Abstract
The role of the immune response to oncolytic Herpes Simplex viral (oHSV) therapy for glioblastoma is controversial. Within hours of oHSV infection of human or syngeneic glioblastoma in mice, activated natural killer (NK) cells are recruited to the site of infection. This response significantly diminished the efficacy of glioblastoma virotherapy. oHSV-activated NK cells coordinated macrophage and microglia activation within tumors. In vitro, human NK cells preferentially lysed oHSV-infected human glioblastoma cell lines. This enhanced killing depended on NK cell natural cytotoxicity receptors (NCR) NKp30 and NKp46, whose ligands were up-regulated in oHSV-infected glioblastoma cells. HSV titers and oHSV efficacy were increased in Ncr1−/− mice and in a Ncr1−/− NK cell adoptive transfer model of glioma, respectively. These in vitro and in vivo (mouse) results demonstrate that glioblastoma virotherapy is partly limited by an antiviral NK cell response involving specific NCRs, uncovering novel potential targets to enhance cancer virotherapy.
Keywords: Herpes simplex virus, gene therapy, oncolytic virus, brain tumor, microglia, macrophages
Current standard of therapy for glioblastoma is palliative1. Attempts to improve survival involve various novel approaches, including oncolytic virotherapy1. In virotherapy, naturally occurring or genetically engineered viruses selectively replicate in and lyse tumor cells while engendering an immune response to virally-infected and uninfected tumor. One virus is oncolytic Herpes Simplex virus (oHSV), genetically modified to replicate in and lyse tumor cells through multiple rounds of viral replication2. To date, oHSV injected into human glioblastomas have been well tolerated, but efforts to demonstrate efficacy in early phase trials have been disappointing3. We have hypothesized that the host immune response to oHSV therapy for glioblastoma is a barrier towards achieving clinical success4. This is based on animal studies in which transient depletion of phagocytic macrophage populations or pharmacologic suppression of immunity improves the effect of oHSV5-7. However, the hypothesis that innate immunity is deleterious to virotherapy5-11 runs counter to the argument that such immune responses are beneficial. In fact, the host immune response following oHSV administration in vivo has been shown to provoke an antitumor immune response against oHSV infected cells and bystander tumor cells12-15. Resolution of these apparently discordant views16,17 is significant since one would attempt to either evade or increase immunity to improve efficacy.
In this context, natural killer (NK) cells are the perfect foe or friend of virotherapy. NK cells are rapidly recruited to the site of viral infection and mediate viral clearance, thus making them a foe11. However, they also possess tumor—clearing properties whereby stimulating NK cell infiltration by oHSV could facilitate antitumor efficacy18-22. In the context of oHSV therapy, the antiviral vs. antitumor role of NK cells has been undefined. The mechanism by which NK cells eradicate virally infected cells is currently a field of intense investigation23. Human NK cells possess a variety of receptors including the natural cytotoxicity receptors (NCR) NKp30, NKp44, and NKp46 that mediate NK cytotoxic functions; however, the key receptor—ligand interactions that coordinate these responses are not known.
In this report, we show that NK cell recruitment to the site of oHSV infection of experimental glioblastoma is rapid and characterized by an activated phenotype that occurs locally in the brain. This response does not facilitate antitumor effects; rather, it leads to premature viral clearance and limits oHSV anticancer efficacy. In vitro, NK mediated killing was primarily dependent on NKp30 and NKp46 which recognize ligands up-regulated in oHSV infected human glioblastoma cell lines. These findings were supported with studies in mice lacking NKp46 (Ncr1−/−) that demonstrated the antiviral role of NKp46. These results demonstrate that the initial in vivo antiviral NK response to oHSV is detrimental in mouse models and suggests NKp30 and NKp46 as potential clinical targets to improve virotherapy.
Results
oHSV induces rapid NK cell recruitment and activation
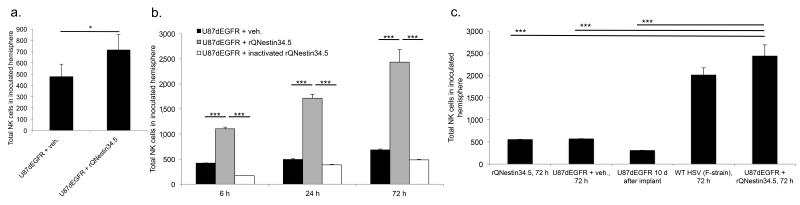
We asked if there was an increase in NK cell infiltration after administering rQNestin34.524 into orthotopic human glioblastoma (U87dEGFR) xenografts and syngeneic mouse glioblastoma (KR158dEGFR). rQNestin34.5 replicates based on the mutational insertion of GFP into the HSV-1 ICP6 locus, providing selectivity for p16−/− cells25 and on nestin promoter transcriptional regulation of the HSV1 γ1 34.5, providing selectivity for nestin-positive glioblastomas24. We observed a significant increase in the total number of NK cells two hours after oHSV infection (Fig. 1a). Compared to mice treated with vehicle or heat inactivated oHSV, NK cell recruitment required viral replication and increased up to 72 hours after rQNestin34.5 (Fig. 1b–c, Suppl. Fig. 1a–c). We confirmed the presence of NK cells in the brains of rQNestin34.5 treated mice that were perfused prior to mononuclear cell isolation (Suppl. Fig. 1b). NK cell recruitment also occurred after intracranial administration of wild-type HSV1 (WT HSV) (Suppl. Fig. 1b). These results thus showed that active oHSV replication elicited a rapid elevation of NK cells into xenograft and syngeneic mouse glioblastomas.
Figure 1. oHSV administration induces natural killer cell recruitment to tumor bearing brain.
(a) FACS quantification of NK cells into the brains of athymic mice bearing U87dEGFR tumors, 2 hours after inoculation with rQNestin34.5 vs. vehicle treated mice (n = 3/group). (b) FACS quantification, at 6, 24, or 72 hours after rQNestin34.5 inoculation of athymic mice bearing U87dEGFR tumors, of the total number of NK cells in tumor bearing hemispheres vs. mice treated with vehicle or heat inactivated virus (n = 4–5/group). (c) NK cell quantification into the brains of mice following either WT HSV or rQNestin34.5 injection into intracranial U87dEGFR, in mice lacking tumor, in tumor bearing mice treated with vehicle, or untreated tumor bearing mice (n = 3–5/group). * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars represent +/− standard deviation.
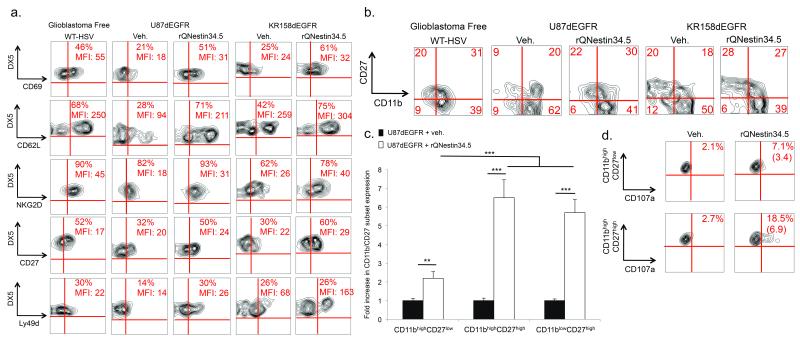
We next characterized the activation status of recruited NK cells by evaluating the expression of 11 different surface antigens. oHSV administration induced a unique NK cell phenotype for cells recruited to the site of infection (Fig. 2a, Suppl. Table 1a) that was not present in peripheral NK cells (Suppl. Fig. S2). CD27 and CD11b denote distinct NK cell functional subpopulations26: immature CD11blowCD27high, cytotoxic CD11bhighCD27high, and senescent CD11bhighCD27low. While NK cells recruited to vehicle-treated mice were mostly CD11bhighCD27low, oHSV administration recruited CD11bhighCD27high or CD11blowCD27high NK cells (Fig. 2b, c, Suppl. Table 1b. oHSV induced a 3 and 7-fold increase in the degranulation marker CD107a in CD11bhighCD27low and CD11bhighCD27high NK cells, respectively (Fig. 2d). Therefore, oHSV significantly enhances the recruitment of distinct NK subsets expressing cytotoxic NK cell markers.
Figure 2. NK cells are activated following oHSV therapy.
(a) FACS assessment of the mean fluorescent intensities (MFI) and percentage of NK cells (CD3–DX5+) expressing various NK cell activation markers (CD69, CD62L, NKG2D, CD27, or Ly49D), 72 hours after intracranial inoculation of rQNestin34.5 into athymic mice bearing U87dEGFR human glioblastomas. Additionally, FACS quantification of the above markers is shown in KR158dEGFR syngeneic tumors and following WT HSV infection into the brains of athymic mice lacking glioblastoma (glioblastoma-free). Refer to Supplementary table 1 for average and ranges summarizing the total number of experiments. (b, c) FACS analysis of NK cell markers CD11bhighCD27high (cytotoxic) or CD11blowCD27high (immature) compared to CD11bhighCD27low (senescent), 72 hours after rQNestin34.5 or vehicle inoculation in both xenograft and syngeneic tumor models in addition to WT HSV infection into the brains of athymic mice lacking glioblastoma (glioblastoma-free). This is presented as both representative dotplots (b) and a fold increase in the expression of each NK cell population compared to vehicle treated mice (c) (n = 4–6 mice/group). (d) Percentage positivity and fold increase (in parentheses) of the degranulation marker CD107a in the CD11bhighCD27high and CD11bhighCD27low NK cell subsets 72 hours after rQNestin34.5 or vehicle treatment of mice with glioblastoma xenografts. * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars represent +/− standard deviation.
Macrophage activation occurs in a NK cell dependent manner
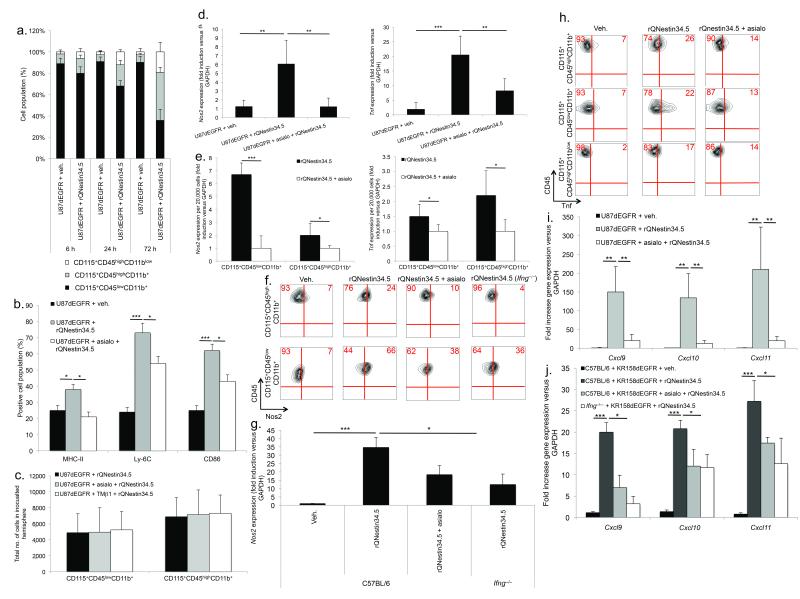
Since NK cells are thought to coordinate macrophage activation27, we examined whether recruited NK cells orchestrate this response in the context of virotherapy. oHSV reduced the percentage of CD115+CD45lowCD11b+ cells (microglia) and concomitantly increased both CD115+CD45highCD11b+ mononuclear (macrophages) and CD115+CD45highCD11blow lymphocyte populations compared to vehicle (Fig. 3a). By selectively gating upon CD115+CD45highCD11b+, these cells displayed significantly enhanced expression of macrophage activation markers. However, NK depletion28 attenuated this response (Fig. 3b, Suppl. Fig. 3a). In order to assess retention of macrophages and microglia after NK depletion, we gated on CD115, a marker for monocyte derived cells29. We subsequently quantified total cell numbers of CD115+CD45lowCD11b+ and CD115+CD45highCD11b+ cells. Notably, NK depletion with either asialo–GM1 or TMβ1 prior to oHSV inoculation did not alter the total number of these cell populations compared to mock depleted mice (Fig. 3c). We further confirmed an equal number of intracranial macrophages and microglia after NK depletion by staining for CD45+F4/80+Gr1+ (Suppl. Fig. 3b).
Figure 3. NK cells mediate macrophage and microglia activation following oHSV therapy.
(a) FACS of time-dependent changes in CD115+CD45highCD11b+, CD115+CD45highCD11blow and CD115+CD45lowCD11b+ cells in glioblastomas following rQNestin34.5 (n = 4–6 mice/group). (b) FACS of CD115+CD45highCD11b+ cells expressing macrophage activation markers (MHC-II, Ly-6C, or CD86), 72 hours after vehicle or rQNestin34.5 infection as a function of NK cell presence (n = 4/group). (c) FACS of CD115+CD45lowCD11b+ or CD115+CD45highCD11b+ cells 72 hours after rQNestin34.5 inoculation as a function of NK cell presence. (d) Nos2 and Tnf expression in tumor bearing hemispheres, 72 hours following vehicle or rQNestin34.5 inoculation in the presence or absence of NK cells (n = 4–5/group). (e) Nos2 and Tnf expression, as a function of NK cell depletion, in FACS sorted CD115+CD45lowCD11b+ or CD115+CD45highCD11b+ cells 72 hours after rQNestin34.5 inoculation of U87dEGFR. (f, g) Intracellular protein staining with FACS quantification (f) and Nos2 mRNA expression (g) in intracranial KR158dEGFR tumors, 72 hours after rQNestin34.5 inoculation as a function of NK cells and Ifng production. (h) FACS of intracellular Tnf 72 hours after rQNestin34.5 treatment of U87dEGFR as a function of NK cell presence. (i, j) Cxcl9, Cxcl10, and Cxcl11 expression 72 hours after rQNestin34.5 treatment of both xenograft (i) and syngeneic (j) tumors as a function of NK cell presence (n = 3–5/group). The dependence of Ifng in gene expression within the KR158dEGFR was also assessed. * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars represent +/− standard deviation.
We subsequently assessed for markers of macrophage and microglia activation. oHSV infection of glioblastoma in vivo significantly increased gene expression and protein production of Nos2 and Tnf, while NK depletion attenuated this response (Fig. 3d–h). By adoptively transferring wild-type NK cells into Ifng−/− mice, we demonstrated that Nos2 induction depended on NK derived Ifng (Fig. 3f–g, Suppl. Fig. 3c). Finally, gene expression of the Ifng inducible chemokines (IIC) Cxcl9, Cxcl10, and Cxcl11, significantly increased following oHSV therapy (Fig. 3i, j). This increase did not occur if NK cells were depleted or if an Ifng−/− mouse was used (Fig. 3i, j). Consistent with the notion that IIC expression is associated with macrophage and microglia activation but is not necessarily derived from these cells, IIC expression was not significantly altered within these cell populations following NK depletion (Suppl. Fig. 3d). Taken collectively, virotherapy results in NK cell mediated macrophage and microglia activation which has been reported to correlate with anticancer and antiviral properties30.
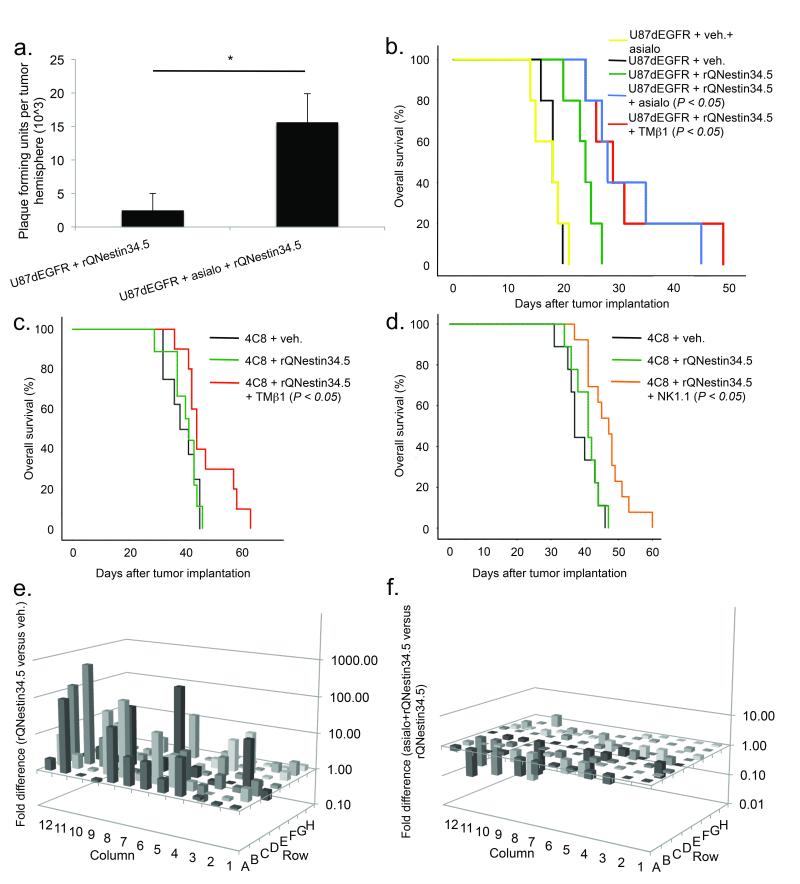
Depletion of NK cells in vivo enhances efficacy of oHSV
The observed NK cell and macrophage activation could potentially hinder virotherapy by eliminating oHSVs. After confirming our ability to deplete NK cells in glioblastoma bearing mice (Suppl. Fig. 4a–c), NK depletion led to significantly elevated titers of rQNestin34.5 compared to non-NK depleted mice (Fig. 4a). Importantly, NK depletion (by antibodies to either asialo-GM1 or TMβ1) significantly increased survival of glioblastoma xenografts treated with oHSV (Fig. 4b). We recapitulated these findings by depleting NK cells with either TMβ-1 or NK1.1 in a mouse syngeneic model31 (Fig. 4c–d).
Figure 4. NK depletion enhances oHSV efficacy.
(a) Viral titers as a function of NK cell depletion, 72 hours after rQNestin34.5 inoculation into athymic mice bearing U87dEGFR glioma (n = 4–5/group). (b) Kaplan-Meier survival curves for athymic mice bearing U87dEGFR tumors treated with rQNestin34.5 vs. vehicle as a function of NK cell depletion (with antibody to either asialo-GM1 or TMβ1) (n = 5/group). (c, d) Kaplan-Meier survival curves for the syngeneic 4C8 mouse glioblastoma model. NK depletion was carried out with TMβ1 specific antibody (c) or NK1.1 specific antibody (d) prior to rQNestin34.5 or vehicle inoculation into brain tumors (n = 8–14/group). (e, f) Differences in gene expression of 84 mouse inflammatory genes, in the presence (e) or absence (f) of NK cells, 72 hours after intracranial inoculation of rQNestin34.5 into athymic mice bearing U87dEGFR cells (n = 4–5/group). Each row and column is represented by a unique number denoting a different transcript. The identity of the transcript following tumor treatment is listed in Supplementary tables 2a (for vehicle vs rQNestin34.5 treatment) and 2b (for asialo-GM1+rQNestin34.5 vs. rQNestin34.5 treatment). * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars represent +/− standard deviation.
To assess the inflammatory response induced by oHSV, we used a mouse inflammatory gene expression array. We observed significant induction in 30 out of 84 genes in tumors treated with oHSV. This included over 100-fold induction of Cxcl9, Cxcl10, Cxcl11, Ccl2, and Ccl7. Strikingly, NK cell depletion reduced oHSV-induction of all 30 genes (Fig. 4e, f, Suppl. Table2). These results indicate that NK cell recruitment following oHSV reduces viral propagation and attenuates its antitumor efficacy.
NKp30 and NKp46 are mediators of oHSV viral clearance
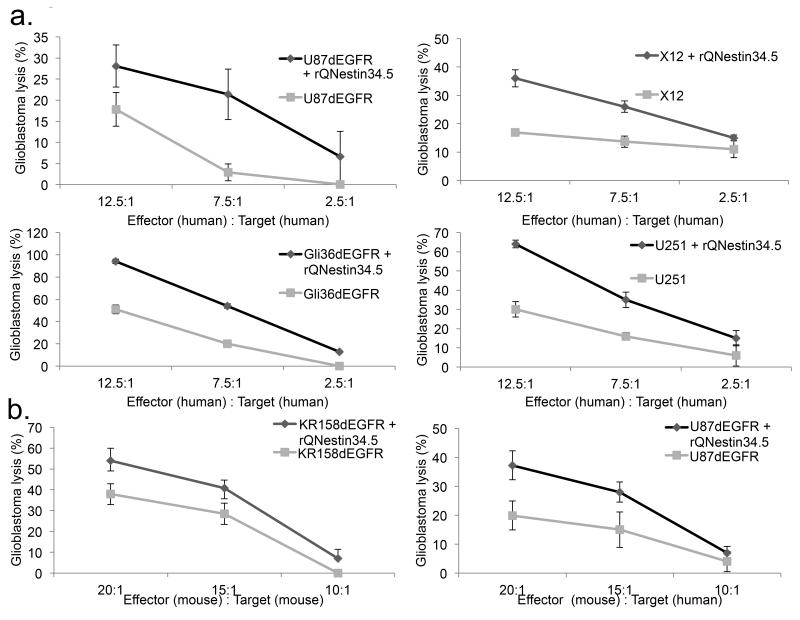
Since IL-15 was up-regulated in tumors by oHSV (Suppl. Fig. 5a), we used IL-15 activated human NK cells to determine their cytotoxicity. Indeed, activated human NK cells displayed enhanced toxicity against oHSV-infected human glioblastoma cells (Fig. 5a) and this was also observed to a reduced level with unstimulated NK cells (Suppl. Fig. 5b). Mouse NK cells also preferentially killed oHSV infected glioblastoma cells (Fig. 5b). Notably, this antiviral response was unique to NK cells as cytotoxic T lymphocytes (CTLs) from human donors were unable to recapitulate this cytotoxicity profile (Suppl. Fig. 5c). Collectively, activated NK cells were more cytotoxic against oHSV infected glioblastoma.
Figure 5. NK cells preferentially lyse oHSV infected human and mouse glioblastoma.
(a) Glioblastoma cell lines (U87dEGFR, Gli36dEGFR, or U251) or primary human glioblastoma cells enriched for stem-like cell properties (X12) were employed in NK cell killing assays. The extent of glioblastoma cell cytotoxicity was assayed as a function of infection with rQNestin34.5, prior to co-culturing with varying numbers of IL-15 stimulated human NK cells, compared to co-cultures of NK cells and mock infected tumor. (b) Splenocyte derived mouse NK cells from athymic mice or C57BL/6 wild type mice were cultured with U87dEGFR or KR158dEGFR glioblastomas, respectively, to assess for NK mediated cytotoxicity. In both (a) and (b), NK mediated killing was tested at varying effector:target ratios. Error bars represent +/− standard deviation.
To explore the mechanism of enhanced cytotoxicity, oHSV infection led to cell contact- and perforin-dependent32,33 killing (Suppl. Fig. 6a), minor alterations in HLA-ABC staining (Suppl. Fig. 6b), and no change in expression of NK activating receptors DNAM-1 and NKG2D (Suppl. Fig. 6c). Blockade of NKG2D inhibited killing in one of four glioblastoma cells, but analyzed collectively this was not significant (P = 0.34) (Suppl. Fig 6a). DNAM-1 blockade achieved moderate inhibition of cell killing in both oHSV (P = 0.03) and mock infected cells (P = 0.01) (Suppl. Fig. 6d). Therefore, it appeared that NK cell cytotoxicity was only partially dependent on canonical NK cell receptor recognition of oHSV-infected tumor cell lines.
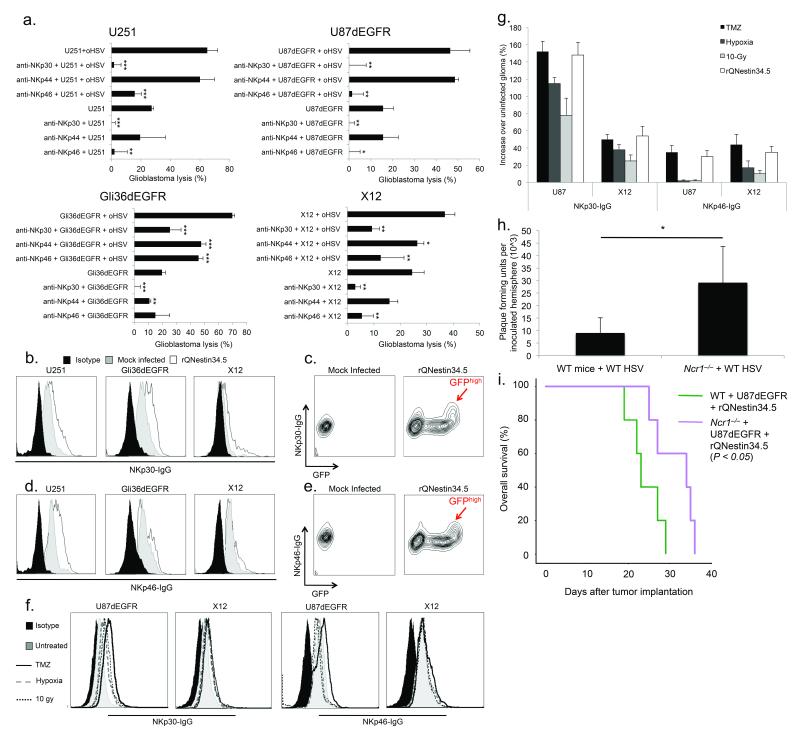
We thus determined if NCR mediated the observed lysis of oHSV infected glioblastoma34,35. We significantly inhibited NK mediated killing by blocking either NKp30 (P = 0.003) or NKp46 (P = 0.02) (Fig. 6a). In a mouse in vitro model, NKp46 (the sole NCR present in mice) mediated NK cell killing of oHSV infected glioblastoma cells (Supp. Fig. 6e). NCR fusion proteins NKp30-Ig and NKp46-Ig also detected enhanced ligand expression in oHSV-infected glioblastoma cells in vitro (Fig. 6b, d). GFP is used to detect rQNestin34.5 infection and the highly infected (GFPhigh) population exhibited maximum NCR ligand staining (Fig. 6c, e). Notably, the up-regulated NCR ligand was not the recently described NKp30 ligand, B7-H636 (data not shown).
Figure 6. NKp30 and NKp46 orchestrate NK mediated killing of oHSV infected glioblastoma cells in vitro and NKp46 mediates viral clearance in vivo.
(a) Glioblastoma cell lines (U87dEGFR, Gli36dEGFR, or U251) or primary human glioblastoma cells enriched for stem-like cell properties (X12) were used for NK killing assays. Glioblastoma cells were infected with rQNestin34.5 or vehicle prior to co-culturing with IL-15 stimulated human NK cells, at a ratio of 12.5:1. The effect of blocking antibodies against NKp30, NKp46 and NKp44 was also assessed. (b–e) Following infection with rQNestin34.5 or mock, ligand expression for NKp30 (b, c) and NKp46 (d, e) was assayed with FACS. Additionally, the expression of GFP by rQNestin34.5 was also assayed in virally infected cells as a function of NKp30 or NKp46 ligand (c, e). (f, g) NKp30 or NKp46 ligand expression in glioma cells in response to temozolomide, radiation, hypoxia (f), or rQNestin34.5 infection (g). (h) WT HSV titers in Ncr1−/− mice compared to WT C57BL/6 mice, 72 hours after infection (n = 3–4/group) (i) Kaplan-Meier survival curves for U87dEGFR brain tumor bearing SCID γc−/− mice adoptively transferred with either Ncr1−/− or wild type NK cells and intracranially inoculated with rQNestin34.5 (n = 5/group). * P < 0.05; ** P < 0.01; *** P < 0.001. Error bars represent +/− standard deviation.
We also asked if NCR ligands were up-regulated after various modes of cellular stress, including temozolomide (TMZ) exposure which is the chemotherapeutic agent used in the treatment of glioblastoma. Although radiation or hypoxia induced moderate expression of NKp30 but not NKp46 ligand, TMZ induced both NKp30 and NKp46 ligand to levels seen with oHSV (Fig. 6f, g). Therefore, NK mediated cytotoxicity of oHSV-treated glioblastoma cells is coordinated primarily through the NK receptors NKp30 and NKp46 that recognize cognate ligands on tumors that are up-regulated by oHSV or TMZ.
To assess the role of NKp46 in viral clearance in vivo, we quantified WT HSV replication after intracerebral inoculation in the brains of Ncr1−/− and WT mice. Compared to wild-type mice, WT HSV titers were significantly enhanced in Ncr1−/− mice (Fig. 6h). We next investigated whether NKp46 deficient NK cells lead to improved survival in our virotherapy tumor model. We adoptively transferred enriched NK cells from WT or Ncr1−/− mice into sublethally irradiated SCID γc null mice (lacking host B, T and NK cells), followed by intracranial tumor implantation. We found that oHSV treatment led to prolonged survival in the mice lacking NKp46 compared to the mice transferred with WT NK cells (Fig. 6i).
Discussion
Here, we explored the response of innate immunity to oHSV therapy of glioblastoma. We discovered a rapid NK cell response to tumor infection with oHSV. This in vivo antiviral NK cell response depends on specific cytotoxic NK cell subsets unique to the brain that also coordinate macrophage activation. This NK cell response leads to killing of oHSV-infected glioblastomas. NK cell depletion improves survival of glioblastoma bearing mice treated with oHSV. We have discovered that oHSV-infected human glioblastomas up-regulate ligands for the NK cell natural cytotoxicity receptors, NKp30 and NKp46 in vitro. Recognition of these ligands is critical for NK cell-mediated cytotoxicity of oHSV-infected cells and viral clearance in vivo. Collectively, we demonstrate that the rapid NK cell response to oHSV therapy cooperates with other innate immune cells to reduce, rather than enhance, the efficacy of oHSV treatment via NKp30 and NKp46. We argue that these findings, combined with clinical reports of patients with deficiencies in NK cell function being susceptible to HSV1 encephalitis37, suggest that NK cells represent a significant barrier to oHSV therapy of glioblastoma.
The role of NK cells in mediating the efficacy of virotherapy is controversial. Most studies show enhanced oHSV efficacy by eliciting an NK mediated anti-tumor response18-21. Only Altomonte et al. described the antiviral properties of NK cells as detrimental to vesicular stomatitis virus therapy for hepatocellular carcinoma38. Our evidence shows that rapidly responding NK cells to oHSV infection of glioblastoma provide a barrier to therapeutic efficacy. First, there is a decline in viral titers within days of oHSV administration5-8. Second, in preclinical studies the clearance of oHSV approaches 80%, corresponding with rapid peripheral macrophage recruitment into the site of viral infection5,7, suggesting a mechanism of oHSV clearance. These findings were validated through a study demonstrating that macrophage depletion enhanced the efficacy of oHSV therapy in glioblastoma5. Third, a recent clinical trial of oHSV for glioblastoma noted prominent inflammatory infiltration within the tumor, correlating with reduced oHSV replication3. Fourth, there are reports of congenital NK cell deficiencies linked to HSV1 encephalitis37. Lastly, mathematical modeling has previously shown the timing of the antiviral innate immune response is detrimental to oHSV therapy9. Collectively, this evidence strongly suggests that initial innate immunity to oHSV therapy is detrimental to anticancer efficacy. However, other studies have shown that immune responses to vesicular stomatitis virus (VSV) virotherapy for metastatic non-CNS tumors are highly desirable20,39,40. Therefore, the effect of immunity on virotherapy’s efficacy may require a balance between virus replication and antitumor responses that differs based on the oncolytic virus and tumor.
For the first time, we show that recruited mouse NK cells up-regulate markers of an activated phenotype. Knowing that NK cells produce significant Ifng and that oHSV is enhanced in both Ifng and macrophage depleted mice5,7, led us to hypothesize that NK cells mediate oHSV-induced macrophage and microglia activation41. We demonstrated that this activation, occurring after oHSV administration, is NK cell and Ifng dependent. Our results argue that the loss of macrophage and microglia activation products was more likely due to loss of NK-mediated activation rather than to loss of macrophage and microglia cell numbers. Interestingly, oHSV infection led to a robust NK cell and Ifng dependent induction of Cxcl9, Cxcl10, and Cxcl11. These chemokines are associated with macrophage activation and herpes encephalitis41. Future clinical studies should assess whether the recruitment of IFN-γ producing CD3– CD56brightCD94bright human NK cells42 is associated with both oHSV inoculation and activation of macrophages and microglia. Similarly, the CD3–CD56dimCD94dim NK subset42, associated with cell-mediated killing, may translate into preferential killing of virally infected glioblastoma cells before sufficient oncolysis has occurred. Future clinical trials could clarify developmental NK subsets, by examining CD56 and CD94 expression42, along with their associated functional properties.
We also assessed the effect of NK depletion in a fully immunocompetent mouse model. After screening several mouse glioblastomas, the KR158dEGFR glioblastoma line demonstrated oHSV replication and cytotoxicity in vitro approximating that observed with human glioblastomas (data not shown). However, KR158dEGFR is syngeneic to C57BL/6 mice, a strain that is notoriously impervious to HSV143 . Due to minimal in vivo viral replication in this model (data not shown), we proceeded to employ the mouse 4C8 model31. Although oHSV replicates about 1.5 log times less in 4C8 cells compared to human glioma cells (data not shown), its syngeneic B6D2F1/J mouse strain is more sensitive to HSV15. Yet, even in this model, significant survival from syngeneic gliomas with oHSV was observed only in the absence of NK cells (Fig. 4c, d), further underscoring the importance of NK cells in limiting glioma virotherapy.
A common feature of glioblastoma is the endogenous expression of ligands for activating NK cell receptors34,35. Recent studies have explored the role of HSV-1 infection in modulating NK activating ligand expression. In human glioblastoma cell lines infected with oHSV, we observed up-regulation of ligands for NKp46 and NKp30. Blockade of these two receptors significantly reduced NK cell cytotoxicity. Additionally, the intensity of NKp30 and NKp46 ligands is increased with oHSV infection, thus indicating that these ligands are better recognized (Fig. 6). Increases in tumor cell ligand expression occur with oHSV infection or Temozolomide but not other methods of tumor killing. These data argue for relatively specific increased recognition of the ligands for NKp30 and NKp46.
The identity of ligands for NCRs is a field of intense investigation44 with influenza hemagglutinin (HA) being identified as an activating ligand for NKp46 and NKp4445. NKp30 is involved in viral eradication by binding to an unknown ligand on immature dendritic cells (imDCs)46 and viral evasion through its recognition of human cytomegalovirus tegument protein pp6547,48. Additionally, NKp30 recognizes B7-H636, a cell surface protein associated with tumor formation, and Bat349, a released cellular stress protein. While NKp30 and NKp46 mediated NK lysis of oHSV infected glioblastoma, B7-H6 did not appear to be involved. Moreover, cell surface expression of NKp30 and NKp46 ligands was similar following either oHSV infection or treatment with TMZ. These findings suggest that the ligands for NKp30 and NKp46 are of cellular origin. Once the identity of the NKp30 and NKp46 ligand is uncovered, future work will investigate whether they can predict sensitivity to oncolysis for personalized treatment.
Since NCR expression is coordinated as either NCRbright or NCRdim 50, future clinical studies should examine the NCR phenotype following oHSV treatment and assess if their expression correlates with diminished viral replication. By clarifying the critical receptor—ligand interactions that mediate human NK killing of oHSV infected glioblastoma, we have uncovered a potential therapeutic target that, when blocked in vivo, could limit NK clearance of these infected cells in future clinical trials.
Online Materials and Methods for NKp30 and NKp46 natural cytotoxicity receptors impede oncolytic virotherapy of glioblastoma
Cell culture
We used human glioblastoma cell lines (U87dEGFR, U251, and Gli36dEGFR), primary human glioblastoma cells enriched for stem-like cell properties (X121), mouse glioblastoma cell lines (KR158dEGFR2 and 4C83), and African green monkey kidney (Vero) cells. We cultured cells in Dulbeco’s Modified Eagle’s Medium (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (100 U ml−1), and streptomycin (100 mg ml−1). We cultured human and mouse derived NK cells in RPMI-1640 (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (100 U ml−1), and streptomycin (100 mg ml−1). We cultured cells at 37 °C supplemented with 5% CO2.
Animal studies
We anesthetized athymic, C57BL/6 (Charles River Laboratories), B6.129S7-Ifngtm1Ts/J (Ifng−/−), B6D2F1/J mice, and NOD-scid IL2Rgnull (SCID gc−/−) 8 week old, female mice4 (The Jackson Laboratory) with intraperitoneal ketamine (100 mg kg−1)/xylazine (20 mg kg−1) and stereotactically injected glioblastoma cells into the right frontal lobe (2 mm lateral and 1 mm anterior to bregma at a depth of 3 mm). We injected a total of 1 × 105 human U87dEGFR, 4 × 105 mouse KR158dEGFR, and 2 × 105 mouse 4C8 glioblastoma cells. For NK cell depletion experiments, either 200 mg of TMβ15 or 50 ml asialo-GM1 antibody combined with 50 ml water (Wako; 986-10001) was injected intraperitoneally two days prior to oHSV administration. For NK1.1 depletion, we injected 200 mg of antibody (PK136) intravenously four days before, one day before, and two days after virus inoculation6. We allowed U87dEGFR cells to grow for 9 days, the KR158dEGFR cells for 10 days, and the 4C8 cells for 21 days. At these times, we injected animals intratumorally with either rQNestin34.5 or vehicle. For experiments with WT HSV1 (F Strain), we injected 104 plaque forming units of virus into the right frontal lobe.
Adoptive transfer experiments
For adoptive transfer into Ifng−/− mice, we prepared donor NK cells using 8-week old Ly5.1 WT and Ly5.2 Ifng−/− mice. We enriched NK cells from splenocytes using NK Cell Isolation Kit (Miltenyi Biotech) and we purified these by fluorescence activated cell sorting (FACS) by sorting CD3-FITC (17A2) and NK1.1-PE (PK136) (BD) with antibody dilutions of 1:200. We used Ly5.2 Ifng−/− mice as recipients that received 2.2 million purified NK cells per mouse from either Ly5.1 WT or Ly5.2 Ifng−/− mice, 4 hours after 4 Gray (Gy) irradiation (RS 2000 X-Ray Irradiator, Rad Source Technologies, Inc.). For adoptive transfer into SCID γc−/− mice, we treated splenocytes from Ncr1−/− 7 and C57BL/6 WT with NK Negative Selection Cocktails (Miltenyi Biotech) and MACS LS Column purification. We then transplanted the isolated NK cells into irradiated SCID γc−/− mice (1.5 Gy dose) with 5 million cells per mouse. We then implanted tumors, 4 weeks after transplantation.
Flow cytometric analysis
We isolated mononuclear cells from oHSV-infected brains using a previously described procedure8. For analysis of splenocyte-derived NK levels, we collected and homogenized tissues through a 70 mm strainer. We lysed erythrocytes using RBC Lysis Buffer (Biolegend). We treated cells, isolated from either the brain or the spleen, with Fc Block (BD). We then stained cells with mouse-specific immune cell surface markers for 30 min at 4 °C. We utilized the following mouse-specific antibodies at a dilution of 1:200: CD3-FITC (17A2), CD49b-PE (DX5), CD3-PercP (145-2C11), CD62L-APC (MEL-14), CD11b-PE (M1/70), CD27-PE (LG.3A10), and Nos2-FITC (6/iNOS/NOS Type II) (BD); CD62L-FITC (MEL-14), CD69-FITC (H1.2F3), NKG2D-APC (CX5), CD27-FITC (LG.7F9), Ly49d-APC (4E5), CD94-FITC (18d3), NKp46-FITC (29A1.4), CD127-FITC (A7R34), CD117-FITC (2B8), NKG2A-FITC (20d5), MHCII-FITC (M5/114.15.2), CD86-PercP (GL1), CD11b-PercP (M1/70), CD3-APC (17A2), CD45-APC (30-F11), Tnf-FITC (MP6-XT22), CD107a-FITC (1D4B) (eBioscience); Ly-6c-FITC (HK1.4) (Biolegend); and CD49b-APC (DX5) (Miltenyi Biotec). For CD107a staining, we cultured mononuclear cells in 10% RPMI with monensin (eBioscience) for 4 hours prior to cell surface staining. For Tnf staining, we cultured mononuclear cells in 10% RPMI with Golgi-Plug (BD) for 4 hours prior to intracellular staining. For staining of both Tnf and Nos2, we followed initial surface staining with cytofix/cytoperm (BD) and finally with intracellular staining. A FACS Calibur (Becton Dickinson) was used for analysis.
Viral yield assay
We measured titers of infectious virus particles recovered from virus inoculated brains as follows. We inoculated athymic mice bearing 9 day old U87dEGFR tumors intratumorally with 5 × 105 plaque forming units of rQNestin34.5. C57BL/6 or Ncr1−/− mice were intracranially inoculated with 104 WT HSV. Seventy-two hours later, we recovered titers of infectious virus particles, as described9.
Quantitative real-time reverse transcriptase PCR
We isolated total RNA from tumor bearing hemispheres using the RNeasy Lipid Tissue Midi kit (Qiagen) with quantitative real-time PCR performed as previously described using SYBR Green PCR Master Mix and an ABI PRISM 7500 sequence detection system (Applied Biosystems)10. We used the following primers: 5’-GAACGGAGATCAAACCTGCCT-3’ and 5’-TGTAGTCTTCCTTGAACGACGA-3’ for Cxcl9; 5’-TGGAGGAACTGGCAAAAGGA-3’ and 5’-TGTTGCTGATGGCCTGATTG-3’ for Ifng; 5’-CAGCTGGGCTGTACAAACCTT-3’ and 5’-CATTGGAAGTGAAGCGTTTCG-3’ for Nos2; 5’-CATATGGAATCCAACTGGATAGATGTAAGATA-3’ and 5’-CATATGCTCGAGGGACGTGTTGATGAACAT-3’ for Il15; 5’AAGCCTGTAGCCCACGTCGTA-3’ and 5’-GGCACCACTAGTTGGTTGTCTTTG-3’ for Tnf; 5’- TGAATCCGGAATCTAAGACCATCAA-3’ and 5’-AGGACTAGCCATCCACTGGGTAAAG-3’ for Cxcl10; 5’-GGCTGCGACAAAGTTGAAGTGA-3’ and 5’-TCCTGGCACAGAGTTCTTATTGGAG-3’ for Cxcl11; and 5’- AAATGGTGAAGGTCGGTGTG-3’ and 5’-TGAAGGGGTCGTTGATGG-3’ for GAPDH internal control. We sorted brain leukocytes with antibodies to mouse CD45-APC and CD11b-PE using FACS (BD FACSAria). We isolated total RNA from twenty-thousand cells of each population with RNeasy Micro kit (Invitrogen) and analyzed by quantitative real-time RT-PCR. To assess inflammatory gene expression, we used Mouse Inflammatory Cytokines & Receptors RT2 Profiler PCR Array (Super Array Bioscience Corporation) following the manufacturer’s instructions.
NK cell isolation
We enriched NK cells or CTLs from peripheral blood leukopacks of healthy donors (American Red Cross, Columbus, Ohio) using RosetteSep cocktail (StemCell Technologies) and either CD56 or CD8 magnetic bead sorting (Miltenyi Biotec). Mouse NK cells were isolated from mouse splenocytes using positive selection bead sorting for DX5.
Cytotoxicity assay
We plated a panel of human glioblastoma cells overnight and infected them the following day with rQNestin34.5 (MOI 1.0) or mock. Eight hours later, we added human NK cells or CTLs at different effector:target ratios in the presence of human IL-15 (for NK cells) or IL-2 (for CTLs) (Miltenyi Biotec). The co-culture was allowed to proceed for 4 hours at 37 °C. We assessed glioblastoma lysis with Vybrant Cytotoxicity Assay Kit (Molecular Probes). For studies evaluating pharmacological inhibitors of cytolysis, we preincubated NK cells for 1 hour with: EGTA/Mg2+ (2mM/4mM) or Chloroquine (100 mg mL−1). For NCR masking experiments, we preincubated NK cells for 1 hour with the appropriate blocking antibody. We treated human NK cells with the following blocking antibodies at 10 mg mL−1: DNAM-1 (F5), NKp30 (F252), NKp44 (KS38), NKp46 (KL247), NKG2D (BAT221)11. Mouse NK cells were treated with blocking antibody against mouse NKp46 (BAB281)12. We then added NK cells to infected or mock infected glioblastomas and co-cultured them for 4 hours at 37 °C.
Analysis of NK ligand expression
We used recombinant human NKp30-IgG or NKp46-IgG fusion proteins (R & D Systems) to investigate the expression of NK ligands on glioblastoma cells, 8 hours following oHSV infection, or following treatment with temozolomide (200 mM), or with 10 Gray radiation, or with hypoxia (1% O2, 5% CO2). We stained for NK ligands by the addition of 10 μl of reconstituted IgG-fusion proteins followed by incubation with APC-conjugated mouse antibody to human IgGfc (Jackson ImmunoResearch) at a 1:100 dilution13. We also analyzed the expression of B7-H6 following oHSV infection of glioblastoma. Eight hours after infection, we also tested B7-H6 expression with 10 mg mL−1 of mouse antibody to human B7-H6 (gift from ZymoGenetics). We assessed cell surface staining of oHSV infected or mock infected glioblastoma with the following human-specific antibodies at a dilution of 1:10: MICA/B-PE (159207), ULBP-1-PE (170818), ULBP-2-PE (165903), CD155-PE (300907) (R&D); HLA-ABC-PE (W6/32) (eBioscience); and CD48-PE (BJ40), CD112-PE (TX31) (Biolegend).
Statistical analysis
All statistical tests were two sided. We used ANOVA models or t-tests to study group differences for all the continuous outcome variables. Chi-square test was used to compare counts or proportions. We used log-rank tests to compare group effects on time to event variables. Total NK and % NK were log transformed (natural log) to achieve normality. We used two-way ANOVA models to study the association between groups (U87dEGFR+vehicle, U87dEGFR+rQNestin34.5, and U87dEGFR+Inactivated rQNestin34.5), time and total or % NK. The interaction term treatment x time was also included in the models. The interaction term was significant in the model for Figure 1b and Supplementary figure 1a, and this is due to the larger slope or rate of increase in total and % NK, respectively, across time observed in the U87dEGFR+rQNestin34.5 group. We performed pairwise comparisons when the group main effect was significant in the ANOVA model (Fig. 1b, and Suppl. Fig. 1a). We used two sample t-tests to compare outcome variables between groups (Fig. 1c; Suppl. Fig. 1c; Suppl. Table 1a, b; Suppl. Fig. 2b, c; Suppl. Table 2a, b; Fig. 3b, 3d, 3e, 3g, 3i and 3j). We used two-way ANOVA to compare the NK fold increase (natural log) across different phenotypes and different groups. The interaction term was included and found to be highly significant (Fig. 2c). We compared percent glioblastoma lysis using pairwise comparisons (Chloroquine, EGTA/Mg vs untreated) and found to be significant (Suppl. Fig. 6a). We used chi square tests (3 by 2 tables) to study the differences in cell type proportions between groups (treated, untreated) at each time point (6, 24 and 72 hours).
Supplementary Material
Acknowledgements
This work was supported by US National Institutes of Health grants 7U01NS061811 (to E.A.C.), CA069246 (to E.A.C.), CA68458 (to M.A.C.), and CA98472 (to M.A.C.), and TL1RR025753 (to C.A.A.-B.). C.A.A.-B. was supported by an American Medical Association Foundation Seed Grant. This work was also supported by the Dardinger Neuro-oncology Laboratory.
Footnotes
Author Contributions C.A.B., J.Y., R.P., J.W., J.P., H.M, M.W., Y.W., S.H., J.H. performed experiments; C.A.B, J.Y., B.K., S.L., A.M., M.A.C., E.A.C conceived the experimental approach, directed experiments, and interpreted data; S.A.F. performed statistical analysis; E.V., O.M, A.M. provided reagents; C.A.B., J.Y., M.A.C., E.A.C. wrote the manuscript.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 3.Markert JM, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre- and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- 5.Fulci G, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda K, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 7.Fulci G, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurozumi K, et al. Effect of Tumor Microenvironment Modulation on the Efficacy of Oncolytic Virus Therapy. J Natl Cancer Inst. 2007 doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 9.Friedman A, Tian JP, Fulci G, Chiocca EA, Wang J. Glioma virotherapy: effects of innate immune suppression and increased viral replication capacity. Cancer Res. 2006;66:2314–2319. doi: 10.1158/0008-5472.CAN-05-2661. [DOI] [PubMed] [Google Scholar]
- 10.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altomonte J, et al. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2008 doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varghese S, Rabkin SD, Nielsen PG, Wang W, Martuza RL. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin Cancer Res. 2006;12:2919–2927. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- 14.Farrell CJ, et al. Combination immunotherapy for tumors via sequential intratumoral injections of oncolytic herpes simplex virus 1 and immature dendritic cells. Clin Cancer Res. 2008;14:7711–7716. doi: 10.1158/1078-0432.CCR-08-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellums EK, et al. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005;7:213–224. doi: 10.1215/S1152851705000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestwich RJ, et al. The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon. Hum Gene Ther. 2009;20:1119–1132. doi: 10.1089/hum.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanford MM, Breitbach CJ, Bell JC, McFadden G. Innate immunity, tumor microenvironment and oncolytic virus therapy: friends or foes? Curr Opin Mol Ther. 2008;10:32–37. [PubMed] [Google Scholar]
- 18.Errington F, et al. Reovirus Activates Human Dendritic Cells to Promote Innate Antitumor Immunity. J Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 19.Prestwich R, et al. Reciprocal Human Dendritic Cell-Natural Killer Cell Interactions Induce Antitumor Activity Following Tumor Cell Infection by Oncolytic Reovirus. J Immunol. 2009 doi: 10.4049/jimmunol.0901074. [DOI] [PubMed] [Google Scholar]
- 20.Kottke T, et al. Use of Biological Therapy to Enhance Both Virotherapy and Adoptive T-Cell Therapy for Cancer. Mol Ther. 2008 doi: 10.1038/mt.2008.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottke T, et al. Improved systemic delivery of oncolytic reovirus to established tumors using preconditioning with cyclophosphamide-mediated treg modulation and interleukin-2. Clin Cancer Res. 2009;15:561–569. doi: 10.1158/1078-0432.CCR-08-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derubertis BG, et al. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 2007;14:590–597. doi: 10.1038/sj.cgt.7701053. [DOI] [PubMed] [Google Scholar]
- 23.Chisholm SE, Howard K, Gómez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195:1160–1168. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- 24.Kambara H, Okano H, Chiocca EA, Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 25.Aghi M, Visted T, Depinho RA, Chiocca EA. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene. 2008;27:4249–4254. doi: 10.1038/onc.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 27.Dalton DK, et al. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino H, et al. Natural killer cell depletion by anti-asialo GM1 antiserum treatment enhances human hematopoietic stem cell engraftment in NOD/Shi-scid mice. Bone Marrow Transplant. 2000;26:1211–1216. doi: 10.1038/sj.bmt.1702702. [DOI] [PubMed] [Google Scholar]
- 29.Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol. 2008;20:52–60. doi: 10.1016/j.coi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savarin C, Bergmann CC. Neuroimmunology of central nervous system viral infections: the cells, molecules and mechanisms involved. Curr Opin Pharmacol. 2008;8:472–479. doi: 10.1016/j.coph.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiner NE, et al. A syngeneic mouse glioma model for study of glioblastoma therapy. J Neuropathol Exp Neurol. 1999;58:54–60. doi: 10.1097/00005072-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Taylor MA, Ward B, Schatzle JD, Bennett M. Perforin- and Fas-dependent mechanisms of natural killer cell-mediated rejection of incompatible bone marrow cell grafts. Eur J Immunol. 2002;32:793–799. doi: 10.1002/1521-4141(200203)32:3<793::AID-IMMU793>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 33.Trotta R, et al. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- 34.Castriconi R, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 35.Wu A, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
- 36.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009 doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 38.Altomonte J, et al. Exponential Enhancement of Oncolytic Vesicular Stomatitis Virus Potency by Vector-mediated Suppression of Inflammatory Responses In Vivo. Mol Ther. 2008;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galivo F, et al. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum Gene Ther. 2010;21:439–450. doi: 10.1089/hum.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galivo F, et al. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010;17:158–170. doi: 10.1038/gt.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marques CP, Hu S, Sheng W, Lokensgard JR. Microglial cells initiate vigorous yet non-protective immune responses during HSV-1 brain infection. Virus Res. 2006;121:1–10. doi: 10.1016/j.virusres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Yu J, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK cell subsets. Blood. 2009 doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundberg P, et al. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J Virol. 2003;77:11661–11673. doi: 10.1128/JVI.77.21.11661-11673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Bloushtain N, et al. Membrane-associated heparan sulfate proteoglycans are involved in the recognition of cellular targets by NKp30 and NKp46. J Immunol. 2004;173:2392–2401. doi: 10.4049/jimmunol.173.4.2392. [DOI] [PubMed] [Google Scholar]
- 46.Ferlazzo G, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnon TI, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 48.Degli-Esposti MA, Smyth MJ. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat Rev Immunol. 2005;5:112–124. doi: 10.1038/nri1549. [DOI] [PubMed] [Google Scholar]
- 49.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Augugliaro R, et al. Selective cross-talk among natural cytotoxicity receptors in human natural killer cells. Eur J Immunol. 2003;33:1235–1241. doi: 10.1002/eji.200323896. [DOI] [PubMed] [Google Scholar]
- 51.Giannini C, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 53.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 54.Yu J, et al. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J Clin Invest. 121:1456–1470. doi: 10.1172/JCI43242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45:33–45. doi: 10.1016/s0166-3542(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 56.Gazit R, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 57.Marques CP, et al. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitale M, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 59.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Giannini C, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 3.Hellums EK, et al. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005;7:213–224. doi: 10.1215/S1152851705000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shultz LD, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, et al. NKp46 identifies an NKT cell subset susceptible to leukemic transformation in mouse and human. J Clin Invest. 121:1456–1470. doi: 10.1172/JCI43242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 2000;45:33–45. doi: 10.1016/s0166-3542(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 7.Gazit R, et al. Lethal influenza infection in the absence of Therapy natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 8.Marques CP, et al. Prolonged microglial cell activation and lymphocyte infiltration following experimental herpes encephalitis. J Immunol. 2008;181:6417–6426. doi: 10.4049/jimmunol.181.9.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulci G, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurozumi K, et al. Effect of Tumor Microenvironment Modulation on the Efficacy of Oncolytic Virus Therapy. J Natl Cancer Inst. 2007 doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- 11.Vitale Mv, et al. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 12.Walzer T, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu A, et al. Expression of MHC I and NK ligands on human CD133+ glioma cells: possible targets of immunotherapy. J Neurooncol. 2007;83:121–131. doi: 10.1007/s11060-006-9265-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.