Abstract
Background
Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CS + HIPEC) is a treatment combining cytoreductive surgery with hyperthermic chemotherapy directly into the peritoneal cavity. Recipients may gain extended life when compared with best supportive care; yet results often are achieved with substantial morbidity and health-related quality of life (HRQOL) deficits. The purpose of this study was to record patient rated outcomes and the HRQOL of long-term survivors.
Methods
One hundred and two patients living 12+ months post-treatment completed a survey including the Medical Outcomes Study 36-item Short Form Health Survey (SF-36), Functional Assessment of Cancer Therapy-Colon (FACT-C), and Pittsburgh Sleep Quality Index.
Results
SF-36 Physical Component scores were significantly lower than general population norms (46.7, z = −2.943, P = 0.003), while Mental Component scores were significantly higher (53.6, z = 4.208, P ≤ 0.001). FACT scores were higher than general FACT normative scores. The majority (56%) of these survivors reported significant sleep quality impairment.
Conclusion
Although most HRQOL scores were comparable to or higher than those of the general population, long-term physical and functional deficits remain. These deficits, along with the poor sleep quality of recipients, may be improved by survivorship programs or targeted psychosocial interventions.
Keywords: health-related quality of life, peritoneal carcinomatosis, long-term survivorship
INTRODUCTION
Peritoneal dissemination of intra-abdominal malignancy is a uniformly fatal condition. Despite advances in systemic chemotherapy, long-term survival without surgery is essentially unknown in this setting. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy (CS + HIPEC) has allowed for long-term survival in a subset of patients undergoing the procedure. CS + HIPEC combines cytoreductive surgery with chemoperfusion directly into the peritoneal cavity [1]. CS + HIPEC offers individuals with peritoneal disease the possibility of achieving extended survival, although it can be associated with substantial morbidity (e.g., 27–56%) [1,2]. Quality of life (QOL) research has revealed additional post-operative complications, including anxiety, sleep disturbance, and depressive symptoms [3–5]. Despite a 20-year history, there are few methodologically sound health-related QOL (HRQOL) and symptom management studies in the CS + HIPEC literature [6].
There are three important reasons to collect HRQOL and symptom data in the HIPEC context. First, post-treatment functioning data can inform expectations and pre-treatment decision-making. Despite detailed efforts to educate patients, most do not, perhaps cannot, fully comprehend the potential impact of CS + HIPEC on all aspects of functioning. Next, routine systematic monitoring could improve patient care. After hospital discharge, patients may be geographically distant and have only one follow-up appointment with their HIPEC surgeon. Likewise, patients may report symptoms only when asked about them directly, posing a risk that certain symptoms will go undetected without systematic data collection [6]. Finally, systematic monitoring of symptoms and HRQOL can assist with survivorship planning [7].
HRQOL and troubling symptoms, including sleep disturbances, are under-investigated in CS + HIPEC survivors. Sleep concerns in the context of cancer may be related to predisposing (e.g., psychological disorder), novel (e.g., pain), or maintaining (e.g., drugs) factors [8]. In addition to its association with HRQOL, sleep quality has been associated with numerous health problems, including immunosuppression and cardiovascular disease [8,9]. In one of the few studies examining the HRQOL of long-term CS + HIPEC survivors (i.e., ≥3 years), poor sleep was one of the top three psychosocial concerns [3], and approximately 35% of participants indicated current or past concerns with sleep. Utilization of a brief, psychometrically sound instrument can clarify the prevalence and nature of sleep disturbances.
The purpose of this study was to collect HRQOL, sleep quality, and symptom data from long-term CS + HIPEC survivors. We hypothesized that the majority of survivors would report HRQOL deficits relative to that of the general population as well as significant physical symptoms and sleep quality impairment.
MATERIALS AND METHODS
This is a cross-sectional, descriptive study designed to gather information about long-term survivors of CS + HIPEC. Participants were selected from a prospectively maintained comprehensive database of recipients at Wake Forest Baptist Health (WFBH). Eligibility criteria included: (1) recipient of CS + HIPEC 12 or more months prior to study initiation; (2) updated contact information; and (3) English literate. This study was approved by the WFBH Institutional Review Board.
A telephone script was used to make recruitment phone calls. Investigators explained the purpose of the study and obtained preliminary verbal consent. Subsequently, a cover letter, written consent, and instruments were mailed. Phone calls were made if instruments were incomplete or missing. Gas cards ($25) and signed consent forms were mailed to participants.
The Medical Outcomes Study 36-Item Short-Form Health Survey
The Short Form Health Survey (SF-36) is a 36 item, generic health measure that assesses perceived health in eight areas: physical functioning (PF), role-physical (RP), role-emotional (RE), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), and mental health (MH) [10–12]. The Mental Component Summary (MCS) and Physical Component Summary (PCS) are the comprehensive components derived from the eight scales. Higher scores indicate better functioning. High scores on the MCS indicate minimal psychological distress and functional limitations due to emotional problems. High scores on the PCS denote minimal physical or role limitations and good GH. The results from the general U.S. population census were utilized as norm anchors, permitting ease of comparison between clinical and general populations.
The Functional Assessment of Cancer Therapy-Colon (FACT-C) Scale
The Functional Assessment of Cancer Therapy-General (FACT-G) is a 27-item self-report questionnaire that measures QOL [13]. The FACT-C is the FACT-G plus the 9-item colon subscale, selected because its items best address symptoms associated with Disseminated Peritoneal Carcinomatosis (DPC). The FACT consists of four sub-scales measuring physical (PWB), functional (FWB), social/familial (SFWB), and emotional well-being (EWB), and provides a total QOL score as well. Patients rate how they feel over the past 7 days on a 5-point Likert-type scale. Higher scores indicate better QOL. FACT scores were compared to published norms for cancer survivors. A Trial Outcome Index (TOI) representing PWB + FWB + C subscale was used to assess treatment impact on PF. Cronbach’s alpha is: PWB (0.82), FWB (0.80), SFWB (0.69), EWB (0.74), FACT-G (0.89) [13].
Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) assesses sleep quality and disturbances over the past 4 weeks [14]. Nineteen of the 24 items are client-rated and used in score tabulation. The scorer assigns an ordinal score, ultimately deriving a global score and seven component scores (sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction). Component scores (i.e., 0–3) are given equal weight, indicating a possible global score of 0–21. Lower scores reflect better sleep quality. A component score of “0” indicates no difficulty; “3” reflects severe difficulties. A global cut-off score of 5 was established to denote clinically significant sleep impairment [15]. A score above this cut-off suggests severe problems in a minimum of two areas or moderate-intensity problems in three or more. Scores, therefore, inherently reflect both the number and severity of sleep–wake disturbances [14].
Data Analysis
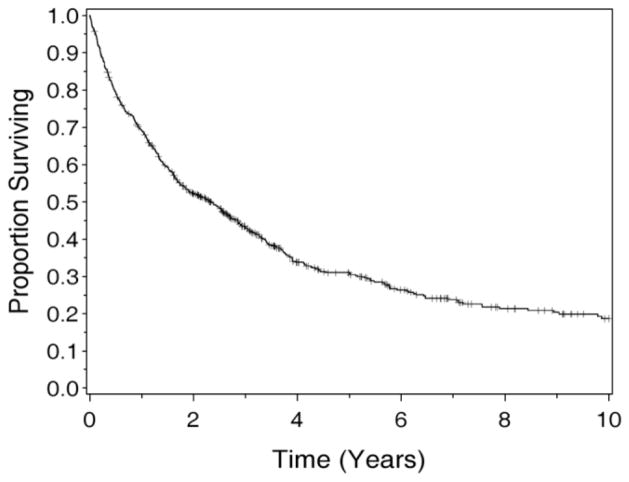
Descriptive statistics, including means and standard deviations for continuous measures and frequencies and proportions for categorical data, were calculated for all measures. Differences between participants and eligible non-participants were compared on: age, gender, resection status, primary tumor site, and performance status. Norm-based scores (NBS) and their associated z-statistics were calculated with general population parameters for the SF-36 data (Table II). Scores that differed significantly from 50 (the general population mean for NBS) with a P-value <0.05 are considered significantly different from general population means. Additionally, the sleep quality of participants was examined. Relationships between sleep quality and HRQOL were examined with Pearson’s product moment correlations. Those relationships strong enough to be considered at least moderate in intensity (r ≥ 0.50) are reported. Finally, the contributions of two independent, categorical surgical variables (i.e., resection status and primary tumor site) to HRQOL PCS and MCS were examined in an analysis of covariance (ANCOVA); this ANCOVA also adjusted for age at the time of surgery and length of time since surgery. The respective groups were combined into resection status groups of R0/R1, R2a, and R2b/c (R2c status had only one subject), while primary tumor sites were grouped into appendix, colon, and “other”. To determine which groups varied significantly, pair-wise comparisons of mean PCS levels by primary disease and resection status were performed if the overall effect was found to be significant. The Kaplan–Meier method was used to estimate survival for the entire CS + HIPEC cohort; estimates are displayed graphically (Fig. 1).
TABLE II.
SF-36 Subscale and Component Scores
| Variable | Mean | SD | Min | Max | Lower 95% for mean | Upper 95% for mean | z-Score | P-value |
|---|---|---|---|---|---|---|---|---|
| PF_NBS | 46.0 | 11.1 | 15.2 | 57.1 | 43.8 | 48.1 | −3.676 | <0.001 |
| RP_NBS | 46.6 | 12.0 | 28.0 | 56.2 | 44.2 | 49.0 | −2.844 | 0.004 |
| BP_NBS | 52.2 | 10.6 | 28.9 | 62.8 | 50.1 | 54.3 | 2.084 | 0.037 |
| GH_NBS | 49.1 | 10.8 | 24.2 | 64.0 | 46.9 | 51.2 | −0.877 | 0.380 |
| VT_NBS | 52.0 | 10.9 | 23.0 | 70.4 | 49.8 | 54.1 | 1.836 | 0.066 |
| SF_NBS | 50.8 | 9.5 | 13.7 | 57.1 | 48.9 | 52.6 | 0.801 | 0.423 |
| RE_NBS | 50.1 | 10.0 | 23.7 | 55.3 | 48.1 | 52.0 | 0.074 | 0.941 |
| MH_NBS | 53.6 | 9.0 | 25.5 | 64.1 | 51.8 | 55.4 | 4.002 | <0.001 |
| PCS | 46.7 | 11.2 | 19.8 | 62.5 | 44.6 | 48.9 | −2.943 | 0.003 |
| MCS | 53.6 | 8.7 | 25.9 | 68.9 | 51.9 | 55.3 | 4.208 | <0.001 |
NBS, norm-based score; PF, physical functioning; RP, role-physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role-emotional; MH, mental health; PCS, physical component score; MCS, mental component score.
P-values represent comparison between the study sample and population norms.
Fig. 1.
Kaplan–Meier survival curve: all recipients of CS + HIPEC at least 12 months prior to the study.
RESULTS
A total of 649 patients were at least 1-year status post-CS + HIPEC. Of those, 224 (35%) were alive, 425 (65%) deceased. Of the 224 eligible, 2 died mid-study; 35 had inaccurate telephone numbers. Of the remaining 187, 136 individuals consented verbally, while the remaining 51 prospective participants were not reached or declined. A total of 102 survivors returned completed questionnaires (refer to Table I for sociodemographic characteristics). Age was the only variable tested that differed significantly between participants and non-participants, with non-responders (mean age at CS + HIPEC = 50.1) averaging approximately 5 years younger than responders (54.7; P = 0.005). When data from the entire sample of CS + HIPEC recipients were examined, approximately 50% were alive 2 years post-treatment (Fig. 1). A Kaplan–Meier survival curve (Fig. 1) provides context for the performance of these long-term survivors relative to all peers who received CS + HIPEC at least 12 months prior to the study (N = 649; CS + HIPEC date range= 12/1991–1/2009). When all 649 recipients are included, the median (midpoint) survival is 2.3 years. Estimates of survival for all recipients (Fig. 1) are: year 1: 69.1% (SE = 1.8%), 2: 52.2% (2.0%), 3: 43.1% (2.0%), 5: 31.0% (2.0%), 10: 18.6% (2.1%). As a basis of comparison, the mean years since surgery for the long-term survivors included in this study is 4.2 (range = 1.1–16.5).
TABLE I.
Participant Characteristics
| Variable | n | % | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|---|
| Age at HIPEC treatment | 101 | 54.7 | 12.0 | 56.0 | 21.0 | 80.0 | |
| Age (current) | 102 | 58.5 | 12.6 | 61.0 | 23.0 | 87.0 | |
| Years since surgery | 99 | 4.2 | 3.5 | 2.9 | 1.1 | 16.5 | |
| Sex | |||||||
| Female | 53 | 52.5 | |||||
| Male | 48 | 47.5 | |||||
| Race | |||||||
| Asian | 2 | 2 | |||||
| African-American | 14 | 14 | |||||
| Native American | 1 | 1 | |||||
| White | 81 | 79 | |||||
| White, Native American | 4 | 4 | |||||
| Primary diagnosis site | |||||||
| Appendix | 66 | 65.4 | |||||
| Colon | 14 | 13.9 | |||||
| Mesothelioma | 6 | 5.9 | |||||
| Ovary | 6 | 5.9 | |||||
| “Other” | 9 | 8.9 | |||||
| Resection status | |||||||
| R0/R1 | 62 | 62 | |||||
| R2a | 27 | 27 | |||||
| R2b | 10 | 10 | |||||
| R2c | 1 | 1 | |||||
To assess population differences attributable to time-since-surgery, patients 13–24 months post-CS + HIPEC were compared to those greater than 24 months post-CS + HIPEC. Those chronologically closer to the procedure (<2 years) rated their health 1 year prior (SF-36) as significantly worse than those more removed (>2 years). No other group differences were noted, so data were analyzed in aggregate form for all subsequent analyses.
HRQOL and Daily Activities
Complete SF-36 results are reported in Table II. The PF [NBS = 46.0, z = −3.676, P ≤ 0.001], RP (NBS = 46.6, z = −2.844, P = 0.004), and PCS (NBS = 46.7, z = −2.943, P = 0.003) scores were significantly lower than those of the general population, while the MH (NBS = 53.6, z = 4.002, P <0.001), BP (NBS = 52.2, z = 2.084, P = 0.037), and the MCS (NBS = 53.6, z = 4.208, P ≤ 0.001) were significantly higher. Table III includes patient-rated limitations on daily activities. A significant percentage (i.e., >15%) of patients indicated that they were “limited a lot” in four areas, including their ability to: climb several flights of stairs (16%), walk several blocks (18%), walk >1 mile (26%), and in vigorous activities (43%).
TABLE III.
Patient-Reported Functional Limitations
| Variable | Limited a lot (%) | Limited a little (%) | Not limited (%) |
|---|---|---|---|
| Vigorous activities | 43 | 41 | 16 |
| Moderate activities | 9 | 36 | 55 |
| Lift/carry groceries | 6 | 20 | 75 |
| Climb flights of stairs | 16 | 33 | 51 |
| Climb 1 flight of stairs | 4 | 22 | 75 |
| Bend/kneel/stoop | 12 | 30 | 58 |
| Walk >1 mile | 26 | 23 | 51 |
| Walk several blocks | 18 | 17 | 65 |
| Walk one block | 6 | 18 | 76 |
| Bathe/dress self | 3 | 8 | 89 |
Scores (SD) on the FACT-C were: PWB, 23.4 (4.8); SWB, 24.5 (4.0); EWB, 20.5 (3.4); FWB, 22.2 (5.8); C, 27.7 (6.4); and overall FACT-G, 90.3 (13.9). TOI was 71.4 (12.2).
Resection Status and HRQOL
The contributions of resection status and primary tumor site to PCS and MCS were examined. Although resection status did not produce significant main effects in the model, primary tumor site showed a significant main effect on the PCS of participants (F(2, 95) = 3.16, P = 0.047). To determine the origin of this effect, pairwise comparisons of mean PCS levels by primary disease were performed. Those with a primary tumor site of appendix (estimated mean = 46.5) versus “other” (39.5) demonstrated significant differences (better) in PF (P = 0.014).
Self-Report of Specific Symptoms
In an open-ended format, patients were asked to identify and rate their most troubling current symptoms [i.e., 1 (very mild) to 10 (as severe as you could imagine)]. Investigators categorized symptom clusters. Patients reported a total of 156 symptoms, averaging 1.5 symptoms reported per patient. A total of 17 participants wrote “none,” while 13 left the question blank. The most common unifying symptom constellation was related to GI distress (e.g., bloating, diarrhea). Symptoms from this category were identified 56 times by responders with an average intensity rating of 5.8.
Similarly, on the FACT-C, a significant percentage (i.e., >15%) of patients indicated that they have problems with diarrhea (18%). On the global QOL question on the FACT, 74% reported “quite a bit” or “very much” to the question “I am satisfied with the quality of my life right now.”
Sleep Quality
On the PSQI, a total of 56% (N = 57) of participants scored above the clinical cut-off score of 5, indicating a high presence of significant sleep disturbances. The mean PSQI score was 6.8 (SD = 4.4, range = 0.0–19.6). The highest scale score mean was on the Sleep Disturbances Scale (m = 1.5), the lowest on Daytime Dysfunction (m = 0.7).
Relationships between sleep quality and HRQOL were examined. Positive relationships (better sleep was associated with higher QOL) were noted between global sleep quality scores and RP (r = 0.54), GH (r = 0.51), VT (r = 0.57), and PCS (r = 0.51), P <0.0001 for all comparisons. Moderate correlations also were noted between Sleep Disturbance and BP (r = 0.54), P <0.0001.
DISCUSSION
Our hypotheses that long-term survivors report HRQOL deficits relative to the general population as well as significant physical symptoms and sleep impairment proved accurate, within the context of four major findings.
First, a number of physical symptoms remain yet patients are adapting. The PF subscale scores on the SF-36 fell significantly below the general population norms, while the MH and MCS were significantly higher than norms. Together, these results suggest that long-term survivors may achieve acceptable emotional well-being 1 or more years post-treatment while physical and functional deficits remain.
All of these participants share the fact that they were presented with the stark possibility of limited lifespan and few treatment options. CS + HIPEC is a serious, perhaps traumatic event for many, if not all, patients. Although not measured directly or over time within this cohort, it is possible that this confrontation with mortality was shocking and produced a type of post-traumatic growth (PTG). PTG has been described as the gradual internal paradigm shifts that may occur following internal disruption spurred by trauma [15,16]. Enhancement of self regard and life philosophy are only a few areas that may be susceptible to gradual growth after trauma. The concept of response shift, suggesting that people will accommodate to new norms and experience shifts in internal standards, also may help to explain these seemingly contradictory findings of decrements in physical well being accompanied by improved MH [17,18]. Compared to their immediate pre- and post-treatment QOL, these survivors may perceive their current mental health as relatively high.
When compared to FACT normative data from a general adult sample [i.e., PWB 22.7 (5.4), SWB 19.1 (6.8), EWB 19.9 (4.8), FWB 18.5 (6.8), and FACT-G 80.1 (18.1)], the scores for CS + HIPEC recipients are higher and suggest comparatively good overall QOL [19]. However, the PCS on the SF-36 indicate that recipients score low relative to the norm in the general population. Differences between QOL instruments may explain discrepancies. The SF-36 includes additional questions on activities of daily living, whereas the FACT is measuring more global aspects of HRQOL. It is only in the Colon subscale of the FACT, a measure of specific symptoms, where we see the QOL deficits and specific symptom clusters more clearly. Similarly, even though a significant percentage of people rated their health as excellent (Table III), 43% reported being “limited a lot” with vigorous activities. In conclusion, reports of lasting physical decrements and symptoms alongside concurrent reports of high HRQOL may reflect a response shift and process of adaptation over the course of many months and years. Specific symptoms reported both formally and informally have implications for ongoing symptom management efforts.
Second, the mean sleep quality score of 6.8 was above the clinical cut-off score that marks the presence of significant sleep quality impairment [14]. A total of 56% of participants endorsed clinically impaired sleep quality, compared to a normal population estimate (e.g., 26%) of sleep impairment [20]. Significant positive relationships between global sleep quality scores and HRQOL subscales suggest the interrelated nature of sleep and alternate domains of functioning. Interestingly, when presented with the opportunity to self-identify troubling symptoms, few identified sleep disturbance specifically. This underscores the importance of presenting people with symptom checklists or inquiring directly about symptoms in order to achieve accurate diagnoses and corresponding treatment plans to treat impaired sleep aggressively. Targeted sleep interventions could help improve physical and MH outcomes as well.
Third, resection status breakdown included 65% = R0/R1, 27% = R2a, 10% = R2b, 1% = R2c. This finding is similar to previous work that suggested resection status was correlated significantly with improved survival status and that patients who underwent a complete resection (i.e., R0/R1) had better outcomes than their incompletely resected counterparts, regardless of primary diagnosis [1]. Relative to long-term HRQOL, however, resection status did not seem to produce significant effects on component scores. Tuttle et al. [21] reported similarly that resection status was not a significant contributor to HRQOL. In conclusion, those with a more desirable resection status may have improved survival estimates but do not demonstrate improved overall physical and mental HRQOL long-term [1].
Fourth, unlike resection status, primary tumor site did demonstrate a significant main effect on the PCS of survivors, with individuals with a primary diagnosis of appendiceal cancer (N = 65% of sample) reporting higher PF. Already supported in the literature, Levine et al. [1] noted the differential clinical outcomes experienced by patients based on the pathological characteristics of their primary diagnosis and concluded that primary tumor site is correlated with improved survival.
Several study limitations exist. First, participants were patients of one hospital and four surgeons. Only those with accurate contact information and with adequate health completed the survey. Those who lived less than 12 months post-surgery or who were too ill to complete the instruments are not represented. Second, ethnic and racial statistics highlight the sample’s homogeneity. Intense recruitment efforts were extended to all patients, however, suggesting that this sample adequately represents long-term survivors. Third, although participants ranged from 1.1 to 16.5 years post-CS + HIPEC, data were collected at one time point along their recovery trajectories. Factors unrelated to CS + HIPEC (e.g., aging, alternative treatments, recurrence) may have impacted responses. Previous CS + HIPEC researchers have demonstrated significant changes in HRQOL scores across recovery, with lowest scores reported immediately post-procedure [22]. To clarify the role of other variables, comparisons were made to population norms, and time-since-surgery differences were assessed. Yet it remains important to acknowledge the possible contributions of other influential variables, such as additional health problems not identified in this study. The inclusion of a non-CS + HIPEC comparison group in future studies could provide greater clarity as to the extent to which QOL, sleep, and reported symptoms are due to CS + HIPEC.
In summary, we believe that this study represents the largest survey of long-term survivors post-CS + HIPEC to date. Recipients living 1 or more years post-treatment report physical and functional HRQOL limitations, including sleep impairment. The opportunity to self-identify troubling symptoms revealed that GI distress is a prominent area for symptom management. Overall MH functioning was improved relative to that expected in the general population. These findings suggest, paradoxically, that long-term survivors may experience both distress and emotional growth. These survivors identified specific areas of concern that could improve with attention from the healthcare team. Ongoing monitoring of symptoms (including GI distress), sleep quality and HRQOL over the months following treatment can improve patient functioning and assure that increased survival is not achieved at the expense of a life of quality.
The next steps for psychosocial researchers include the design of methodologies that include control groups. Such findings permit an enhanced ability to delineate the extent to which reported symptoms are due to CS + HIPEC and have direct implications for the establishment of patient education efforts, monitoring, psychosocial interventions, and follow-up care.
Acknowledgments
We acknowledge Mary Cromer, Joyce Fenstermaker, Dr. Adrienne Hill, and the brave participants whose contributions lead to the chance for improved QOL for those who follow.
Footnotes
These data were published in part in the dissertation of the first author and were presented and published in Abstract form at the 2011 American Psychosocial Oncology Society Conference.
References
- 1.Levine EA, Stewart JH, Russell GB, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Experience with 501 procedures. J Am Coll Surg. 2007;204:943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 2.Stewart JH, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy: An evolving paradigm for the treatment of peritoneal surface malignancies. Expert Rev Anticancer Ther. 2008;8:1809–1818. doi: 10.1586/14737140.8.11.1809. [DOI] [PubMed] [Google Scholar]
- 3.McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2003;10:155–162. doi: 10.1245/aso.2003.03.067. [DOI] [PubMed] [Google Scholar]
- 4.McQuellon RP, Danhauer SC, Russell GB, et al. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:1105–1113. doi: 10.1245/s10434-006-9304-5. [DOI] [PubMed] [Google Scholar]
- 5.McQuellon RP, Russell GB, Shen P, et al. Survival and health outcomes after cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol. 2008;15:125–133. doi: 10.1245/s10434-007-9678-z. [DOI] [PubMed] [Google Scholar]
- 6.McQuellon R, Duckworth KE. Health-related quality of life and cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Curr Probl Cancer. 2009;33:203–218. doi: 10.1016/j.currproblcancer.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ. Follow-up of adult cancer survivors: New paradigms for survivorship care planning. Hematol Oncol Clin North Am. 2008;22:201–210. doi: 10.1016/j.hoc.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 9.Colten HR, Altevogt BM. Institute of Medicine (US) committee on sleep medicine and research. Washington, DC: National Academies Press (US); 2006. Sleep disorders and sleep deprivation: An unmet public health problem. [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 11.Ware JE, Jr, Kosinski M, Bjorner JB. Users manual for the SF-36v2 health survey. Lincoln, RI: Quality Metric Incorporated; 2007. [Google Scholar]
- 12.Ware JE, Jr, Snow KK, Kosinski M. Manual and interpretation guide. Boston: Nimrod Press; 1993. SF-36 health survey. [Google Scholar]
- 13.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–471. doi: 10.1007/BF02103658. [DOI] [PubMed] [Google Scholar]
- 16.Stanton AL, Bower JE, Low CC. Posttraumatic growth after cancer. In: Calhoun LG, Tedeschi RG, editors. Handbook of postraumatic growth: Research and practice. London: Lawrence Erlbaum; 2006. pp. 138–175. [Google Scholar]
- 17.Sprangers MA, Schwartz CE. The challenge of response shift for quality-of-life-based clinical oncology research. Ann Oncol. 1999;10:747–749. doi: 10.1023/a:1008305523548. [DOI] [PubMed] [Google Scholar]
- 18.Sprangers MA, Moinpour CM, Moynihan TJ. Assessing meaningful change in quality of life over time: A users’ guide for clinicians. Mayo Clin Proc. 2002;77:561–571. doi: 10.4065/77.6.561. [DOI] [PubMed] [Google Scholar]
- 19.Brucker PS, Yost K, Cashy J, et al. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28:192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 20.Strine TW, Chapman DP. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med. 2005;6:23–27. doi: 10.1016/j.sleep.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Tuttle TM, Zhang Y, Greeno E, et al. Toxicity and quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2006;13:1627–1632. doi: 10.1245/s10434-006-9186-6. [DOI] [PubMed] [Google Scholar]
- 22.McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol. 2001;27:65–73. doi: 10.1053/ejso.2000.1033. [DOI] [PubMed] [Google Scholar]