 RT p51 C terminus that contribute to accommodating the RNA/DNA hybrid. For simplicity, the model in a highlights only the connection subdomains of p66 and p51 (yellow), the p66 RNase H domain (orange), and the p51 thumb (green). A portion of the RNA/DNA hybrid from Lapkouski et al. (23) is indicated, with DNA represented in blue and RNA represented in red. The peptide scanning Phe-416–Pro-421 of the p51 connection, which is in close contact to both strands of the RNA/DNA hybrid, is depicted in magenta, and α-helix M′ is in cyan. Residues Tyr-427 of α-helix M′ and Asn-348 of β-strand 20′, shown from our recent work to be involved in hydrogen bonding, are depicted in black. In b, this structure has been rotated 90° counterclockwise, and the p66 connection has been deleted. Residues Phe-416–Pro-421 are again highlighted in magenta.
EXPERIMENTAL PROCEDURES
Site-directed Mutagenesis and Enzyme Purification
Alanine substitutions were introduced into the p51 HIV-1 RT gene using the QuikChange protocol (Agilent Technologies Inc., Santa Clara, CA). Selectively mutated and selectively deleted p66/(His)6p51 heterodimers were purified from recombinant Escherichia coli by a combination of immobilized metal affinity and ion exchange chromatography. Purified, concentrated enzymes were stored at −20 °C in a buffer of 50 mm Tris/HCl, pH 7.0, 25 mm NaCl, 1 mm EDTA, and 50% (v/v) glycerol.
Enzyme Assays
Sequence-independent RNase H activity was evaluated on a 40-nt RNA/30-nt DNA hybrid, whereas PPT cleavage was determined on a 39-nt DNA/29-nt RNA hybrid. In both instances, the RNA strand was 5′ end-labeled with Cy5. Hydrolysis was initiated by adding 2 μl of 100 mm MgCl2 to 18 μl of mixture containing 1 ng of enzyme, 200 nm substrate, 50 mm Tris, pH 8.0, 80 mm KCl, and 2 mm DTT at 37 °C. Aliquots were removed at the indicated time points and combined with an equal volume of 8 m urea in 1× Tris/borate/EDTA. Hydrolysis products were resolved by high voltage and denaturing polyacrylamide gel electrophoresis and visualized by fluorescent imaging (Typhoon Trio+, GE Healthcare).
RNA-dependent DNA polymerase activity was determined on a viral RNA corresponding to nt 1–363 of the HIV-1 NL4-3 (+) strand genome. RNA was prepared by in vitro transcription utilizing an immobilized metal affinity-purified His6-tagged T7 RNA polymerase. A fluorescently labeled DNA primer (5′-Cy5 CAGACGGGCACACACTAC-3′) was combined with template RNA at a ratio of 1:1.2 in 10 mm Tris/HCl, pH 7.6, 25 mm KCl and heated in a thermal cycler at 85 °C for 3 min and then cooled to 4 °C at 0.1 °C/s. DNA polymerase reactions were performed at 37 °C and contained 10 mm Tris/HCl, pH 7.8, 10 mm MgCl2, 80 mm KCl, 1 mm DTT, 0.2 mm dNTPs, 25 nm template/primer, and 12.5 nm RT. Aliquots were removed at the indicated time points and combined with an equal volume of 8 m urea in 1× Tris/borate/EDTA. Before loading, samples were heated to 85 °C for 3 min and immediately placed on ice. Nucleic acids were fractionated by denaturing 5% polyacrylamide gel electrophoresis. Gels were scanned with a GE Healthcare Typhoon Trio + and analyzed with Image Quant Total Lab software.
Electrophoretic Mobility Shift Analysis
Serial dilutions of wild type and mutant enzymes were added to a mixture containing a 5′-Cy5-labeled RNA annealed to a DNA molecule in 25 mm HEPES, pH 7.5, 5 mm NaCl, 1 mm DTT, 0.5 mm EDTA, and 5% glycerol. After 20 min at 4 °C, 0.2 volume of 50% glycerol was added, and the samples were fractionated by nondenaturing 5% polyacrylamide gel (25 mm Tris, pH 8.4, 192 mm glycine, 1 mm EDTA). Following electrophoresis at 4 °C, gels were analyzed by fluorescent imaging (Typhoon Trio+, GE Healthcare), and Kd values were determined by curve-fitting analysis using Prism5 (GraphPad Software)
Differential Scanning Fluorometry (Thermofluor)
To a LightCycler® 480 96-well plate (Roche Applied Science) 50 μl of 300 nm HIV-1 RT in a buffer of 20 mm HEPES, pH 7.5, 10 mm MgCl2, 100 mm NaCl, and a 1:1000 dilution of SYPRO® Orange dye (Invitrogen) was added. The solution was heated from 30 to 80 °C in increments of 0.2 °C. Fluorescence intensity was measured using excitation/emission wavelengths of 483 and 568 nm, respectively. Melting temperatures of the proteins were analyzed by using LightCycler® 480 Software. All assays were performed in triplicate.
RESULTS
C-terminal p51 Truncations Stabilize the HIV-1 RT Heterodimer
In a previous study, we reported inhibition of RNase H and tRNA-primed (−) strand strong-stop DNA synthesis activities of a mutant HIV-1 RT, whose p51 subunit contained a 13-residue C-terminal truncation removing Gln-428–Phe-440 (25). Despite this surprising observation, the structural basis was unclear. Our recently solved cocrystal structure of HIV-1 RT complexed with an RNA/DNA hybrid and in the presence of an NNRTI (23) indicated that Tyr-427 of p51 α-helix M′ and Asn-348 of the β-17/β-18 connecting loop participate in hydrogen bonding, raising the possibility that this important interaction was destabilized when residues Gln-428–Phe-440 were removed from the p51 C terminus p51. As a first step in testing this notion, we used differential scanning fluorometry (Thermofluor (26)) to determine the thermal stability of reconstituted heterodimers whose p51 subunit contained 5-, 9-, and 13-residue C-terminal deletions, the results of which are illustrated in Fig. 2. In the absence of divalent cation, we determined a Tm for wild type RT of 56.8 °C, which increased to 59.2 °C in the presence of 0.5 mm MnCl2 or 10.0 mm MgCl2, indicating stabilization when divalent metal is bound at the DNA polymerase and RNase H active sites. Surprisingly, progressive p51 C-terminal deletions further stabilized the reconstituted heterodimer in both the absence and the presence of divalent metal, raising the Tm by ∼3 °C in the case of p66/p51Δ13 RT. Such observations suggest that removing what has been described as disordered residues from the p51 C terminus may improve packing at the dimer interface, a notion forwarded by Bauman et al. (27) as a means of crystal engineering. However, our previous work (21, 22, 24), and data presented herein, suggest that such stabilization of the RT heterodimer may remove a significant degree of flexibility, which can have important consequences on substrate positioning and RNase H activity. Thus, the observed Tyr-427 and Asn-348 hydrogen bond may correlate with an activated state for RNA degradation.
FIGURE 2.
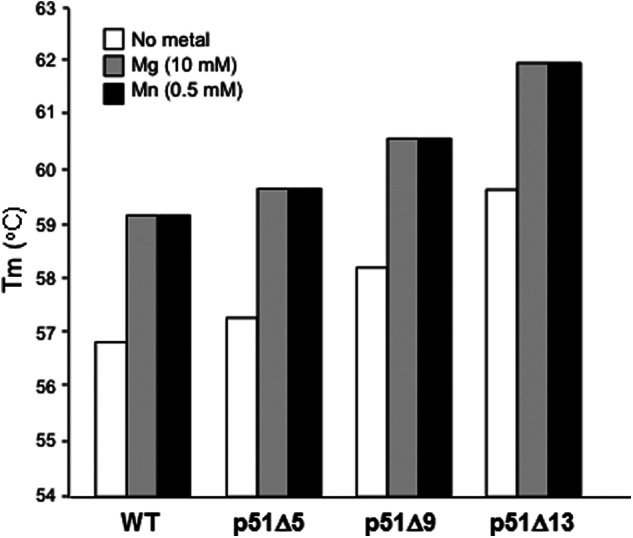
Truncating the C terminus of p51 HIV-1 RT increases thermal stability of reconstituted selectively deleted p66/p51 heterodimers. Differential scanning fluorometry was performed in the absence of divalent metal or in the presence of 10 mm MgCl2 or 0.5 mm MnCl2 as indicated. Results are the average of duplicate analysis. p51 deletions Δ5, Δ9, and Δ13 remove C-terminal residues Gly-436–Phe-440, Glu-432–Phe-440, and Gln-428–Phe-440, respectively.
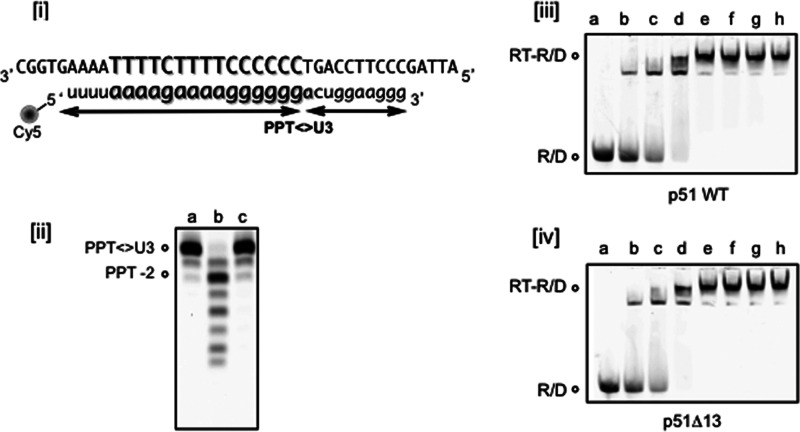
p66/p51Δ13 HIV-1 RT Displays Altered PPT Cleavage Kinetics
When the RNase H activity of p66/p51Δ13 HIV-1 RT determined on a heteropolymeric RNA/DNA hybrid was examined (21, 25), we and others observed retention of polymerization-dependent activity and loss of polymerization-independent function (Fig. 3, panel i). We therefore elected to re-examine RNase H activity of this mutant on a PPT-containing RNA/DNA hybrid, which lacks a recessed DNA primer 3′ terminus for recognition by the DNA polymerase active site. As indicated in Fig. 3, panel ii, wild type RT cleaves at the PPT/U3 junction, and additionally at positions −1 and −2. Although p66/p51Δ13 RT efficiently cleaved the RNA/DNA hybrid at the PPT/U3 junction, the additional −1 and −2 cleavage products were absent. The inability of mutant RT to cleave at positions −1/−2 suggests that after initial cleavage, mutant RT either (a) dissociates and fails to rebind the “nicked” substrate or (b) remains bound to the substrate, but in a “dead-end” conformation that fails to translocate.
FIGURE 3.
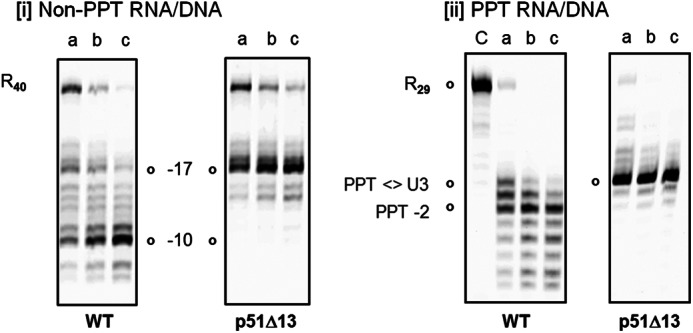
Cleavage of non-PPT (panel i) and PPT-containing RNA/DNA hybrid by wild type and p66/p51Δ13 HIV-1 RT (panel ii). In panel i, polymerization-dependent and -independent RNase H hydrolysis products are represented by −17 and −10, respectively. R40: intact RNA template. In panel ii, the intact, PPT-containing RNA strand is designated as R29. In both cases, lanes a–c represent samples analyzed after 10, 20, and 30 min of incubation, respectively. Lane C indicates control.
In an attempt to distinguish between these possibilities, the experiment of Fig. 3 was repeated, but in this case with a DNA to which two RNA strands were hybridized, as a means of mimicking precleavage at the PPT/U3 junction. To achieve this, the RNA/DNA hybrid of Fig. 4, panel i, was first cleaved at the PPT/U3 with p66/p51Δ13 RT, after which enzyme was removed by phenol extraction and the nucleic acids were precipitated, resuspended, and incubated with a fresh aliquot of wild type or mutant enzyme (this step was necessary based on our past experience that commercially synthesized PPT oligoribonucleotides terminating in an (rG)6 sequence rapidly formed G-quartets and high molecular weight aggregates). Under these conditions, wild type RT retained the capacity to rebind in an orientation that permitted −1/−2 cleavage, whereas in contrast, p66/p51Δ13 RT again could not support additional hydrolysis (Fig. 4, panel ii). Electrophoretic mobility shift analysis was performed with the three-stranded substrate (Fig. 4, panels iii and iv) and indicated that both enzymes bound the nicked duplex with equal affinity, an observation that was also confirmed with a nonspecific heteropolymeric RNA/DNA hybrid (data not shown). Together with the thermal stability analysis of Fig. 1, the data of Figs. 3 and 4 suggest that the altered RNase H activity of p66/p51Δ13 RT may be the consequence of a stabilized heterodimer in the ground state that lacks the conformational flexibility to permit translocation following initial polymerization-dependent hydrolysis.
FIGURE 4.
p66/p51Δ13 HIV-1 RT fails to process a model “precut” PPT RNA template beyond the PPT/U3 junction. The sequence of the three-stranded, precut PPT is indicated in panel i, and the breakpoint between the PPT and U3 sequences is delineated by arrows. RNase H hydrolysis profiles obtained with wild type and p66/p51Δ13 RT are shown in panel ii, lanes b and c, respectively. Lane a, precut PPT RNA. Panels iii and iv, gel electrophoretic mobility shift analysis of binding of wild type (panel iii) and p66/p51Δ13 HIV-1 RT (panel iv) to the precut, PPT-containing RNA/DNA hybrid of panel i. Migration positions of RNA/DNA hybrid and the nucleoprotein complex are denoted as R/D and RT-R/D, respectively.
Mutating p51 Residue Tyr-427 Induces a Translocation Defect
As suggested by Fig. 1, a structural consequence of removing p51 residues Gln-428-Phe-440 might be disruption of hydrogen bonding between Tyr-427 of α-helix M′ and Asn-348 of the β-17/β-18 connecting loop. This notion was next investigated by substituting each of these residues with Ala, either individually or in tandem, in the context of a full-length p51 subunit. In addition, and based on data implicating the p51 mutation N348I in both NRTI and NNRTI resistance (13, 14, 16, 29), we also constructed and purified a reconstituted, selectively mutated p66/p51N348I RT variant. The data of Fig. 5, panel i, indicate that, on a nonspecific RNA/DNA hybrid, both p66/p51Y427A and RT p66/p51Y427A,N348A RT, analogous to mutant p66/p51Δ13, retained equivalent levels of polymerization-dependent activity, whereas polymerization-independent activity was significantly reduced. Likewise, the same mutants efficiently cleaved the PPT-containing RNA/DNA hybrid at the PPT/U3 junction, but failed to translocate to support cleavage at positions −1 and −2 (Fig. 5, panel ii). With respect to Tyr-427, such observations indeed lend support to the notion that a bulky aromatic side chain may be required to preserve the architecture of the p51 C terminus to facilitate RNA/DNA hybrid binding and impose the correct orientation on the RNA strand for hydrolysis in the p66 RNase H active site (23). At the same time, the data of Figs. 3–5 indirectly suggest that this important interaction is similarly disrupted as a consequence of removing p51 residues Gln-428–Phe-440.
FIGURE 5.
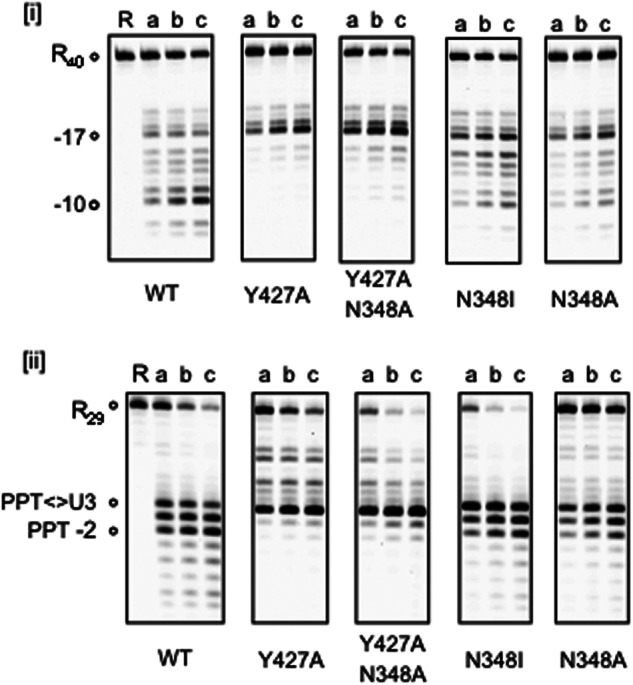
Effects of disrupting hydrogen bonding between p51 connection residues Tyr-427 (α-helix M′) and Asn-348 (β-strand 20′) on RNase H activity. Panel i, non-PPT RNA/DNA hybrid. Uncut RNA and polymerization-dependent and -independent hydrolysis products are represented by the notations R40, −17, and −10, respectively. The nature of the single or dual p51 mutation in the reconstituted p66/p51 heterodimer is indicated below each panel. Lane R indicates RNase H. Panel ii, PPT-containing RNA/DNA hybrid. Uncut PPT RNA is designated as R29, and the PPT hydrolysis products are indicated. Lanes a—c represent 10, 20, and 30 min time points. Lane R indicates Control.
Interestingly, replacing p51 residue Asn-348 with either Ile or Ala was less severe with respect to RNase H activity than Y427A. However, such observations are consistent with a previous study of Shuckmann et al. (29), who observed only a slight difference in RNase H activity with enzymes containing single or dual Asn-348 substitutions. Although supportive evidence is lacking, one possibility to explain the observations of Fig. 5 is that replacing Asn-348 with Ile or Ala could be compensated by hydrophobic, albeit weaker, interactions between these residues and the aromatic ring of Tyr-427. Alternatively, in the absence of hydrogen bonding, Asn-348 substitutions might induce π-π interaction between the aromatic side chains of Phe-346 and Tyr-427, conferring a similar stabilization of the p51 C terminus. Finally, although their RNase H phenotypes are different, the data of supplemental Table 1 indicate that mutating Tyr-427, either alone or in the presence of an N348A substitution, decreased nevirapine sensitivity as much as 3-fold for the double mutant.
Alanine Scanning Mutagenesis of the p51 β-20′-α-M′ Connecting Loop
In addition to a contribution of hydrogen bonding between Tyr-427 and Asn-348 toward stabilizing the p51 C terminus, our recent RT-RNA/DNA/NNRTI cocrystal structure (23) showed that a peptide spanning p51 residues Phe-416–Pro-421 (comprising the β-20′-α-M′ connecting loop) interacts with the DNA strand, crosses the shallow minor groove (where the hybrid bends), and forms van der Waals contacts with the RNA strand 3 nt from the scissile phosphate. Of these residues, the main chain C=O of Phe-416 is ∼3.5 Å of the deoxyribose of base T10. To evaluate the contribution of this peptide to substrate recognition, Phe-416–Pro-421 were individually replaced with alanine, and the properties of reconstituted, selectively mutated heterodimers (31) were examined. Although not shown here, all p51 mutants were successfully and quantitatively reconstituted into p66/p51 heterodimers, indicating that mutagenesis did not adversely alter their dimer interface.
Fig. 6 indicates the thermal stability of the reconstituted enzymes, determined in the presence of 10 mm MgCl2. In contrast to C-terminal p51 truncations (Fig. 2), which progressively stabilized HIV-1 RT, the Tm of reconstituted heterodimer containing a p51 F416A mutation decreased by ∼3.2 °C, indicating significant destabilization. Additional p51 mutants were destabilized to a lesser extent (ΔTm = from −0.8 to −1.2 °C), whereas slight stabilization was observed for p66/p51N418A RT (ΔTm = + 0.3 °C). Substrate dissociation constants for reconstituted enzymes, determined by electrophoretic mobility shift analysis using an RNA/DNA hybrid (Table 1), indicated a 3.3-fold decrease for RT mutant p66/p51F416A (218.3 ± 35.3 nm) when compared with the wild type enzyme (66.1 ± 10.5 nm), suggesting that Ala substitution destabilized the RT heterodimer and interactions with the nucleic acid substrate. Although not shown here, polymerization-dependent and -independent RNase H activities of p51 mutants V417A, N418A, P420A, and P421A were similar to wild type RT. However, we observed accumulation of polymerization-dependent hydrolysis products and a significant reduction in polymerization-independent RNase H activity for mutants p66/p51F416A and p66/p51T419A (Fig. 7, middle and right panels). To determine whether the phenotype of these two mutants was similar to that of enzymes containing either a 13-residue C-terminal p51 deletion (24) or substitution of Tyr-427 (Figs. 4 and 5), Fig. 7, lane d–f, shows the outcome of increasing the amount of input enzyme and extending the incubation time. Under these conditions, the expected polymerization-independent (position −10) RNase H hydrolysis product rapidly accumulates with wild type RT. Although the gradual accumulation of such products with p66/p51F416A and p66/p51T419A RT indicates an ability to reposition on the RNA/DNA hybrid following the initial hydrolysis event, this step appears to be significantly impaired, possibly indicating unfavorable repositioning of the β-20′-α-M′ connecting loop with respect to the RNA/DNA hybrid.
FIGURE 6.
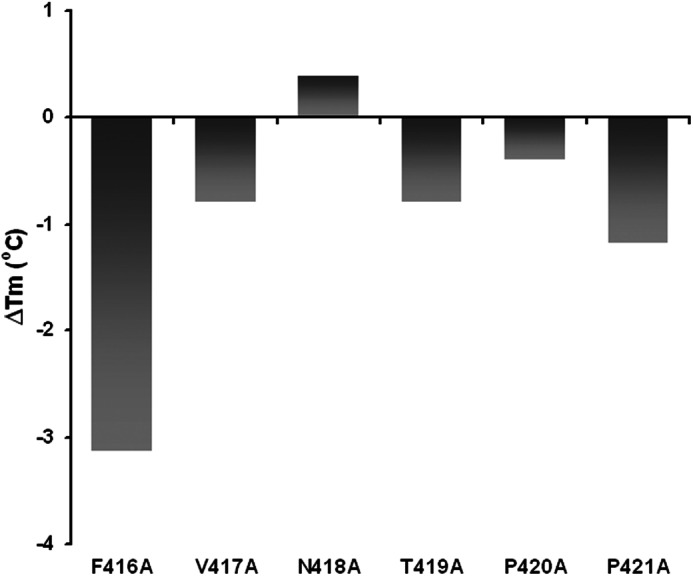
Thermal stability of reconstituted HIV-1 RT mutants containing alanine substitutions spanning Phe-416–Pro-421 of the p51 connection subdomain, which are in close contact with the RNA/DNA hybrid. In this instance, the change in melting temperature relative to wild type RT is presented, and the data represent the average of duplicate analyses.
TABLE 1.
Dissociation constants for p51 connection subdomain mutants p66/p51F416A to p66/p51P421A
Values represent the average of triplicate experiments and were determined on an RNA/DNA hybrid.
| p51 mutation | KD |
|---|---|
| nm | |
| WT | 66.1 ± 10.5 |
| F416A | 218.3 ± 35.3 |
| V417A | 65.0 ± 15.2 |
| F416A | 40.4 ± 8.3 |
| N419A | 131.2 ± 13.9 |
| P420A | 92.4 ± 12.0 |
| P421A | 114.8 ± 10.8 |
FIGURE 7.
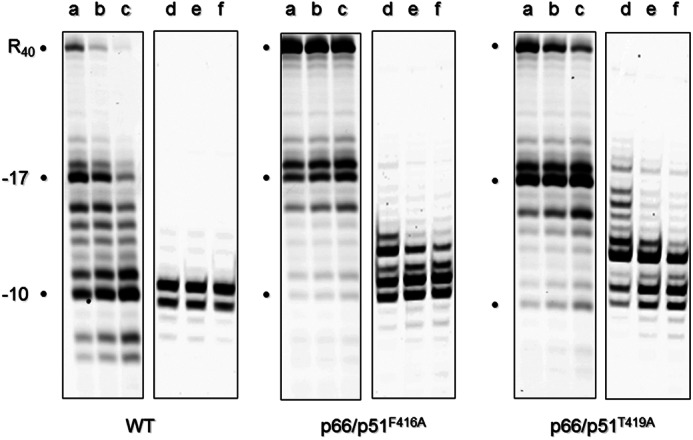
RNase H activity of p51 connection subdomain mutants p66/p51F416A and p66/p51T419A. Lanes a–c represent an input enzyme of 10 ng and incubation times of 10, 20, and 30 min, respectively, whereas lanes d–f represent an input enzyme of 50 ng and incubation times of 30, 60, and 90 min, respectively. The intact RNA template is designated as R40, and polymerization-dependent and -independent hydrolysis products are represented by the notations −17 and −10, respectively.
Mutating p51 Residue Phe-416 Inhibits RNA-dependent DNA Synthesis
Finally, RNA-dependent DNA polymerase activity of the selectively mutated β-20′-α-M′ connecting loop mutants was determined using a synthetic viral RNA template corresponding to nt 1–363 of HIV-1 NL4-3 DNA (Fig. 8, Supplemental Fig. 1, and “Experimental Procedures”). As outlined in Fig. 8, panel i, the DNA primer was hybridized to a region ahead of the natural primer binding site and preceding the poly(A) hairpin and TAR stem-loop. As a consequence, this template also monitors the capacity for intramolecular strand displacement synthesis, which Kim et al. (32) have shown is ∼8-fold slower than DNA synthesis on single-stranded templates. Major pause sites were observed for all mutants immediately following initiation of DNA synthesis, which we interpret as the replication complex stalling within the partially destabilized poly(A) stem (Fig. 8, panel ii). Thus, to a first approximation, the extent of pausing at this position indicated that each p51 mutant initiated RNA-dependent DNA synthesis with equal efficiency. Although the next secondary structure element, the TAR hairpin, induces substantial pausing, strand displacement activity of wild type RT is sufficient to disrupt this structure and synthesize the expected, full-length (−) strong-stop DNA product as well as a larger species that results from self-priming on reannealed, nascent cDNA. Although slightly less active, mutant p66/p51T419A, whose RNase H activity was compromised (Fig. 7), retained sufficient displacement activity to synthesize through the TAR hairpin to generate both the single-stranded and the self-primed cDNA products. In contrast, p66/p51F416A RT failed to catalyze cDNA synthesis beyond the TAR hairpin, whereas at the same time giving rise to slightly elevated pause products in the immediate vicinity of the primer. Thus, in support of our structural data (23), disrupting interactions between Phe-416 of the p51 connection subdomain and the DNA strand of the RNA/DNA hybrid exerts subtle alterations in the manner through which the RNA/DNA product of reverse transcription is accommodated by the replicating enzyme, possibly resulting in stalling or dissociating in the context of strand displacement synthesis.
FIGURE 8.
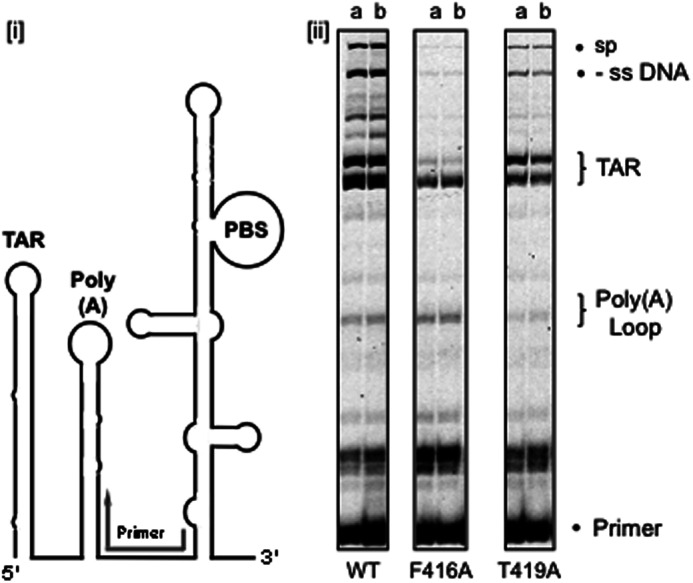
RNA-dependent DNA polymerase activity of p51 connection subdomain mutants p66/p51F416A and p66/p51T419A. A schematic representation of the HIV-1 genomic RNA substrate is presented in panel i. PBS, primer binding site; Poly(A), poly(A) hairpin; TAR, transactivation hairpin. The position to which the synthetic primer was hybridized is indicated in gray. In the RNA-dependent DNA synthesis analyses of panel ii, the positions of these secondary structure elements are indicated. -ss DNA indicates the expected DNA synthesis product, and sp reflects the results of a self-priming event. The nature of the p51 mutation is indicated below each panel. Lanes a and b represent 5 and 20 min time points, respectively
DISCUSSION
Although a crystallographic interpretation has until recently been lacking, several biochemical studies have supported and continue to support a contribution from C-terminal structural elements of the noncatalytic p51 HIV-1 RT subunit to correctly position nucleic acid at the DNA polymerase and RNase H active sites. As an early example, the selectively deleted RT mutant p66/p51Δ19, lacking residues Leu-422–Phe-440, retained dimerization function, but exhibited significantly diminished processivity of DNA synthesis and negligible RNase H activity (24). Although both activities were recovered by restoring p51 residues Leu-422–Tyr-427, the resulting RT mutant, p66/p51Δ13, failed to support tRNA-primed (−) strong-stop DNA synthesis (25) and lacked polymerization-independent RNase H, a consequence of which was the inability to mediate DNA strand transfer (21). Finally, a recent study demonstrated that RT mutants p66/p51Δ9 and p66/p51Δ5 exhibited increased sensitivity to thienopyrimidinone-based RNase H inhibitors (22), shown by mass spectrometry to interact with structural elements of the p51 thumb (30). Our recent cocrystal structure of HIV-1 RT containing a non-PPT RNA/DNA hybrid and an NNRTI, which captured the catalytic configuration at the RNase H catalytic center (23), may provide a unifying hypothesis for such biochemical observations inasmuch as they could destabilize the activated conformation of RT supported by the hydrogen bond between Tyr-427 and Asn-348. In this conformation, the β-20′-α-M′ connecting loop is positioned to contact the RNA/DNA hybrid, rendering RT proficient for RNA degradation. Given their location at either extremity of p51 α-helix M′, our mutagenesis studies collectively suggest that these two structural features are intimately linked and critical determinants of RNase H activity.
The observation that mutants p66/p51Δ13 and p66/p51Y427A display equivalent RNase H hydrolysis patterns on non-PPT and PPT-containing RNA/DNA hybrids (Figs. 3 and 5), and in particular loss of polymerization-independent hydrolysis, suggests a common inability, in the absence of DNA synthesis, to advance along the substrate following initial polymerization-dependent hydrolysis. That translocation per se is not affected can be inferred from previous studies showing that, in a model DNA strand transfer system, p66/p51Δ13 RT efficiently extends a DNA primer to the 5′ terminus of the RNA template, but fails to transfer nascent DNA due to loss of polymerization-independent RNase H activity (21). Although defects in RNase H activity are less severe for mutants p66/p51N348I and p66/p51N348A, our observations are in line with those of others who have studied the role of connection subdomain mutations on NRTI resistance (15, 16, 29). If, as suggested here, the p51 mutation N348I manifests itself in reduced translocation and polymerization-independent RNase H activity, this may promote “dislocation” from the RNase H active site, favoring positioning of nucleic acid at the DNA polymerase catalytic center, where increased residency time would promote NRTI excision, as suggested by Nikolenko et al. (15). The severity of the RNase H defect introduced by a Y427A mutation predicts that this should be present in isolates from patients demonstrating high level NRTI resistance. The fact that such a mutation has never been observed could reflect the critical role this residue plays in the viral enzyme, where the mutation would be present in both subunits. Because Tyr-427 of p66 β-strand 22 immediately precedes the RNase H domain, we suspect that its substitution with Ala could also influence substrate binding and catalysis. Moreover, using QuickAlign of the Los Alamos HIV-1 sequence database (www.hiv.lanl.gov/content/sequence/QUICK_ALIGN/QuickAlign.html) indicates that the only substitutions observed at this position are Ser and Thr, re-enforcing the importance of hydrogen bonding in stabilizing p51 α-helix M′.
Mutagenesis data of this study, in conjunction with crystallographic data of Lapkouski et al. (23) may also help explain our recent observations of increased sensitivity to thienopyrimidinone-based RNase H inhibitors as the p51 C terminus is progressively truncated (22). 5,6-Dimethyl-2-(4-nitrophenyl)thieno(2,3-d)pyrimidin-4(3H)-one (DNTP) was proposed by mass spectrometry and site-directed mutagenesis to interact with α-helix I′ of the p51 thumb (30). Because p51 thumb residues Cys-280–Thr-290 and p66 RNase H residues Pro-537–Glu-546 constitute ∼33% of the buried surface at the p51-p66 dimer interface (28), we proposed that DNTP induced a conformational change at the active site that was incompatible with catalysis. Surprisingly, RT mutants p66/p51Δ5 (lacking p51 residues Gly-436–Phe-440) and p66/p51Δ9 (lacking p51 residues Glu-432–Phe-440), exhibited 3- and 10-fold increased DNTP sensitivity, respectively. Thermofluor analysis of Fig. 2 indicates that these C-terminal deletions have a stabilizing effect on the RT heterodimer, suggesting that they could, additionally, stabilize inhibitor binding.
In summary, mutagenesis data presented here confirm and extend the crystallographic findings of Lapkouski et al. (23) that structural elements close to the C terminus of p51 HIV-1 RT play a critical, and hitherto unappreciated, role in accommodating the RNA/DNA substrate for correct positioning in the RNase H active site. Our data also highlight the importance of retaining a correctly sized p51 subunit for crystallographic analysis with RNA/DNA hybrids despite benefits that stabilizing mutations have been shown to provide to cocrystals containing NRTIs and NNRTIs for structure-based drug design (27).
Supplementary Material
This work was supported, in whole or in part, by the Intramural Research Programs of the NIDDK and NCI, respectively, of the National Institutes of Health (to W. Y. and S. F. J. L. G.)

This article contains supplemental Table 1.
- NRTI
- nucleoside RT inhibitor
- NNRTI
- non-nucleoside RT inhibitor
- PPT
- polypurine tract
- nt
- nucleotide(s)
- TAR
- transactivation response element
- DNTP
- 5,6-dimethyl-2-(4-nitrophenyl)thieno(2,3-d)pyrimidin-4(3H)-one.
REFERENCES
- 1. di Marzo Veronese F., Copeland T. D., DeVico A. L., Rahman R., Oroszlan S., Gallo R. C., Sarngadharan M. G. (1986) Characterization of highly immunogenic p66/p51 as the reverse transcriptase of HTLV-III/LAV. Science 231, 1289–1291 [DOI] [PubMed] [Google Scholar]
- 2. Le Grice S. F., Mills J., Mous J. (1988) Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 7, 2547–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. (1992) Crystal structure at 3.5 Ä resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256, 1783–1790 [DOI] [PubMed] [Google Scholar]
- 4. Harris D., Lee R., Misra H. S., Pandey P. K., Pandey V. N. (1998) The p51 subunit of human immunodeficiency virus type 1 reverse transcriptase is essential in loading the p66 subunit on the template primer. Biochemistry 37, 5903–5908 [DOI] [PubMed] [Google Scholar]
- 5. Ding J., Das K., Tantillo C., Zhang W., Clark A. D., Jr., Jessen S., Lu X., Hsiou Y., Jacobo-Molina A., Andries K., et al. (1995) Structure of HIV-1 reverse transcriptase in a complex with the non-nucleoside inhibitor α-APA R 95845 at 2.8 Ä resolution. Structure 3, 365–379 [DOI] [PubMed] [Google Scholar]
- 6. Ren J., Esnouf R., Hopkins A., Ross C., Jones Y., Stammers D., Stuart D. (1995) The structure of HIV-1 reverse transcriptase complexed with 9-chloro-TIBO: lessons for inhibitor design. Structure 3, 915–926 [DOI] [PubMed] [Google Scholar]
- 7. Hsiou Y., Ding J., Das K., Clark A. D., Jr., Hughes S. H., Arnold E. (1996) Structure of unliganded HIV-1 reverse transcriptase at 2.7 Ä resolution: implications of conformational changes for polymerization and inhibition mechanisms. Structure 4, 853–860 [DOI] [PubMed] [Google Scholar]
- 8. Huang H., Chopra R., Verdine G. L., Harrison S. C. (1998) Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282, 1669–1675 [DOI] [PubMed] [Google Scholar]
- 9. Sarafianos S. G., Das K., Tantillo C., Clark A. D., Jr., Ding J., Whitcomb J. M., Boyer P. L., Hughes S. H., Arnold E. (2001) Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20, 1449–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Himmel D. M., Sarafianos S. G., Dharmasena S., Hossain M. M., McCoy-Simandle K., Ilina T., Clark A. D., Jr., Knight J. L., Julias J. G., Clark P. K., Krogh-Jespersen K., Levy R. M., Hughes S. H., Parniak M. A., Arnold E. (2006) HIV-1 reverse transcriptase structure with RNase H inhibitor dihydroxy benzoyl naphthyl hydrazone bound at a novel site. ACS Chem. Biol. 1, 702–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirschberg T. A., Balakrishnan M., Huang W., Hluhanich R., Kutty N., Liclican A. C., McColl D. J., Squires N. H., Lansdon E. B. (2008) Triazole derivatives as non-nucleoside inhibitors of HIV-1 reverse transcriptase—structure-activity relationships and crystallographic analysis. Bioorg. Med. Chem. Lett. 18, 1131–1134 [DOI] [PubMed] [Google Scholar]
- 12. Das K., Bauman J. D., Rim A. S., Dharia C., Clark A. D., Jr., Camarasa M. J., Balzarini J., Arnold E. (2011) Crystal structure of tert-butyldimethylsilyl-spiroaminooxathioledioxide-thymine (TSAO-T) in complex with HIV-1 reverse transcriptase (RT) redefines the elastic limits of the non-nucleoside inhibitor-binding pocket. J. Med. Chem. 54, 2727–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehteshami M., Beilhartz G. L., Scarth B. J., Tchesnokov E. P., McCormick S., Wynhoven B., Harrigan P. R., Götte M. (2008) Connection domain mutations N348I and A360V in HIV-1 reverse transcriptase enhance resistance to 3′-azido-3′-deoxythymidine through both RNase H-dependent and -independent mechanisms. J. Biol. Chem. 283, 22222–22232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hachiya A., Kodama E. N., Sarafianos S. G., Schuckmann M. M., Sakagami Y., Matsuoka M., Takiguchi M., Gatanaga H., Oka S. (2008) Amino acid mutation N348I in the connection subdomain of human immunodeficiency virus type 1 reverse transcriptase confers multiclass resistance to nucleoside and nonnucleoside reverse transcriptase inhibitors. J. Virol. 82, 3261–3270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nikolenko G. N., Delviks-Frankenberry K. A., Palmer S., Maldarelli F., Fivash M. J., Jr., Coffin J. M., Pathak V. K. (2007) Mutations in the connection domain of HIV-1 reverse transcriptase increase 3′-azido-3′-deoxythymidine resistance. Proc. Natl. Acad. Sci. U.S.A. 104, 317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yap S. H., Sheen C. W., Fahey J., Zanin M., Tyssen D., Lima V. D., Wynhoven B., Kuiper M., Sluis-Cremer N., Harrigan P. R., Tachedjian G. (2007) N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 4, e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rausch J. W., Lener D., Miller J. T., Julias J. G., Hughes S. H., Le Grice S. F. (2002) Altering the RNase H primer grip of human immunodeficiency virus reverse transcriptase modifies cleavage specificity. Biochemistry 41, 4856–4865 [DOI] [PubMed] [Google Scholar]
- 18. Julias J. G., McWilliams M. J., Sarafianos S. G., Alvord W. G., Arnold E., Hughes S. H. (2003) Mutation of amino acids in the connection domain of human immunodeficiency virus type 1 reverse transcriptase that contact the template-primer affects RNase H activity. J. Virol. 77, 8548–8554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nowotny M., Gaidamakov S. A., Crouch R. J., Yang W. (2005) Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell 121, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 20. Nowotny M., Gaidamakov S. A., Ghirlando R., Cerritelli S. M., Crouch R. J., Yang W. (2007) Structure of human RNase H1 complexed with an RNA/DNA hybrid: insight into HIV reverse transcription. Mol. Cell 28, 264–276 [DOI] [PubMed] [Google Scholar]
- 21. Cameron C. E., Ghosh M., Le Grice S. F., Benkovic S. J. (1997) Mutations in HIV reverse transcriptase which alter RNase H activity and decrease strand transfer efficiency are suppressed by HIV nucleocapsid protein. Proc. Natl. Acad. Sci. U.S.A. 94, 6700–6705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chung S., Miller J. T., Johnson B. C., Hughes S. H., Le Grice S. F. (2012) Mutagenesis of human immunodeficiency virus reverse transcriptase p51 subunit defines residues contributing to vinylogous urea inhibition of ribonuclease H activity. J. Biol. Chem. 287, 4066–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lapkouski M., Tian L., Miller J. T., Le Grice S. F., Yang W. (2013) Complexes of HIV-1 RT, NNRTI, and RNA/DNA hybrid reveal a structure compatible with RNA degradation. Nat. Struct. Mol. Biol. 20, 230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacques P. S., Wohrl B. M., Howard K. J., Le Grice S. F. (1994) Modulation of HIV-1 reverse transcriptase function in “selectively deleted” p66/p51 heterodimers. J. Biol. Chem. 269, 1388–1393 [PubMed] [Google Scholar]
- 25. Arts E. J., Ghosh M., Jacques P. S., Ehresmann B., Le Grice S. F. (1996) Restoration of tRNA3Lys-primed(−)-strand DNA synthesis to an HIV-1 reverse transcriptase mutant with extended tRNAs. Implications for retroviral replication. J. Biol. Chem. 271, 9054–9061 [DOI] [PubMed] [Google Scholar]
- 26. Phillips K., de la Peña A. H. (2011) The combined use of the Thermofluor assay and ThermoQ analytical software for the determination of protein stability and buffer optimization as an aid in protein crystallization, In Current Protocols in Molecular Biology, Chapter 10, Unit 10.28, John Wiley & Sons, Inc., New York: [DOI] [PubMed] [Google Scholar]
- 27. Bauman J. D., Das K., Ho W. C., Baweja M., Himmel D. M., Clark A. D., Jr., Oren D. A., Boyer P. L., Hughes S. H., Shatkin A. J., Arnold E. (2008) Crystal engineering of HIV-1 reverse transcriptase for structure-based drug design. Nucleic Acids Res. 36, 5083–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J., Smerdon S. J., Jäger J., Kohlstaedt L. A., Rice P. A., Friedman J. M., Steitz T. A. (1994) Structural basis of asymmetry in the human immunodeficiency virus type 1 reverse transcriptase heterodimer. Proc. Natl. Acad. Sci. U.S.A. 91, 7242–7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuckmann M. M., Marchand B., Hachiya A., Kodama E. N., Kirby K. A., Singh K., Sarafianos S. G. (2010) The N348I mutation at the connection subdomain of HIV-1 reverse transcriptase decreases binding to nevirapine. J. Biol. Chem. 285, 38700–38709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wendeler M., Lee H. F., Bermingham A., Miller J. T., Chertov O., Bona M. K., Baichoo N. S., Ehteshami M., Beutler J., O'Keefe B. R., Götte M., Kvaratskhelia M., Le Grice S. (2008) Vinylogous ureas as a novel class of inhibitors of reverse transcriptase-associated ribonuclease H activity. ACS Chem. Biol. 3, 635–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le Grice S. F., Naas T., Wohlgensinger B., Schatz O. (1991) Subunit-selective mutagenesis indicates minimal polymerase activity in heterodimer-associated p51 HIV-1 reverse transcriptase. EMBO J. 10, 3905–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S., Schroeder C. M., Xie X. S. (2010) Single-molecule study of DNA polymerization activity of HIV-1 reverse transcriptase on DNA templates. J. Mol. Biol. 395, 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.