Hematopoietic stem cell (HSC) biology studies have shown that several adhesion molecules such as CXCR4, VLA4, VLA5, CD44, Cd11a and L-selectin are involved in self-renewal, trafficking, survival and adhesion of HSC to their niches (1). Similarly to adult HSC, umbilical cord blood (UCB) HSC have been shown to highly express CXCR4 and other adhesion molecules involved in engraftment and trafficking (2, 3).
The applicability of UCB transplantation had been historically limited by the small cell number available from a single unit (4). Over the last decade the utilization of double UCB (dUCB) unit grafts was shown to, at least in part, overcome the cell dose limitation and has been adopted by several institutions (5, 6). Our group (7) and others (5, 8) have shown that despite the infusion of two UCB units, in the vast majority of cases only a single unit will predominate and provide long-term lympho-hematopoietic recovery. Available data shows that UCB unit predominance is influenced by the T-cell content an interaction between T-cells from both donor units (5, 7). Identifying additional factors that determine the long-term predominant unit after dUCB transplantation will allow us to further refine UCB graft selection, and potentially improve outcomes. Thus, we studied whether the expression of CXCR4 on UCB CD34+ cells and different CD34+ cells subsets were also involved in unit predominance after dUCB transplantation.
We studied patients with hematologic malignancies undergoing dUCB transplantation after myeloablative (MA) or nonmyeloablative (NMA) conditioning regimens at the University of Minnesota between 2005 and 2009. To be included in this analysis, patients were required to have achieved neutrophil engraftment with chimerism and had post-thaw flow cytometry data for the 2 UCB donor units. Patient demographics, laboratory and engraftment data were prospectively collected and available from the University of Minnesota Blood and Marrow Transplant Database. Graft selection, conditioning, immunosuppressive regimens and supportive care have been previously reported (4, 9) Sixty-eight patients were included in this analysis. Forty-one patients (60%) were male. The majority of the patients (62%, n=42) were older than 35 years old. The median recipient weight was 82 kg (range: 22–145). Most patients received a unit with at least 1 HLA mismatch (54%, n=37), and in 37% of the cases (n=25), both units were 4/6 HLA matched with the recipient. Acute leukemias were the indication for transplantation in 52% of the cases (n=35), and lymphoid malignancies in 23% (n=33), and other diagnoses in 25% (n=10). Forty-two patients (62%) received a NMA conditioning regimen. In 54 patients (80%) there was gender mismatch between the 2 units and in 55 patients (81%) there was ABO incompatibility between the 2 units. The median follow up time was 2 years (range 1.0–3.2 years).
Cord predominance, investigated descriptively by looking the median dose between the predominant and non-predominant units among the study population, and was defined as ≥ 70% of chimerism by day +100 post-transplantation by PCR of informative polymorphic variable number tandem repeat (VNTR) and more commonly, short tandem repeat (STR) regions in the recipient and donor as previously described (7). Donor chimerism was determined in BM at days 21, 100, 180, 360, and 720 after transplantation, with additional time points as needed. Engraftment studies showed that by day +100 and subsequent time points after transplantation a median chimerism from the predominant unit >70% was observed in 97% and 94% of MA and NMA, respectively (p=0.35).
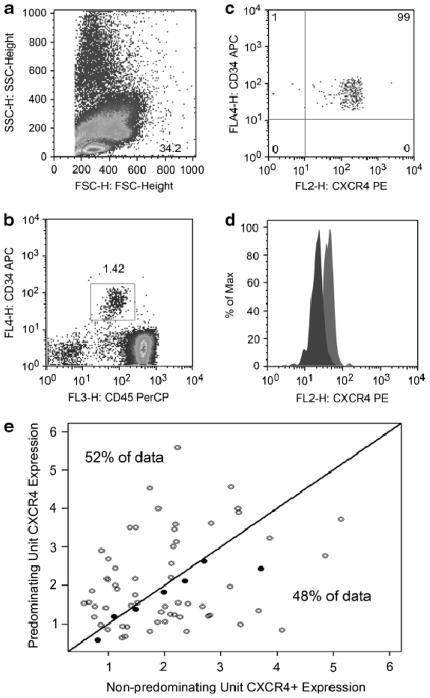
CXCR4 was highly expressed in 98% of the CD34+ cells in UCB grafts, with no significant differences between the predominant and non-predominant units regarding MFI (Figure 1A–D). It has been reported that UCB CD34+CXCR4+ cell subset ranges from 8 to 80% (2, 10). Discrepancies may, at least in part, be due to technical differences on UCB processing, staining and source. In our study, we only included clinically utilized UCB units obtained from 6 cord blood banks (CBB) in contrast to Timeus et al. (10) and Ohno et al. (2) that used ‘research’ cord blood units. In part, cryopreservation may have been produced a slight decrease on CXCR4 expression levels as reported (10). In our study, thawing and staining process were uniform for all units, and CXCR4 expression should not have been significantly influenced by this factors.
Figure 1.
Panels A through D describe the gating strategy for CXCR4 expression. Flow cytometry was performed on the FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) using CellQuest Pro Software (BD Biosciences, San Jose, CA). Samples for flow cytometric analysis were obtained from the final product prior to its release to the transplant floor and were stained and analyzed within 24 hours of thaw without fixation. Briefly, aliquots containing 5×105 CB cells were incubated with fluorochrome-conjugated monocolonal antibodies at 4°C for 20 minutes. To lyse the red blood cells the samples were treated with Optilyse B (Beckman Coulter, Brea, CA) per manufacturer’s instructions. Samples were stained for CD45 (PerCP, BD Biosciences, San Jose, CA), CD34 (APC, BD Bioscience, San Jose, CA), CXCR4 (PE, BD Bioscience, San Jose, CA), CD33 (FITC, BD Bioscience, San Jose, CA) and the corresponding fluorochrome-conjugated isotype controls (BD Biosciences, San Jose, CA). CXCR4 was substituted by CD38 (PE, BD Bioscience, San Jose, CA) in other tube set. Results were analyzed using FlowJo Software (TreeStar, Ashland, OR). The live lymphoid CD34+/CD45+ subpopulation was determined by creating a gate on the lymphocyte population (A). Next, a plot of CD34 (y-axis)/CD45 (x-axis) cells was determined to select the double positive population that represents progenitor/stem cell subpopulation (B). The double positive population was analyzed for median CXCR4 fluorescence intensity (MFI) and percentage of positive cells (C). Panel D shows a representative MFI of 2 cords infused to a single patient. CD34 subpopulation was determined by creating a gate on the lymphocyte population followed by a plot of CD34 (y-axis)/forward scatter to select the single CD34 positive population. Similarly, following the lymphocyte population subpopulation, a plot of CD34 (y-axis)/CD33 (x-axis) cells was determined to select the double positive population. Another plot of CD34 (y-axis)/CD38 (x-axis) cells was determined to select the CD34+/CD38− subpopulation. In panel E we show the proportion of cord blood units that predominate long-term by their expression, as measured by the mean fluorescence intensity, of CXCR4 in CD34+ cells. The open circles represent patient for whom the predominating unit had a similar or similar HLA-match as compared to the non-predominant one; the open circles represent predominating unit that were better HLA matched as compared to the non-predominant one.
It has been shown that CXCR4 is involved in homing and engraftment (11). In addition, priming of hematopoietic progenitors with reagents, such as complement fragment 3a (12) and prostaglandins(13), leads to CXCR4 activation with increased migration towards a SDF1 gradient and improved engraftment. The proportion of CD34+CXCR4+ cells in the predominant UCB unit was 1.7% (range 0.5–6.5%) vs. 2.0 (0.6–5.2) in the non-predominant. The intensity of expression of CXCR4 on CD34+ cells, as measured by MFI, was similar for the predominant (36.5; range 3.7–214) and non-predominant unit (44.1; range 3.7–219) (p=.34). Thus, both the proportion of CD34+CXCR4+ cells and the intensity of CXCR4 expression on CD34+ cells were found to be similar between the predominant and non-predominant units (Table 1). We also found no difference in the time to neutrophil recovery whether the CXCR4 expression on the predominant unit was above (18 days, range 6–40) or below (22 days; range 0–39) the median MFI (p=0.26).
Table 1.
Logistic regression showing the univariate odds of the 1st Unit randomly chosen UCB unit predominating depending on the expression of surface markers
| Factors | N | Odds Ratio of 1st Unit Winning (95% CI) | P-value |
|---|---|---|---|
| Proportion CD34+ | |||
| 1st Unit < 2nd Unit | 28 | 1.0 | |
| 1st Unit > 2nd Unit | 33 | 0.9 (0.3–2.6) | 0.91 |
| 1st Unit = 2nd Unit | 7 | 0.8 (0.1–4.0) | 0.74 |
| Proportion CD34+/CD38+ | |||
| 1st Unit < 2nd Unit | 26 | 1.0 | |
| 1st Unit > 2nd Unit | 39 | 1.4 (0.5–3.9) | 0.43 |
| 1st Unit = 2nd Unit | 3 | 2.7 (0.2–34.0) | 0.48 |
| Proportion CD34+/CD33+ | |||
| 1st Unit < 2nd Unit | 23 | 1.0 | |
| 1st Unit > 2nd Unit | 40 | 1.2 (0.4–3.4) | 0.72 |
| 1st Unit = 2nd Unit | 5 | 0.3 (0.1–2.8) | 0.28 |
| Proportion CD34+/CXCR4+ | |||
| 1st Unit < 2nd Unit | 22 | 1.0 | |
| 1st Unit > 2nd Unit | 20 | 0.6 (0.2–1.9) | 0.40 |
| 1st Unit = 2nd Unit | 26 | 0.5 (0.2–1.6) | 0.36 |
| CXCR4 MFI in CD34+ | |||
| 1st Unit < 2nd Unit | 22 | 1.0 | |
| 1st Unit > 2nd Unit | 20 | 0.6 (0.2–1.9) | 0.40 |
| 1st Unit = 2nd Unit | 26 | 0.5 (0.2–1.6) | 0.36 |
CD, cluster of differentiation; MFI, mean fluorescent intensity
While homing of HSCs depends the CXCR4/SDF1 axis, the expression of CXCR4 in UCB CD34+ cells was not associated with unit predominance (Figure 1E). Subsequent to homing and engraftment, as shown by our group (7) and others (8), the unit that will provide long-term lympho-hematopoietic recovery is largely determined by T-cell content and interactions between the T-cells of the 2 donor UCB units (5). Although CD34+ viability has also been shown to predict predominance after dUCB transplantation, it is only predictive when one of the two units’ viability is below 75% (6) and was not a factor in our study.
In summary, our data shows that in dUCB transplantation CXCR4 expression is not a predictor of long-term predominant UCB unit. While CXCR4 expression on UCB CD34+ cells did not influence unit predominance in our study, it is possible that function/activation rather than expression alone may influence homing, engraftment and possibility long term predominance. Thus, as most CD34+ cell express CXCR4, functional responsiveness to a SDF-1 gradient in a transwell system may be better parameter to be considered in future studies. An ongoing phase I/II studies, based on pre-clinical data (12, 13), is testing this hypothesis by priming one of two UCB unit composing the graft with C3a and Prostaglandin E2 (14, 15).
Acknowledgments
This work was supported in part by grants from the National Cancer Institute CA65493 (J.E.W., J.S.M., C.G.B), the Children’s Cancer Research Fund (J.E.W., T.E.D.), the American Cancer Society Audrey Meyer Mars International Fellowship in Clinical Oncology (P.R.) and Pontificia Universidad Católica de Chile (P.R.), American Society of Blood and Marrow Transplantation Robert A. Good New Investigator Award (C.G.B.), and Leukemia and Lymphoma Society Scholar in Clinical Research Award (C.G.B.).
Footnotes
Conflict of interest: The authors declare NO competing financial interests in relation to the work described.
References
- 1.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005 Sep 15;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 2.Ohno N, Kajiume T, Sera Y, Sato T, Kobayashi M. Short-term culture of umbilical cord blood-derived CD34 cells enhances engraftment into NOD/SCID mice through increased CXCR4 expression. Stem Cells Dev. 2009 Oct;18(8):1221–6. doi: 10.1089/scd.2008.0298. [DOI] [PubMed] [Google Scholar]
- 3.Timeus F, Crescenzio N, Basso G, Ramenghi U, Saracco P, Gabutti V. Cell adhesion molecule expression in cord blood CD34+ cells. Stem Cells. 1998;16(2):120–6. doi: 10.1002/stem.160120. [DOI] [PubMed] [Google Scholar]
- 4.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007 Oct 15;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010 Jan 28;115(4):757–65. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scaradavou A, Smith KM, Hawke R, Schaible A, Abboud M, Kernan NA, et al. Cord blood units with low CD34+ cell viability have a low probability of engraftment after double unit transplantation. Biol Blood Marrow Transplant. 2010 Apr;16(4):500–8. doi: 10.1016/j.bbmt.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez P, Wagner JE, Defor TE, Blazar BR, Verneris MR, Miller JS, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2011 Sep 26; doi: 10.1038/bmt.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanda J, Rizzieri DA, Gasparetto C, Long GD, Chute JP, Sullivan KM, et al. Adult dual umbilical cord blood transplantation using myeloablative total body irradiation (1350 cGy) and fludarabine conditioning. Biol Blood Marrow Transplant. 2011 Jun;17(6):867–74. doi: 10.1016/j.bbmt.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005 Feb 1;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 10.Timeus F, Crescenzio N, Saracco P, Doria A, Fazio L, Albiani R, et al. Recovery of cord blood hematopoietic progenitors after successive freezing and thawing procedures. Haematologica. 2003 Jan;88(1):74–9. [PubMed] [Google Scholar]
- 11.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008 Jan;15(1):49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 12.Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004 Mar 15;103(6):2071–8. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- 13.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009 May 28;113(22):5444–55. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler CS, Shoemaker D, Ballen KK, Robbins D, Desponts C, Kao GS, et al. FT1050 (16,16-dimethyl Prostaglandin E2)-Enhanced Umbilical Cord Blood Accelerates Hematopoietic Engraftment After Reduced Intensity Conditioning and Double Umbilical Cord Blood Transplantation. ASH Annual Meeting Abstracts; 2011 November 18; 2011. p. 653. [Google Scholar]
- 15.Brunstein CG, McKenna DH, DeFor TE, Sumstad D, Ratajczak M, Laughlin MJ, et al. Priming of Hematopoietic Progenitor Cells (HPC) with Complement Fragment 3A (C3A) to Promote Homing of Umbilical Cord Blood (UCB): Safety Profile. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18(2):S210. doi: 10.1016/j.bbmt.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]