Abstract
This is a cross-sectional, observational study to evaluate the hypothesis that HIV-seropositive (HIV+) apolipoprotein E4 (APOE4) carriers are at increased risk for HIV-associated Neurocognitive Disorders (HAND) compared to APOE4 noncarriers with HIV in the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) Group sample. APOE genotype was determined in 466 CHARTER participants with varying disease stages and histories of antiretroviral treatment who did not have severe psychiatric or medical comorbid conditions that preclude diagnosis of HAND. HAND diagnoses were based on results of comprehensive neurobehavioral evaluation and use of current neuroAIDS diagnostic criteria. HAND status consisting of two levels: neuropsychologically normal status (i.e., no HAND) and any HAND diagnosis (i.e., asymptomatic neurocognitive impairment, minor neurocognitive disorder, HIV-associated dementia). Logistic regression analyses revealed no association between APOE4 carrier status and HAND, and there were no interactions between APOE4 carrier status and ethnicity, age, substance use disorders, duration of infection, or nadir CD4. Results did not differ when analysis was restricted to symptomatic HAND, and no APOE4 gene dose-dependent relationship to HAND emerged. APOE4 status was not associated with concurrent HAND in this large, well-characterized sample. This does not preclude emergence of an association between APOE4 status and HAND as this population ages. Prospective, longitudinal studies are needed to examine APOE4 as a risk factor for neurocognitive decline, incident HAND at older ages, and potential associations with CSF amyloid.
Keywords: HIV/AIDS, genetics, neuropsychological assessment
INTRODUCTION
HIV-associated neurocognitive disorders (HAND) remain prevalent in the era of combined antiretroviral therapy (cART), affecting up to half of all infected individuals across the spectrum of HAND1. Although the rate of HIV-associated dementia (HAD) has decreased considerably, among medically-asymptomatic HIV-seropostive (HIV+) patients the prevalence of HIV-associated neurocognitive impairment is significantly higher since the introduction of cART (i.e., 35% versus 25%, p=.001;2), suggesting that HIV remains neurovirulent despite medical treatment advances. As such, identification of HIV+ individuals at risk for neurocognitive impairment is still important, especially considering that even milder forms of HAND have been associated with functional impairment (e.g.,3), morbidity, and mortality (e.g.,4).
In addition to HIV disease (e.g., low nadir CD4 count;2) and demographic (e.g., age;5, 6) risk factors, host genetics are increasingly viewed as an important factor in determining who is at risk for developing HAND. The apolipoprotein E4 allele (APOE4) is a reliable genetic risk factor for sporadic Alzheimer’s disease (e.g.,7), and has been suggested to have a role in several other neurological conditions, including central nervous system (CNS) response to chronic infection such as HIV8, 9. Clinical reports examining the association of APOE4 and HAND have been mixed. HIV-associated dementia (HAD) has been reported to be twice as prevalent in APOE4 carriers as compared to non-carriers even when disease characteristics were controlled10. Valcour and colleagues11 observed that presence of an APOE ε4 allele was associated with a threefold independent increase in risk for HAD in a group of older HIV+ individuals (i.e., 50 years of age and older), but no increased APOE4-related risk was demonstrated in a group of younger HIV+ counterparts. In contrast, several studies have failed to demonstrate a relationship between APOE4 and HAD12-14, and two studies that related APOE4 status to risk for the full spectrum of HAND (i.e., including milder forms, such as Asymptomatic Neurocognitive Impairment or ANI) have reported contradictory results, with a positive association reported in a sample from China15, but a negative finding in South Africa16.
Importantly, small sample sizes (i.e., typically fewer than 200 participants; cf.12), restricted age ranges, and differences in approach to HAND diagnosis may have contributed to the inconsistent findings reported across these studies, and therefore a thorough investigation of the potential APOE4-related risk for HAND in a large, well-characterized HIV+ sample is warranted. Accordingly, the present study aimed to extend prior reports of an association between APOE4 and cognition in HIV, with the hypothesis that APOE4 carriers would be at greater risk for HAND.
METHOD
Participants
Participants included 466 individuals with HIV infection who were enrolled in the larger, ongoing CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study, which investigates neurological complications of HIV infection in the era of cART. Confirmed HIV+ participants in the CHARTER cohort received comprehensive neurobehavioral and neuromedical examinations. The study excluded individuals with severe comorbid psychiatric, medical, and neurological disorders likely to adversely affect cognitive functioning (i.e., those rated as “confounding;” see1) while still yielding a representative sample of HIV-seropositive individuals.
For this study, participants were also excluded if they were not fluent in English and/or if their self-reported ethnicity was identified as any category other than Caucasian or African American. Restriction of the sample to these ethnicity groups, which were most the prevalent groups within the larger CHARTER sample, better allowed for consideration of the potentially confounding effects of population stratification (i.e., the possibility that results may reflect false positive findings or true associations may be masked due to genetic background;17).
Procedure
Neurobehavioral assessment
The neurobehavioral evaluation included the Composite International Diagnostic Interview (CIDI;18) for documentation of current and lifetime psychiatric disorders and a neuropsychological battery that met the standard of practice for neuropsychological research in HIV by providing a comprehensive yet relatively brief (i.e., approximately 3 hours) evaluation of the cognitive domains affected by HIV19, including the following: (1) Verbal Fluency: Controlled Oral Word Association Test (COWAT-FAS;20, 21) and semantic verbal fluency (animals;21); (2) Speed of Information Processing: Trail Making Test-Part A (TMT-A; 22, 23), Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Symbol and Symbol Search24, 25, Stroop Color-Word Test26; (3) Attention/Working Memory: Paced Auditory Serial Addition Test (PASAT; 27, 28), Wechsler Adult Intelligence Scale-III (WAIS-III;24, 25) Letter-Number Sequencing; (4) Executive Functions: Halstead Category Test22, 23, Wisconsin Card Sorting Test-64 Card Version (WCST-64; 29) Perseverative Responses, Trail Making Test Part B (TMT-B; 22, 23), Stroop Interference Ratio26; (5) Learning: Hopkins Verbal Learning Test-Revised (HVLT-R;30, 31) and Brief Visuospatial Memory Test-Revised (BVMT-R; 31, 32) Total Trial 1-3 Recall, Delayed Recall; (6) Memory: HVLT-R and BVMT-R30-32 Delayed Recall; (7) Motor: Grooved Pegboard Dominant and Non-dominant hand22, 33.
In an effort to minimize the effect of demographic characteristics, such as age, education, sex, and ethnicity, on neuropsychological test performance, raw scores from the measures listed above were converted to demographically-corrected T-scores. Trained clinical neuropsychologists used the T-score to assign clinical ratings based on a highly specified algorithm described previously [see 34 ] and also determined case conference HAND diagnoses using the recently published guidelines for classifying HAND (i.e., Frascati criteria; 19).
Neuromedical evaluation
The following information was gathered during the neuromedical evaluation: Medical history, current medications and medication history, neurological examination, physical examination, and laboratory evaluations (e.g., blood and cerebrospinal fluid collected for banking and testing).
Genetic characterization
As part of the neuromedical examination for the CHARTER Host Genetics project, participants underwent a blood draw, for which 10-12 mL of plasma and 5 mL of serum are collected, and 4 mL of peripheral mononuclear blood cells (PBMCs) are stored (providing an adequate quantity for genetic analysis). DNA was extracted from the PBMCs using standard procedures. rs7412 and rs429358 (which define the APOE ε2, ε3, and ε4 isoforms), were genotyped using TaqMan predesigned SNP genotyping assays (Applied Biosystems; Foster City, California). Assays (C_904973_10 (rs7412) and C_30846793_20 (rs429358)) were performed following the manufacturers recommended protocol.
RESULTS
The demographic and HIV disease characteristics of the total sample and the APOE4 carrier status groups (carrier versus non-carrier) are presented in Table 1 and psychiatric characteristics are presented in Table 2. The groups were largely comparable, with the exception of a higher percentage of Caucasian participants in the APOE4 noncarrier group relative to the carrier group (p=.002). Also, there were no group differences on a rating that represents the degree to which comorbidities may affect cognitive performance, which is also shown in Table 1; that is, the number of cases rated as “incidental” (indicating that neurocognitive deficits were most likely due to HIV infection) or “contributing” (reflecting the presence of conditions that likely contribute to the observed deficits) did not statistically differ between the APOE4 carriers and noncarriers (see 1 for additional information regarding classification of comorbid conditions).
Table 1.
Demographic and Disease Characteristics of the Sample
| Variable | Total Sample N = 466 | APOE4 Carrier n = 144 | APOE4 Noncarrier n = 322 | p |
|---|---|---|---|---|
| Age (M, SD) | 44.1 (8.4) | 44.3 (8.1) | 44.0 (8.6) | .75 |
| Education (M, SD) | 13.0 (2.5) | 12.8 (2.5) | 13.0 (2.4) | .38 |
| % Male | 78.8% | 77.1% | 79.5% | .56 |
| % Caucasian | 50% | 39.6% | 55.0% | .002 |
| % cART | 69.5% | 68.8% | 69.9% | .92 |
| % AIDS | 61.1% | 58.7% | 62.1% | .49 |
| % Detectable CSF VL | 30.1% | 30.2% | 30.0% | .97 |
| % Detectable Plasma VL | 53.1% | 50.7% | 54.2% | .49 |
| Duration infection (Mo)1 | 124.8 [56.9, 183.4] | 122 [59, 180] | 126 [52, 185] | .68 |
| Nadir CD41 | 175 [50.3, 299] | 193 [45, 333] | 162 [55, 286] | .39 |
| % HCV | 27.0% | 31.5% | 24.9% | .15 |
| Comorbidity rating (% Incidental) | 68.2% | 66.7% | 68.9% | .63 |
Note. cART = combined antiretroviral therapy; CSF VL = cerebrospinal fluid viral load; plasma VL = plasma viral load; HCV = hepatitis C infection; Incidental comorbidity rating reflects likelihood that observed neurocognitive impairment was due to HIV infection versus contributing or confounding comorbid conditions [35];
data are presented as median [interquartile range] and p-values are based on Wilcoxon Rank-Sum test
Table 2.
Psychiatric Characteristics of the Sample
| Disorder | Total N = 466 | APOE4 Carrier n = 144 | APOE4 Noncarrier n = 322 | p |
|---|---|---|---|---|
| Current MDD | 13.7% | 11.8% | 14.6% | .40 |
| Current Dysthymia | 0.2% | 0% | 0.3% | .10 |
| LT MDD | 51.3% | 51.4% | 51.2% | .98 |
| LT Dysthymia | 1.1% | 1.4% | 0.9% | .65 |
| LT Alcohol Dependence | 29.4% | 32.6% | 28.0% | .31 |
| LT Meth Dependence | 12.0% | 12.5% | 11.8% | .83 |
| LT Cocaine Dependence | 33.5% | 38.9% | 31.1% | .10 |
| LT Cannabis Dependence | 10.9% | 11.8% | 10.6% | .69 |
| LT Opioid Dependence | 15.2% | 20.1% | 13.0% | .05 |
| LT Sedative Dependence | 2.6% | 4.9% | 1.6% | .05 |
| LT Inhalant Dependence | 1.1% | 1.4% | 0.9% | .65 |
| LT PCP Dependence | 1.5% | 2.1% | 1.2% | .68 |
| LT Hallucinogen Dependence | 1.3% | 0% | 1.9% | .18 |
| LT Other Dependence | 0.6% | 0% | 0.6% | .56 |
Note. Current = diagnostic criteria were met within 30 days of evaluation; MDD = Major Depressive disorder; LT = lifetime diagnosis
Results of a logistic regression analysis in which APOE4 carrier status, ethnicity, age, and interaction terms for APOE4 carrier status by ethnicity and age were included in a model predicting HAND revealed that the model was significant (df=5, X2=12.1, p=.03), and the only significant predictor was age (p=0.03; all other predictors ps > .10). Given that no interaction effects were observed, Figure 1 shows a follow-up contingency analysis collapsed across ethnicity and age in which there is no difference in the prevalence of HAND in the APOE4 carrier and noncarrier groups (X2=0.42, p=0.52, OR=0.87, 95% CI = 0.58 - 1.31). It is important to note that for this analysis, and for all subsequent analyses reported below, the pattern of results did not differ when the analyses were restricted to the Caucasian group only. Furthermore, the results were similar when APOE4 dose (i.e., noncarrier; heterozygous carrier, n=126, 27%; homozygous carrier, n=18, 0.04%) was substituted for APOE4 carrier status (i.e., carrier versus non-carrier).
Figure 1. No Differences in Rate of HIV-associated Neurocognitive Disorder (HAND) By APOE4 Carrier Status.
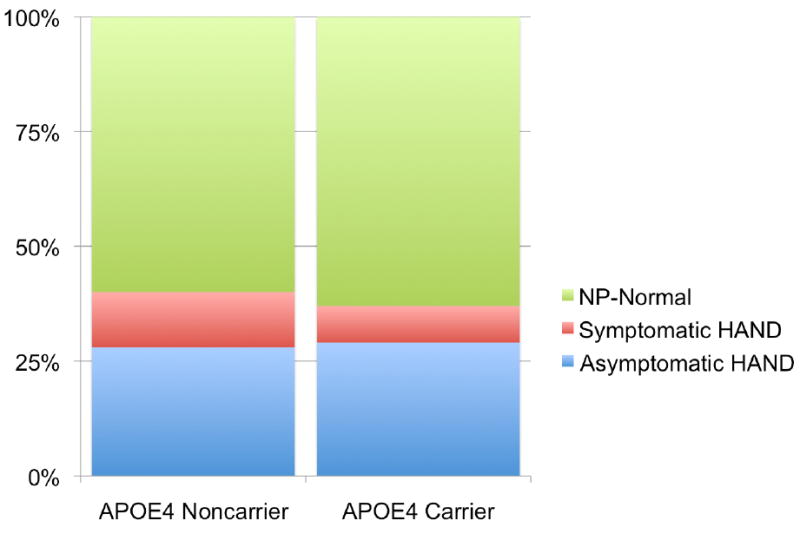
There was no difference in the prevalence of HAND by APOE4 carrier status, even when the analysis was restricted to symptomatic HAND (i.e., Minor Neurocognitive disorder and HIV-associated dementia, excluding asymptomatic neurocognitive impairment); all ps > .10.
Since studies reporting an APOE4 effect in HIV infection have typically demonstrated increased risk for HAD, a similar logistic regression was run but the criterion was restricted to symptomatic HAND, comprised of participants diagnosed with MND (n=46) and HAD (n=6) versus normal cognition (n=281). The overall model was null (df=5, X2=8.0, p=0.16) and all predictors were nonsignificant (ps > .10), suggesting that APOE4 carriers were not more likely to have symptomatic HAND than noncarriers. Figure 1 shows a contingency analysis in which no difference in the rate of symptomatic HAND was observed across the APOE4 carrier groups (X2=1.66, p=.20, OR=0.64, 95% CI = 0.32, 1.27).
In a series of planned follow-up analyses, separate logistic regression analyses were conducted in which potentially important predictors were included with APOE4 carrier status, including demographic characteristics (i.e., years of education, comorbidity status), HIV disease characteristics (i.e., nadir CD4 count, CSF VL detectability, cART status, and AIDS diagnosis), and psychiatric characteristics (i.e., current and lifetime Major Depressive disorder and lifetime substance dependence diagnoses), as were interaction terms between all of these variables and APOE4 carrier status. Notably, all main effects and interactions with APOE4 carrier status in predicting HAND were nonsignificant (ps > .10). Finally, a series of chi-square analyses conducted for seven separate cognitive domains revealed no differences in the prevalence of domain-level impairment (based on domain clinical ratings) by APOE4 carrier status (all ps > .10), including the following domains: learning, memory, executive functions, working memory, speed of information processing, verbal ability, and motor skills.
DISCUSSION
We found no significant associations between HAND and APOE4 allele status. Moreover, there was no significant interaction of APOE4 carrier status by age, which might be expected based on prior findings by Valcour and colleagues11, who demonstrated APOE4-related risk for HAD among older but not younger adults in their sample. Furthermore, there were no interaction effects between APOE4 and HIV disease characteristics that are believed to be associated with cognition, including nadir CD4, detectible CSF viral load, use of antiretrovirals (cART), and AIDS diagnosis. Finally, no relationships were revealed in investigations of whether APOE interacts with current or lifetime history of Major Depressive disorder or history of substance dependence, years of education, or degree of comorbidity.
These results are consistent with four studies that have reported no association between APOE4 and cognitive outcomes (i.e.,12-14, 16). Prior studies have had significant limitations that temper their conclusions, but the strengths of our study include a relatively large sample size of 466 HIV+ individuals representative of American HIV patients, and a systematic and extensive neuromedical and neurobehavioral characterizaton of HAND diagnoses19. We were also able to investigate the potential contributions of demographic, disease, and psychiatric factors that might have confounded the primary observations, and still could find no evidence of an effect of APOE4 status on HAND.
With regard to the potential effects of ethnicity, it is known that APOE4 is more common among African Americans in comparison to Caucasians but the risk relationship of APOE4 to poor cognitive outcomes (e.g., AD) has been shown to be weaker in this group35. Although population stratification could therefore potentially have influenced our findings, it is unlikely because we accounted for this possibility in two ways; namely, we included ethnicity and its interaction with APOE4 carrier status in our regression models and verified our findings among our Caucasian sample separately, both of which yielded negative results.
Since two of three prior studies that suggested an APOE4 effect on neurocognitive outcomes in HIV used HAD as their criterion10, 11, we also conducted analyses in which we restricted the comparison to those with no HAND diagnosis versus those with symptomatic HAND, including MND and HAD. In contrast to both Corder and colleagues10 and Valcour and colleagues11, we did not observe any relationship between these outcomes and APOE4. However, our study did not have the power to consider risk for HAD alone given is rarity in the current therapeutic era. Similarly, Corder and colleagues’10 sample largely included HAD associated with uncontrolled HIV viral infection, a condition that was rare in the present sample.
Another important factor that was considered in our analyses was the potential influence of age. APOE4 is a risk factor for sporadic AD that is expressed in advanced age. Valcour and colleagues11 observed a significant APOE4 effect on risk for HAD among older but not younger HIV+ individuals. We did not observe a significant interaction between APOE4 carrier status and age in our sample, but it is possible that too few older adults were included in our sample to observe the effect. Specifically, our sample included 118 HIV-seropositive individuals (25.3% of the sample) whose age exceeded the standard age cut-point defining older adult status in neuroAIDS (i.e., age 50 or older), 50% of whom had a HAND diagnosis, but nevertheless there were relatively few individuals aged 60 or older (i.e., 17 participants; 3.7% of the sample). Valcour and colleagues36 raised the possibility that carrying APOE4 may have a detrimental effect on survival given their observation that the older group of HIV+ individuals in their sample had a significantly lower APOE4 carrier rate (23%) relative to their younger group of individuals less than 40 years of age (37%, p = .02), a factor which may influence APOE4-related findings. In contrast, in our sample the APOE4 carrier rate among the older adults (aged 50 and older) was 31%, which is similar to the APOE4 carrier rate of the total sample and not statistically different from the rate observed among participants younger than 40 (25.6%, p = .32), suggesting that our findings were not likely influenced by survivor bias.
These findings do not necessarily indicate that accelerated aging does not occur in HIV infection. It is known that beta amyloid accumulation occurs for decades prior to manifestation of neurobehavioral deficits37. Thus, the current negative findings regarding neurocognitive impairment in a sample with an average age of 44.1 (SD = 8.4) do not preclude the possibility that neurocognitive deficits may develop prematurely, or that neuropathological and biomarker changes might be observed in our sample38, 39. It may also be the case that the specific APOE4-related risk is expressed at more advanced ages, similar to uninfected individuals, but that other factors may result in manifestation of neurocognitive impairment at younger ages, such as earlier onset of cardiovascular and metabolic disease (e.g.,40). Additionally, age was a significant predictor in the first model presented above, despite the use of age-corrected normative scores for determination of HAND. Although not directly tested in this study, this finding may be evidence of an interaction between HIV and aging on expression of HAND, which may be further elucidated through examination of interactions between age and HIV disease factors (e.g., nadir CD4 count, duration of infection and ART) and comorbidities (e.g., substance dependence) in future work.
There are several limitations to the present study, suggesting future directions of study. The limited number of >60 year-old subjects limits our ability to detect impairment associated with the development of AD. Ideally, this analysis should be conducted in a sample with a larger number of older individuals with varying duration of infection to assess the role of survivor bias. Furthermore, with only six individuals with HAD in our sample, we cannot effectively study this advanced neurocognitive disorder, which may have had a different mechanism from our current mild HAND presentation. Nevertheless, although it is possible that our findings may have been different if a larger number of HAD cases were available, it is unlikely given that APOE4 did not predict symptomatic HAND, which included HAD and MND (n=52). Additionally, the cross-sectional design of the study did not allow for examination of APOE4 effects on longitudinal outcomes such as neurocognitive decline, rate of progression to HAND, and risk for functional decline. Although the present study examined many cofactors in order to account for their potential influence, such as demographic factors (e.g., age), HIV disease characteristics (e.g., nadir CD4 count), and psychiatric factors (e.g., substance dependence), there are many other variables that may be critical to neurocognitive impairment in HIV that warrant consideration, such as cardiovascular risk factors (e.g., hypertension, diabetes mellitus), markers of cerebrovascular disease, effectiveness of antiretroviral CNS penetration, other genetic risk factors (e.g., monocyte gene expression), survivor bias, and potential unmeasured gene-environment interactions. Furthermore, the current study relied upon self-reported ethnicity to evaluate the potential effects of population stratification, and therefore it is possible that a different pattern may emerge if ancestry-informative markers (AIM) were used to define the populations and correct for potential effects of stratification (e.g.,17).
Regarding future directions, there are several potential avenues for further elucidating the relationship between APOE4 carrier status and neurocognitive outcomes in HIV infection. The role of cognitive reserve in expression of HAND as a function of APOE4 carrier status could be explored with a more sophisticated measure of cognitive reserve than education alone (which yielded no significant interaction in the present study). Future studies may also include biomarker or imaging data, which would allow for more direct examination of the potential APOE4-related manifestations of HIV-associated neuropathology in relation to neurocognitive performance. Given that amyloid deposition may precede clinical manifestation of AD by a period of decades, future work could also examine whether APOE4 is associated with beta amyloid levels in the CHARTER sample of HIV+ individuals through measurement of CSF beta amyloid or imaging techniques such as 11C-PiB (e.g.,37).
Acknowledgments
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) is supported by award N01 MH22005 from the National Institutes of Health. Dr. Morgan is supported by T32 AA013525.
* The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University, Mount Sinai School of Medicine, University of California, San Diego, University of Texas, Galveston, University of Washington, Seattle, Washington University, St. Louis and is headquartered at the University of California, San Diego and includes: Director: Igor Grant, M.D.; Co-Directors: J. Allen McCutchan, M.D., Ronald J. Ellis, M.D., Ph.D., Thomas D. Marcotte, Ph.D.; Center Manager: Donald Franklin, Jr.,.; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), J. Allen McCutchan, M.D., Terry Alexander, R.N.; Laboratory, Pharmacology and Immunology Component: Scott Letendre, M.D. (P.I.), Edmund Capparelli, Pharm.D.,; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Matthew Dawson; Virology Component: Joseph K. Wong, M.D. (P.I.); Imaging Component: Terry Jernigan, Ph.D. (Co-P.I.), Michael J. Taylor, Ph.D. (Co-P.I.), Rebecca Theilmann, Ph.D.; Data Management Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman,; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Christopher Ake, Ph.D., Florin Vaida, Ph.D.; Protocol Coordinating Component: Thomas D. Marcotte, Ph.D. (P.I.), Rodney von Jaeger, M.P.H.; Johns Hopkins University Site: Justin McArthur (P.I.), Gilbert Mbeo, MBChB; Mount Sinai School of Medicine Site: Susan Morgello, M.D. (Co-P.I.) and David Simpson, M.D. (Co-P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.), Susan Ueland, R.N.; University of Washington, Seattle Site: Ann Collier, M.D. (Co-P.I.) and Christina Marra, M.D. (Co-P.I.), Trudy Jones, M.N., A.R.N.P.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Heckendorn, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Muhammad Al-Lozi, M.D., Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
References
- 1.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc. 2004;10:317–331. doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 4.Vivithanaporn P, Heo G, Gamble J, et al. Neurologic disease burden in treated HIV/AIDS predicts survival: a population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in HIV virus infection. AIDS. 2004;18(Suppl 1):S11–18. [PubMed] [Google Scholar]
- 6.Woods SP, Dawson MS, Weber E, Grant I. The semantic relatedness of cue-intention pairings influences event-based prospective memory failures in older adults with HIV infection. J Clin Exp Neuropsychol. 2010;32:398–407. doi: 10.1080/13803390903130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlmann I, Minihane AM, Huebbe P, Nebel A, Rimbach G. Apolipoprotein E genotype and hepatitis C, HIV and herpes simplex disease risk: a literature review. Lipids Health Dis. 2010;9:8. doi: 10.1186/1476-511X-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urosevic N, Martins RN. Infection and Alzheimer’s disease: the APOE epsilon4 connection and lipid metabolism. J Alzheimers Dis. 2008;13:421–435. doi: 10.3233/jad-2008-13407. [DOI] [PubMed] [Google Scholar]
- 10.Corder EH, Robertson K, Lannfelt L, et al. HIV-infected subjects with the E4 allele for APOE have excess dementia and peripheral neuropathy. Nat Med. 1998;4:1182–1184. doi: 10.1038/2677. [DOI] [PubMed] [Google Scholar]
- 11.Valcour V, Shikuma C, Shiramizu B, et al. Age, apolipoprotein E4, and the risk of HIV dementia: the Hawaii Aging with HIV Cohort. J Neuroimmunol. 2004;157:197–202. doi: 10.1016/j.jneuroim.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression. Proc Natl Acad Sci USA. 2008;105:8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlop O, Goplen AK, Liestol K, et al. HIV dementia and apolipoprotein E. Acta Neurol Scand. 1997;95:315–318. doi: 10.1111/j.1600-0404.1997.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 14.Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9:677–680. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 15.Spector SA, Singh KK, Gupta S, et al. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. AIDS. 2010;24:1471–1479. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joska JA, Combrinck M, Valcour VG, et al. Association between apolipoprotein E4 genotype and human immunodeficiency virus-associated dementia in younger adults starting antiretroviral therapy in South Africa. J Neurovirol. 2010;16:377–383. doi: 10.3109/13550284.2010.513365. [DOI] [PubMed] [Google Scholar]
- 17.Enoch MA, Shen PH, Xu K, Hodgkinson C, Goldman D. Using ancestry-informative markers to define populations and detect population stratification. J Psychopharmacol. 2006;20:19–26. doi: 10.1177/1359786806066041. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Composite International Diagnostic Interview. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 19.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1983. [Google Scholar]
- 21.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 22.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Lutz, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 23.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 24.Heaton RK, Taylor M, Manly JJ. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. In: Tulsky DS D, Heaton RK, Chelune G, Ivnik R, Bornstein RA, Prifitera A, Ledbetter M, editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego, CA: Academic Press; 2002. pp. 183–210. [Google Scholar]
- 25.Psychological Corporation. WAIS-III and WMS-III technical manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 26.Golden CJ. Stroop Color and Word Test Chicago: Stoelting. 1998 [Google Scholar]
- 27.Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- 28.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 29.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test- 64 Card Computerized Version. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 30.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12:43–55. [Google Scholar]
- 31.Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benedict RH. Brief Visuospatial Memory Test- Revised. Odessa, Florida: Psychological Assessment Resources; 1997. [Google Scholar]
- 33.Kløve H. Grooved Pegboard. Lafayette, IN: Lafayette Instruments; 1963. [Google Scholar]
- 34.Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- 35.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279:751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 36.Valcour V, Shiramizu B, Shikuma C. Frequency of apolipoprotein E4 among older compared with younger HIV patients: support for detrimental effect of E4 on survival. Proc Natl Acad Sci USA. 2008;105:E66. doi: 10.1073/pnas.0806919105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21:117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clifford DB, Fagan AM, Holtzman DM, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magalhaes MG, Greenberg B, Hansen H, Glick M. Comorbidities in older patients with HIV: a retrospective study. J Am Dent Assoc. 2007;138:1468–1475. doi: 10.14219/jada.archive.2007.0083. [DOI] [PubMed] [Google Scholar]


