Abstract
Although clinical benefits of dietary fiber supplementation seem to depend partially on the extent of fiber degradation and fermentation by colonic bacteria, little is known about the effect of supplemental fiber type on bacterial metabolism. In an experiment using a non-adapted human bacterial population from three normal subjects, extent of in vitro fermentation was greater for gum arabic (GA) than for psyllium (PSY), which was greater than that for carboxymethylcellulose (CMC). In a separate experiment, in vitro incubation with feces from 52 subjects with fecal incontinence, before and after random assignment to and consumption of one of three fiber (GA, PSY, or CMC) supplements or a placebo for 20-21d, indicated that prior consumption of a specific fiber source did not increase its degradation by fecal bacteria. Results suggest that the colonic microbial community enriched on a particular fiber substrate can rapidly adapt to the presentation of a new fiber substrate. Clinical implications of the findings are that intake of a fiber source by humans is not expected to result in bacterial adaptation that would require continually larger and eventually intolerable amounts of fiber to achieve therapeutic benefits.
Keywords: dietary fiber, fiber degradation and fermentation in humans
INTRODUCTION
A variety of health benefits have been attributed to dietary fiber consumption including improvements in glucose control, serum lipid profiles, and bowel function.1-3 These effects depend in part on the type of fiber supplemented and the extent of fiber degradation and fermentation by colonic bacteria. An increase in intake of a particular fiber may alter the normal metabolism of colonic bacteria, yet there is little information about this relationship. This relationship has potential clinical significance in that adaptation by colonic bacteria to a dietary fiber substrate that has a clinical benefit might increase the amount of dietary fiber that is required to maintain the effect. Furthermore, consuming a dietary fiber that colonic bacteria prefer as a fermentation substrate, in addition to one having a clinical benefit, might interfere with a desired clinical benefit. Alternatively, fiber supplementation may have additional benefits if such fiber supported the growth of colonic bacteria that had other benefits for the host or the colonic bacterial community.
The purpose of this study was to assess the in vitro fermentation capability of the colonic bacterial population present in human feces before and after fiber supplementation with one of three dietary fibers, carboxymethylcellulose (CMC), gum arabic (GA), and psyllium (PSY), compared to a placebo control. The specific objectives were to: 1) determine the in vitro degradability of CMC, GA, and PSY by human fecal bacterial populations; 2) assess if supplementation of human subjects with CMC, GA, or PSY alters the ability of their fecal bacterial populations to degrade fiber sources and, if so, whether or not the response is similar for all fiber source supplements, and 3) determine the fermentation time course of CMC, GA, and PSY by a non-adapted human bacterial community.
MATERIALS AND METHODS
Fiber Sources
Bulk samples of GA (TIC Gums, Bellcamp, MD), sodium CMC, and PSY (both from Gallipot, St. Paul, MN) were used in this study. A subsample of each fiber source used for supplementation of human subjects also served as substrate for the in vitro digestibility studies. Fiber sources were analyzed for soluble and insoluble fiber fractions by the Uppsala Dietary Fiber method.4 In brief, fiber samples were treated with heat-stable α-amylase at 90° C for 1 h in a 0.1 M acetate buffer (pH 5.0), followed by addition of α-amyloglucosidase and incubation at 50° C for 3 h, in order to hydrolyze any starch that might be present. Upon completion of the starch hydrolysis step, samples were centrifuged to separate soluble and insoluble fiber fractions. The soluble fiber fraction in the supernatant was precipitated by addition of sufficient 95% (vol/vol) ethanol to reach an 80% ethanol concentration. After centrifugation the precipitated soluble fiber was washed 3 times with 80% ethanol. The insoluble fiber fraction obtained above was similarly washed 3 times with 80% ethanol. Both fiber fractions were finally washed with acetone, dried under a N2 gas stream, and subjected to a two-stage sulfuric acid hydrolysis as described by Theander et al.4
Neutral sugar components of the cell wall polysaccharides in the dietary fiber were converted to alditol-acetate derivatives and identified and quantified by GC using inositol as an internal standard.4 Total uronic acids (glucuronic, galacturonic, and 4-O-methylglucuronic acids) were quantified colormetrically in an aliquot from the first stage of the acid hydrolysis, using galacturonic acid as the standard.5 Klason lignin was measured gravimetrically as the ash-free insoluble residue remaining after the two-stage acid hydrolysis.4
Human Fiber Supplementation
In a randomized clinical trial, 189 community-living subjects supplemented their usual diet with one of the three dietary fibers or a placebo to assess their effects on fecal incontinence and adverse gastrointestinal (GI) symptoms.6, 7 All supplements were prepared as a small muffin and juice mixture, both consumed twice daily with the morning and evening meals; the placebo supplement consisted of the basic muffin recipe and plain diluted juice to which the fibers were added for the other supplements. Adherence to consuming the supplements was monitored by subjects’ report of a color change of their stools after being clandestinely administered a marker dose of a food dye among daily decoy dye amounts with their supplements as well as by self-report and return of unconsumed supplement portions.6 Initial analysis of the fiber sources suggested that 16 g of total fiber/d were administered; subsequent analysis of supplement subsamples collected during the study using the Uppsala method4 found that 16.6 g, 14.6 g, and 16.2 g of dietary fiber from GA, PSY, and CMC, respectively, were administered daily on average. As part of that study, 13 subjects from each group (n = 52) were randomly selected over the entire study to collect a fresh stool twice for the in vitro fiber analysis of this study. Stools were collected during the baseline period prior to fiber supplementation and again after 20 to 21 d of steady dose supplementation. Stools were collected in plastic bags, using procedures previously reported,2 and used within 12 h as inoculum sources for in vitro degradation of the three fiber sources.
In Vitro Fiber Degradation
Each subject’s stool sample was used as an inoculum for the in vitro degradation of each of the three fiber sources. The fresh stool samples were mixed with anaerobic McDougall’s buffer.8 Duplicate 50 ml centrifuge tubes that contained 100 mg of the individual fiber sources (CMC, GA, and PSY) were inoculated with the stool preparations. Tubes were purged with N2 prior to capping. Tubes were gently swirled immediately after inoculation and immediately placed in a 39°C water bath. Tubes were incubated for 24 h. After completion of the incubation period, the entire contents of the tubes were frozen and subsequently freeze-dried. The dry incubation residues were then analyzed for residual soluble and insoluble fiber remaining after the incubation by the Uppsala Dietary Fiber method described earlier. A set of tubes containing buffer and inoculum but no substrate was treated similarly in order to determine the amount of fiber contributed to the incubations by each individual stool sample inoculum.
Fiber degradation was determined for the major cell wall monosaccharide components and total cell wall polysaccharides of each fiber source and fraction (soluble or insoluble). Extent of degradation of the fiber sources was calculated as the difference between the amount of each individual cell wall monosaccharide added to the tubes from each fiber source minus the amount of the monosaccharide in the incubation residue, corrected for the amount of the monosaccharide contributed by the stool sample. Data for the duplicate incubations of each fiber source were averaged.
In Vitro Gas Production
In vitro fermentations and measurements of gas production were conducted by a modification of the method of Weimer et al.9 Stools were provided by three adult donors (two female and one male) with no known GI problems. In order to reduce known variation in in vitro fermentation activity of colonic microflora among individual subjects,10 fecal inocula were prepared by compositing the stool samples with McDougall’s buffer8 in a ratio of 1:3 (w/w) under CO2 in a sealed plastic zipper bag, followed by thorough squeezing of the bag to obtain a homogenized mixture. The inoculum was prepared within 12 h of stool collection, and was used immediately for setting up the in vitro fermentations. Setup and incubation of the fermentations were conducted at 39 C. Serum vials (Wheaton, 50 mL nominal volume, volume calibrated to 0.01 mL) contained ~ 0.1 g of fiber sample (weighed to 0.0001 g) hydrated with 1.0 mL of buffer. After gassing with CO2 to remove oxygen, vials were inoculated under a CO2 headspace with 12 mL of diluted fecal inoculum from a stirred and CO2-sparged vessel. Inoculated vials were immediately sealed with flanged butyl stoppers and aluminum crimp seals. Gas pressure measurements were made immediately after sealing each vial, and at 3, 6, 9, 12, 18, 24, and 36 h thereafter. Briefly, gas pressure (in lb/in2) was measured with a digital pressure gauge, and was converted to volume of gas by multiplying the headspace volume of each vial (determined individually) by a factor determined from a calibration curve at 39° C. After subtraction of the average ml gas produced in blank vials containing inoculum but lacking fiber substrate, volumetric gas production was corrected to a per gram substrate added basis (measured to 0.1 mg for each fermentation vial). At each time point, vials (in pairs for each fiber source) were sacrificed for analysis of pH, fermentation end products and residual fiber. Short-chain (C1-C5) organic acid acids were determined by HPLC.11 Net production of gas and acids at each time point was calculated by subtracting amounts produced in blank vials containing buffer and inoculum but no fiber substrate. The University of Minnesota Institutional Review Board approved the human studies.
Statistical Analysis
Means and standard deviations were used to describe normally distributed interval data, medians and ranges for non-normal interval data, and frequencies for categorical data. Demographics were compared between groups using an analysis of variance (ANOVA) for interval data with post hoc comparisons using Tukey’s LSD if needed, and a chi-square test of association for categorical data.
For objective 1, degradability of total polysaccharides was compared in the baseline period, prior to supplementation, with a one-way ANOVA with Tukey’s LSD used for post hoc comparisons. For objective 2, the analysis used mixed modeling, comparing each specific fiber source before and after supplementation and the degradability of the cell wall polysaccharide sugar components of interest. This mixed modeling assessed the effect of the fiber supplemented, the period, and the interaction of the fiber supplemented by period. Mixed effects models are considered superior to ANOVA for analysis of measures over time as they accommodate correlated and non-homogeneous residuals,12 which would be expected in repeated measures, and can deal with missing data points, which ANOVA cannot.
Models with several within-subject error covariance structures were explored and an autoregressive covariance structure was ultimately chosen for the analysis as this had the best model fit. Post hoc comparisons with appropriate adjustments (e.g., studentized maximum modulus) were used to determine if there was a significant change from baseline to supplement degradability values for each specific subject fiber supplementation group. The following effects are detectable in this model: 1) a significant period effect that means values significantly changed from baseline to the supplement period, 2) a significant supplementation group effect that means, overall, at least one of the supplementation groups had significantly different values than the other supplementation groups, and 3) a supplementation group by period interaction that means the amount of change from the baseline to the supplement period is significantly different for at least one of the supplementation groups compared to the others.
Objective 3 was also assessed using mixed models, accommodating time and fiber substrate. The PSY supplemented group was always the reference group. Gas production was assessed as ml/g of organic matter added to each incubation vial and production of total SCFA was expressed as the net millimolar concentration at each incubation time point. Graphs were employed to visually assess the change in gas and SCFA concentrations over time and to choose the correct regression model form. Graphs appeared to follow a basically quadratic pattern for gas production, at least over the 36 hours of data collection. CMC appeared to deviate slightly from this pattern at 36 hours, but did not impact the fit of the model. For SCFA concentrations over 36 hours, accumulation curves of total SCFA, propionate and butyrate displayed a quadratic component, the others did not. The best fitting covariance structure for these analyses was also autoregressive. Model fit diagnostics included residual analysis (model errors pattern) and influence analysis (compare estimates of model parameters with cases removed one by one).13-16
Molar fractions of acetate and propionate, which together ultimately accounted for 85% to 91% of the SCFAs on a molar basis, were averaged across time points and compared between substrates. The averaging allows the direct comparison of the partitioning of substrate into fermentation products using an ANOVA with post hoc comparisons using Tukey’s LSD. Analyses were performed using SAS v 9.2. Final results were considered significant at the p < .05 level.
RESULTS
Fiber Composition
While all three fiber sources contained both soluble and insoluble dietary fiber, the GA and PSY fiber sources contained predominantly soluble (88%) and insoluble (93%) fiber respectively (Table 1). The CMC appeared to contain low concentrations of both soluble (115 g/kg dry matter) and insoluble (207 g/kg dry matter) fiber, but the Uppsala Dietary Fiber method could only quantify neutral sugar residues in CMC that did not include a carboxymethyl substitution. Because this substitution confers increased solubility to CMC salts,17 we concluded that the majority of the CMC was soluble, and non-measurable, fiber. It should also be noted that while the GA and PSY fiber sources contained little ash (33 and 52 g/kg dry matter, respectively), the CMC contained a substantial amount of ash (248 g/kg dry matter) which was mostly sodium. Given the ash and measureable fiber content of the CMC, we estimated that approximately 55% of the glucose residues in the CMC had at least one carboxymethyl group substitution and, therefore, would not be available for fermentation by the gut microflora.
Table 1.
Concentration and Composition of Soluble and Insoluble Fiber Fractions of the Supplemental Fibers
| Fiber | Total | Araa | Fuc | Gal | Glu | Man | Rha | Xyl | UA | Lignin |
|---|---|---|---|---|---|---|---|---|---|---|
| g/kg DM | ---------------------------- g/kg fiber---------------------------------------- | |||||||||
| Soluble Fiber | ||||||||||
| CMCb | 115 | 0 | 0 | 0 | 706 | 64 | 0 | 103 | 26 | 101 |
| GAc | 843 | 387 | 4 | 383 | 14 | 5 | 36 | 0 | 157 | 13d |
| PSYe | 63 | 76 | 0 | 1 | 88 | 14 | 103 | 367 | 245 | 106 |
| Insoluble Fiber | ||||||||||
| CMC | 207 | 7 | 0 | 1 | 635 | 47 | 0 | 68 | 25 | 217 |
| GA | 118 | 136 | 5 | 158 | 70 | 3 | 8 | 0 | 111 | 509 |
| PSY | 805 | 210 | 3 | 41 | 58 | 24 | 26 | 536 | 55 | 49 |
Ara=arabinose, Fuc=fucose, Gal=galactose, Glu=glucose, Man=mannose, Rha=rhamnose, Xyl=xylose, UA=uronic acids;
CMC= carboxymethylcellulose (only polysaccharide sugar residues not substituted with CMC groups could be quantified by the Uppsala Dietary Fiber method. It was assumed that the dry matter not included in total fiber consisted of carboxymethyl-glucose residues and ash in CMC);
GA= gum arabic;
the very small proportion of DM of GA that is lignin may be due to contamination.
PSY= psyllium.
The sugar composition of the GA soluble cell wall polysaccharides indicated this fiber source was primarily an arabinogalactan consisting of roughly equal amounts of arabinose and galactose residues, with a substantial amount of uronic acids (Table 1). In contrast, the PSY fiber source was predominately an insoluble arabinoxylan polysaccharide based on its sugar composition. Both the soluble and insoluble CMC fiber fractions consisted mostly of glucose-containing polysaccharide, as expected for substituted cellulose. As a proportion of dry matter, none of the fiber sources contained substantial amounts of lignin, but lignin accounted for half of the fiber in the very small insoluble GA fiber fraction. The lignin in GA was probably an artifact due to large amounts of insoluble arabinogalactan proteins and other glycoproteins in GA preparations18 rather than actual lignin.
Characteristics of Human Subjects with Fecal Incontinence
There were no significant differences in characteristics among subjects in the supplementation groups at baseline (Table 2). The majority of subjects was White non-Hispanic, female, married, and had at least a high school education. On average the sample was middle-aged (overall mean age = 58 years (SD = 13 years) and overweight (average body mass index was 29 (SD = 6)).
Table 2.
Demographic Characteristics of the Human Subjects Providing Fecal Inocula for Assessing In Vitro Dietary Fiber Degradation Before and After Fiber Supplementation
| Supplement Group | Placebo Control (n=13) | Carboxy methyl cellulose (n=13) | Gum Arabic (n=13) | Psyllium (n=13) |
|---|---|---|---|---|
| Agea | 56.5 (11.0) | 60.6 (14.9) | 51.0 (11.8) | 63.6 (11.6) |
| Body mass indexa | 28.5 (3.9) | 30.2 (6.7) | 30.1 (8.9) | 27.9 (4.4) |
| n (%) | ||||
| Female | 8 (62) | 9 (69) | 10 (77) | 11 (85) |
| Race and Ethnicity | ||||
| White, not Hispanic | 12 (92) | 13 (100) | 12 (92) | 12 (92) |
| Black, not Hispanic | 0 | 0 | 1(8) | 0 |
| American Indian | 0 | 0 | 0 | 1 (8) |
| Asianb | 0 | 0 | 0 | 0 |
| More than 1 raceb | 1(8) | 0 | 0 | 0 |
| Hispanicb | 0 | 1(8) | 1(8) | 0 |
| Married/Partnered | 6 (46) | 10 (77) | 10 (77) | 8 (62) |
| Employed | 9 (69) | 7 (54) | 10 (77) | 6 (46) |
| Education- highest level | ||||
| Less than high school | 0 | 0 | 1 (8) | 0 |
| High school graduate, equivalent, or higher | 6 (46) | 6 (46) | 7 (54) | 4 (31) |
| College graduate or higher | 7 (54) | 7 (54) | 5 (39) | 9 (69) |
mean (sd); all among group comparisons were not statistically significant (p>.05);
too few observations per cell to analyze statistically
Degradabilities of Fiber Sources at Baseline and among Groups after Supplementation
Table 3 presents the in vitro degradability by human fecal inocula, before and after supplementation with dietary fiber, of the following: total cell wall polysaccharides and the most abundant cell wall polysaccharide sugar components in the dominant fractions (soluble or insoluble fiber) for each fiber source. There was no significant difference in degradability by fecal microflora of total polysaccharides or any of the component sugars among supplementation groups in the baseline period. The degradability of the component sugars of all fiber sources differed between the baseline and supplement periods; however, a significant fiber group by period interaction (i.e., the change from the baseline to the supplement period was different for at least one fiber source) was also observed for some sugars. The main effect of period (i.e., change from baseline to supplement period) was significant for degradability of nine of the 16 polysaccharide sugar components; however, for four of these cases, there was a significant period × supplemented fiber interaction (i.e., the change from baseline to supplement period differed among the fiber sources) (Table 3). For those fiber polysaccharide sugar components which only exhibited a significant main effect due to period (i.e., all fiber sources changed similarly from baseline to supplement period), degradability of insoluble CMC glucose, and total insoluble CMC and GA polysaccharides were greater in the baseline period than after supplementation; however, degradability of total soluble PSY polysaccharides was greater in the supplement period than during baseline.
Table 3.
In Vitro Degradability of Soluble (SOL) and Insoluble (INS) Fractions of Total Polysaccharides and the Most Abundant Cell Wall Polysaccharide Sugar Components of Three Dietary Fibers (Carboxymethylcellulose (CMC), Gum Arabic (GA), and Psyllium (PSY)) Before and After Their Supplementation.a Data Are the Proportion of Sugars Degraded at 24 h by Human Fecal Inocula.
| Fiber Source/Component | Fiber Fraction | Human Subject Supplementation Groups Baseline and Supplement (Suppl) Study Periods | p-value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Control | CMC | GA | PSY | Fiber Group Baseline Period | Fiber Group Overall | Period | Fiber Group Supplement Periodb | ||||||
| Baseline | Suppl | Baseline | Suppl | Baseline | Suppl | Baseline | Suppl | ||||||
| CMC | |||||||||||||
| Glucose | SOL | .36(.15) | .39(.10) | .42(.07) | .50(.11) | .38(.18) | .34(.13) | .41(.10) | .41(.11) | .66 | .09 | .36 | .22 |
| INS | .77(.20) | .69(.21) | .77(.19)C | .58(.13)d | .76(.14) | .64(.12) | .80(.19) | .64(.14) | .95 | .77 | <.001 | .50 | |
| Xylose | SOL | .52(.10) | .49(.12) | .59(.09) | .61(.08) | .58(.10) | .51(.12) | .53(.10) | .52(.24) | .23 | .11 | .35 | .55 |
| INS | .77(.19) | .83(.21)A | .72(.27) | .76(.12)A | .79(.15) | .72(.21)A | .73(.29)c | .39(.25)dB | .85 | .004 | .04 | .002 | |
| Total | INS | .75(.20) | .67(.21) | .78(.23)C | .59(.17)d | .75(.13) | .59(.20) | .77(.21) | .54(.12) | .96 | .82 | <.001 | .17 |
| SOL | .41(.19) | .43(.10) | .45(.20) | .56(.12) | .42(.12) | .40(.15) | .48(.11) | .43(.10) | .78 | .06 | .34 | .09 | |
| GA | |||||||||||||
| Arabinose | SOL | .93(.11) | .91(.13)A | .99(.07)c | .89(.15)dB | .92(.11) | .94(.10)A | .98(.11) | .92(.10)A | .20 | .89 | .005 | .009 |
| Galactose | SOL | .92(.11) | .92(.12)A | .98(.07)c | .89(.14)dB | .91(.11) | .94(.09)A | .97(.10) | .91(.09)B | .21 | .93 | .04 | .01 |
| Uronics | SOL | .97(.12) | .94(.08) | .93(.14) | .97(.07) | .94(.13) | .97(.05) | .92(.13) | .92(.06) | .80 | .64 | .61 | .51 |
| Total | INS | .59(.24) | .50(.27) | .68(.21)c | .31(.15)d | .64(.13) | .20(.38) | .42(.36) | .21(.44) | .43 | .54 | .004 | .10 |
| SOL | .94(.10) | .91(.10) | .97(.04) | .88(.22) | .92(.08) | .95(.07) | .95(.07) | .92(.08) | .45 | .97 | .52 | .07 | |
| PSY | |||||||||||||
| Xylose | INS | .84(.06)d | .89(.04)c | .88(.04) | .86(.06) | .85(.06) | .87(.05) | .83(.09)C | .87(.06)d | .19 | .80 | .009 | .003 |
| Arabinose | INS | .72(.08)d | .79(.05)c | .74(.06) | .76(.06) | .73(.05) | .73(.10) | .73(.06) | .75(.04) | .75 | .75 | .009 | .08 |
| Uronics | INS | .89(.19) | .94(.16) | .99(.08) | .89(.06) | .97(.07) | .95(.11) | .92(.10) | .91(.08) | .15 | .67 | .12 | .02 |
| Total | INS | .79(.06) | .84(.07) | .83(.05) | .79(.06) | .81(.05) | .79(.06) | .78(.07) | .80(.02) | .20 | .69 | .92 | .003C |
| SOL | .18(.11) | .30(.26) | .36(.09) | .47(.21) | .25(.14) | .32(.20) | .15(.13) | .70(.31) | .18 | .26 | .02 | .92 | |
Values are means (sds); supplement period means presented are unadjusted for baseline values; bolded values show where the substrate was the same as the fiber that the group ingested.
Because baseline values serve as the reference time point and are coded as 0, these p-values also test the significance of group × period interaction effect.
Values for in vitro degradability of a fiber source component for baseline and supplementation periods within a fiber supplementation group that do not share a superscript within a row differ significantly (p <0.05).
Values for in vitro degradability of a fiber source component in the supplementation period that do not share a superscript within a row differ significantly between fiber supplementation groups (p <0.05).
For this row, none of the comparisons between the values during the supplementation period were significantly different after adjustment for values in the baseline period.
Insoluble xylose in CMC was degraded to a significantly lesser extent by those who consumed the PSY supplement than by those who consumed the other supplements (Table 3). Soluble arabinose and galactose in GA were degraded to a greater extent by those who consumed GA than by those who consumed PSY. Insoluble xylose in PSY was degraded to a lesser extent by those who consumed CMC than by those who consumed PSY. The degradability of the uronic acids in PSY by those who consumed CMC was less than those who consumed PSY. For degradability of total polysaccharides, the only significant interaction was a greater degradability of total polysaccharides in the insoluble fraction of PSY by the placebo control group than by the CMC group. For degradability of total polysaccharides in PYS, a significant interaction effect was seen for the insoluble fraction; however, pair-wise comparisons between supplementation groups in the supplement period showed no significant differences when adjusted for baseline and multiple comparisons.
Degradabilities of fiber sources within supplement group
After adjustment for multiple comparisons, significant differences were seen in fiber degradability from the baseline to the supplement period within supplementation groups (Table 3). When stools from subjects who ingested CMC were used as the inoculum, degradability of the CMC fiber source in the supplement period was significantly lower than the baseline period for insoluble glucose and insoluble total polysaccharide. Similarly, the degradability of xylose in the insoluble fraction of CMC was significantly lower in the supplement period than the baseline period for stool inoculums from subjects ingesting PSY. For stools from subjects who ingested CMC, degradability of arabinose and galactose in the soluble GA fraction decreased significantly from the baseline period. The degrabability of total polysaccharide in the insoluble fraction of GA decreased significantly from the baseline to the supplement period in stools from subjects ingesting CMC or GA. One of the sugars of PSY, insoluble xylose, showed an increase in degradability after PSY consumption. Stools of the placebo group in the supplement period resulted in significantly increased degradability of xylose and arabinose in the insoluble fraction of PSY compared to baseline. Total polysaccharides in the soluble fraction of PSY showed a significant change from the baseline to the supplement period for all supplementation groups combined. However, total polysaccharides in the soluble fraction of PSY did not differ significantly between supplementation groups in the supplement period after adjusting for multiple comparisons.
Fermentation of Fiber Sources
Figures 1a and 1b illustrate the raw and modeled amounts of gas produced from fermentation of the three dietary fiber sources over time. Estimates from the modeled data compared to the raw data agreed well except after 24 h for CMC. Statistical diagnostic testing showed that overall model fit was good: 148 of the 150 residuals were below the standard acceptable value of the absolute value of 2 (-2.05 and 2.18); covariance ratios remained very close to the standard of 1 (.9 to 1.1); and the range of fixed effects deletion estimates for the parameters were tight: intercept 41.6 (39 to 44), hours 50.3 (48.5 to 52), hours2 -1.7 (-1.9 to- -1.4), CMC compared to PSY -74.3(-78 to -70), and GA compared to PSY (32.7 (30 to 37). None of the diagnostics indicated that the models did not fit the data.
Figure 1.
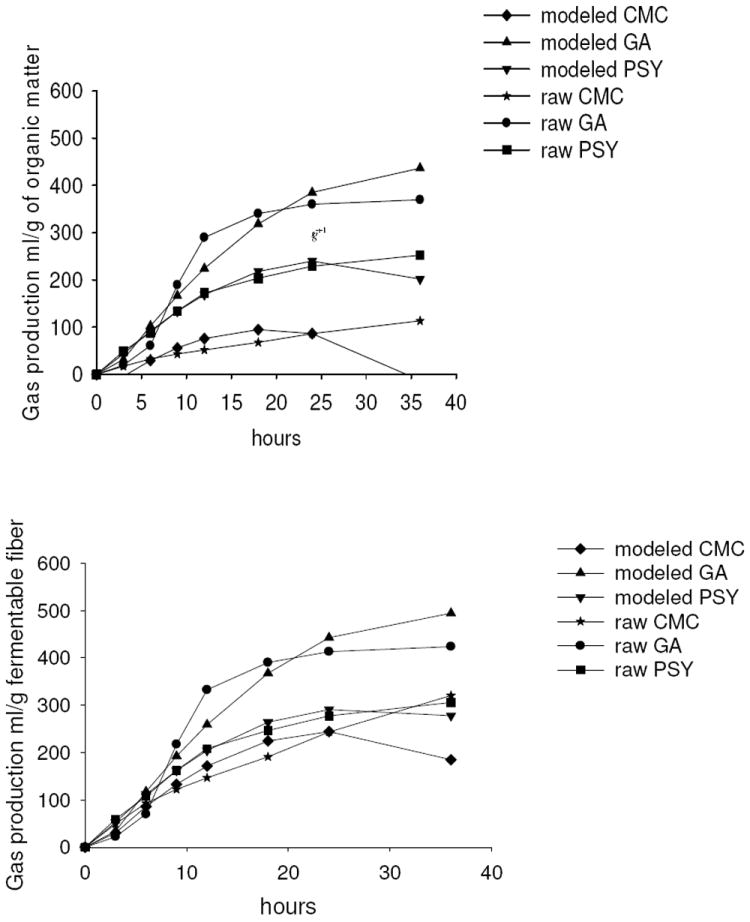
Graphs show raw and modeled data of gas production over time calculated per g of total organic matter of the fibers (Figure 1a) and measured dietary fiber content of the fiber sources (Figure 1b).
When gas production was calculated per unit of total organic matter (Figure 1a), initiation of gas production was most rapid for the PSY fiber substrate, but fermentation of GA fiber quickly overtook gas production from PSY by the 9 h time point and then gas production from GA fermentation continued to be greatest for the remainder of the fermentation. Specifically, the first measure (3 hours) of gas production from CMC was 32.1 ml gas/g added organic matter (OM) lower than PSY, the reference group (p < .001). Gas production of GA was 34.2 ml gas/g added OM lower than PSY, p < .001. Over time, production of gas from CMC averaged 5.1 ml gas/g added OM per hour lower than PSY, and GA averaged 7.5 ml gas/g added OM higher than PSY, both p < .001. At the 36 h time point, gas accumulation was still linear with time from CMC fermentation whereas gas accumulation from GA and PSY fermentation had begun to plateau. Adjusted pair-wise comparisons showed that differences in gas production at each time point were statistically significant at p < .001 except for GA vs. CMC at 3 hours (p = .39). However, when gas production was calculated based on measured dietary fiber content of the fiber sources (Figure 1b) rather than per unit of total organic matter, gas production from PSY and CMC fiber sources were quite similar.
As expected, concentrations of total and individual short-chain fatty acids produced by the fermentation of the three fiber sources (Figure 2) followed accumulation profiles very similar to those observed for total gas production on an organic matter basis. Production of SCFAs from CMC at 3 h was 15.7 mM below that of PSY, the reference group, (p = .04). Production of SCFAs from GA at 3 h was 22.4 mM above PSY (p = .006). Adjusted pair-wise comparisons indicated SCFA production remained significantly different over time. Overall, SCFA production increased .86 mM per hour (p = .007). The differences in SCFA production among fiber sources were not affected by incubation time.
Figure 2.
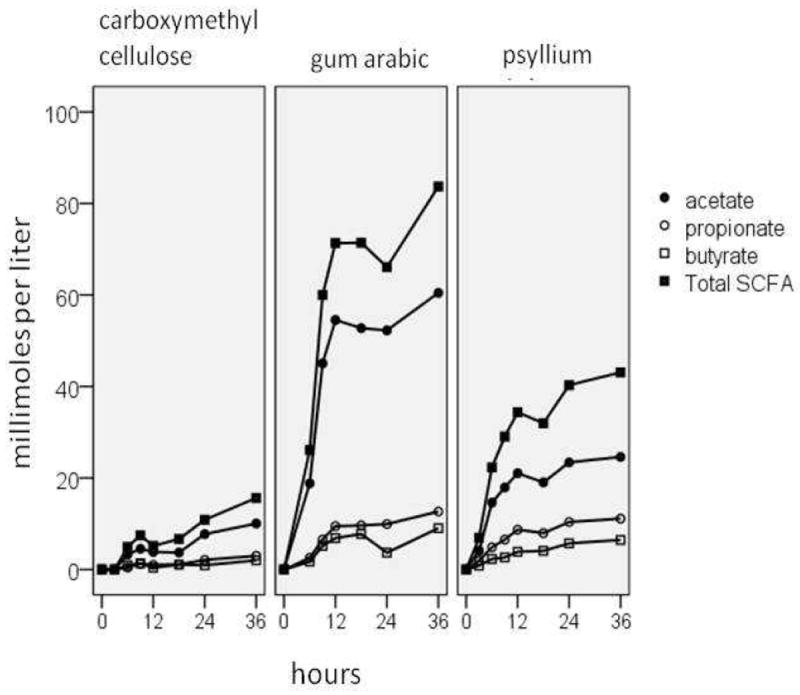
Short chain fatty acid (SCFA) concentrations from in vitro fermentation of fiber sources with a composite fecal inoculum from three non-supplemented human subjects are shown.
Regarding individual SCFAs, acetate was the primary fermentation product with propionate being of secondary abundance, although only slightly more so than butyrate, for all three fiber sources (Figure 2). Small amounts of other products (formate, succinate, and valerate) were also produced by the fiber fermentations (data not shown). The molar proportions of the individual SCFAs within fiber source did not change with incubation time (p > .05); therefore, the molar proportions were averaged over time. The averaged (sd) molar proportion of acetate decreased in the order GA (.77 (.04)) > CMC (.65 (.07)) > PSY (.62 (.04)), p < .001 with GA significantly different from CMS and PSY, but CMC and PSY not significantly different. The averaged molar proportion of propionate displayed the opposite relationship, although these differences, while statistically significant, were fairly small (GA (.13 (.02)) < CMC (.16(.04)) < PSY (.25 (.02)), p < .001, all significantly different).
DISCUSSION
In this study, we examined degradation of supplemental dietary fibers by examining production of microbial fermentation products (gas and SCFAs) resulting from in vitro incubation with mixed human colonic bacteria. In vitro fermentations have been shown to simulate the colonic fermentation of fiber to SCFAs,10, 11, 19, 20 and gas production has been shown to display good correlation to SCFA production during in vitro fermentation of other substrates (e.g., oligosaccharides) by human fecal microflora.21
The results show that supplementation of humans with a dietary fiber did not increase degradability of that fiber source in vitro using the fecal microflora of the subjects. Of the 15 comparisons for degradability between baseline and supplementation periods of the fiber source that was used to supplement subjects, two cases of declines in degradability were found and only one case of an increase. These findings suggest that the colonic fiber degrading microflora did not shift their metabolic capacity to degrade the additional fiber substrate more completely. Bacteria in the human colon have numerous substrates and nutrients available to metabolize, from both exogenous (i.e., the host’s diet) and endogenous (e.g., intestinal mucin, bile acids) sources.22 Changes in diet have been reported to modify the composition of the colonic microbial community in some human groups23-25 but our understanding of what influences the metabolism of bacteria in this complex environment is only at an early stage.22, 26, 27 Proposed factors influencing bacterial function range from the type and amount of substrate ingested,28, 29 health of the colon, baseline profile of the fecal microbiota,23 and genetics of the individual.30
A finding similar to ours has been shown in ruminant animals. Jung and Varel31 fed three ruminally cannulated steers a mixture of alfalfa, smooth bromegrass, and switchgrass hays in equal proportions and then each of the hays separately at 1.8% body weight for six weeks using a Latin square design. Ruminal samples were used as inocula in an in vitro fiber digestion study with the forages as the substrates. Improved in vitro fermentability of a particular forage species was not observed after feeding the same forage to the donor animals. While some experiments with ruminant animals have indicated that the rumen microflora do adapt to the feed source,32, 33 most studies of fiber degradability have not found any adaptation that increases fiber degradation.34-36 The implication of these findings for human fiber supplementation is that increased intake of a fiber source is not expected to result in bacterial adaptation that would require continually larger and eventually intolerable amounts of fiber to achieve a clinically important outcome associated with fiber ingestion.
The fecal degradation of some fiber fractions after supplementation with CMC or PSY was lower compared to baseline. A possible explanation is that although the mixed basal diet of humans results in colonic bacterial populations capable of degrading multiple types of fiber, heavy supplementation with a single fiber source in some subjects may have shifted the colonic bacterial population towards those bacteria capable of utilizing that fiber source, resulting in fewer bacteria able to degrade the other fibers and whose reduced presence somehow altered the microbial community’s capacity to degrade the fiber source in vitro. If this were the case, a clinical implication is that providing a supplement containing a mixture of different dietary fibers to humans, as has been reported,37, 38 might result in less degradation and fermentation of some fibers affecting desired clinical effects. This finding offers a hypothesis for future studies, which could be addressed with 16S pyrotag sequencing of the colonic bacterial community in fresh feces. In this regard, Metzler-Zebeli et al.39 have reported that feeding fiber supplements to pigs resulted in identifiable shifts in specific microbial taxa within the colon.
Because there were slight changes in SCFA molar proportions between treatments, the differences in gas production between fiber sources would be expected to estimate differences in fiber fermentation, but the estimation is likely to be fairly good, given the large differences in gas production compared to the small differences in SCFA molar proportions. Because fermentation end product formation is stoichiometrically coupled to the formation of specific SCFAs, the potential exists that measurement of gas production alone may not reflect true quantitative differences in the degradation of different substrates. In this study, however, substrate-dependent differences in the proportions of the main individual SCFAs were much smaller than substrate-dependent differences in observed gas production, suggesting that gas production provides a good estimate of differences in the fermentability of individual fiber substrates.
The poor fermentability of CMC is in part a reflection of the extensive substitution with carboxymethyl groups on the cellulose chain.40 These substitutions give CMC its desired water solubility but limit the ability of the products of CMC hydrolysis to serve as fermentable substrates for the colonic population. The high rate and extent of GA degradation were expected based on similar findings in our previous study2 and are in accord with the data of Bourquin et al.20 who reported that human fecal microflora not previously exposed to dietary fiber displayed greater in vitro degradation of GA than of CMC or PSY.
One question that arises is whether the clinical results were influenced by differences in digesta transit time among the groups which might have impacted degradation by the colonic microflora. In the parent study of all 189 subjects, there was no significant difference in the oral-anal transit time among the groups during the supplement period adjusting for baseline transit. Transit was estimated at two different times points (15 and 25 d after taking a steady amount of the fibers or placebo) by a change in color of stool after a capsule of dye was swallowed and ranged from <1 to 6 d at both times.6 Therefore, we do not believe the effects of digesta transit time on the colonic microflora account for the general decline in degradability observed between the baseline and supplement periods.
The greater amount of gas produced by GA in vitro suggests that a dietary supplement of GA might produce uncomfortable symptoms of flatus and low tolerance in vivo. In the parent study, the amount of flatus reported by all the groups during the supplement period was small, and the only significant difference was a greater amount of flatus in the CMC group compared to the placebo control group.7 There may be several explanations for this difference between in vitro and in vivo findings. Flatus was estimated by self-report of subjects and not measured, so estimates may lack precision. There was considerable variation in flatus amount within each supplement group which may have been due to actual differences in flatus or only the perception of flatus. Flatus responses may be different between subjects with normal GI function who provided stools for the in vitro experiment in this study and subjects with fecal incontinence in the parent study who consumed the supplements and estimated flatus amount. Finally, conditions in an in vitro system may be different than in the colon; for example, gas accumulation in the in vitro system was not released during the course of the experiment, while gas produced in the colon may be released gradually.
There are other limitations to this study. The general lack of effect of fiber supplementation on the rate or extent of in vitro fermentation of the fibers was determined on subjects who had fecal incontinence and responses of those with normal GI function may differ. Colonic transit time may be more important than oral-anal transit time for explaining fiber fermentation but was not measured in this study.
The findings of this study provide evidence from humans about effects of dietary fiber supplementation on fermentation previously reported only in ruminant animals. The human response shows no improvement in in vitro dietary fiber degradability after supplementation with the same fiber source, suggesting that beneficial clinical effects of dietary fiber might be sustained with continued supplementation.
Acknowledgments
This study was funded by the National Institute of Nursing Research, NIH, R01 NR07756.
Abbreviations used
- CMC
carboxy-methylcellulose
- GA
gum arabic
- PSY
psyllium
- SCFA
short chain fatty acid
Footnotes
Supporting information descriptions
A copy of a manuscript cited in this paper that is in review and not yet published: Bliss, D. Z.; Savik, K.; Jung, H. J.; Whitebird, R.; Lowry, A.; Sheng, X. Dietary fiber supplementation for fecal incontinence: A randomized clinical trial. In review
Safety
There are no special safety concerns.
References
- 1.Klosterbuer A, Roughead ZF, Slavin J. Benefits of dietary fiber in clinical nutrition. Nutr Clin Pract. 2011;26:625–635. doi: 10.1177/0884533611416126. [DOI] [PubMed] [Google Scholar]
- 2.Bliss DZ, Jung HJ, Savik K, Lowry A, LeMoine M, Jensen L, Werner C, Schaffer K. Supplementation with dietary fiber improves fecal incontinence. Nurs Res. 2001;50:203–213. doi: 10.1097/00006199-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- 4.Theander O, Aman P, Westerlund E, Andersson R, Pettersson D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): collaborative study. J AOAC Int. 1995;78:1030–1044. [PubMed] [Google Scholar]
- 5.Ahmed AER, Labavitch JM. A simplified method for accurate determination of cell wall uronide content. J Food Biochem. 1977;1:361–365. [Google Scholar]
- 6.Bliss DZ, Savik K, Jung HJ, Whitebird R, Lowry A, Sheng X. Dietary fiber supplementation for fecal incontinence: A randomized clinical trial. doi: 10.1002/nur.21616. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss DZ, Savik K, Jung HJ, Whitebird R, Lowry A. Symptoms associated with dietary fiber supplementation over time in individuals with fecal incontinence. Nurs Res. 2011;60:S58–67. doi: 10.1097/NNR.0b013e3182186d8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall EI. Studies on ruminant saliva. I. The composition and output of sheep’s saliva. Biochem J. 1948:99–109. [PMC free article] [PubMed] [Google Scholar]
- 9.Weimer PJ, Dien BS, Springer TL, Vogel KP. In vitro gas production as a surrogate measure of the fermentability of cellulosic biomass to ethanol. Appl Microbiol Biotechnol. 2005;67:52–58. doi: 10.1007/s00253-004-1844-7. [DOI] [PubMed] [Google Scholar]
- 10.Bourquin LD, Titgemeyer EC, Garleb KA, Fahey GC., Jr Short-chain fatty acid production and fiber degradation by human colonic bacteria: effects of substrate and cell wall fractionation procedures. J Nutr. 1992;122:1508–1520. doi: 10.1093/jn/122.7.1508. [DOI] [PubMed] [Google Scholar]
- 11.Weimer PJ, Shi Y, Odt CL. A segmented gas/liquid delivery system for continuous culture of microorganisms on solid substrates, and its use for growth of Ruminococcus flavefaciens on cellulose. Appl Microbiol Biotechnol. 1991:178–183. [Google Scholar]
- 12.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 13.Pan Z, Lin DY. Goodness-of-fit methods for generalized linear mixed models. Biometrics. 2005;61:1000–1009. doi: 10.1111/j.1541-0420.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 14.Bell BA, Schoeneberger JA, Morgan GB, Kromrey JD, Ferron JM. Fundamental diagnistics for two-level mixed models: The SAS Macro Mixed_DX. SAS Global Forum. 2010 http://support/SAS.com/usergroups/
- 15.Schabenberger O. Mixed model influence diagnostics. Proceedings of the SAS User group International. 2013 http://support/SAS.com/usergroups/
- 16.Keselman HJ, Algina J, Kowalchuk RK. The analysis of repeated measures designs: a review. Br J Math Stat Psychol. 2001;54:1–20. doi: 10.1348/000711001159357. [DOI] [PubMed] [Google Scholar]
- 17.Barba C, Montane D, Rinaudo M, Farriol X. Synthesis and characterization of carboxymethylcellulose (CMC) from non-wood fibers. I. Accessibility of celluose fibers and CMC synthesis. Cellulose. 2002:319–326. [Google Scholar]
- 18.Showalter AM. Arabinogalactan proteins: structure, expression and function. Cell Mol Life Sci. 1999:1399–1417. doi: 10.1007/PL00000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titgemeyer EC, Bourquin LD, F GC, Jr, Garleb KA. Fermentability of various fiber sources by human fecal bacteria in vitro. Am J Clin Nutr. 1991;53:1418–24. doi: 10.1093/ajcn/53.6.1418. [DOI] [PubMed] [Google Scholar]
- 20.Bourquin LD, Titgemeyer EC, Fahey GC, Jr, Garleb KA. Fermentation of dietary fiber by human colonic bacteria: disappearance of, short-chain fatty acid production from, and potential water-holding capacity of, various substrates. Scand J Gastroenterol. 1993;28:249–255. doi: 10.3109/00365529309096081. [DOI] [PubMed] [Google Scholar]
- 21.Hernot DC, Boileau TW, Bauer LL, Middelbos IS, Murphy MR, Swanson KS, Fahey GC., Jr In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. J Agric Food Chem. 2009;57:1354–1361. doi: 10.1021/jf802484j. [DOI] [PubMed] [Google Scholar]
- 22.Gilland MG, Young VB, Huffnagle GB. Gastrointestinal Microbial Ecology with Perspectives on Health and Disease. Elsevier Inc.; Philadelphia, PA: 2012. [Google Scholar]
- 23.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. The Isme Journal. 2011;5:220–230. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelan K, Judd PA, P V, Simmering R, Jann A, Taylor MA. Fructooligosaccharides and fiber partially prevent the alterations in fecal microbiota and short-chain fatty acid concentrations caused by standard enteral formula in healthy humans. J Nutr. 2005;135:1896–902. doi: 10.1093/jn/135.8.1896. [DOI] [PubMed] [Google Scholar]
- 26.Duncan SH, Louis P, Flint HJ. Cultivable bacterial diversity from the human colon. Lett Appl Microbiol. 2007;44:343–350. doi: 10.1111/j.1472-765X.2007.02129.x. [DOI] [PubMed] [Google Scholar]
- 27.Egert M, de Graaf AA, Smidt H, de Vos WM, Venema K. Beyond diversity: functional microbiomics of the human colon. Trends Microbiol. 2006;14:86–91. doi: 10.1016/j.tim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Bouhnik Y, Vahedi K, Achour L, Attar A, Salfati J, Pochart P, Marteau P, Flourie B, Bornet F, Rambaud JC. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J Nutr. 1999;129:113–116. doi: 10.1093/jn/129.1.113. [DOI] [PubMed] [Google Scholar]
- 29.Majid HA, Emery PW, Whelan K. Faecal microbiota and short-chain fatty acids in patients receiving enteral nutrition with standard or fructo-oligosaccharides and fibre-enriched formulas. J Hum Nutr Diet. 2011;24:260–268. doi: 10.1111/j.1365-277X.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 30.Arora T, Sharma R. Fermentation potential of the gut microbiome: implications for energy homeostasis and weight management. Nutr Rev. 2011;69:99–106. doi: 10.1111/j.1753-4887.2010.00365.x. [DOI] [PubMed] [Google Scholar]
- 31.Jung HG, Varel VH. Influence of forage type on ruminal bacterial populations and subsequent in vitro fiber digestion. J Dairy Sci. 1988;71:1526–1535. doi: 10.3168/jds.S0022-0302(88)79716-7. [DOI] [PubMed] [Google Scholar]
- 32.Bezeau LM. Effect of source on inoculum on digestibility of substrate in in vitro digestion trials. J Animal Sci. 1965:823–825. [Google Scholar]
- 33.Horton GMJ, Christensen DA, Steacy GM. In vitro fermentation of forages with inoculum from cattle and sheep fed different diets. Agron J. 1980:601–605. [Google Scholar]
- 34.Grant RJ, Van Soest PJ, McDowell RE. Influence of rumen fluid source and fermentation time on in vitro true dry matter digestibility. J Dairy Sci. 1974;57:1201–1205. doi: 10.3168/jds.S0022-0302(74)85037-X. [DOI] [PubMed] [Google Scholar]
- 35.Jung HG, Fahey GC, Jr, Garst JE. Simple phenolic monomers of forages and effects of in vitro fermentation on cell wall phenolics. J Animal Sci. 1983;57:1294–1305. [Google Scholar]
- 36.Nik-Khan A, Tribe DE. A note on the effect of diet on the inoculum used in digestibility determination in vitro. J Animal Prod. 1977;25:103–106. [Google Scholar]
- 37.Schneider SM, Girard-Pipau F, Anty R, van der Linde EG, Philipsen-Geerling BJ, Knol J, Filippi J, Arab K, Hebuterne X. Effects of total enteral nutrition supplemented with a multi-fibre mix on faecal short-chain fatty acids and microbiota. Clin Nutr. 2006;25:82–90. doi: 10.1016/j.clnu.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Linetzky Waitzberg D, Alves Pereira CC, Logullo L, Manzoni Jacintho T, Almeida D, Teixeira da Silva ML, Matos de Miranda Torrinhas RS. Microbiota benefits after inulin and partially hydrolized guar gum supplementation: a randomized clinical trial in constipated women. Nutricion Hospitalaria. 2012;27:123–129. doi: 10.1590/S0212-16112012000100014. [DOI] [PubMed] [Google Scholar]
- 39.Metzler-Zebeli BU, Hooda S, Zilstra RT, Mosenthin R, Ganzle MG. Dietary supplementation of viscous and non-starch polysaccharides (NSP) modulates microbial fermentation in pigs. Livestock Sci. 2010;133:95–97. [Google Scholar]
- 40.Miller GL, Blum R, Glennon WE, Burton AL. Measurement of carboxymethylcellulase activity. Anal Biochem. 1960;1:127–132. [Google Scholar]


