Abstract
Background
The phosphatidylinositol 3-kinase (PI3K), a critical intracellular pathway, is negatively regulated by phosphatase and tensin homologue (PTEN). Integrin-linked kinase (ILK) induces phosphorylation of Akt leading to an increase in cell survival. However, a potential interaction between ILK and PTEN activity in neuroblastoma cells is unknown. We sought to examine the relationship between ILK and PTEN in the PI3K/Akt signaling pathway in neuroblastoma tumorigenesis.
Methods
The human neuroblastoma cell line, BE(2)-C, was transfected with small interfering (si) or short hairpin (sh) RNA to silence ILK expression. A plasmid containing the ILK wild type (ILK wt) gene was transfected to over express ILK. Cell proliferation was assessed, and anchorage independence was measured by soft agar assay. Insulin-like growth factor (IGF)-1 was used to stimulate the PI3K/Akt pathway. Protein levels were determined by Western blotting.
Results
Transient silencing of ILK produced correlative decreases in PTEN expression, cell proliferation and soft agar colony formation. Conversely, stably-transfected ILK knockdown cells showed an increase in phospho-Akt levels, leading to cell proliferation.
Conclusions
ILK plays an important role in the regulation of PI3K/Akt pathway via PTEN or an upstream effector of PTEN. The effects of ILK silencing on PTEN expression appear to be critically dependent on duration of ILK dysregulation.
Keywords: ILK, PTEN, Neuroblastoma, PI3K, Akt
Introduction
Advanced-stage neuroblastoma is a highly lethal pediatric malignancy with mortality rates exceeding 50%.1 We2 and others3 have shown that phosphatidylinositol 3-kinase (PI3K) pathway plays a critical role in promoting neuroblastoma cell growth. In many human malignancies, integrin-linked kinase (ILK) is aberrantly expressed, and in one particular study, it has also been detected in up to one third of neuroblastomas.4, 5 ILK provides a link between the integrin β1 cytoplasmic tail and the actin cytoskeleton to mediate cell signaling, thus regulating many cellular processes necessary for cancer progression including survival, proliferation, and migration.6, 7 Over expression of ILK in select neuroblastoma cell lines has been shown to decrease apoptosis, increase tumor formation in nude mice, and increase in vitro phosphorylation of Akt.5
The phosphorylated (phospho)-Aktpromotes cell survival of various cancer types.8 ILK phosphorylates Akt on serine 473 in a PI3K-dependent manner.9 ILK activity is dependent upon interactions between phosphatidylinositol triphosphate (PIP3) with ILK's pleckstrin homology domain.10 PIP3 levels are regulated by phosphatase and tensin homologue (PTEN) and PI3K.11 PTEN, an endogenous negative regulator of PI3K, is a phosphatase that dephosphorylates phosphatidylinositol phosphate (PIP) on the 3-position of the inositol ring. PTEN counteracts the action of PI3K, which phosphorylates PI(4,5)P2 on the 3-position.12 PIP3 levels regulate the phosphorylation of Akt, providing a survival signal that protects cells from apoptosis.13, 14 The balance between PI3K and PTEN is critical in the regulation of neuroblastoma progression, and in particular, PTEN has been shown to be down regulated in many poorly differentiated cancers, including neuroblastomas.2, 15
It has been suggested that ILK and PTEN play opposite roles in gastric cancer progression, whereby their expressions are inversely correlated in gastric carcinoma.16 Others have reported that α-parvin interacts with ILK, and that it is necessary for the stimulation of ILK's kinase activity.17 Furthermore, PTEN and inhibitors of PI3K disrupt the interaction between ILK and α-parvin.17 Therefore, the purpose of our study was to investigate the relationship between ILK and PTEN, and also to determine the effects of ILK on neuroblastoma tumorigenesis.
Materials and Methods
Materials&cell culture
All reagents of molecular biology grade were purchased from Sigma (St. Louis, MO) or Amresco (Solon, OH). Insulin-like growth factor (IGF)-1 was purchased from Bachem (Torrance, CA). X-ray film was purchased from Eastman Kodak (Rochester, NY). Human neuroblastoma cell line, BE(2)-C, was purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 medium with L-glutamine (CellgroMediatech, Inc. Herndon, VA) supplemented with 10% fetal bovine serum (Sigma). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
RNA silencing and over expression
MISSION® Non-Target shRNA Control Vector,shc002, and MISSION® shRNA Bacterial Glycerol Stock lentiviral plasmid TRCN0000000968, shILK (human NM_001014795)were purchased from Sigma. ILK ON-TARGETplusSMARTpool siRNA (siILK) and non-targeting control (siNTC) were purchased from Dharmacon, Inc (Lafayette, CO). Plasmid pcDNA3.1-ILK-wild type-His (ILK wt) was kindly provided by Dr. ShoukatDedhar (The University of British Columbia, Vancouver, BC, Canada). Transfections were carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Four μg of plasmid DNA and 100 nM of siRNA were used for transfection. Stably-silenced shILK and over expressing ILK cells were selected by puromycin (2.5 μg/ml) and G418 (450 μg/ml), respectively.
Western blot analysis
Protein samples were collected using cell lysis buffer (Cell Signaling, Boston, MA) with 1mM PMSFat indicated time points. Whole-cell lysates (20-30 μg) were run on a Tris-Bis gel (Invitrogen Corp., Carlsbad, CA) and transferred onto polyvinylidenedifluoride membranes. Membranes were blocked with 5% milk in TBST (120 mMTris–HCl, pH 7.4, 150 mMNaCl, and 0.05% Tween 20) for 1 hr at room temperature. Proteins were detected by incubating with rabbit or mouse anti-human antibodies overnight at 4°C or 3 hrs at room temperature. Membranes were then washed three times with TBST and incubated for 1 hr with secondary antibody conjugated to HRP. Blots were developed using Western Lightning® Plus-ECL substrate (PerkinElmer, Inc). β-actin served as a loading control.
IGF-1stimulation
IGF-1 was used to stimulate the PI3K/Akt pathway. Stably- and transiently-transfected cells were serum-starved overnight, and then stimulated with 100 nM IGF-1 for 15 min. Cells were subsequently collected using cell lysis buffer. Transiently-transfected cells were stimulated at 48, 72, and 96 hrs post-transfection.
Cell proliferation assay
Cells were seeded in 96-well plates at a density of 5×103 cells/well in RPMI 1640 culture medium with 10% FBS and grown for up to 96 hrs post-transfection. Cell number was assessed daily using Cell-Counting Kit-8 (Dojindo Molecular Technologies, Inc., Gaithersburg, MD). Each time point assay was performed in triplicate. The values, corresponding to the number of viable cells, were read at OD450 with EL808 Ultra Microplate Reader (Bio Tek Instrument, Inc., Winooski, VT).
Soft agar assay
Anchorage-independent cell growth was measured by assessing for colony formation in a soft agar suspension. Cells were trypsinized and resuspended in RPMI 1640 medium containing 0.4% agarose and 7.5% FBS. Cells were overlaid onto a bottom layer of solidified 0.8 % agarose in RPMI 1640 medium containing 5% FBS, at a concentration of 2×103 cells per well. After solidifying, 500 μl of media was plated on top of the second agar layer to prevent over-drying. Assays were incubated for 2 weeks before being stained with 0.005% crystal violet, then imaged with the Bio-Rad Gel Doc ™ XR+ Imaging System. Quantity One Gel Doc version 4.6.9 software from Bio-Rad was used to count the colonies. Anchorage-independence was measured for transient cell lines by plating 24 hrs post-transfection.
Results
ILK silencing down regulates PTEN expression
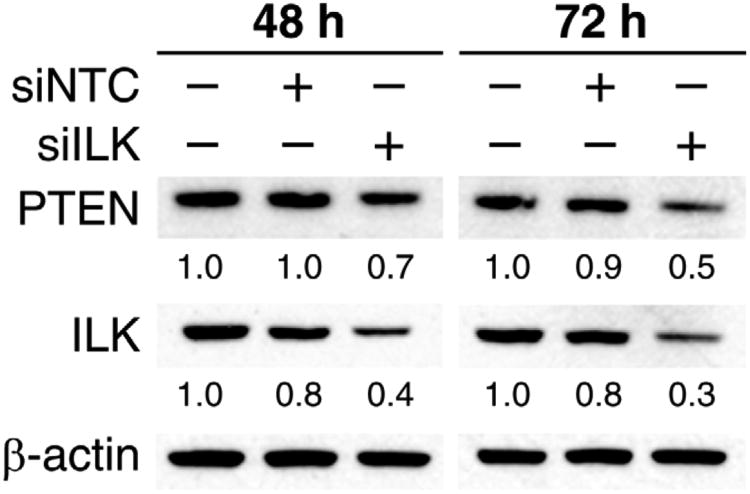
We examined the effects of silencing ILK on PTEN protein expression. We observed a correlative decrease in ILK and PTEN expression at 72 hrs post-transfection following ILK knockdown with siILK (Fig. 1). The effect of ILK silencing on PTEN protein levels was time-dependent, where a moderate decrease in PTEN expression was noted at 48 hrs post-transfection with the most significant decrease at 72 hrs time point.
Fig. 1. Transient ILK silencing decreased PTEN expression.
PTEN protein expression was decreased in ILK-silenced cells, as assessed by. Western blot analysis at 48 and 72 hrs after BE(2)-C cells were transiently transfected with either siILK or non-targeted control (siNTC). Numbers indicate densitometry values relative to β-actin.
Transient ILK silencingdecreases phospho-Akt and PTEN expression, cell proliferation and anchorage-independent cell growth
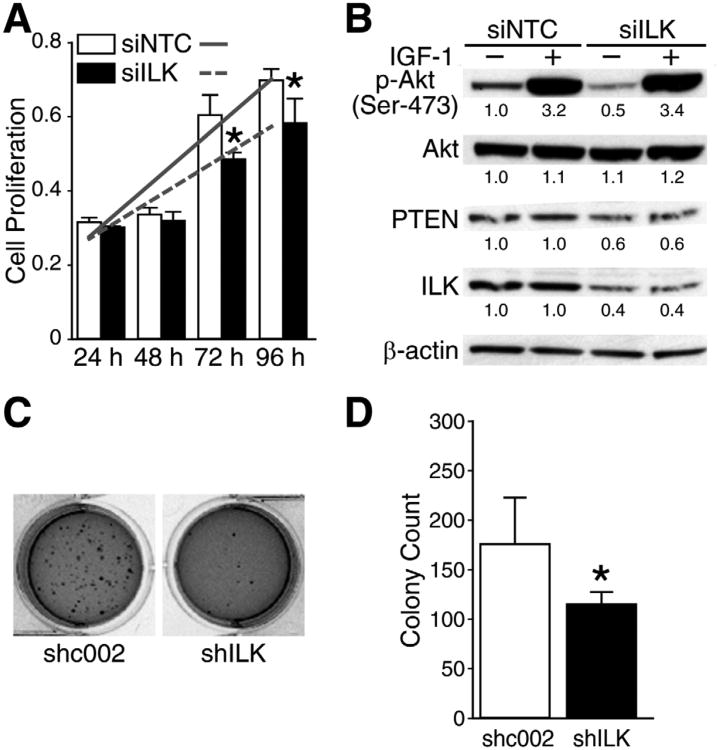
We wanted to determine the effects of transient ILK knockdown on PI3K pathway and cellular growth. BE(2)-C cells demonstrated anti-proliferative effect when transiently-transfected with siRNA targeted at ILK. Inhibitory effects on cell proliferation was noted at 72 and 96 hrs after siILK transfection with the most significant decrease in cell proliferation at only 70% of the control cells at 96 hrs time point (Fig. 2A). We next determined the effects of ILK silencing on the PI3K pathway. We performed the experiment with or without pretreating the cells with exogenous IGF-1 to stimulate the PI3K pathway. We then examined the effect of IGF-1 and ILK silencing on PTEN and phospho-Akt expression. We had anticipated observing that ILK silencing would negate IGF-1-induced phosphorylation of Akt since both IGF-1-mediated cell signaling and ILK convergeat the PI3K/Akt pathway. Our results show that IGF-1 pretreatment had no significant effects on phospho-Akt expression after silencing of ILK; however, cells without IGF-1 yielded attenuation of phospho-Akt after ILK knockdown (Fig. 2B). Interestingly, we also observed a decrease in PTEN expression at 72 hrs post-transfection following ILK knockdown with siILK. At 48 hrs post-transfection, there was no correlative change between ILK and PTEN expression (data not shown). Anchorage-independent cell growth in soft agar represents tumorigenicity of cells in vitro and correlates to the malignant potential of cells in vivo. 18BE(2)-C cells were transiently-transfected with either control plasmid (shc002) or shILK. They were then plated in soft agar for 2 weeks. BE(2)-C cells plated 24 hrs post-transfection with shILK showed a 35% decrease in the number of colonies formed (Figs. 2C, D).
Fig. 2. Transient ILK silencing suppressed phospho-Akt and PTEN expression and inhibited BE(2)-C cell proliferation and soft agar colony formation.
(A) BE(2)-C cells were transiently transfected with siILK or siNTC, and replated to 96-well plate at 5 × 103 cells/well. Cell proliferation was measured (mean ±SEM; *=P<0.05 vs. siNTC; slope lines represent changes in cell growth rate). (B) Western blot analysis showed decrease in PTEN expression at 72 hrs in ILK silenced cells. IGF-1 was used to stimulate the PI3K pathway. Numbers indicate densitometry values relative to β-actin. (C) A representative photograph of soft agar plates at 2 weeks. Transient silencing of ILK (shILK) resulted in an increase in BE(2)-C colony formation when compared to controls (shc002). (D) Quantitative assessment of soft agar colony growth [*=P< 0.05 vs. shc002 (control)].
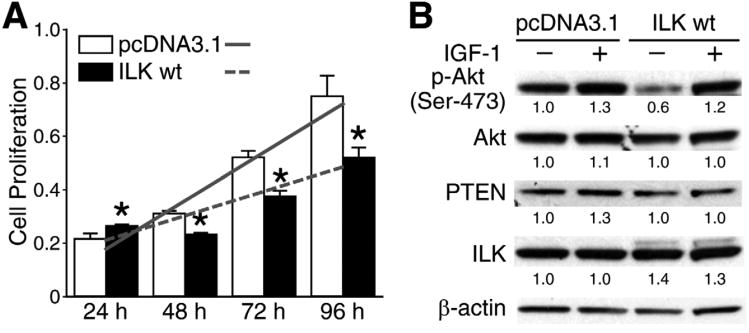
ILK over expression increases phospho-Akt, cell proliferation and anchorage independent cell growth
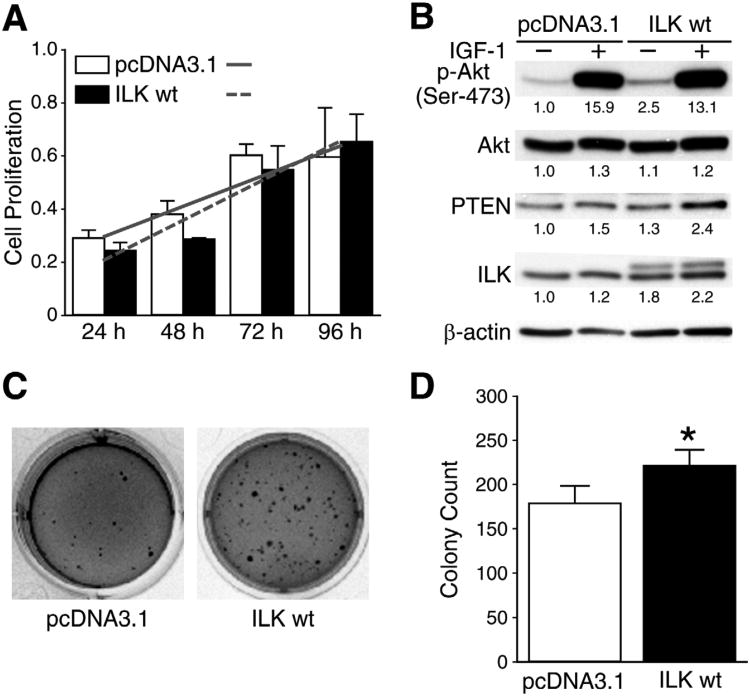
In order to determine the exact role of ILK on tumorigenic properties of neuroblastoma cells, BE(2)-C cells were transiently transfected with ILK wt or control (pcDNA3.1) plasmids. Our data show that ILK over expression resulted in approximately 31% increase in BE(2)-C cell proliferation, as determined by the slope of the growth trend (Fig. 3A, solid vs. dotted lines). However, there were no statistically significant increases in cell proliferation in ILK wt at each of the time points when compared to controls (pcDNA3.1) (Fig. 3A). We next examined the effects of IGF-1 and ILK over expression on phospho-Akt protein levels. Similar to our ILK knockdown experiment results (Fig. 2A), we did not observe any changes in phospho-Akt expression after IGF-1 stimulation in ILK wt transfected cells. However, IGF-1untreated cells yielded significant increases in phospho-Akt. The ILK wt cells expressed exogenous ILK wt gene with His-tag as seen by the larger protein band above the endogenous ILK protein band. ILK wt cells showed no change in PTEN expression (Fig. 3B). Although ILK over expression did not significantly alter cell proliferation, ILK wt transfection led to an increase in BE(2)-C soft agar colonies by 21% as well as an increase in colony size when plated 24 hrs after transfection (Figs. 3C, D).
Fig. 3. Transient ILK over expression increased cell proliferation, phospho-Akt levels,and soft agar colony formation.
(A) BE(2)-C cells were transiently transfected with ILK-wt (wild type) orpcDNA3.1 (control vector) plasmid alone, then replated to 96-well plate at 5 × 103 cells/well. Cell proliferation was measured (mean ±SEM; *=P <0.05 vs. pcDNA3.1; slope lines represent changes in cell growth rate). (B) Western blot analysis showed an increase in phospho-Akt in BE(2)-C cells over expressing ILK at 48 hrs. Numbers indicate densitometry values relative to β-actin. (C) BE(2)-C cells transiently transfected to over express ILK (ILK wt) showed greater colony growth at 2 weeks when compared to controls (pcDNA3.1). (D) Quantitative assessment of soft agar colony growth (*=P< 0.05 vs. pcDNA3.1).
Stable knockdown of ILK increases phospho-Akt and cell proliferation
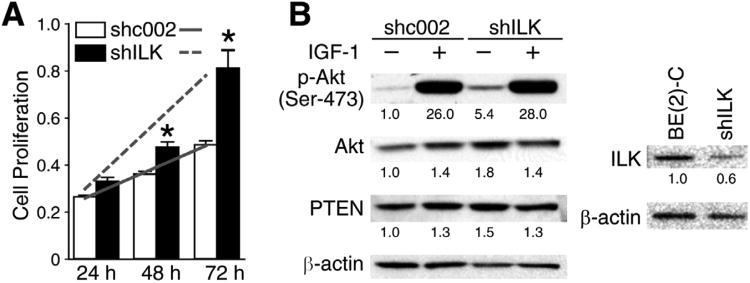
Cells stably transfected with shILK underwent a dramatic proliferative effect, growing at a rate that is 217% that of the control cells (Fig. 4A). We next examined the effect of IGF-1 and ILK silencing on PTEN and phospho-Akt expression. Similarly, ILK silencing had no effect on IGF-1-induced stimulation of phospho-Akt levels with no observable differences between control (shc002) and shILK cells. However, untreated cells once again yielded significant results. BE(2)-C shRNA cells showed an increase in the amount of phospho-Akt levels compared to the control cells (Fig. 4B). Interestingly, PTEN expression was not altered by stable silencing of ILK.
Fig. 4. ILK knockdown in stable cells increased phospho-Akt expression and BE(2)-C cell proliferation.
(A) BE(2)-C cells were stably transfected with shILK or shc002 (control) plasmid. Cells were then selected with puromycin (2.5 μg/ml) to generate a stably-transfected cell line, and plated to a 96-well plate at 5 × 103 cells/well. Cell proliferation was measured (mean ±SEM; *=P<0.05 vs. shc002; slope lines represent changes in cell growth rate). (B) Western blot analysis showed an increase in phospho-Akt in shILK cells. Numbers indicate densitometry values relative to β-actin.
Stable over expression of ILK decreases phospho-Akt as well as cell proliferation
Cells stably-transfected with ILK wt plasmid grew at a rate that is only 50% of the control cells, which represented a 2-fold decrease in rate of growth (Fig. 5A). We next examined the effect of IGF-1 and increased ILK protein levels on PTEN and phospho-Akt expression. While IGF-1 stimulated increases in phospho-Akt levelsin control and ILK over expression cells uniformly, we again observed significant results in untreated cells. BE(2)-C cells stably-transfected with ILK wt plasmid demonstrated a significant drop in phospho-Akt expression. These ILK wt cells showedlittle changes in PTEN expression when compared to the controls (Fig. 5B).
Fig. 5. Stable cell lines over expressing ILK decreased phospho-Akt expression and BE(2)-C cell proliferation.
(A) BE(2)-C cells were transfected with ILK-wt (wild type) plasmid or pcDNA3.1 vector alone as control. Cells were then selected with G418 (450 μg/ml) to generate a stably-transfected cell line, and replated to a 96-well plate at 5 × 103 cells/well. Cell proliferation was measured (mean±SEM; *=P<0.05 vs. pcDNA3.1; slope lines represent changes in cell growth rate). (B) Western blot analysis showed a decrease in phospho-Akt expression in stable ILK over expressing cells. Numbers indicate densitometry values relative to β-actin
Discussion
In this study, we investigated the relationship between ILK and PTEN as well as the effect of ILK on human neuroblastoma cell proliferation and anchorage-independent cell growth. We anticipated that the knockdown of ILK would lead to an enhancement of PTEN expression. Much to our surprise, we discovered that the down regulation of ILK led to a decrease in PTEN expression. We also observed differences in proliferation and anchorage-independent cell growth, which correlated to changes in phospho-Akt levels. Interestingly, these results varied when ILK was silenced via transient or stable transfection. Our data further support the need to investigate the long-term effects of deregulating ILK expression, as results vary based on the length of time that ILK expression is silenced. As expected, BE(2)-C cells transiently-transfected to silence ILK exhibited a decrease in phospho-Akt expression levels, with resultant decreases in cell proliferation and anchorage-independent cell growth. This was markedly different in our stable knockdown cell lines, which showed higher expression levels of phospho-Akt and increased cell proliferation. When we established ILK over expression cellsusing transient and stable transfection techniques, we observed results that were the opposite of respective knockdown cell lines with correlative changes in phospho-Akt expression. Additionally, siILK cells demonstrated a more pronounced decrease in PTEN at the 72 hr time point when compared to 48 hr time point. These results also suggest that the effect of ILK silencing on PTEN expression is a time-dependent downstream process. It is likely that ILK does not act on PTEN directly, but instead acts on an upstream effector of PTEN, thereby explaining the delay in PTEN down regulation following ILK knockdown.
Our data call into question the straight-forward description of ILK's involvement in Akt phosphorylation and prolonged tumor cell survival. It is well documented that a decrease in PTEN and an increase in phospho-Akt often translates to increases in cell invasion and survival.8, 13, 19 One could argue that the down regulation of ILK and subsequent decreases in the tumor suppressor PTEN observed in our study should cause an increase in neuroblastoma cell survival in the short-term as well as the long-term. However, ILK activates Akt by phosphorylating it on serine 473 in a PI3K-dependent manner,9 and consequently, one observes an initial decrease in neuroblastoma cell growth shortly following the knockdown of ILK and the resultant drop in phospho-Akt levels. On the other hand, Akt can also be phosphorylated by other proteins such as PDK1 and PDK28, and is therefore, not solely dependent on ILK for its phosphorylation. This ability for cells to compensate for the loss of ILK activity may partially explain long-term increases in phospho-Akt following ILK and PTEN suppression. Thus, a decrease in ILK expression followed by a decrease in PTEN expression would have a longer and more far reaching effect on tumor progression than the initial and short-lived effect of lowered Akt expression.
The mechanisms by which ILK regulate PTEN expression is unclear. It is possible that ILK influences PTEN expression via interactions with other proteins that bind ILK. ILK-binding protein β-parvin interacts with α-PIX, a guanine nucleotide exchange factor and activator for Cdc42, a small Rho GTPase.20 Recent reports have shown that members of the Rho family of small GTPases, Cdc42 and RhoA, are involved in the intracellular localization of PTEN. They also found that Cdc42 and RhoA act synergistically to stimulate PTEN's phosphatase activity in a RhoA-associated kinase dependent manner in leukocytes and human transfected embryonic kidney cells.21 Perhaps ILK influences PTEN expression via β-parvin and α-PIX and their interactions with small Rho GTPases such as Cdc42. At this time, further studies are needed to elucidate this mechanism.
In conclusion, our study demonstrates the importance of ILK in the PI3K/Akt pathway, not only in its role as a kinase acting on Akt, but also as a regulator of PTEN. In addition, the functional changes that arise in neuroblastoma cells as a result of ILK dysregulation show that down regulation of ILK on a long-term basis may serve to promote neuroblastoma tumor growth. Further investigation is required in order to better understand the relationship between ILK and PTEN.
Acknowledgments
We extend special thanks to Karen Martin for manuscript preparation and to Dr. ShoukatDedhar for providing the ILK wt plasmid used in this study.
This work was supported by grant R01 DK61470 from the National Institutes of Health.
Footnotes
We have no financial or other interests, which may be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung NK. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–92. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 2.Ishola TA, Kang J, Qiao J, Evers BM, Chung DH. Phosphatidylinositol 3-kinase regulation of gastrin-releasing peptide-induced cell cycle progression in neuroblastoma cells. Biochim Biophys Acta. 2007;1770:927–32. doi: 10.1016/j.bbagen.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender A, Opel D, Naumann I, Kappler R, Friedman L, von Schweinitz D, et al. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene. 2010 doi: 10.1038/onc.2010.429. [DOI] [PubMed] [Google Scholar]
- 4.McDonald PC, Dedhar S. The role of integrin-linked kinase in cancer development and progression. In: Zent R, Pozzi A, Zent R, Pozzi AS, editors. Cell-extracellular matrix interactions in cancer. New York: Springer; 2010. pp. 245–73. [Google Scholar]
- 5.Meyer A, van Golen CM, Boyanapalli M, Kim B, Soules ME, Feldman EL. Integrin-linked kinase complexes with caveolin-1 in human neuroblastoma cells. Biochemistry. 2005;44:932–8. doi: 10.1021/bi048619r. [DOI] [PubMed] [Google Scholar]
- 6.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 7.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379:91–6. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 8.Weinberg RA. The biology of cancer. London: Garland Science; 2006. [Google Scholar]
- 9.Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–9. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]
- 10.Eke I, Hehlgans S, Cordes N. There's something about ILK. Int J Radiat Biol. 2009;85:929–36. doi: 10.3109/09553000903232892. [DOI] [PubMed] [Google Scholar]
- 11.Bunney TD, Katan M. Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer. 2010;10:342–52. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 12.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–6. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–7. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 14.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Yu D. PI(3)king apart PTEN's role in cancer. Clin Cancer Res. 2010;16:4325–30. doi: 10.1158/1078-0432.CCR-09-2990. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Wang X, Li J, Sun X, Wang X. The expression significance and associativity of ILK and PTEN in gastric cancer tissue. The Chinese-German Journal of Clinical Oncology. 2008;7:701–3. [Google Scholar]
- 17.Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;14:4813–25. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponten J. Spontaneous and virus induced transformation in cell culture. Virol Monogr. 1971;8:1–253. doi: 10.1007/978-3-7091-8258-1_1. [DOI] [PubMed] [Google Scholar]
- 19.Tamura M, Gu J, Tran H, Yamada KM. PTEN gene and integrin signaling in cancer. J Natl Cancer Inst. 1999;91:1820–8. doi: 10.1093/jnci/91.21.1820. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger G, Jantke I, Gal A, Kutsche K. Interaction of alphaPIX (ARHGEF6) with beta-parvin (PARVB) suggests an involvement of alphaPIX in integrin-mediated signaling. Hum Mol Genet. 2003;12:155–67. doi: 10.1093/hmg/ddg019. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]