Abstract
Background
The overall survival for neuroblastoma remains dismal, largely in part, due to emergence of resistance to chemotherapeutic drugs. We have demonstrated that gastrin-releasing peptide (GRP), a gut peptide secreted by neuroblastoma, act as an autocrine growth factor. We hypothesized that knockdown of GRP will induce apoptosis in neuroblastoma cells to potentiate the cytotoxic effects of chemotherapeutic agents.
Methods
The human neuroblastoma cell lines (JF, SK-N-SH) were transfected with small interfering (si) RNA targeted at GRP. Apoptosis was assessed by DNA fragmentation assay. Immunoblotting was used to confirm molecular markers of apoptosis, and flow cytometry was performed to determine cell cycle arrest after GRP knockdown.
Results
siGRP resulted in an increase in apoptosis in the absence of chemotherapeutic interventions. A combination of GRP silencing and chemotherapeutic drugs resulted in enhanced apoptosis when compared to either of the treatment alone. GRP silencing led to increased expression of proapoptotic proteins, p53 and p21.
Conclusions
Silencing of GRP induces apoptosis in neuroblastoma cells; it acts synergistically with chemotherapeutic effects of etoposide and vincristine. GRP knockdown-mediated apoptosis appears to be associated with upregulation of p53 in neuroblastoma cells. Targeting GRP may be postulated as a potential novel agent for combinational treatment to treat aggressive neuroblastomas.
Introduction
Neuroblastoma is the most common extracranial solid tumor in infants and children. Despite advances in multi-modality therapy, survival rates for all stages remain a dismal 50%, and therefore, novel therapeutic options are needed to improve patient outcomes. Acquisition of chemo-resistance represents a significant issue concerning the failure to achieve long-term survival in the treatment of neuroblastoma.1 Failure to respond to conventional chemotherapy may indicate a shift to the malignant phenotype of the disease, and may impose altered molecular regulation involving apoptosis and cell cycle regulation signaling pathways.2 Hence, novel molecular approaches that upregulate apoptotic pathways in neuroblastoma cells may potentiate the effect of existing anticancer drugs, such as vincristine and etoposide; this would allow for use of lower dosages, thus minimizing potentially serious complications that are associated with chemotherapeutic agents.
Vincristine is a vinca alkaloid that disrupts microtubule assembly and arrests cells in metaphase, preventing cell replication. It is part of an arsenal of chemotherapeutic agents commonly used to treat solid tumors based on its mechanism of action to induce apoptosis, which is, in part, is mediated by the inactivation of Raf1/MEK/ERK cascade.3 Some of the unwarranted side effects of vincristine include neuropathy.4 Etoposide, an epipodophyllotoxin, interferes with topoisomerase II activity and arrests cell division in the late S-G2 phase of the cell cycle; it induces a caspase-3-dependent apoptosis in neuroblastoma cells.5 Although etoposide is highly cytotoxic for neuroblastoma, the side effects as a result of myelosuppression makes it dose limiting in the treatment of this childhood cancer.
One of the hallmarks of neuroblastoma is increased cell survival and evasion of apoptosis; this is partly attributed to the synthesis and response to various growth factors and cytokines. Gastrin-releasing peptide (GRP), the mammalian equivalent of bombesin, is both a gut peptide and a neuropeptide that has the potential to induce mitogenic response in normal as well as various cancer cell types of intestine, lung, pancreas, breast, and prostate.6 We have previously shown that the GRP is notably increased in undifferentiated human neuroblastomas when compared to its benign counterpart, ganglioneuromas.7 Moreover, we have also demonstrated that GRP treatment induces G1-S phase cell cycle progression in neuroblastoma cells.8 Suppression of GRP activity with cell surface receptor antagonists or neutralizing antibodies has been shown to inhibit growth9, 10 and induce apoptosis in cancer cells;11, 12 however, the molecular mechanisms involved in the inhibition of neuroblastoma cell proliferation upon GRP downregulation are not known. Furthermore, combining conventional chemotherapy with GRP receptor antagonist appears to significantly enhance cancer cell death by a mechanism termed as “receptor enhanced chemosensitivity”.13 Therefore, the purpose of our current investigation was to demonstrate and elucidate, in broader detail, the mechanism by which GRP inhibition induces apoptosis and potentiates the cytotoxic effects of chemotherapeutic drugs in the treatment of aggressive, refractory neuroblastomas.
In this study, we report that silencing of GRP induced apoptosis in neuroblastoma cell lines of JF and SK-N-SH, when administered alone or in combination with chemotherapeutic drugs, vincristine and etoposide. Moreover, GRP silencing decreased cell proliferation and induced cell cycle exit followed by apoptosis in the neuroblastoma cells. We also observed, at the molecular level, that p53 and its downstream target p21 are upregulated by GRP knockdown, leading to a decreased activation of cell proliferation regulator, ERK. Our findings demonstrate that silencing of GRP promotes apoptosis in neuroblastoma cells and enhances the cytotoxic effects of chemotherapeutic agents by activation of p53-mediated cell death mechanisms.
Materials and Method
Materials
Cleaved caspase-3 antibodies, cleaved PARP antibodies and cell lysis buffer were obtained from Cell Signaling Technology (Beverly, MA). Anti β-actin monoclonal antibody and fetal bovine serum (FBS) were from Sigma (St. Louis, MO). NuPAGE Novex 4–12% Bis–Tris Gel and Lipofectamine 2000 were purchased from Invitrogen (Carlsbad, CA). Horseradish Peroxidase (HRP)-conjugated secondary antibodies against mouse and rabbit IgG were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Cell Death Detection ELISAPlus was purchased from Roche Applied Science (Indianapolis, IN).
Cell Culture
Human neuroblastoma cell line, SK-N-SH, was purchased from American Type Culture Collection (Manassas, VA). JF, a primary neuroblastoma cell line established from resected tumor, was a gift from Dr. Jason M. Shohet (Baylor College of Medicine, Houston, TX). Cells were maintained in RPMI 1640 medium with L-glutamine (Cellgro Mediatech, Inc. Herndon, VA) supplemented with 10% FBS. The cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Small Interfering (si) RNA Transfection
siRNA against GRP (siGRP) and non-targeting control (siNTC) were purchased from Dharmacon, Inc (Lafayette, CO). Transient transfection was carried out with Lipofectamine 2000 transfection reagent according to the manufacturer's protocol. Cells were seeded on 6-well plates for RNA or protein preparation and 96-well plates for DNA fragmentation or cell growth assays. After 24 h incubation, media were replaced to serum-free RPMI 1640 containing siRNA (150 nM) and transfection reagent. Cells were harvested for assays daily for three consecutive days after transfection with the siRNA duplexes. The experiments were repeated on at least three separate occasions.
RNA Isolation and Real-Time RT-PCR
The total cellular RNA extraction was carried out using RNAqueous kit (Ambion, Inc., Austin, TX) according to manufacturer's instructions. Applied Biosystems assays-on-demand 20× assay mix of primers and TaqMan MGB probes (FAM™ dye-labeled) for target gene, human GRP (NCBI Accession No. NM_002091 [GenBank]), and pre-developed 18S rRNA (VIC™-dye labeled probe) TaqMan® assay reagent (P/N 4319413E) for endogenous control were utilized. Singleplex one-step reverse transcription (RT)-PCR was performed with 80 ng RNA for both target gene and endogenous control. The reagent used was TaqMan one-step RT-PCR master mix reagent kit (P/N 4309169). The probe for GRP was purchased from Applied Biosystems Inc., CA. The cycling parameters for one-step RT-PCR were as follows: reverse transcription 48°C for 30 min, AmpliTaq activation 95°C for 10 min, denaturation 95°C for 15 s, and annealing/extension 60°C for 1 min (repeat 40×) on ABI7000. Duplicate CT values were analyzed in Microsoft Excel using the comparative CT (ΔΔCT) method as described by the manufacturer (Applied Biosystems). The amount of target (2-ΔΔCT) was obtained by normalized to endogenous reference (18 s) and relative to a calibrator (one of the experimental samples).
Western Blot Analysis
Whole-cell lysates were prepared using cell lysis buffer with 1 mM PMSF and incubated on ice for 30-60 min. Total protein (50 mg/lane) was resolved on NuPAGE Novex 4-12% Bis–Tris gels and electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA). Nonspecific binding sites were blocked with 5% milk in TBST (120 mM Tris–HCl, pH 7.4, 150 mM NaCl, and 0.05% Tween 20) for 1 h at room temperature or overnight at 4 °C. Target proteins were detected by using rabbit or mouse anti-human antibodies (1:500-1000 dilution) for 3 h at room temperature or overnight at 4°C. The membranes were washed three times and incubated with secondary antibodies (1:5000 dilution) conjugated with HRP. Immune complexes were visualized using the enhanced chemiluminescence system (Amersham Biosciences, Arlington, IL). Equal loading and transfer were confirmed by blotting the same membrane with β-actin antibody (1:5000 dilution). Data are representative of three independent experiments with nearly identical results.
DNA Fragmentation Assay
Apoptosis was measured using a DNA fragmentation assay as previously described.14 Briefly, cells (100 μl; 5-10 × 103 cells/well) were plated in triplicate 24 h before transfection. Cells were then treated with siRNA for control (NTC) or GRP for 48 and 72 h. Cytoplasmic histone-associated DNA fragments (mono- and oligonucleosomes) were detected using a Cell Death Detection ELISAplus kit according to manufacturer's recommended protocol. The experiments were repeated on at least three separate occasions.
Cell Proliferation Assay
Cells were seeded in 96-well plates at a density of 5-10 × 103 cells/well in RPMI 1640 culture medium with 10% FBS and grown for up to 3 days after transfection. Cell numbers were assessed by using Cell-Counting Kit-8 (Dojindo Molecular Technologies, Inc., Gaithersburg, MD) daily. Each assay point was performed in triplicate, and the experiment was repeated three times for each cell line. The values, corresponding to the number of viable cells, were read at OD450 with EL808 Ultra Microplate Reader (Bio Tek Instrument, Inc., Winooski, VT).
Cell Cycle Analysis
Cell cycle distribution was analyzed using flow cytometry. Cells were trypsinized, washed once with PBS, and fixed in 70% ethanol. Fixed cells were washed with PBS, incubated with 100 mg/ml RNAse for 30 min at 37°C, stained with Propidium Iodide (50 mg/ml) and approximately 1 × 106 cells were analyzed on a 5-laser BD LSRII. The percentages of cells in different cell cycle phases were analyzed using FACSDiva version 6.1.3.
Statistical Analysis
Scoring index, relative DNA fragmentation, relative mRNA expression, and cell proliferation were expressed as means ± SEM; statistical analyses were performed using one-way analysis of variance for comparisons between the treatment groups. A P value of <0.05 was considered significant.
Results
GRP Transcript Depletion by GRP siRNA
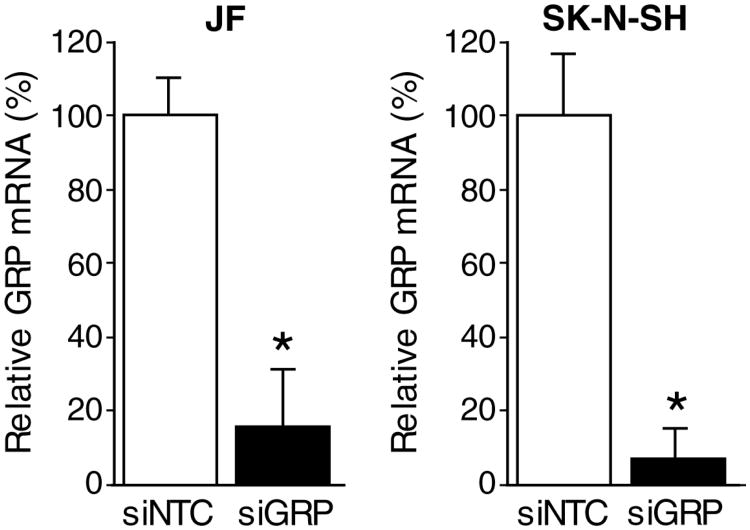
To examine the effects of GRP siRNA on GRP mRNA expression, we used constitutively GRP-amplified (JF and SK-N-SH) human neuroblastoma cell lines. To assess for the reduction in gene expression, cells were treated with siGRP or siNTC over a time course (24, 48, and 72 h) and total cellular RNA was extracted for analysis of GRP transcripts by real-time RT-PCR. As shown in Fig. 1, siGRP resulted in significant GRP mRNA reduction of approximately 80-90% in both JF and SK-N-SH cells after 48 h treatment indicating the specificity of siGRP in our study. Comparable results were also observed at 24 and 72 h post treatment (data not shown).
Figure 1. Knockdown of GRP expression using siRNA in GRP-amplified neuroblastoma cell lines.
JF and SK-N-SH cells were transfected with siGRP or siNTC, and gene expression was analyzed using RT-PCR. GRP expression was effectively silenced with siGRP when compared to controls (siNTC) in both cell lines examined (mean ± SEM; * = p < 0.05 vs. siNTC).
GRP Silencing Induces Apoptosis
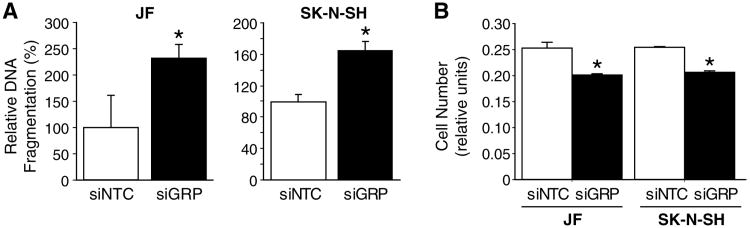
Since we have previously demonstrated that GRP acts as a mitogen in human neuroblastoma cell lines, we next examined the effect of GRP inhibition by siGRP on cell growth and viability. A remarkable increase in apoptosis was detected in both human neuroblastoma cell line, JF and SK-N-SH, at 48 h after siGRP treatment, as measured by levels of DNA fragmentation, a hallmark of apoptosis (Fig. 2A). Increases in apoptosis were noted to a maximum of 2.5 fold change. Conversely, siGRP also significantly decreased neuroblastoma cell proliferation, as measured by the Cell Counting Kit-8 (CCK-8) (Fig. 2B), thus demonstrating dual cellular effects of siGRP on proapoptotic as well as anti-proliferative responses in neuroblastoma cells.
Figure 2. GRP silencing-induced apoptosis.
(A) Cells treated with siGRP exhibited an increase in apoptosis in comparison to control cells (siNTC). (B) GRP silencing (siGRP) resulted in a significant decrease in cell proliferation when compared to control cells. Apoptosis and cell proliferation were analyzed using Cell Death ELISA and CCK-8, respectively (mean ± SEM; * = p < 0.05 vs. siNTC).
Vincristine- and Etoposide-Induced Apoptosis
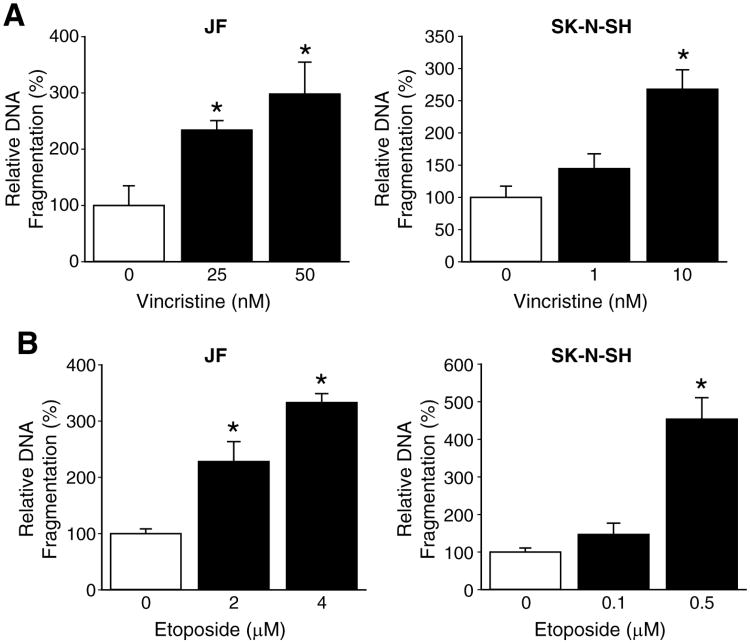
Chemotherapeutic drugs exert lethality on tumor cells by induction of apoptosis. So, we determined apoptotic dose-response curves of two commonly used chemotherapeutic agents, vincristine and etoposide. Human neuroblastoma cell lines, JF and SK-N-SH, were treated for 48 h with varying dosages of vincristine and etoposide. Treatment with chemotherapeutic drugs resulted in significant cell death of JF and SK-N-SH cells in a dose-dependent manner. The lowest dosages of vincristine that produced significant apoptosis were determined to be 25 nM and 1 nM for JF and SK-N-SH cells, respectively (Fig. 3A). Whereas, these values for etoposide were 2 μM for JF cells and 0.1 μM for SK-N-SH cells (Fig. 3B).
Figure 3. Chemotherapy-induced apoptosis.
A dose-response curve for the effects of chemotherapeutic agents on apoptosis was assessed using Cell Death ELISA for JF and SK-N-SH cells at 48 h time point. (A) The lowest effective dosage of vincristine was determined as 25 nM for JF cells and 1 nM for SK-N-SH cells. (B) The lowest effective dosage of etoposide was determined as 2 μM for JF cells and 0.1 μM for SK-N-SH cells (mean ± SEM; * = p < 0.05 vs. without treatment).
GRP Knockdown Enhances Chemotherapy-Induced Apoptosis
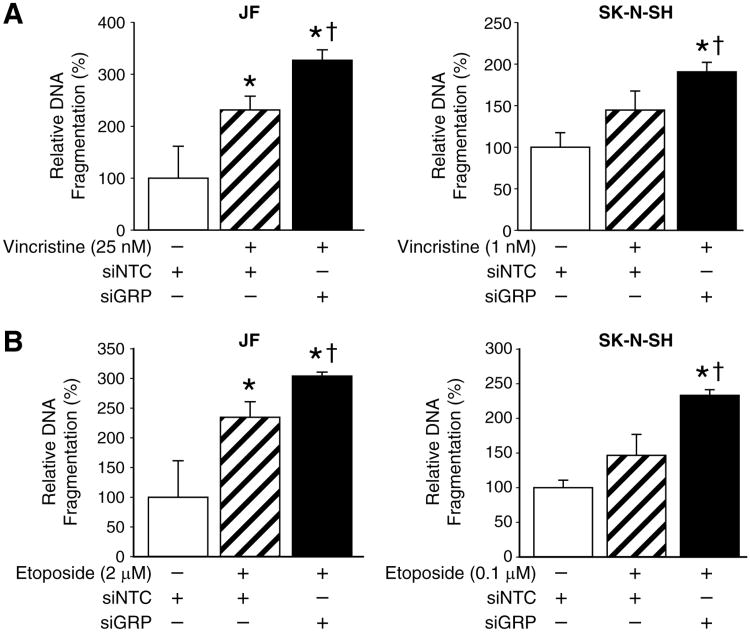
Individually, GRP inhibition and administration of chemotherapeutic drugs have the same end point – apoptosis of human neuroblastoma cells. Therefore, it is conceivable that GRP silencing could serve as an adjuvant therapy to chemotherapy. Hence, we combined the two approaches and measured the level of apoptosis in human neuroblastoma cell lines. JF and SK-N-SH cells were transfected with siGRP, and then subsequently exposed to the lowest dosages of either vincristine or etoposide that produced apoptosis. A significant augmentation in apoptosis was observed for the combination treatment using siGRP and either vincristine (Fig. 4A) or etoposide (Fig. 4B) when compared to chemotherapeutic agent alone. These findings suggest an important role for siGRP as an effective adjuvant therapy to be used in combination with lower concentrations of current chemotherapeutic regimen, thus potentially decreasing the incidence of chemotherapy-associated complications.
Figure 4. GRP knockdown enhances chemotherapy-induced apoptosis.
(A) Combination treatment with vincristine and siGRP resulted in augmentation of apoptosis in JF and SK-N-SH cells when compared to vincristine alone. (B) GRP silencing, in addition to treatment with etoposide, resulted in an increase in apoptosis in comparison to drug treatment alone in both JF and SK-N-SH cells (mean ± SEM; * = p < 0.05 vs. siNTC alone, †= p < 0.05 vs. siNTC plus drug).
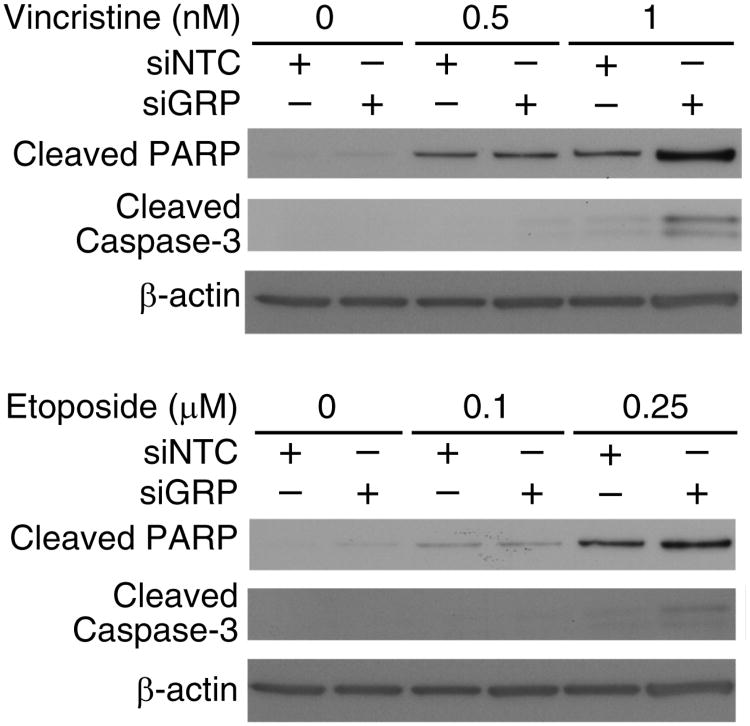
siGRP-Mediated Cleavage of PARP And Caspase-3
To further validate our hypothesis that GRP silencing results in human neuroblastoma cell death via an apoptotic pathway, we measured cleavage of PARP and caspase-3, as markers of apoptosis. SK-N-SH cells were transfected with siGRP, and then treated to varying concentration of either vincristine or etoposide for 48 h. siNTC transfected cells served as controls. As shown in Figure 5, dose-dependent increases in cleaved PARP protein levels were observed with both chemotherapeutic drugs; these effects were further enhanced when combined with GRP silencing. Similarly, siGRP also produced additive effects on caspase-3 activity, but only with higher dosages of vincristine and etoposide.
Figure 5. Activation of apoptotic pathway after siGRP treatment.
Combination treatment of SK-N-SH cells with chemotherapeutic drugs and siGRP resulted in increased cleavage of PARP and caspase-3 suggesting activation of apoptosis as the mechanism of cell death by GRP silencing. β-actin levels indicate equal sample loading.
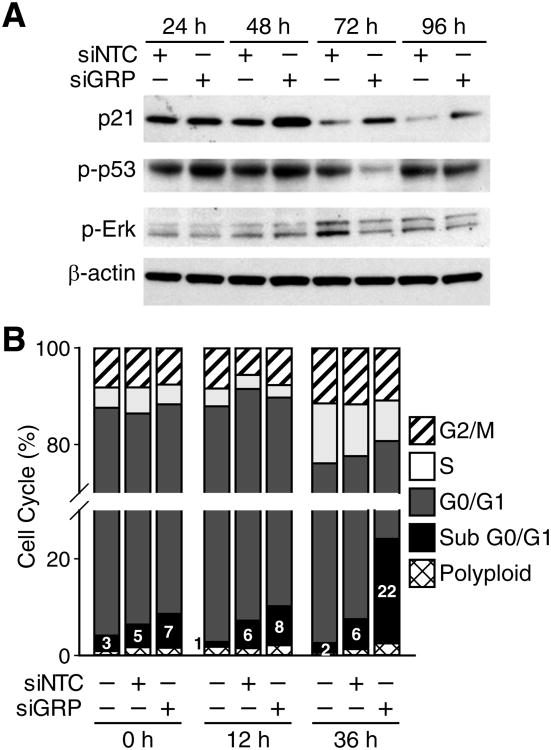
GRP Inhibition Blocks Cell Proliferation and Induces Cell Cycle Arrest
The mechanisms of action for the chemotherapeutic drugs, vincristine and etoposide, have been well elucidated. The cytotoxic effects of vincristine are associated with a cell cycle arrest in the G2/M phase and induction of apoptosis in target cells.3 On the other hand, studies have reported that etoposide induces apoptosis in neuroblastoma cells in caspase-dependent fashion and upregulation of p21.5, 15 In order to investigate the mechanism by which GRP silencing enhances the capacity of these drugs to induce apoptosis, we next examined cell cycle regulators, namely, p53, p21, and pERK, after siGRP treatment alone. As expected, GRP silencing led to increased expression of p53 and its transcriptional target p21 at 48 h after treatment in SK-N-SH cells (Fig. 6A). A delayed yet significant decrease in the expression of pERK was observed at 72 h after treatment. In order to determine the effects of GRP silencing on cell cycle progression, we also analyzed SK-N-SH cells using flow cytometry after siGRP treatment. A significant increase in the percentage of cells in apoptotic sub G0/G1 phase was observed when compared to control cells (Fig. 6B). This demonstrates that upon GRP silencing neuroblastoma cells undergo cell cycle exit, and subsequent apoptosis.
Figure 6. GRP silencing-induced apoptosis is mediated by p53 and G0-G1 cell cycle arrest.
(A) Treatment of SK-N-SH cells with siGRP over a time course (24-96 h) resulted in an increase in phosphorylation of p53 and its downstream target p21. A delayed decrease in phospho-ERK was observed at 72 and 96 h time points (B) siGRP or siNTC transfected SK-N-SH cells (1 × 106 cells/well) were plated and analyzed for cells in different phases of the cell cycle using flow cytometry. The percentage of cells in the apoptotic sub G0/G1 phase showed a significant increase after siGRP treatment.
Discussion
In this study, we show that specific, selective silencing of GRP by siRNA leads to increased apoptosis in human neuroblastoma cells that constitutively express high levels of GRP; cell death was strongly associated with attenuation of cell proliferation, and cell cycle arrest in the G0-G1 phase. Moreover, GRP knockdown enhanced the cytotoxic effects of chemotherapeutic drugs routinely used in the treatment of neuroblastomas. Increases in caspase-3 activation and p53 expression after treatment also further validated activation of apoptotic pathway. These observations underscore the significance of silencing GRP, and suggest a potential novel combinational treatment for refractory, chemoresistance neuroblastomas.
siRNA-directed gene silencing is a routinely used molecular tool in laboratories for down-regulating a target oncogene. Recent reports have shown effective in vitro delivery of siRNA in neuroblastoma cells.14 In the present study, we demonstrated that siGRP could be delivered efficiently in neuroblastoma cells to produce significant silencing of GRP mRNA and also its protein products (data not shown). Transient transfection of neuroblastoma cells with siGRP produced specific knockdown of GRP gene expression as early as 24 h; this effect was most notable at 48 h after transfection.
GRP and its equivalent bombesin act as autocrine growth factor to promote cell proliferation in various cancer cell types16, 17; these mitogenic effects of GRP on tumor cells have been well established. Similarily, we have previously shown that overexpression of GRP receptors increases the proliferative capacity of SK-N-SH human neuroblastoma cells.18 Moreover, we have also demonstrated that GRP treatment induces G1-S phase progression.8 In this study, we found that silencing of GRP in JF and SK-N-SH cell lines induced significant apoptosis. We also showed that cell proliferation and cell cycle progression are intricately related to GRP expression, as silencing of GRP led to inhibition of both of these processes. Our findings are consistent with reports published by others, where inhibition of GRP using GRP/Bombesin analogs inhibited cell proliferation in a variety of cancer cell types.19-21
Evaluating drug toxicities and mechanism of action becomes extremely important when designing treatment regimens; this is especially critical for patients with advanced-stage neuroblastoma with a potential to develop drug resistance to conventional chemotherapy. Microtubule-damaging agents, such as vincristine, induce apoptosis in cancer cells via inhibition of ERK/MAPK pathway. On the other hand, topoisomerase II inhibitors like etoposide activate a p53-dependent cell death mechanism in cancer cells.15 In the current study, we report that both of these chemotherapeutic agents induced apoptosis in human neuroblastoma cells. When the lowest effective dosages of either drug were combined with siGRP treatment, the level of apoptosis was enhanced in both cell lines examined when compared to either drug alone. Targeting neuroblastoma cells with simultaneous use of sublethal dose of chemotherapeutic drugs and GRP silencing has not been reported previously. Thus, our findings in this study suggest a potential novel regimen to overcome resistance in patients suffering with aggressive phenotypes of neuroblastomas.
Failure to activate apoptotic pathways in response to drug treatment as a result of development of chemoresistance has been one of the major hurdles in anticancer therapies. Previous studies have reported a correlation between silencing of caspase activation with that of chemoresistance in neuroblastoma patients with unfavorable outcomes.22, 23 Here, we demonstrated the activation of caspase-3 and PARP cleavage when JF and SK-N-SH cell lines were subjected to a combination treatment of chemotherapeutic drugs and GRP silencing, indicating additive effects on activation of apoptotic pathway. Consequently, induction of apoptosis via activation of caspase cascade upon treatment with siGRP would allow for potentially bypassing chemoresistance, and thus providing significant therapeutic benefit to patients with advanced-stage neuroblastomas.
Although previous reports suggest the presence of a wild-type p53 gene in neuroblastomas, the functional competence of this tumor suppressor protein in this form of pediatric cancer is controversial.24 It has been suggested that sequestration of p53 in the cytoplasm leads to an attenuated DNA-damage induced G1 arrest in neuroblastomas.25 Our data demonstrated that silencing of GRP leads to stabilization of p53 protein in neuroblastoma cells, and thus leading to activation of p21, a transcriptional target of p53. This finding is in agreement with reports by others on reactivation of p53 function in neuroblastomas.24, 26 However, contrary to previous reports, our cell cycle analysis data suggests an enhancement in cell cycle arrest followed by apoptosis in SK-N-SH cells after GRP silencing. Hence, we postulate that GRP inhibition acts synergistically and/or in parallel with the chemotherapeutic drugs to enhance G1 -arrest in neuroblastoma cells, thereby leading to the induction of apoptosis in these cells.
In summary, chemoresistance in patients with refractory neuroblastomas and toxicities associated with conventional chemotherapeutic drugs necessitates the need for novel therapeutics in advanced-stage neuroblastomas. Our findings from this study indicate that silencing of GRP, an autocrine growth factor for neuroblastoma, induces significant apoptosis, allowing for chemosensitization. This could potentially allow for use of lower, safer doses of conventional chemotherapeutic drugs in multi-regimen treatment modality for aggressive neuroblastomas.
Acknowledgments
We thank Karen Martin for her assistance with the manuscript preparation.
This work was supported by grant R01 DK61479 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chesler L, Goldenberg DD, Collins R, Grimmer M, Kim GE, Tihan T, et al. Chemotherapy-induced apoptosis in a transgenic model of neuroblastoma proceeds through p53 induction. Neoplasia. 2008;10:1268–74. doi: 10.1593/neo.08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelis M, Klassert D, Barth S, Suhan T, Breitling R, Mayer B, et al. Chemoresistance acquisition induces a global shift of expression of aniogenesis-associated genes and increased pro-angogenic activity in neuroblastoma cells. Mol Cancer. 2009;8:80. doi: 10.1186/1476-4598-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–5. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 4.Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr. 2009 doi: 10.1007/s12098-009-0254-3. [DOI] [PubMed] [Google Scholar]
- 5.Day TW, Wu CH, Safa AR. Etoposide induces protein kinase Cdelta- and caspase-3-dependent apoptosis in neuroblastoma cancer cells. Mol Pharmacol. 2009;76:632–40. doi: 10.1124/mol.109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel O, Shulkes A, Baldwin GS. Gastrin-releasing peptide and cancer. Biochim Biophys Acta. 2006;1766:23–41. doi: 10.1016/j.bbcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Hu W, Kelly DR, Hellmich MR, Evers BM, Chung DH. Gastrin-releasing peptide is a growth factor for human neuroblastomas. Ann Surg. 2002;235:621–9. doi: 10.1097/00000658-200205000-00003. discussion 9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishola TA, Kang J, Qiao J, Evers BM, Chung DH. Phosphatidylinositol 3-kinase regulation of gastrin-releasing peptide-induced cell cycle progression in neuroblastoma cells. Biochim Biophys Acta. 2007;1770:927–32. doi: 10.1016/j.bbagen.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Y, Halmos G, Cai RZ, Szoke B, Ertl T, Schally AV. Bombesin antagonists inhibit in vitro and in vivo growth of human gastric cancer and binding of bombesin to its receptors. J Cancer Res Clin Oncol. 1994;120:519–28. doi: 10.1007/BF01221028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J, Ishola TA, Baregamian N, Mourot JM, Rychahou PG, Evers BM, et al. Bombesin induces angiogenesis and neuroblastoma growth. Cancer Lett. 2007;253:273–81. doi: 10.1016/j.canlet.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Chen J, Zhong R, Mokotoff M, Shultz LD, Ball ED. Targeting gastrin-releasing peptide receptors on small cell lung cancer cells with a bispecific molecule that activates polyclonal T lymphocytes. Clin Cancer Res. 2006;12:2224–31. doi: 10.1158/1078-0432.CCR-05-1524. [DOI] [PubMed] [Google Scholar]
- 12.Stangelberger A, Schally AV, Letsch M, Szepeshazi K, Nagy A, Halmos G, et al. Targeted chemotherapy with cytotoxic bombesin analogue AN-215 inhibits growth of experimental human prostate cancers. Int J Cancer. 2006;118:222–9. doi: 10.1002/ijc.21292. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Chen J, Mokotoff M, Zhong R, Shultz LD, Ball ED. Bombesin/gastrin-releasing peptide receptor: a potential target for antibody-mediated therapy of small cell lung cancer. Clin Cancer Res. 2003;9:4953–60. [PubMed] [Google Scholar]
- 14.Kang JH, Rychahou PG, Ishola TA, Qiao J, Evers BM, Chung DH. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells. Biochem Biophys Res Commun. 2006;351:192–7. doi: 10.1016/j.bbrc.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brantley-Finley C, Lyle CS, Du L, Goodwin ME, Hall T, Szwedo D, et al. The JNK, ERK and p53 pathways play distinct roles in apoptosis mediated by the antitumor agents vinblastine, doxorubicin, and etoposide. Biochem Pharmacol. 2003;66:459–69. doi: 10.1016/s0006-2952(03)00255-7. [DOI] [PubMed] [Google Scholar]
- 16.Rozengurt E, Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983;80:2936–40. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander RW, Upp JR, Jr, Poston GJ, Gupta V, Townsend CM, Jr, Thompson JC. Effects of bombesin on growth of human small cell lung carcinoma in vivo. Cancer Res. 1988;48:1439–41. [PubMed] [Google Scholar]
- 18.Qiao J, Kang J, Cree J, Evers BM, Chung DH. Gastrin-releasing peptide-induced down-regulation of tumor suppressor protein PTEN (phosphatase and tensin homolog deleted on chromosome ten) in neuroblastomas. Ann Surg. 2005;241:684–91. doi: 10.1097/01.sla.0000161173.47717.71. discussion 91-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szepeshazi K, Schally AV, Nagy A, Halmos G. Inhibition of growth of experimental human and hamster pancreatic cancers in vivo by a targeted cytotoxic bombesin analog. Pancreas. 2005;31:275–82. doi: 10.1097/01.mpa.0000175892.97036.a7. [DOI] [PubMed] [Google Scholar]
- 20.Kanashiro CA, Schally AV, Zarandi M, Hammann BD, Varga JL. Alterations of EGFR/HER, angiogenesis and apoptosis pathways after therapy with antagonists of growth hormone releasing hormone and bombesin in non-small cell lung cancer. Int J Oncol. 2007;30:1019–28. [PubMed] [Google Scholar]
- 21.Zhang Q, Bhola NE, Lui VW, Siwak DR, Thomas SM, Gubish CT, et al. Antitumor mechanisms of combined gastrin-releasing peptide receptor and epidermal growth factor receptor targeting in head and neck cancer. Mol Cancer Ther. 2007;6:1414–24. doi: 10.1158/1535-7163.MCT-06-0678. [DOI] [PubMed] [Google Scholar]
- 22.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–90. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–35. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 24.McKenzie PP, Guichard SM, Middlemas DS, Ashmun RA, Danks MK, Harris LC. Wild-type p53 can induce p21 and apoptosis in neuroblastoma cells but the DNA damage-induced G1 checkpoint function is attenuated. Clin Cancer Res. 1999;5:4199–207. [PubMed] [Google Scholar]
- 25.Moll UM, Ostermeyer AG, Haladay R, Winkfield B, Frazier M, Zambetti G. Cytoplasmic sequestration of wild-type p53 protein impairs the G1 checkpoint after DNA damage. Mol Cell Biol. 1996;16:1126–37. doi: 10.1128/mcb.16.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKenzie PP, Danks MK, Kriwacki RW, Harris LC. P21Waf1/Cip1 dysfunction in neuroblastoma: a novel mechanism of attenuating G0-G1 cell cycle arrest. Cancer Res. 2003;63:3840–4. [PubMed] [Google Scholar]