Abstract
Purpose.
Treatment with light in the far-red to near-infrared region of the spectrum (photobiomodulation [PBM]) has beneficial effects in tissue injury. We investigated the therapeutic efficacy of 670-nm PBM in rodent and cultured cell models of diabetic retinopathy.
Methods.
Studies were conducted in streptozotocin-induced diabetic rats and in cultured retinal cells. Diabetes-induced retinal abnormalities were assessed functionally, biochemically, and histologically in vivo and in vitro.
Results.
We observed beneficial effects of PBM on the neural and vascular elements of retina. Daily 670-nm PBM treatment (6 J/cm2) resulted in significant inhibition in the diabetes-induced death of retinal ganglion cells, as well as a 50% improvement of the ERG amplitude (photopic b wave responses) (both P < 0.01). To explore the mechanism for these beneficial effects, we examined physiologic and molecular changes related to cell survival, oxidative stress, and inflammation. PBM did not alter cytochrome oxidase activity in the retina or in cultured retinal cells. PBM inhibited diabetes-induced superoxide production and preserved MnSOD expression in vivo. Diabetes significantly increased both leukostasis and expression of ICAM-1, and PBM essentially prevented both of these abnormalities. In cultured retinal cells, 30-mM glucose exposure increased superoxide production, inflammatory biomarker expression, and cell death. PBM inhibited all of these abnormalities.
Conclusions.
PBM ameliorated lesions of diabetic retinopathy in vivo and reduced oxidative stress and cell death in vitro. PBM has been documented to have minimal risk. PBM is noninvasive, inexpensive, and easy to administer. We conclude that PBM is a simple adjunct therapy to attenuate the development of diabetic retinopathy.
Keywords: photobiomodulation, diabetic retinopathy, retinal ganglion cells
Low-intensity far-red light inhibited diabetes-induced abnormalities in ERG, number of apoptotic retinal ganglion cells, and parameters related to retinal oxidative stress and inflammation. Photobiomodulation may be a simple adjunct therapy to help inhibit the development of diabetic retinopathy.
Introduction
Diabetes produces a spectrum of retinal abnormalities that result in damage to the vasculature and neurons, and in severe cases, loss of vision itself. The pathogenesis of diabetic retinopathy remains to be elucidated, although reduction in hyperglycemia has been shown to exert positive effects on the development and progression of diabetic retinopathy. Nevertheless, achievement and maintenance of glycemic control has been difficult or impossible in many patients, therefore effective therapies are needed to inhibit the retinopathy.
Efforts to inhibit diabetic retinopathy and other complications of diabetes have focused on highly specific therapeutic approaches. For example, studies in animals have targeted specific pharmacologic targets1 or used genetic approaches to alter expression of single enzymes, such as iNOS (inducible isoform of nitric oxide synthase) or superoxide dismutases.2–4 These approaches have successfully inhibited the early stages of diabetic retinopathy in animals,5,6 but their translation to the clinic may be problematic.
An alternative approach would be to identify innovative noninvasive treatment modalities that act by multiple potential mechanisms. Light in the far-red to near-infrared region of the spectrum (630–1000 nm) has been reported to be beneficial in the treatment of infected, ischemic, and hypoxic wounds and other soft tissue injuries.7–11 This light therapy, or photobiomodulation (PBM), also has been found to rescue damaged neurons from apoptotic cell death.12,13
The objective of this study was to determine if PBM might have value against diabetes-induced damage to the retina. We investigated the effects of transient (4 minutes per day, 25 mW/cm2, 6 J/cm2) phototherapy with far-red light on in vivo and in vitro pathologic changes relevant to the development of diabetic retinopathy. Our study found that PBM exerted significant beneficial effects on the retina in diabetes. In streptozotocin (STZ)-diabetic rats, PBM attenuated diabetes-induced abnormalities of retinal function and reduced retinal ganglion cell (RGC) death. At the cellular level, PBM decreased retinal superoxide generation and inhibited diabetes-induced abnormalities of electroretinograms (ERGs), RGC viability, superoxide generation, leukostasis, and expression of MnSOD and ICAM-1 in vivo. In retinal cells incubated in diabeticlike concentrations of glucose, PBM attenuated oxidative stress and improved cell survival.
Methods
In Vivo Studies
Animals.
All animal studies were approved by the institutional animal care and use committee and all experiments were conducted in accordance with the ARVO statement for the use of animals in ophthalmic and vision research and with the National Institutes of Health regulations. Male Lewis rats (225 g) were randomly assigned to four treatment groups: (1) nondiabetic controls (N), (2) nondiabetic controls exposed to 670 nm light (N+PBM), (3) diabetic controls (D), and (4) diabetic rats exposed to 670 nm light (D+PBM). Diabetes was induced by the administration of STZ (intraperitoneal injection of a freshly prepared solution of STZ in citrate buffer [pH 4.5] at 55 mg/kg of body weight). Insulin was given as needed to achieve slow weight gain without preventing hyperglycemia and glucosuria (0–2 units of insulin subcutaneously, 0–3 times per week), so that diabetic rats were insulin deficient but not catabolic. Food consumption and body weight were measured weekly. Glycated hemoglobin (GHb) was measured at 8 to 9 weeks of diabetes. All animals were treated identically, with the exception of exposure to the 670-nm light. All animals had free access to food and water, and were maintained under a 14-hour on/10-hour off light cycle for the 10 weeks.
To ensure that the light therapy did not influence the severity of diabetes, PBM therapy was not initiated until diabetes was established (defined as three consecutive measures of nonfasting blood glucose > 275 mg/dL). Thus, PBM therapy was initiated 7 to 10 days after STZ. Approximately 20 animals were studied per experimental group, divided among the various tests. All analyses were conducted in a masked manner; although diabetic animals tended to be smaller than nondiabetic animals, investigators did not know whether diabetic animals were treated with PBM or not.
Near-Infrared PBM.
Animals assigned to PBM treatment were treated once per day between 9 and 11 AM. Animals were placed in an open-top plexiglass restrainer with the light-emitting diode (LED) source approximately 1 inch above the animal, and exposed to whole-body irradiation at 670 nm for 240 seconds per day. The near-infrared light was generated by a 75-cm2 LED array (SpectraLife; Quantum Devices, Barneveld, WI) which was configured to output 25 mW/cm2 (50% intensity setting). This generated a fluence (or radiant exposure) of 6.0 J/cm2 per session over the whole body. This regimen was selected because similar treatment paradigms (670 nm 4–9 J/cm2 ) have been documented to attenuate retinal toxicity induced by methanol14 and retinal light damage.15 No ill effects from this treatment were noted. Of importance for this ocular study, the animals were not forced or encouraged to look at the light source, and the animals were not found to do so spontaneously. For comparison, some diabetic animals were treated only three times per week with 670 nm PBM (4 minutes at 25 mW/cm2, 6 J/cm2) for the duration of the study.
Prior to autopsy, ERGs were measured on animals in each group. Animals were euthanized at 10 weeks diabetes, and some animals were perfused for the leukostasis assay as described previously.1,2
ERG.
ERG responses were measured before animals were euthanized by using methods previously described.16,17 Briefly, rats were dark-adapted overnight, anesthetized with ketamine and xylazine (4 and 1 mg/100 g body weight, respectively; intraperitoneal injection), and placed on a warming pad during the recording session. Pupils were dilated with 1.0% tropicamide, 1.0% cyclopentalate hydrochloride, and 2.5% phenylephrine hydrochloride. Recordings were made using a stainless steel wire loop that contacted the corneal surface through a thin layer of 1% methylcellulose. Needle electrodes placed in the tail and cheek served as ground and reference electrodes, respectively. Responses were amplified (1–1000 Hz), averaged, and stored on an LKC signal-averaging system (UTAS; LKC Technologies, Inc., Gaithersburg, MD). A dark-adapted intensity-response series was recorded using a series of Ganzfeld flashes with intensities ranging from 0 to 5 log cd s/m2. Cone ERGs were obtained using a series of flash intensities (−0.22 to 0.52 log cd s/m2) after 10 minutes of light adaptation in which the animals were exposed to a steady rod-desensitizing background light of 0.8 log cd/m2 presented in the Ganzfeld bowl. For each intensity, the average response to 25 flashes was calculated. The amplitude and latency of the ERG a and b waves were measured.
Western Blots.
Proteins were fractionated by SDS-PAGE, and probed with antibodies for Intercellular Adhesion Molecule-1 (ICAM-1; 1:500 dilution; R&D Systems, Minneapolis, MN), iNOS (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), heme oxygenase-1 (HO-1; 1:200 dilution; Santa Cruz Biotechnology), manganese superoxide dismutase (MnSOD; 1:600 dilution; Millipore, Billerica, MA), total Akt and p-Akt (Ser473) (both 1:1000 dilution; Cell Signaling, Beverly, MA), total HSP-27 and p-HSP-27 (Ser82) (both 1:1000 dilution; Cell Signaling), total p38 MAPK and p-p38 MAPK (Thr180/Tyr182) (both 1:1000 dilution; Cell Signaling), and mitochondrial cytochrome oxidase complex IV subunit I (MT-CO-1; 1:2000 dilution; Abcam, Cambridge, MA). After extensive washing, protein bands detected by the antibodies were visualized by enhanced chemiluminescence (ECL; Santa Cruz Biotechnology) and evaluated by densitometry (Bio-Rad, Hercules, CA). Transferred nitrocellulose membranes were stained with washable Ponceau S solution (Sigma Chemical Company, St. Louis, MO) to visually ensure that protein loading was similar among lanes, and this was later confirmed by staining for beta-actin (1:10,000 dilution) in Western blots. The densitometry results of Western blots were expressed as mean ± SD.
TUNEL.
The transferase-mediated dUTP nick-end labeling (TUNEL) reaction (In Situ Cell Death Detection kit, fluorescein; Roche, Mannheim, Germany) was performed to detect apoptosis of RGCs. The TUNEL-positive RGCs were quantitated in retinal sections (formalin-fixed, paraffin-embedded) as per our previous reports.18,19 As a positive control for the TUNEL reaction, one retinal section was treated with DNase (50 units/100 μL) for 10 minutes to fragment DNA each time the assay was performed.
Leukostasis.
Leukostasis was measured using methods previously described.1,2 Rats were anesthetized with sodium pentobarbital, and a 20-gauge perfusion needle was inserted through the left ventricle into the base of the aortic arch, making sure that the needle did not obstruct the carotid arteries. The needle was clamped in place, and the right atrium was cut to allow outflow. PBS (60 mL) was perfused into the aorta at the normal cardiac output rate for a rat (60 mL/min) to clear erythrocytes and nonadherent leukocytes. Fluorescein isothiocyanate–coupled Concanavalin A lectin (20 μg/mL in PBS, pH 7.4; total concentration, 5 mg/kg body weight; Vector Laboratories, Burlingame, CA) then was perfused to stain adherent leukocytes and vascular endothelium, followed by another wash with PBS (60 mL) at the same perfusion rate to remove excess Concanavalin A. The retina with attached vitreous was separated from the choroid and sclera, flat-mounted on a microscope slide, covered with antifading medium and a coverslip, and imaged via fluorescence microscopy. Only whole retinae in which the entire vascular network was stained were used for analysis. The total number of adherent leukocytes per retina was counted.
Retinal Superoxide.
Fresh retinas from animals were analyzed for superoxide production as previously described.20 Briefly, retinas were placed in 0.2 mL Krebs/HEPES buffer and allowed to equilibrate in the dark at 37°C under 95% O2/5% CO2 conditions for 30 minutes. To each tube, 0.5 mM lucigenin (Sigma Chemical Company) was added, and the photon emission was detected for 10 seconds by a luminometer (Analytical Luminescence Laboratory, San Diego, CA). Retinal protein was quantified (Bio-Rad), and the luminescence was expressed per milligram protein.
Nitric Oxide.
Nitrite and nitrate released into the culture media were measured as an estimate of nitric oxide generation and release. The assay was performed using the previously reported Greiss reaction.21
Cytochrome Oxidase Activity.
Cytochrome oxidase activity was determined in retinal cross-sections (10 weeks diabetes). The histochemical method for cryosections was performed as reported previously.12 The data were measured on one eye from each of five animals in each group (three sections per animal).
In Vitro Studies
Cell lines representing RGCs (RGC522), photoreceptors (661W23), Müller (glia) cells (rMC-124), and retinal pigment epithelium (ARPE1925) were used. Cells were passaged in Dulbecco's modified Eagle's medium containing 5 mM glucose and 10% fetal bovine serum (FBS). For the experiments, cells were incubated in 5 mM or 30 mM glucose in a dark incubator, with or without PBM treatment (200 seconds at 25 mW/cm2 twice per day resulting in a fluence of 5 J/cm2) for 4 days. This duration of PBM treatment was selected following preliminary tests of exposure to 50, 100, and 200 seconds in 661W cells to assess effects on superoxide generation and cell death in 30 mM glucose. For experiments, FBS was reduced to 2%, and media was changed every other day. Cells were harvested by treating with a trypsin-EDTA solution (0.5% and 0.02%, respectively, wt/vol). The NO scavenger, Carboxy-PTIO,26 was added to the media of some plates at a concentration of 0.2 mM. Comparison of NO standard curves with and without this scavenger indicated that this concentration of scavenger removed NO from the media (lowered a NO standard curve) by an average of 58% (not shown). Cell death was measured as trypan blue exclusion,27 and superoxide generation in vitro was measured with luciginen as above.
Cytochrome Oxidase Activity
Cytochrome oxidase activity was determined in cultured RGC-5 cells. The assay was performed as per manufacturer's instructions (Complex IV Rodent Enzyme Activity Microplate Assay Kit; MitoSciences, Eugene, OR).
Statistical Analysis
Data are expressed as means ± SD. Statistical analysis was performed using analysis-of-variance followed by Fisher's test. P values less than or equal to 0.05 were considered statistically significant. Repeated measures ANOVA was used to test the significance of ERG differences between diabetic-treated and nontreated groups.
Results
Diabetic Animals
Glycemia was elevated in all diabetic groups to a similar degree, and body weight was less than normal in all diabetic animals (Table 1), but this was secondary to the animals not growing at the normal rate. Diabetic animals were not losing weight. The animals tolerated the daily light treatments with no noticeable effects.
Table 1. .
GHb and Body Weight in Animal Groups With and Without 670 nm PBM
| HbA1c, % |
Final Body Weight, g |
|
| Nondiabetic without PBM | 2.2 ± 0.2 | 421 ± 58 |
| Nondiabetic with PBM, 3 times/wk | 2.2 ± 0.2 | 426 ± 38 |
| Nondiabetic with PBM, 7 times/wk | 2.2 ± 0.2 | 448 ± 10 |
| Diabetic without PBM | 5.5 ± 0.4 | 301 ± 12 |
| Diabetic with PBM, 3 times/wk | 6.0 ± 0.3 | 281 ± 15 |
| Diabetic with PBM, 7 times/wk | 5.6 ± 0.5 | 299 ± 21 |
Death and Dysfunction of Retinal Neurons, and Correction by PBM
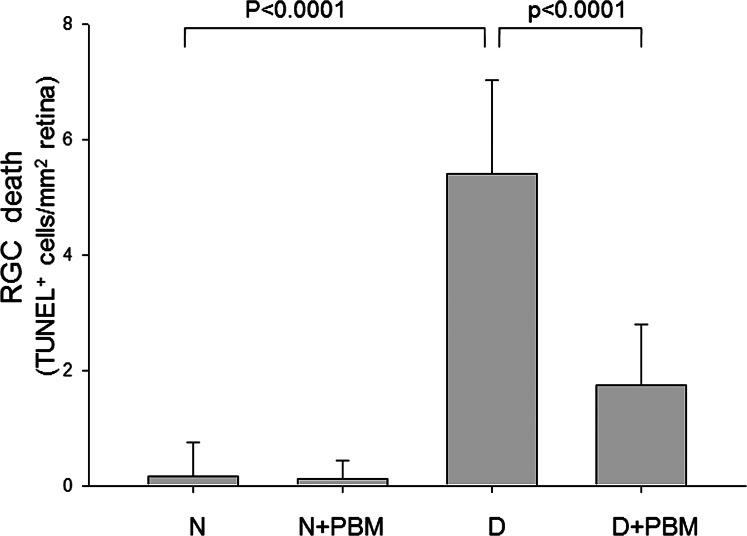
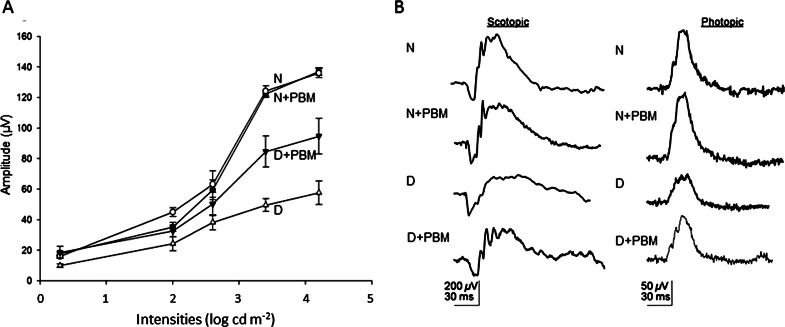
Consistent with previous studies in diabetic rats, diabetes of 10 weeks' duration in rats produced a significant increase in the number of TUNEL-positive cells in the RGC layer (Fig. 1), as well as an impairment of dark-adapted ERG (Fig. 2) compared with nondiabetic controls. Daily exposure to 4 minutes 670-nm PBM throughout the study resulted in significant inhibition in the diabetes-induced death of RGCs, as well as a 50% improvement of the diabetes-induced reduction in ERG amplitude (photopic b wave responses) (both P < 0.01). Diabetes-induced reductions in photopic (a wave) and scotopic (a and b waves) responses were partially attenuated (not shown) by the light therapy. Cataract was variably present at 2 months of diabetes, but cataract severity did not predict the ERG responses to PBM.
Figure 1. .
Far-red PBM therapy significantly reduced diabetes-induced death of cells in the RGC. Rats that had been made experimentally diabetic (D) or remained nondiabetic (N) were exposed to 670-nm light (PBM) each day (4 minutes, 25 mW/cm2, 6 J/cm2), or no treatment. After 10 weeks diabetes, retinal sections were assayed with TUNEL kit to assess death of cells in the RGC layer (n = 5 per experimental group).
Figure 2.
(A) PBM for 4 minutes per day (25 mW/cm2, 6 J/cm2) inhibited diabetes-induced decrease in ERG. ERG responses were measured following approximately 2 months of PBM. Recordings on test animals were obtained after dark adaptation overnight using standard methods. Treatment of diabetic animals with PBM partially preserved photopic b wave responses (D is significantly different from N, N+PBM, and D+PBM, all P < 0.05) (n = 5 per experimental group). (B) Representative ERG waveforms (max intensity) under scotopic and photopic conditions are shown.
We conducted in vitro studies to investigate effects of PBM on the death of several retinal cell types in high glucose. Cell lines were incubated in 5 mM glucose to simulate nondiabetic conditions or 30 mM glucose to simulate diabetic conditions, with or without 670 nm light treatment. For all the cell lines studied, cells maintained in 30 mM glucose exhibited increased cell death compared with cells grown in 5 mM glucose, although the extent of the increase varied by cell type (Table 2). To select an exposure duration for use in vitro, the effect of PBM on 661W cells was tested in 30 mM glucose. Effects of PBM on superoxide generation and cell death in high glucose were found to be exposure dependent, PBM having no significant effect on either parameter at 50 seconds exposure, but significantly inhibiting both at 100- and 200-second exposures (inhibiting the glucose-induced increase in superoxide at the two exposure durations by 65% and 103%, respectively, and inhibiting the glucose-induced increase in cell death by 83% and 73%, respectively). The PBM treatment regime selected for study was 200 seconds (25 mW/cm2 resulting in a fluence of 5 J/cm2) two times each day for 4 days. Application of PBM to cells in hyperglycemic conditions produced a significant reduction in cell death to levels at or below controls in all cell types except ARPE19 cells (Table 2). We appreciate that results of such in vitro studies cannot be clearly extrapolated to the in vivo situation, but include these studies to begin to dissect the contribution of various cell types to the effects of hyperglycemia and response to PBM.
Table 2. .
Effect of Elevated Glucose and 670-nm PBM on Cell Death In Vitro
|
Cell Line |
Reported Cell Derivation |
Cell Death, % of Value in 5 mM Glucose |
||
|
30 mM Glucose |
30 mM Glucose + PBM |
30 mM Glucose + PBM + Carboxy-PTIO |
||
| RGC5 | Retinal ganglion cells | 189 ± 11* | 63 ± 17† | 87 ± 19† |
| 661W | Photoreceptors | 136 ± 25* | 97 ± 6† | 85 ± 4† |
| ARPE19 | RPE | 151 ± 23* | 131 ± 29 | 135 ± 25 |
P < 0.05 compared to 5 mM glucose.
P < 0.05 compared to 30 mM glucose.
In an effort to explore the mechanism of the beneficial effect of PBM on retinal cells in hyperglycemic conditions, we examined several mechanisms that have been previously postulated to account for the beneficial actions of PBM therapy.
Cytochrome Oxidase
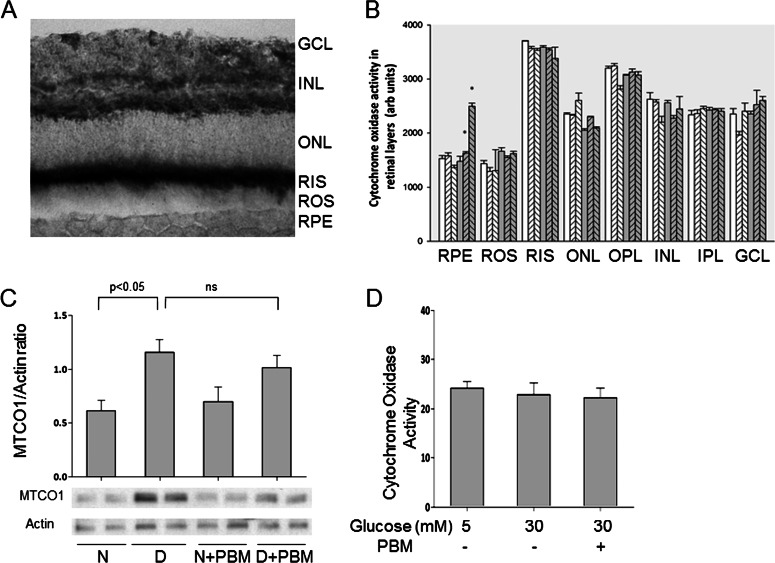
Previous investigations have focused on the effects of near infrared light on cytochrome oxidase (CO) as an explanation for observed beneficial effects of the PBM,12,28 so we investigated effects of elevated glucose and PBM on CO activity and expression in retinas and in cell culture. Quantitation of CO activity in cryosections of frozen retina from nondiabetic or diabetic (2–3 months) rats revealed no significant effect of diabetes on CO activity in any layers of retina (Figs. 3A, 3B). Diabetes did increase expression of mitochondrial CO in retina (complex IV subunit I; MTCO1; Fig. 3C), but that change did not alter activity of the enzyme in the various retinal layers (Fig. 3B). Cell culture of RGC5 cells (Fig. 3D) or rMC-1 cells (not shown) in 30-mM glucose had no significant effect on activity of CO activity.
Figure 3. .
Effect of diabetes or 670-nm PBM on CO activity (A, B) or expression (C) in retinas from rats diabetic 10 weeks. (A) Micrograph shows a representative cryosection demonstrating sites of CO activity. Black color indicates more activity. (B) The figure summarizes these reaction intensity results for layers of the retina. N animals are indicated in white bars, and D animals are indicated by gray bars. Cross-hatching to right and left represent PBM daily or 3 days per week, respectively. Asterisk demonstrates P < 0.05 different from D control. In none of the retinal layers was CO activity in diabetic controls significantly different from that in nondiabetic controls. (C) The figure summarizes expression of mitochondrial CO (complex IV subunit I). Expression of the protein did increase in diabetes, but PBM had no significant effect on that change. Representative results from Western blots are shown. (D) The figure indicates that high glucose with or without PBM did not alter CO activity in RGC5 cells in vitro. The graphical data in the figures summarize data from five animals per group (A–C), or six dishes per experimental condition (D).
Nitric Oxide
NO is known to reversibly inhibit CO activity,29,30 and some investigators have provided evidence that PBM can act by releasing NO that has been bound to CO (thus resulting in increased activity of the enzyme).31 We estimated NO in cell culture studies before and after PBM, to determine if PBM altered the amount of NO in cells. We also used an NO scavenger (Carboxy-PTIO26) to determine if NO had a role in the observed beneficial effect of PBM on cell death in high glucose. We found no significant evidence that the NO scavenger altered the effect of PBM on cell death (Table 2) and found no effect of either high glucose or PBM on NO in RGC5 or 661W cells (Table 3), thus providing no evidence that beneficial effects of the light therapy were mediated by NO release in these cell types. Only in ARPE-19 cells did high glucose significantly increase NO, and PBM partially restored this value toward values seen in cells incubated in 5 mM glucose.
Table 3. .
Effect of Elevated Glucose and 670 nm PBM on NO Generation In Vitro
|
Cell Line |
Reported Cell Derivation |
NO Generation, nmol/mg Protein |
||
|
5 mM Glucose |
30 mM Glucose |
30 mM Glucose + PBM |
||
| RGC5 | Retinal ganglion cells | 0.98 ± 0.13 | 1.13 ± 0.11 | 1.01 ± 0.03 |
| 661W | Photoreceptors | 2.41 ± 0.12 | 2.18 ± 0.18 | 2.08 ± 0.15 |
| ARPE19 | RPE | 3.36 ± 0.69 | 5.56 ± 0.94* | 4.18 ± 0.74† |
P < 0.05 compared to 5 mM glucose.
P < 0.05 compared to 30 mM glucose.
Oxidative Stress
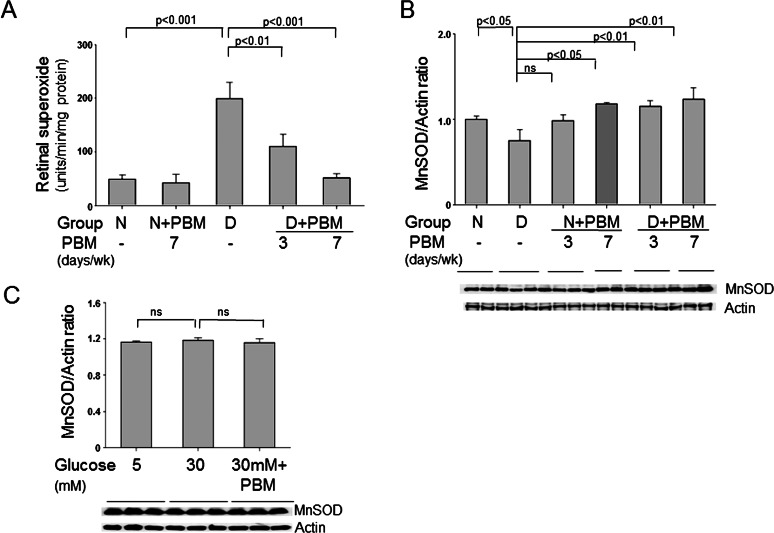
Because previous studies by us and others have implicated oxidative stress in development of the early stages of diabetic retinopathy, we investigated the effects of PBM on diabetes-induced changes in oxidative stress in the retina. Superoxide production was quantitated as a parameter of oxidative stress. Diabetes of 10 weeks' duration increased superoxide production by the retina (Fig. 4), and significantly reduced expression of the antioxidant enzyme, MnSOD. Daily exposure to PBM for 4 minutes per day significantly inhibited the diabetes-induced production of superoxide and corrected the diabetes-induced decrease in MnSOD expression in vivo, and daily therapy was more effective than PBM administered three times per week (Fig. 4). In vitro, high glucose increased superoxide in all cell lines tested, and PBM appreciably inhibited the superoxide generation in this diabeteslike environment (Table 4). The use of carboxy-PTIO to scavenge NO reduced superoxide generation in 661W and ARPE-19 cells, but not in RGC5 cells. Our in vitro studies of RGC5 cells indicated that neither elevated glucose concentration nor PBM had any effect on expression of MnSOD in these cells (Fig. 4C).
Figure 4. .
Effect of diabetes and far-red PBM on oxidative stress in retina and retinal cells. (A) Diabetes (2 months) increased superoxide production by retina, and this increase was inhibited by 4 minutes per day PBM. Administration of PBM either daily or only three times per week both significantly inhibited the superoxide generation, but daily therapy was more effective. (B) Diabetes caused a significant reduction in expression of MnSOD, which was corrected by the PBM therapy. (C) In contrast to intact retina, neither elevated glucose nor PBM (two sessions of 200 seconds each day) had any effect on MnSOD expression in RGC5. Representative results from Western blots are shown under the graphs. The graphical data in the figures summarizes data from three to six animals per group and six dishes per experimental condition.
Table 4. .
Effect of Elevated Glucose and 670-nm PBM on Superoxide Generation by Retinal Cells In Vitro
|
Cell Line |
Reported Cell Derivation |
Superoxide Generation, arb Units/min/9 × 105 Cells |
|||
|
5 mM Glucose |
30 mM Glucose |
30 mM Glucose + PBM |
30 mM Glucose + PBM + Carboxy-PTIO |
||
| RGC-5 | Retinal ganglion cells | 52 ± 17 | 105 ± 21* | 58 ± 13† | 63 ± 6 |
| 661W | Photoreceptors | 102 ± 55 | 351 ± 110* | 154 ± 10† | 121 ± 21†‡ |
| ARPE19 | RPE | 75 ± 8 | 164 ± 53* | 89 ± 11† | 67 ± 15†‡ |
P < 0.05 compared to 5 mM glucose.
P < 0.05 compared to 30 mM glucose.
P < 0.05 compared to 30 mM glucose + PBM.
Inflammation
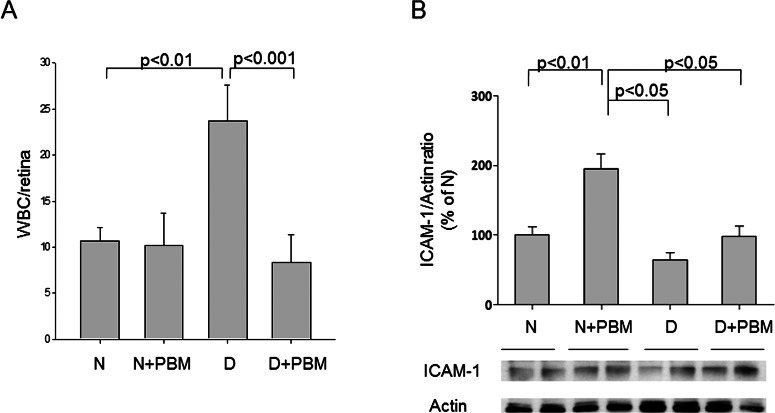
Previous studies by us and others have implicated inflammatory processes in development of the early stages of diabetic retinopathy.6 Based on this, we investigated the effects of PBM on diabetes-induced changes in local inflammation in the retina. Adherence of white blood cells to the retinal vascular wall (leukostasis) and retinal expression of the adhesion molecule, ICAM-1, were quantitated as parameters of inflammation. Diabetes significantly increased both leukostasis and expression of ICAM-1, and PBM essentially prevented both of these abnormalities (Fig. 5).
Figure 5. .
PBM of 670 nm for 4 minutes per day (25 mW/cm2, 6 J/cm2) inhibited the diabetes-induced increase in leukostasis in retinal blood vessels. (A) The number of leukocytes adherent to the capillary walls (after perfusion to remove unbound erythrocytes and white blood cells) is indicated per retina in the figure. (B) Expression of ICAM-1 in retina, the adhesion molecule that leukocytes adhere to, is summarized. Representative results from Western blots are shown. The graphical data in the figures summarizes data from five animals in each experimental group.
Additional Signaling Pathways Affected by High Glucose and Far-Red Light PBM
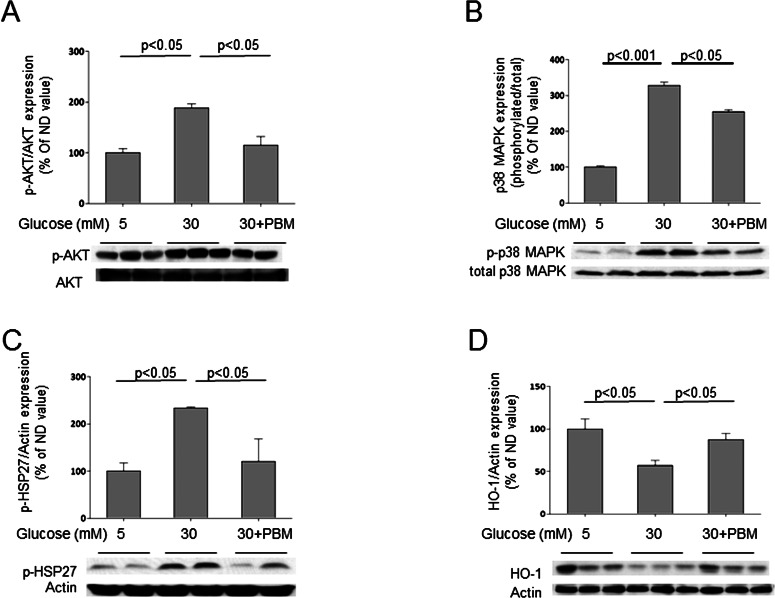
To obtain additional insight into molecular actions that might explain how PBM inhibited degeneration of RGCs in diabetes/elevated glucose, we conducted studies using the RGC5 cell line (Fig. 6). Expression of Akt was used as a marker of cell survival pathways, HSP-27 as a marker of cell stress, p38 MAPK as a marker of inflammation, and HO-1 as a marker of oxidative stress. High (30 mM) glucose significantly increased the phosphorylation of Akt (Ser473), HSP-27 (Ser82), and p38 MAPK (Thr180/Tyr182), and PBM inhibited all these effects. High glucose also decreased expression of HO-1, and PBM significantly inhibited the glucose-induced downregulation of this enzyme.
Figure 6. .
Effects of elevated glucose and 670-nm PBM on RGC5 cells in vitro. Incubation in elevated glucose significantly increased expression of phosphorylated forms of AKT, p38 MAPK, and HSP 27, and downregulated expression of HO-1 (A–D). In each case, PBM (200 seconds, 25 mW/cm2, 5 J/cm2) significantly inhibited these abnormalities toward values seen in normal (5 mM) levels of glucose. The graphical data in the figures summarizes data from six dishes for each experimental condition.
Discussion
High-energy light has been used as a treatment option for ophthalmic diseases, such as in laser photocoagulation for age-related macular degeneration or diabetic retinopathy. In the present study, however, we demonstrate that PBM using low-intensity light in the far-red portion of the spectrum also can inhibit a number of retinal abnormalities caused by diabetes.
PBM is a process in which specific wavelengths of light are absorbed by cellular photoacceptor molecules, resulting in the activation of signaling pathways in the cell. In contrast to high-intensity lasers or ultraviolet light, low-intensity light therapy has the important advantage of being nondestructive and lacking mutagenic properties. PBM previously has been shown to be of benefit in the treatment of infected, ischemic, and hypoxic wounds, and beneficial effects have been demonstrated for wound healing, recovery from ischemic injury in the heart, and retinal degeneration secondary to methanol intoxication and intense light.10–13,15,32–34 The wavelength used by us in the present study is not the only wavelength that has been shown to have therapeutic effects; 810- to 830-nm light has also been shown to be neuroprotective in studies of traumatic brain injury and inflammation.35,36 However, most research on neuroprotection and retinal protection has been conducted with the wavelength (670 nm) used in the present study.
Several studies have suggested that PBM works especially on mitochondria, and specifically via the cellular respiratory chain.12,28,37 Far-red light has effects on metalloproteins, including CO, and this mechanism has been implicated in the effects of PBM on some cell types.28,38 CO is a critical enzyme in the mitochondrial electron transport system, and the correlation between the absorption spectra of CO and the action spectra for biological responses to light suggested that CO was a key photoacceptor in the cellular response to PBM.39–43 Consistent with this hypothesis, far-red light PBM was found to inhibit retinal damage and blindness secondary to methanol toxicity44,45 (metabolism of methanol generates the mitochondrial toxin, formic acid, which specifically inhibits CO). Nevertheless, our studies do not suggest that the observed beneficial effects of PBM on retina in diabetes are mediated via a direct role of PBM on retinal CO activity. Diabetes did increase expression of CO in retina (complex IV subunit I; MTCO1; Fig. 3C), but that change did not alter activity of the enzyme in the various retinal layers (Fig. 3B), possibly suggesting a decrease in CO-specific activity in diabetes. Importantly, PBM affected neither the activity nor expression of the enzyme in retinas of diabetic rats or in retinal cells exposed to diabeteslike concentrations of glucose. A previous study that used a similar histochemical method46 agrees with our conclusion that diabetes did not alter activity of CO in neural retina, but did find that it increased CO activity in retinal pigment epithelium. Our data on activity in RPE is not consistent with that report, perhaps due to differences in strain or severity of hyperglycemia (which was not reported by them).
Although our studies did not show any effect of 670 nm PBM on CO activity in vivo or in vitro, they do not exclude the possibility that CO is acting as a photoacceptor molecule in the effects observed. Previous studies suggest that the interaction of 670-nm light with the CO enzyme complex results in the initiation of a signaling cascade that culminates in cytoprotective effects similar to those observed in our studies.10,13
Our studies indicate that light therapy effectively inhibits the diabetes-induced increase in superoxide production, leukostasis, and ICAM-1 expression by retina. We previously reported that mitochondria are a major source of the diabetes-induced increase in generation of oxidative stress by retina,20,47 but whether or not the beneficial effects of PBM in our studies are solely mediated by effects on mitochondria is not clear. Light therapy with far-red wavelengths also significantly inhibited the diabetes-induced increase in retinal leukostasis and expression of ICAM-1, which we interpret as indicating that the PBM inhibited at least aspects of the diabetes-induced local inflammation in the retina. Far-red light likewise has been found to inhibit production of the proinflammatory cytokines, IFN-γ and TNF-α, and to upregulate anti-inflammatory cytokines, IL-4 and IL-10 in experimental autoimmune encephalomyelitis.48 Oxidative stress and proinflammatory proteins in the retina are important in diabetes, because vascular lesions of early diabetic retinopathy are significantly inhibited if oxidative stress and inflammatory changes are inhibited by overexpressing either mitochondrial or cytoplasmic superoxide dismutases or by administering lipoic acid (an antioxidant that acts in mitochondria) or anti-inflammatory agents are administered.4,6,47,49
Molecular changes detected in the retina after the daily exposure to far-red light for 4 minutes each day also seem consistent with the phenomena known as preconditioning. Preconditioning describes a situation in which exposure to subtoxic levels of some noxious agent increases resistance against subsequent injury from an even more toxic stress.50–57 One well-studied example of beneficial effects of preconditioning came from studies in which subjecting the brain to transient, mild ischemia resulted in tolerance to subsequent stroke-inducing levels of ischemia. Preconditioning also occurs in the retina and other tissues in response to subtoxic ischemia, hypoxia, or cyclic light,56–58 and it seems likely that PBM might be added to this list of agents that cause preconditioning. It is impressive that the observed beneficial effects of PBM resulted from only very transient (4 minutes per day) exposure to the far-red light, perhaps indicating effects of the light on transcription factors or particular genes. PBM is known to cause upregulation of known retinoprotectant proteins, CNTF and FGF-2.15 We show beneficial effects of PBM on HO-1 expression along with de-phosphorylation of Akt (Ser473), HSP-27 (Ser82), and p38 MAPK (Thr180/Tyr182). Induction of these factors has been associated with beneficial effects in diabetes and diabetic retinopathy.1,59,60
PBM had a beneficial effect on retinal function as assessed by ERG. Although diabetes-induced death of RGCs was detected, it was minor at the short duration of diabetes studied, and not likely to account for the observed effects on electrophysiology. It seems likely that the light therapy was minimizing the severity of neuronal dysfunction, perhaps in part via effects on oxidative stress or local inflammation.
Our studies of PBM effects in retina to date have focused primarily on neural retina, and effects on the vasculature remain to be investigated in long-term studies. Nevertheless, the action of PBM to inhibit oxidative stress and inflammation suggest that the light therapy likewise might have beneficial effects on the microvasculature. It seems apparent that far-red light PBM exerts effects on multiple pathways (including oxidative stress, inflammation, and possibly preconditioning) in multiple cell types, and so a single mechanism of this beneficial action might not be identified. Although our in vitro studies of several retinal cells indicates that each can respond to the light therapy, it is important to recognize that the effect of PBM on retina observed in vivo might include also indirect effects. PBM has been found to exert beneficial effects in tissues that are not directly exposed to the light, including liver,61 kidney,62 and bone marrow and peripheral nerves (Tang J, Kern TS, unpublished observations, 2012) of diabetic animals, thus suggesting that the observed retinal effects might not be a result of direct exposure to the light.
PBM has been found to be associated with minimal risk in the studies conducted to date, and it offers opportunities, because it is noninvasive, inexpensive, and can be used essentially anywhere. PBM may offer additional benefits outside of the eye, as it has been found also to inhibit diabetes-induced defects in wound healing, kidney function, and heart function.9,61,62 Given our findings, further study into the mechanisms and potential uses of PBM is warranted. Because PBM has been found to be associated with minimal risk, and is noninvasive, inexpensive, and easy to administer, it may be a simple adjunct therapy to help inhibit the development of diabetic retinopathy.
Acknowledgments
Supported by National Eye Institute Grant EY00300 (TSK), Medical Research Service of the Department of Veteran Affairs grant (TSK), the Foundation Fighting Blindness Grant TA-NP-0709-0465-UWI (JTE), the International Retinal Research Foundation, the CWRU Visual Science Research Center core facility Grant P30EY11373, and supported in part by a CDA2 VA Career Development and VA Foundation Award from the Veteran's Administration (JT).
Disclosure: J. Tang, None; Y. Du, None; C.A. Lee, None; R. Talahalli, None; J.T. Eells, Quantum Devices (C), P; T.S. Kern, None
References
- 1. Du Y, Tang J, Li G, et al. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010; 51: 2158–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng L, Du Y, Miller C, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007; 50: 1987–1996 [DOI] [PubMed] [Google Scholar]
- 3. Kanwar M, Chan PS, Kern TS, Kowluru RA. Oxidative damage in the retinal mitochondria of diabetic mice: possible protection by superoxide dismutase. Invest Ophthalmol Vis Sci. 2007; 48: 3805–3811 [DOI] [PubMed] [Google Scholar]
- 4. Berkowitz BA, Gradianu M, Bissig D, Kern TS, Roberts R. Retinal ion regulation in a mouse model of diabetic retinopathy: natural history and the effect of Cu/Zn superoxide dismutase overexpression. Invest Ophthalmol Vis Sci. 2009; 50: 2351–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng L, Kern T. Role of nitric oxide, superoxide, peroxynitrite and poly(ADP-ribose) polymerase in diabetic retinopathy. Front Biosci. 2009; 14: 3974–3987 [DOI] [PubMed] [Google Scholar]
- 6. Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011; 30: 343–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelan HT, Smits RL Jr, Buchman EV, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001; 19: 305–314 [DOI] [PubMed] [Google Scholar]
- 8. Whelan HT, Connelly JF, Hodgson BD, et al. NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg. 2002; 20: 319–324 [DOI] [PubMed] [Google Scholar]
- 9. Whelan HT, Buchmann EV, Dhokalia A, et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003; 21: 67–74 [DOI] [PubMed] [Google Scholar]
- 10. Eells JT, Wong-Riley MT, VerHoeve J, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004; 4: 559–567 [DOI] [PubMed] [Google Scholar]
- 11. Zhang R, Mio Y, Pratt PF, et al. Near infrared light protects cardiomyocytes from hypoxia and reoxygenation injury by a nitric oxide dependent mechanism. J Mol Cell Cardiol. 2009; 46: 4–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005; 280: 4761–4771 [DOI] [PubMed] [Google Scholar]
- 13. Liang HL, Whelan HT, Eells JT, et al. Photobiomodulation partially rescues visual cortical neurons from cyanide-induced apoptosis. Neuroscience. 2006; 139: 639–649 [DOI] [PubMed] [Google Scholar]
- 14. Eells JT, Henry MM, Summerfelt P, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003; 100: 3439–3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Albarracin R, Eells J, Valter K. Photobiomodulation protects the retina from light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 3582–3592 [DOI] [PubMed] [Google Scholar]
- 16. Ball SL, Powers PA, Shin HS, Morgans CW, Peachey NS, Gregg RG. Role of the beta(2) subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci. 2002; 43: 1595–1603 [PubMed] [Google Scholar]
- 17. Kern TS, Miller CM, Du Y, et al. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes. 2007; 56: 373–379 [DOI] [PubMed] [Google Scholar]
- 18. Kern TS, Tang J, Mizutani M, et al. Response of capillary cell death to aminoguanidine predicts the development of retinopathy: comparison of diabetes and galactosemia. Invest Ophthalmol Vis Sci. 2000; 41: 3972–3978 [PubMed] [Google Scholar]
- 19. Martin PM, Roon P, Van Ells TK, Ganapathy V, Smith SB. Death of retinal neurons in streptozotocin-induced diabetic mice. Invest Ophthalmol Vis Sci. 2004; 45: 3330–3336 [DOI] [PubMed] [Google Scholar]
- 20. Du Y, Miller CM, Kern TS. Hyperglycemia increases mitochondrial superoxide in retina and retinal cells. Free Radic Biol Med. 2003; 35: 1491–1499 [DOI] [PubMed] [Google Scholar]
- 21. Du Y, Smith MA, Miller CM, Kern TS. Diabetes-induced nitrative stress in the retina, and correction by aminoguanidine. J Neurochem. 2002; 80: 771–779 [DOI] [PubMed] [Google Scholar]
- 22. Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001; 86: 1–12 [DOI] [PubMed] [Google Scholar]
- 23. al-Ubaidi MR, Font RL, Quiambao AB, et al. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol. 1992; 119: 1681–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998; 39: 212–216 [PubMed] [Google Scholar]
- 25. Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996; 62: 155–169 [DOI] [PubMed] [Google Scholar]
- 26. Cao BJ, Reith ME. Nitric oxide scavenger carboxy-PTIO potentiates the inhibition of dopamine uptake by nitric oxide donors. Eur J Pharmacol. 2002; 448: 27–30 [DOI] [PubMed] [Google Scholar]
- 27. Du Y, Sarthy V, Kern T. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol. 2004; 287: R735–741 [DOI] [PubMed] [Google Scholar]
- 28. Wong-Riley MT, Bai X, Buchmann E, Whelan HT. Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. Neuroreport. 2001; 12: 3033–3037 [DOI] [PubMed] [Google Scholar]
- 29. Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001; 1504: 46–57 [DOI] [PubMed] [Google Scholar]
- 30. Sarti P, Forte E, Mastronicola D, Giuffre A, Arese M. Cytochrome c oxidase and nitric oxide in action: molecular mechanisms and pathophysiological implications. Biochim Biophys Acta. 2012; 1817: 610–619 [DOI] [PubMed] [Google Scholar]
- 31. Poyton RO, Ball KA. Therapeutic photobiomodulation: nitric oxide and a novel function of mitochondrial cytochrome c oxidase. Discov Med. 2011; 11: 154–159 [PubMed] [Google Scholar]
- 32. Yeager RL, Lim J, Millsap DS, et al. 670 nanometer light treatment attenuates dioxin toxicity in the developing chick embryo. J Biochem Mol Toxicol. 2006; 20: 271–278 [DOI] [PubMed] [Google Scholar]
- 33. Liang HL, Whelan HT, Eells JT, Wong-Riley MT. Near-infrared light via light-emitting diode treatment is therapeutic against rotenone- and 1-methyl-4-phenylpyridinium ion-induced neurotoxicity. Neuroscience. 2008; 153: 963–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albarracin RS, Valter K. Treatment with 670-nm light protects the cone photoreceptors from white light-induced degeneration. Adv Exp Med Biol. 2012; 723: 121–128 [DOI] [PubMed] [Google Scholar]
- 35. Pallotta RC, Bjordal JM, Frigo L, et al. Infrared (810-nm) low-level laser therapy on rat experimental knee inflammation. Lasers Med Sci. 2012; 27: 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu Q, Xuan W, Ando T, et al. Low-level laser therapy for closed-head traumatic brain injury in mice: effect of different wavelengths. Lasers Surg Med. 2012; 44: 218–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karu TI, Afanas'eva NI. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light [in Russian]. Dokl Akad Nauk. 1995; 342: 693–695 [PubMed] [Google Scholar]
- 38. Beauvoit B, Kitai T, Chance B. Contribution of the mitochondrial compartment to the optical properties of the rat liver: a theoretical and practical approach. Biophys J. 1994; 67: 2501–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karu TI, Pyatibrat LV, Afanasyeva NI. A novel mitochondrial signaling pathway activated by visible-to-near infrared radiation. Photochem Photobiol. 2004; 80: 366–372 [DOI] [PubMed] [Google Scholar]
- 40. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005; 23: 355–361 [DOI] [PubMed] [Google Scholar]
- 41. Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005; 81: 98–106 [DOI] [PubMed] [Google Scholar]
- 42. Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008; 84: 1091–1099 [DOI] [PubMed] [Google Scholar]
- 43. Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010; 62: 607–610 [DOI] [PubMed] [Google Scholar]
- 44. Nicholls P. Formate as an inhibitor of cytochrome c oxidase. Biochem Biophys Res Commun. 1975; 67: 610–616 [DOI] [PubMed] [Google Scholar]
- 45. Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976; 430: 13–29 [DOI] [PubMed] [Google Scholar]
- 46. Caldwell RB, Slapnick SM. Increased cytochrome oxidase activity in the diabetic rat retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1989; 30: 591–599 [PubMed] [Google Scholar]
- 47. Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radic Biol Med. 2006; 41: 1191–1196 [DOI] [PubMed] [Google Scholar]
- 48. Muili KA, Gopalakrishnan S, Meyer SL, Eells JT, Lyons JA. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS One. 2012; 7: e30655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004; 53: 3233–3238 [DOI] [PubMed] [Google Scholar]
- 50. Li W, Luo Y, Zhang F, et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006; 26: 181–198 [DOI] [PubMed] [Google Scholar]
- 51. Stetler RA, Zhang F, Liu C, Chen J. Ischemic tolerance as an active and intrinsic neuroprotective mechanism. In: Vinken PJ, Bruyn GW. eds Handbook of Clinical Neurology. 2009; 92: 171–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang F, Jia J, Wu Y, Hu Y, Wang Y. The effect of treadmill training pre-exercise on glutamate receptor expression in rats after cerebral ischemia. Int J Mol Sci. 2010; 11: 2658–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang F, Oswald T, Holt J, Gerzenshtein J, Lei MP, Lineaweaver WC. Regulation of inducible nitric oxide synthase in ischemic preconditioning of muscle flap in a rat model. Ann Plast Surg. 2004; 52: 609–613 [DOI] [PubMed] [Google Scholar]
- 54. Zhang F, Wu Y, Jia J. Exercise preconditioning and brain ischemic tolerance. Neuroscience. 2011; 177: 170–176 [DOI] [PubMed] [Google Scholar]
- 55. Zhang FY, Chen XC, Ren HM, Bao WM. Effects of ischemic preconditioning on blood-brain barrier permeability and MMP-9 expression of ischemic brain. Neurol Res. 2006; 28: 21–24 [DOI] [PubMed] [Google Scholar]
- 56. Chollangi S, Wang J, Martin A, Quinn J, Ash JD. Preconditioning-induced protection from oxidative injury is mediated by leukemia inhibitory factor receptor (LIFR) and its ligands in the retina. Neurobiol Dis. 2009; 34: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ueki Y, Le YZ, Chollangi S, Muller W, Ash JD. Preconditioning-induced protection of photoreceptors requires activation of the signal-transducing receptor gp130 in photoreceptors. Proc Natl Acad Sci U S A. 2009; 106: 21389–21394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roth S. Endogenous neuroprotection in the retina. Brain Res Bull. 2004; 62: 461–466 [DOI] [PubMed] [Google Scholar]
- 59. Jacot JL, Sherris D. Potential therapeutic roles for inhibition of the PI3K/Akt/mTOR pathway in the pathophysiology of diabetic retinopathy. J Ophthalmol. 2011; 2011: 589813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fan J, Xu G, Jiang T, Qin Y. Pharmacologic induction of heme oxygenase-1 plays a protective role in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci. 2012; 53: 6541–6556 [DOI] [PubMed] [Google Scholar]
- 61. Lim J, Ali ZM, Sanders RA, et al. Effects of low-level light therapy on hepatic antioxidant defense in acute and chronic diabetic rats. J Biochem Mol Toxicol. 2009; 23: 1–8 [DOI] [PubMed] [Google Scholar]
- 62. Lim J, Sanders RA, Snyder AC, Eells JT, Henshel DS, Watkins JB III. Effects of low-level light therapy on streptozotocin-induced diabetic kidney. J Photochem Photobiol B. 2010; 99: 105–110 [DOI] [PubMed] [Google Scholar]