Abstract
The heparan sulfate proteoglycan (HSPG) syndecan-1 (SDC1) acts as a major receptor for triglyceride-rich lipoprotein (TRL) clearance in the liver. We sought to identify the relevant apolipoproteins on TRLs that mediate binding to SDC1 and determine their clinical relevance. Evidence supporting ApoE as a major determinant arose from its enrichment in TRLs from mice defective in hepatic heparan sulfate (Ndst1f/fAlbCre+ mice), decreased binding of ApoE-deficient TRLs to HSPGs on human hepatoma cells, and decreased clearance of ApoE-deficient [3H]TRLs in vivo. Evidence for a second ligand was suggested by the faster clearance of ApoE-deficient TRLs after injection into WT Ndst1f/fAlbCre– versus mutant Ndst1f/fAlbCre+ mice and elevated fasting and postprandial plasma triglycerides in compound Apoe–/–Ndst1f/fAlbCre+ mice compared with either single mutant. ApoAV emerged as a candidate based on 6-fold enrichment of ApoAV in TRLs accumulating in Ndst1f/fAlbCre+ mice, decreased binding of TRLs to proteoglycans after depletion of ApoAV or addition of anti-ApoAV mAb, and decreased heparan sulfate–dependent binding of ApoAV-deficient particles to hepatocytes. Importantly, disruption of hepatic heparan sulfate–mediated clearance increased atherosclerosis. We conclude that clearance of TRLs by hepatic HSPGs is atheroprotective and mediated by multivalent binding to ApoE and ApoAV.
Introduction
Accumulation of plasma triglyceride-rich lipoproteins (TRLs) is now considered an independent risk factor for cardiovascular disease (1, 2). TRLs consist of chylomicrons derived from dietary fats in the intestine, VLDLs derived from de novo synthesized lipids in the liver, and remnant particles that arise from lipolysis of these lipoproteins in the peripheral circulation. TRLs vary in size dependent on their origin, but have similar low buoyant density (δ < 1.006 g/ml) due to their high content of triglycerides and cholesteryl esters relative to protein. TRL-associated proteins include ApoB100 (VLDL) or ApoB48 (chylomicrons in humans, chylomicrons and VLDL in rodents), ApoE, ApoCI–ApoCIII, and multiple less-abundant apolipoproteins and lipases, including ApoAI, ApoAII, ApoAIV, ApoAV, lipoprotein lipase (LPL), hepatic lipase (HL), and endothelial lipase. Many of the apolipoproteins have profound effects on lipoprotein metabolism by acting as structural proteins, as cofactors for activation of enzymes involved in lipolysis, or as ligands for receptor-mediated clearance of TRLs in the liver and peripheral tissues (3).
TRLs in the circulation undergo lipolytic processing primarily by LPL immobilized on the capillary endothelial surface by way of its receptor GPIHBP1, resulting in triglyceride hydrolysis and release of free fatty acids for energy production or storage in the surrounding tissue (4, 5). The remnant TRLs then undergo rapid clearance in the liver by receptors located on the basal membrane of hepatocytes facing the space of Disse. The dominant endocytic receptors in the liver for TRL remnants include the LDL receptor (LDLR), the LDLR-related protein 1 (LRP1), and heparan sulfate proteoglycans (HSPGs), most notably syndecan-1 (SDC1; refs. 6, 7). LDLR preferentially clears ApoB-containing lipoproteins, thus mediating the removal of LDL and subclasses of TRLs containing ApoB100 or combinations of ApoB48 and ApoE (8, 9). Consistent with these observations, mice lacking LDLR exhibit mild hypertriglyceridemia under fasting conditions and in the postprandial state (10). LRP1 shows overlapping specificity with LDLR, preferentially interacting with ApoE-bearing lipoproteins. However, inactivation of LRP1 in hepatocytes does not cause hypertriglyceridemia, presumably due to compensation by LDLR (11). Genetic evidence in mice has demonstrated the importance of HSPGs, in particular SDC1, in TRL clearance (12–14). SDC1 works in parallel to, but independently of, LDLR based on the compound effect of altering hepatic heparan sulfate and LDLR deficiency on plasma triglycerides (12). Recent studies of a Turkish population showed that SNPs in glucuronic acid epimerase (GLCE), which encodes an important enzyme involved in heparan sulfate biosynthesis, were associated with triglyceride and HDL cholesterol levels, a finding supported by studies of Glce+/– mice fed a high-fat diet (15).
Studies of mice bearing mutations in enzymes involved in heparan sulfate biosynthesis showed that TRL binding to SDC1 depends on specific subsets of sulfate groups on the heparan sulfate chains, which presumably facilitate interaction of SDC1 with protein ligands involved in lipoprotein clearance (12–14, 16, 17). Proteins associated with TRLs that can interact with heparan sulfate or heparin (a highly sulfated form of heparan sulfate) include ApoB48 and ApoB100 (18, 19), ApoE (20–22), ApoAV (23), and LPL and HL (24, 25). Cell culture studies of model lipoproteins enriched with ApoE, LPL, or HL have demonstrated enhanced binding and uptake by HSPGs (26–30). Mutated lipases lacking enzymatic activity can also act as bridging molecules between lipoproteins and proteoglycans (31, 32). Furthermore, all of these factors are manufactured in the liver and secreted into the space of Disse, where they can potentially bind to HSPGs. These observations led Mahley and coworkers to suggest that TRL clearance might occur through a “secretion-capture” hypothesis, in which TRLs entering the space of Disse become enriched in ApoE or lipases, thus facilitating their binding to HSPGs (reviewed in refs. 6, 7, 33). In spite of all this information, the physiological protein ligands responsible for TRL binding and uptake via HSPGs in vivo remain undefined.
In the current study, we sought to identify the relevant protein ligands on TRLs for HSPG-mediated clearance, and the role of this receptor system in preventing atherosclerosis. We showed that mutants containing undersulfated hepatic heparan sulfate accumulated TRLs enriched in ApoE and ApoAV. Direct evidence for a dominant role of these apolipoproteins was obtained by analysis of apolipoprotein mutants, depletion/reconstitution studies, and competition with mAbs using a novel lipoprotein-proteoglycan flotation assay. Our findings suggest that HSPGs clear a unique subset of particles enriched in both ApoE and ApoAV, and support a model in which the heparan sulfate chains on SDC1 form multivalent contacts with both ApoE and ApoAV on the surface of TRLs. Furthermore, disruption of this clearance mechanism resulted in increased plasma triglycerides and a significant increase in atherosclerosis development in Apoe–/– mice, demonstrating for the first time that proteoglycan-mediated clearance of TRLs is atheroprotective.
Results
ApoE mediates TRL binding and clearance by heparan sulfate.
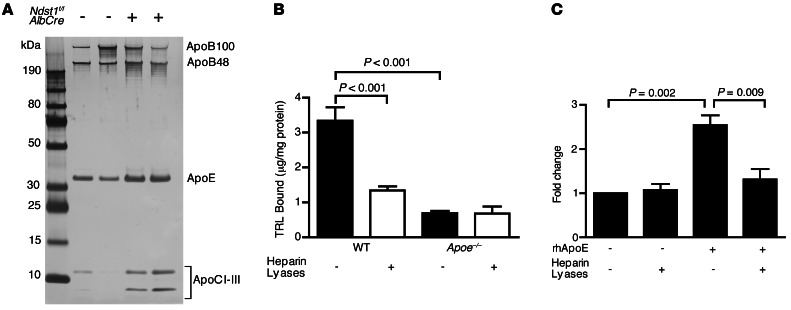
To identify the proteins on TRLs responsible for HSPG-mediated clearance, we analyzed TRLs from mice deficient for the liver heparan sulfate biosynthetic enzyme N-deacetylase-N-sulfotransferase-1 (NDST1; referred to herein as Ndst1f/fAlbCre+ mice). NDST1 deficiency induced in this way results in undersulfation specifically of hepatocyte heparan sulfate and accumulation of TRLs in both the fasted and postprandial state due to altered hepatic clearance (12). We reasoned that if HSPGs preferentially clear subspecies of TRLs, then lipoproteins of unique composition should accumulate in the mutant. Thus, plasma TRLs from fasted WT Ndst1f/fAlbCre– and mutant Ndst1f/fAlbCre+ mice were isolated by density ultracentrifugation (δ < 1.006 g/ml), and equal amounts of protein were analyzed by SDS-PAGE and silver staining (Figure 1A). ApoE and ApoCs were enriched in TRLs from Ndst1f/fAlbCre+ compared with Ndst1f/fAlbCre– mice, whereas no consistent change in ApoB48 or ApoB100 was observed. Similar results were obtained when postprandial TRLs were analyzed. Of the apolipoproteins detected in this way, only ApoE and ApoB bound to heparan sulfate or to heparin; thus, we focused our initial experiments on these apolipoproteins.
Figure 1. Murine TRLs require ApoE for binding to cell surface heparan sulfate.
(A) TRLs (δ < 1.006 g/ml) were isolated from fasted WT Ndst1f/fAlbCre– and mutant Ndst1f/fAlbCre+ mice (n = 4 per group) and analyzed by gradient SDS-PAGE. Individual proteins were visualized by silver staining. A representative gel is shown with n = 2 per genotype. (B) Binding of [3H]TRLs (50 μg/ml) from WT and Apoe–/– mice to Hep3B cells was measured before (filled bars) and after (open bars) treatment with heparin lyases (n = 5). (C) ApoE-deficient [3H]TRLs were reconstituted with recombinant human ApoE3 and purified by ultracentrifugation (δ < 1.006 g/ml). Cell surface binding of the reconstituted particles was measured (n = 8).
To examine the participation of ApoE in binding to hepatic HSPGs, we developed a cell surface receptor binding assay using metabolically labeled postprandial [3H]TRLs derived from mice orally gavaged with [3H]retinol and corn oil. Binding to cell surface HSPGs was assessed by incubation of the radiolabeled TRLs with Hep3B human hepatocarcinoma cells, a cell line expressing an HSPG profile similar to that of primary human and murine hepatocytes (13, 34). Incubation of cells at 4°C with [3H]TRLs derived from WT mice demonstrated saturable binding (maximum binding, 3.8 ± 1.9 μg TRL/mg cell protein; Figure 1B) with an apparent Kd of 38 μg/ml (r2 = 0.83), which compared well with values previously obtained for human TRL binding to primary murine hepatocytes (maximum binding, 8.2 ± 1.1 μg VLDL protein/mg cell protein; Kd, 43 ± 10 μg/ml; ref. 13). Subsequent measurements were made with 50 μg/ml purified TRLs in order to economize the use of radioactive TRLs. Under these conditions, 3.3 ± 0.4 μg TRL/mg cell protein bound to the cells. Treatment of Hep3B cells with heparin lyases degraded the heparan sulfate chains and significantly reduced cell surface binding to 1.3 ± 0.1 μg TRL/mg cell protein (P < 0.001; Figure 1B). The residual binding presumably reflects other receptors (e.g., LDLR) or incomplete removal of heparan sulfate.
In contrast to the behavior of WT TRLs, [3H]TRLs isolated from Apoe–/– mice bound to Hep3B cells poorly (0.7 ± 0.1 μg TRL/mg cell protein), and the residual binding did not depend on heparan sulfate (Figure 1B). Reconstitution of [3H]TRLs from Apoe–/– mice with recombinant human ApoE3 restored binding to Hep3B cells (2.5-fold versus ApoE-deficient particles; P = 0.002), and the enhanced binding was heparan sulfate dependent (P = 0.009; Figure 1C). Analysis of ApoE-deficient TRLs by SDS-PAGE showed that in addition to the loss of ApoE, the particles were enriched with ApoAI and ApoAIV (Supplemental Figure 1A; supplemental material available online with this article; doi: 10.1172/JCI67398DS1). Interestingly, reconstitution of ApoE-deficient TRLs with recombinant human ApoE3 resulted in displacement of ApoAIV, but not ApoAI or other apolipoproteins (Supplemental Figure 1, B and C, and ref. 35).
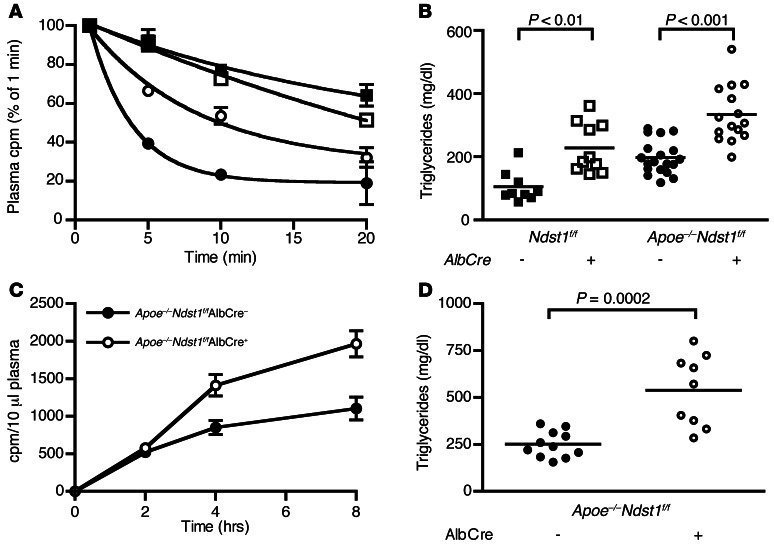
Additional evidence demonstrating the importance of ApoE for HSPG-mediated TRL clearance was obtained by intravenously injecting [3H]TRLs into Ndst1f/fAlbCre– and Ndst1f/fAlbCre+ mice and measuring their clearance from the plasma. Whereas injected [3H]TRLs cleared rapidly in Ndst1f/fAlbCre– mice (t1/2, ∼4 minutes), clearance was much delayed in Ndst1f/fAlbCre+ mice (t1/2, >20 minutes; Figure 2A), confirming the importance of the HSPG-mediated pathway in vivo. ApoE-deficient [3H]TRLs injected into Ndst1f/fAlbCre+ mice showed similarly delayed clearance kinetics (Figure 2A). The lack of an additive effect of compounding these 2 alterations supports the idea that ApoE on TRLs interacts with hepatic HSPGs.
Figure 2. ApoE-independent clearance of TRLs by HSPGs.
(A) Isolated [3H]TRLs from WT (filled symbols) and Apoe–/– (open symbols) mice were injected intravenously into WT Ndst1f/fAlbCre– (circles) or mutant Ndst1f/fAlbCre+ (squares) mice (n = 3). Clearance of [3H]TRLs was assessed by measuring the counts remaining in the plasma relative to counts recovered 1 minute after injection. (B) Plasma triglycerides were measured in plasma samples collected from fasted 8-week-old Ndst1f/fAlbCre– (n = 9, filled squares), Ndst1f/fAlbCre+ (n = 10, open squares), Apoe–/–Ndst1f/fAlbCre– (n = 19, filled circles), and Apoe–/–Ndst1f/fAlbCre+ (n = 15, open circles) mice. (C and D) 10-week-old Apoe–/–Ndst1f/fAlbCre– (n = 11, filled circles) and Apoe–/–Ndst1f/fAlbCre+ (n = 8, open circles) mice were gavaged with corn oil, and (C) [3H]retinol excursion and (D) plasma triglycerides 4 hours after gavage were measured.
ApoE-deficient [3H]TRLs in Ndst1f/fAlbCre+ mice displayed slower clearance compared with those in Ndst1f/fAlbCre– mice (t1/2, ∼20 vs. ∼10 minutes; Figure 2A), which suggests that there might be another ligand on TRLs that can interact with hepatic HSPGs. We reached a similar conclusion by analysis of plasma triglycerides in Ndst1f/fAlbCre+, Apoe–/–, and compound Apoe–/–Ndst1f/fAlbCre+ mice. NDST1 and ApoE deficiency resulted in modest hypertriglyceridemia compared with Ndst1f/fAlbCre– mice (Ndst1f/fAlbCre–, 105 ± 16 mg/dl, n = 9; Ndst1f/fAlbCre+, 228 ± 25 mg/dl, n = 10; Apoe–/–, 197 ± 12 mg/dl, n = 19; Figure 2B), as shown previously (9, 12–14). The absence of ApoE led to increased plasma cholesterol as well, but NDST1 deficiency did not (Supplemental Figure 2). Importantly, plasma triglycerides accumulated to a greater extent in Apoe–/–Ndst1f/fAlbCre+ mice (334 ± 23 mg/dl, n = 15; Figure 2B) than in either single mutant. A significant delay in the clearance of intestinally derived TRLs occurred in Apoe–/–Ndst1f/fAlbCre+ versus Apoe–/– mice (AUC, 9,300 vs. 5,800; Figure 2C), resulting in elevated postprandial plasma triglycerides in Apoe–/–Ndst1f/fAlbCre+ mice compared with their Apoe–/–Ndst1f/fAlbCre– littermates (Apoe–/–Ndst1f/fAlbCre+, 538 ± 63 mg/dl, n = 9; Apoe–/–Ndst1f/fAlbCre–, 251 ± 21 mg/dl, n = 11; P = 0.0002; Figure 2D). The increase in fasting plasma triglycerides and impairment in clearance of dietary lipids in the compound mutant compared with the single mutants, along with the slower clearance of injected ApoE-deficient [3H]TRLs in Ndst1f/fAlbCre+ versus Ndst1f/fAlbCre– mice, strongly suggest that HSPG-mediated TRL clearance does not exclusively depend on ApoE.
The ApoE-independent pathway of TRL clearance by heparan sulfate does not involve ApoB.
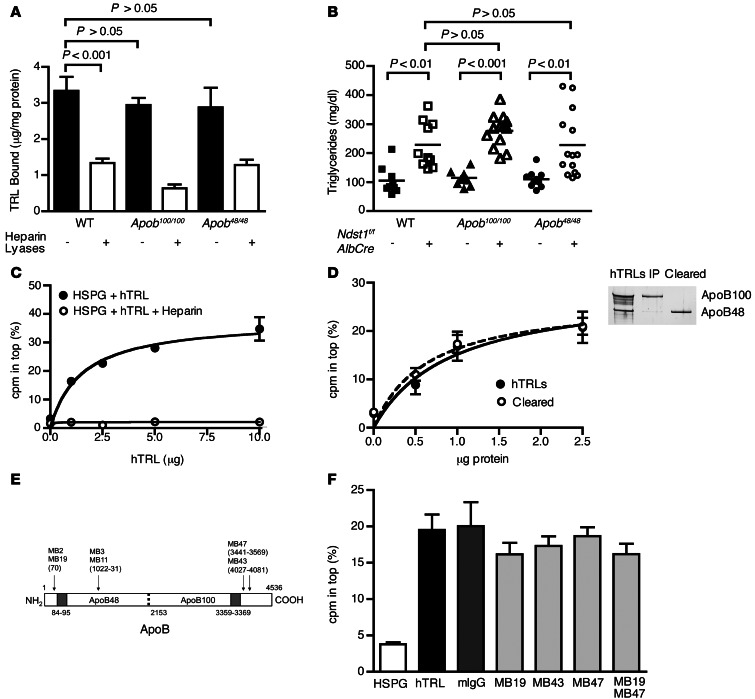
In light of these findings, we examined the contribution of other TRL-associated proteins known to bind to heparin. We excluded any contribution from ApoB based on several criteria. First, binding of [3H]TRLs derived from Apoe–/– mice to hepatocytes did not depend on heparan sulfate (Figure 1B), yet these particles contained ApoB, mostly ApoB48 (Supplemental Figure 1A). Second, binding of [3H]TRLs derived from ApoB100- and ApoB48-deficient mice (referred to herein as Apob48/48 and Apob100/100 mice, respectively; Supplemental Figure 3) was similar to that of WT TRLs (Apob100/100, 2.9 ± 0.2 μg TRL/mg cell protein; Apob48/48, 2.9 ± 0.5 μg TRL/mg cell protein; WT, 3.3 ± 0.4 μg TRL/mg cell protein; Figure 3A). Third, binding of both ApoB-restricted mutant particles was significantly reduced after treatment of Hep3B cells with heparin lyases, but not to any greater extent than WT TRLs (Figure 3A). Fourth, triglyceride accumulation in Apob100/100Ndst1f/fAlbCre+ and Apob48/48Ndst1f/fAlbCre+ mice was not elevated compared with Ndst1f/fAlbCre+ mice (Figure 3B). Finally, binding of TRLs to HSPGs was not affected in vitro by mAbs raised against different domains of ApoB. In these latter experiments, we took advantage of the observation that shed SDC1 ectodomains will form complexes with purified TRLs from human plasma, based on an assay in which 35S-labeled HSPG ectodomains are mixed with human TRLs and subjected to ultracentrifugation (34). Under these conditions, complexes of human TRLs with [35S]ectodomains floated in saline solution containing nonionic iodixanol (δ = 1.019 g/ml), while unbound [35S]ectodomains sedimented. Binding was concentration dependent and saturable (Figure 3C), and under these conditions, about 25%–35% of [35S]ectodomains bound to human TRLs. Heparin, a highly sulfated derivative of heparan sulfate, abolished binding of the ectodomains (Figure 3C). Using this assay, we showed that particles bearing only ApoB48, which were separated by immunoprecipitation with an ApoB100-specific Ab, did not differ in ectodomain binding (Figure 3D). Furthermore, the ApoB mAbs MB19 (which binds to the N-terminal domain of ApoB and reduces LDL binding to heparin), MB43 (ApoB100-specific), and MB47 (ApoB100-specific), or the combination of MB19 and MB47, had no effect on TRL binding to ectodomains (Figure 3, E and F, and refs. 36–38). Other mAbs that recognize epitopes on both ApoB isoforms (MB2, MB3, and MB11) also had no effect (data not shown and refs. 38, 39). Thus, although particles that bind to heparan sulfate contain ApoB, this apolipoprotein appears to be dispensable for the interaction.
Figure 3. TRL binding to heparan sulfate is independent of ApoB.
(A) Binding of [3H]TRLs from Apob100/100 and Apob48/48 mice to Hep3B cells was measured before (filled bars) or after (open bars) treatment with heparin lyases (n = 4). Binding of WT [3H]TRLs done at the same time is repeated here from Figure 1B for comparison. (B) Fasting plasma triglycerides from Apob100/100Ndst1f/fAlbCre– (n = 12, filled triangles), Apob100/100Ndst1f/fAlbCre+ (n = 13, open triangles), Apob48/48Ndst1f/fAlbCre– (n = 8, filled circles) and Apob48/48Ndst1f/fAlbCre+ (n = 13, open circles) mice were measured. Plasma triglycerides from Ndst1f/fAlbCre– animals (squares) are included from Figure 2B for comparison. (C) Binding of purified [35S]HSPG ectodomains to human TRLs (hTRL) was measured by ultracentrifugation (see Methods). Binding of [35S]HSPGs to human TRLs (filled circles) occurred in a saturable manner and was inhibited by heparin (open circles). (D) Binding of [35S]HSPGs to ApoB48 only (open circles) or mixed human TRLs (filled circles) was measured. SDS-PAGE and silver staining for ApoB of purified human TRLs is also shown. (E) Diagram of epitope map for ApoB mAbs. Specific residues recognized by Abs are shown in parentheses, and heparin binding sites are shaded gray. Image is not drawn to scale. (F) [35S]HSPG binding to human TRLs was measured by ultracentrifugation in the absence (black bar) or presence of mouse IgG (dark gray bar) or mAbs specific for domains spanning ApoB (MB19, MB43, and/or MB47; light gray bars). A control experiment done without human TRLs (white bar) is shown for comparison.
The ApoE-independent pathway of TRL clearance by heparan sulfate involves ApoAV.
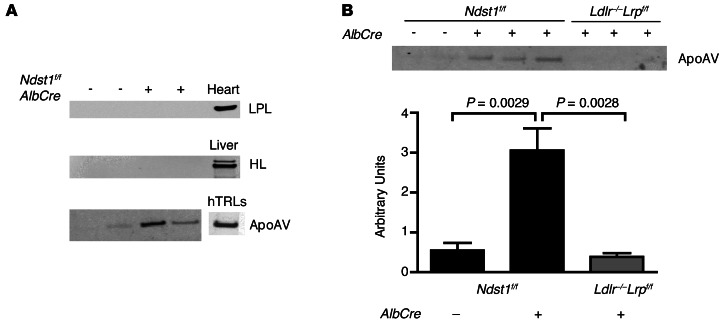
To investigate other candidate heparin-binding proteins, fasting TRLs from Ndst1f/fAlbCre– and Ndst1f/fAlbCre+ mice were subjected to SDS-PAGE and analyzed by Western blotting. Previous work has suggested that LPL and HL can facilitate remnant clearance by acting as bridge between TRLs and hepatic HSPGs (32, 40). However, we did not detect either lipase in TRL preparations derived from Ndst1f/fAlbCre– or Ndst1f/fAlbCre+ mice (n = 4 per genotype; Figure 4A). In contrast, ApoAV was detected and enriched approximately 6-fold on TRLs derived from Ndst1f/fAlbCre+ versus Ndst1f/fAlbCre– mice (Ndst1f/fAlbCre+, 3.1 ± 0.6 AU, n = 13; Ndst1f/fAlbCre–, 0.5 ± 0.2 AU, n = 8; P = 0.0029; Figure 4B). We previously did not observe ApoAV in TRLs accumulating in Ndst1f/fAlbCre+ mice (12), presumably because of a lack of sensitivity of the staining techniques. ApoAV was also enriched in TRLs in Apoe–/– mice (Apoe–/–, 5.1 ± 1.2 AU; WT, 0.5 ± 0.2 AU; data not shown), consistent with the role of ApoE in clearance mediated by HSPGs. Importantly, mice deficient in LDLR and hepatic LRP1 (Ldlr–/–Lrp1f/fAlbCre+ mice) also had elevated plasma triglycerides (361 ± 44 mg/dl, n = 7), but did not accumulate TRLs bearing ApoAV (0.4 ± 0.1 AU, n = 7; Figure 4B). These findings demonstrated that ApoAV accumulation in Ndst1f/fAlbCre+ mice strictly depends on loss of hepatic heparan sulfate, and does not simply reflect impaired TRL clearance.
Figure 4. TRLs from mice with impaired HSPG-mediated TRL clearance accumulate ApoAV.
(A) Western blot of mouse TRLs from Ndst1f/fAlbCre– and mutant Ndst1f/fAlbCre+ mice using Abs against LPL,HL, and ApoAV. Homogenates of heart and liver were used as positive controls. Human TRLs were also probed for the presence of ApoAV with a human anti-ApoAV mAb (right). (B) Fasting TRLs from Ndst1f/fAlbCre–, Ndst1f/fAlbCre+, and Ldlr–/–Lrp1f/fAlbCre+ mice were analyzed by SDS-PAGE and probed for the presence of ApoAV by Western blotting. The blots were scanned by densitometry, and the amount of ApoAV was normalized to ApoB for each sample and presented as AU. n = 8 (Ndst1f/fAlbCre–); 13 (Ndst1f/fAlbCre+); 7 (Ldlr–/–Lrp1f/fAlbCre+).
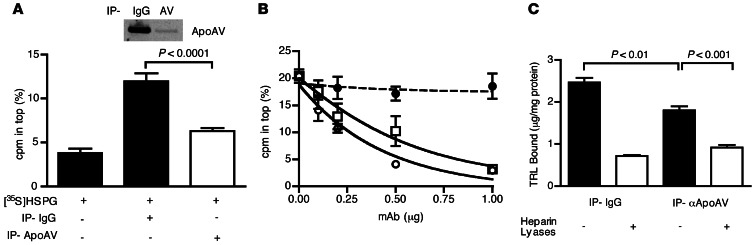
ApoAV deficiency alters peripheral lipolysis, resulting in the accumulation of very large TRLs that would confound the interpretation of binding experiments with ApoAV-deficient particles (41). However, we were able to compare the behavior of comparably sized particles by depleting TRLs of ApoAV. Treatment of human TRLs with a mAb against human ApoAV depleted ApoAV particles by 85% (Figure 5A), without affecting the content of ApoB and ApoE. Removal of ApoAV reduced binding of [35S]ectodomains compared with human TRLs treated with nonspecific murine IgG (P = 0.0001; Figure 5A). Furthermore, anti-ApoAV mAb inhibited binding of unmodified human TRLs in a dose-dependent manner (IC50, ∼0.5 μg/ml), similar to anti-ApoE mAb (IC50, ∼0.25 μg/ml), whereas nonspecific mouse IgG had no effect (Figure 5B).
Figure 5. TRL binding to HSPGs depends on ApoE and ApoAV.
(A) Human TRLs were subjected to 3 rounds of immunoprecipitation using nonspecific mouse IgG or ApoAV-specific mAb, and the cleared, Ab-free solution was collected. [35S]HSPG binding to the preparations was measured by ultracentrifugation. SDS-PAGE and Western blot for ApoAV content of human TRLs after treatment with nonspecific IgG and ApoAV-specific IgG is also shown. (B) [35S]HSPG binding to human TRLs in the presence of increasing amounts of mouse IgG (filled circles) or mouse mAbs specific for ApoE (open squares) or ApoAV (open circles). (C) Murine [3H]TRLs from Ndst1f/fAlbCre+ were subjected to 3 rounds of immunoprecipitation using nonspecific mouse IgG or ApoAV-specific mAb, and the Ab-free solution was collected. Murine [3H]TRL binding to Hep3B cells at 4°C was measured before and after treatment with heparin lyases (n = 3) and expressed as bound TRL (in micrograms) per cell protein (in milligrams).
Treatment of murine TRLs derived from Ndst1f/fAlbCre+ mice with mAb against murine ApoAV also resulted in ApoAV displacement (>95%), allowing us to examine whether clearance of ApoAV-depleted murine [3H]TRLs is impaired after their injection into WT mice. Only a modest difference was observed after depletion of murine [3H]TRLs compared with murine [3H]TRLs treated with nonspecific IgG (t1/2, ∼3.5 minutes vs. 3.2 minutes, n = 4; P = 0.4145; data not shown). The difference was accentuated by depleting ApoAV from TRLs derived from Apoe–/– mice, but still did not reach significance (7.6 minutes vs. 5.6 minutes, n = 4; P = 0.1320; data not shown). To circumvent potential confounding effects of apolipoprotein reconstitution that might occur in vivo, we examined binding of depleted particles to hepatocytes in culture. Binding of ApoAV-depleted murine [3H]TRLs to Hep3B cells was significantly reduced compared with murine [3H]TRLs treated with nonspecific IgG (P < 0.01; Figure 5C).
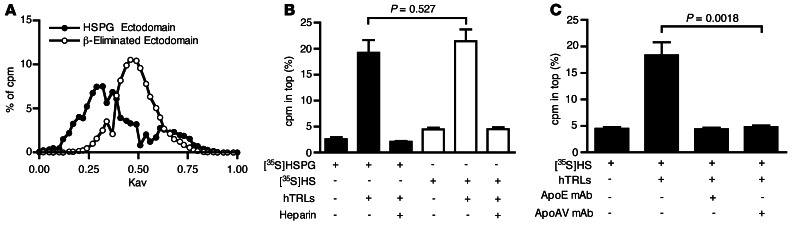
SDC1, like other HSPGs, contains up to 3 attachment sites for heparan sulfate clustered near the N-terminus of the protein. Because binding of TRLs to ectodomains appeared to depend on both ApoE and ApoAV, we examined whether the interaction depends on the presentation of clustered heparan sulfate chains on the ectodomains. Because of their large hydrodynamic volume (42), [35S]ectodomains eluted toward the void volume during gel filtration chromatography on CL-4B resins (Figure 6A). Alkaline treatment of the ectodomains resulted in β-elimination and liberation of the individual chains, as demonstrated by the more retarded elution of individual chains compared with the native ectodomains (Figure 6A). Binding of individual chains to TRLs also occurred in the flotation assay and, like binding to intact ectodomains, binding to individual chains was sensitive to added heparin (Figure 6B). Typically, 20%–35% of the [35S]ectodomains or [35S]heparan sulfate chains would associate with the human TRLs in this assay, which suggests that only a subpopulation of the ectodomains and chains has the capacity to bind under these conditions. mAbs directed against ApoE or ApoAV prevented the formation of complexes to the same extent (Figure 6C). These findings suggest that individual chains contain binding sites for both of ApoE and ApoAV and that simultaneous interaction with these apolipoproteins provides for optimal binding.
Figure 6. ApoE and ApoAV mediate binding of TRLs to heparan sulfate chains.
(A) Gel filtration chromatography of [35S]HSPG ectodomains (filled circles) and [35S]heparan sulfate chains (open circles). (B) Binding of [35S]heparan sulfate ([35S]HS) or [35S]HSPGs to human TRLs was measured by ultracentrifugation. (C) Inhibition of [35S]heparan sulfate binding to human TRLs by mAbs against ApoE or ApoAV.
Clearance of TRLs by HSPGs is atheroprotective.
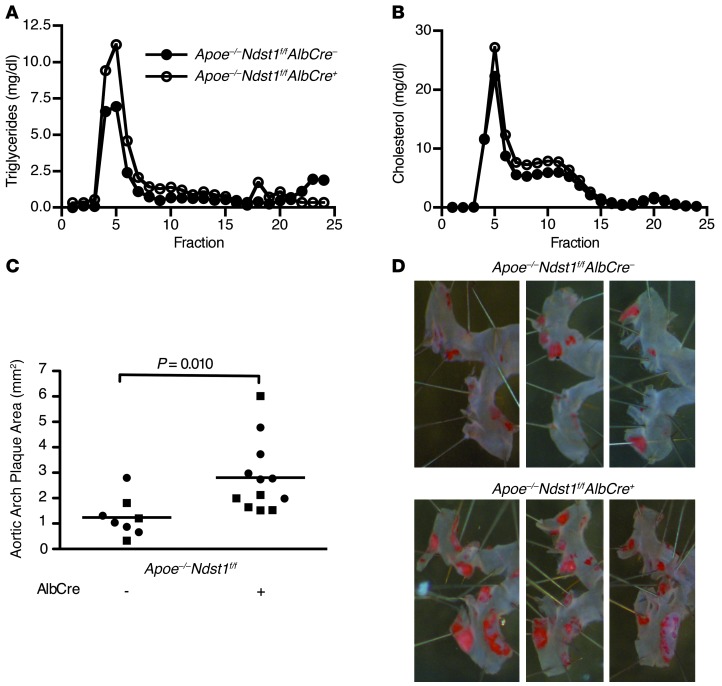
To determine the relevance of hepatic TRL clearance mediated by HSPGs, we examined 6-month-old Apoe–/–Ndst1f/fAlbCre+ and Apoe–/–Ndst1f/fAlbCre– mice for spontaneous atherosclerosis. Like the younger animals described in Figure 2B, the older Apoe–/–Ndst1f/fAlbCre– mice showed elevated plasma triglycerides compared with Apoe–/–Ndst1f/fAlbCre+ mice (Apoe–/–Ndst1f/fAlbCre+, 250 ± 82 mg/dl; Apoe–/–Ndst1f/fAlbCre–, 169 ± 33 mg/dl), and plasma cholesterol remained unchanged (Apoe–/–Ndst1f/fAlbCre–, 356 ± 57 mg/dl; Apoe–/–Ndst1f/fAlbCre+, 378 ± 66 mg/dl; data not shown). Analysis of the plasma lipoproteins by gel filtration fast protein liquid chromatography (FPLC) showed accumulation of triglycerides in large TRLs and no change in cholesterol-rich lipoproteins (Figure 7, A and B). En face analysis of aortas from chow-fed Apoe–/–Ndst1f/fAlbCre+ mice demonstrated a striking 2.25-fold increase in plaque formation in the aortic arch compared with Apoe–/–Ndst1f/fAlbCre– littermates (Apoe–/–Ndst1f/fAlbCre+, 2.81 ± 0.40 mm2; Apoe–/–Ndst1f/fAlbCre–, 1.24 ± 0.27 mm2; P = 0.010; Figure 7C). Both females and males were affected to the same extent. These findings provide the first evidence that HSPG-mediated clearance of TRLs is atheroprotective.
Figure 7. Disruption of HSPG-mediated TRL clearance enhances atherosclerosis development.
(A and B) Lipoproteins from pooled plasma samples (n = 6) from Apoe–/–Ndst1f/fAlbCre– (filled circles) and Apoe–/–Ndst1f/fAlbCre+ (open circles) mice were analyzed by FPLC gel filtration, and the amount of (A) triglyceride and (B) cholesterol was determined. (C and D) 6-month-old Apoe–/–Ndst1f/fAlbCre– (n = 8) and Apoe–/–Ndst1f/fAlbCre+ (n = 12) mice on standard chow diets were analyzed for spontaneous atherosclerosis development. (C) Plaque formation in the aortic arch from males (squares) and females (circles) was quantified. (D) 3 representative images of stained aortas from females for each genotype.
Discussion
Our present findings demonstrated that ApoE and ApoAV are the dominant particle-associated proteins involved in TRL binding to HSPGs in mice. This conclusion is based on (a) accumulation of TRLs bearing ApoE and ApoAV in Ndst1f/fAlbCre+ mice (Figures 1 and 4); (b) failure of TRLs isolated from Apoe–/– mice to bind to hepatocytes in a heparan sulfate–dependent manner, with restoration of binding by reconstitution of ApoE-deficient TRLs with recombinant human ApoE3 (Figure 1); (c) decreased clearance of ApoE-deficient TRLs in Ndst1f/fAlbCre– mice and lack of any further reduction in Ndst1f/fAlbCre+ mice (Figure 2); (d) inhibition of binding of human TRLs to 35S-labeled HSPG ectodomains by mAbs specific to ApoE and ApoAV, but not to ApoB (Figures 3–5); (d) reduction of binding after depletion of ApoAV from human and murine TRLs (Figure 5); and (e) decreased clearance of ApoAV-depleted particles in vivo. Although the in vivo data were derived from genetic studies in mice, we infer from the in vitro studies of human TRLs that these same factors may be relevant to remnant clearance in humans.
Studies of Apoe–/– mice 2 decades ago demonstrated the importance of ApoE as a major determinant for remnant lipoprotein clearance in mice. Apoe–/– mice exhibit impaired lipoprotein clearance, elevated plasma triglycerides and cholesterol, and extreme susceptibility to atherosclerosis (43). Interbreeding of Apoe–/– mice with Ldlr–/– mice showed that plasma triglyceride levels were similar in Ldlr–/–Apoe–/– and Apoe–/– mice, but higher than in Ldlr–/– mice, suggesting a second receptor dependent on ApoE (9). Ji et al. showed that addition of ApoE to rabbit β-VLDLs resulted in enhanced binding to and uptake via cell surface HSPGs (28), and Wilsie et al. showed that ApoE enrichment of human VLDL enhanced binding to SDC1 transfected cells (44). ApoE is found at high concentrations on the sinusoidal side of the basal membrane of hepatocytes (45), which led to the idea that ApoE produced by hepatocytes might enrich lipoproteins in the space of Disse and enhance clearance through proteoglycans (secretion-capture hypothesis; ref. 46). The binding site in ApoE for heparin has been characterized (20, 22). Furthermore, several naturally occurring ApoE variants that exhibit reduced binding to heparin have been found in patients with hyperlipoproteinemia (47–49). These prior findings, coupled with the experimental evidence presented here, firmly establish the importance of ApoE in TRL clearance by HSPGs.
ApoAV, another heparin-binding apolipoprotein (23), also plays a key role in TRL metabolism. Apoav–/– mice accumulate plasma triglycerides 4-fold, whereas transgenic mice overexpressing ApoAV have reduced plasma triglycerides (50). Apoav–/– mice have very large TRLs due to impaired lipolysis caused by diminished interactions with LPL and/or endothelial LPL receptors, such as GPIHBP1 and endothelial HSPGs (41, 51, 52). ApoAV has also been suggested to promote clearance of TRLs by acting as a ligand for receptor-mediated endocytosis (53). The accumulation of TRLs bearing ApoAV in Ndst1f/fAlbCre+ mice and the inhibition of binding to HSPG by depletion of ApoAV or by addition of neutralizing Abs provides experimental evidence for a role of ApoAV in proteoglycan-mediated clearance. Although ApoAV has been suggested to act as a ligand for LDLR family members, we favor the idea that ApoAV facilitates TRL binding to hepatic proteoglycan receptors, which act independently of members of the LDLR family (12, 13). The lack of accumulation of TRLs bearing ApoAV in Ldlr–/–Lrp1f/fAlbCre+ mice, in which HSPG-mediated clearance is intact, provides further support for this conclusion. Importantly, human population studies have shown a positive correlation between SNPs in ApoAV and plasma triglycerides, particularly in individuals with hypertriglyceridemia (50, 54). Our present findings suggest that impairments in HSPG-mediated TRL clearance could account for this positive correlation, a hypothesis that warrants further investigation.
Remarkably, the concentration of ApoAV in plasma was extremely low compared with other apolipoproteins, and its accumulation in Ndst1f/fAlbCre+ mice required Western blotting for detection. It has been estimated that as few as 1 in 24 VLDL particles carries ApoAV in plasma (55). Superficially, the low abundance of ApoAV-bearing particles would appear to be inconsistent with the observation that HSPG receptors, notably SDC1, account for a large proportion of TRL clearance (∼50% under fasting conditions and as much as 70% under postprandial conditions; ref. 13). However, the 6-fold enrichment in ApoAV in TRLs from Ndst1f/fAlbCre+ mice suggests that approximately 25% of TRLs contain ApoAV in the absence of HSPG-mediated clearance. Thus, the low concentration of ApoAV in Ndst1f/fAlbCre– mice most likely reflects efficient clearance of ApoAV-bearing lipoproteins from the circulation. One should also keep in mind that plasma ApoAV concentration might not reflect events occurring in the liver, the site of ApoAV production (50). Although the concentration of ApoAV in the space of Disse is unknown, it is intriguing to speculate that a dual “enrichment-capture” mechanism might exist, in which remnant lipoproteins could become enriched for ApoAV as well as ApoE when they enter the liver (53, 56).
ApoB, which also binds to heparin, does not appear to participate directly in HSPG-mediated clearance. Clearance of ApoB48 and ApoB100 particles occurred normally in Ndst1f/fAlbCre+ mice, and no additive effects were noted in compound mutants. Moreover, Apoe–/– TRLs contained ApoB48, but no longer bound to hepatocytes in a heparan sulfate–dependent manner. Additionally, mAbs directed against ApoB had no effect on binding of human TRLs to proteoglycan ectodomains. Thus, the heparin-binding capacity of ApoB does not appear to facilitate binding of TRLs to HSPGs. Conceivably, domains involved in heparin binding might not be exposed in TRLs, only appearing as the particles are reduced in size or converted to LDL (36). The observation that LDL can form complexes with arterial heparan sulfate (57) appears to be consistent with this idea. With respect to hepatic clearance, we propose that ApoB is primarily responsible for interaction with members of the LDLR family.
Multiple studies have demonstrated that the triglyceride lipases HL and LPL could act as ligands for TRL binding to HSPGs, independent of their catalytic capacity (32, 58–61). Both lipases bind to heparan sulfate, and enrichment of TRLs with either lipase led to enhanced binding to cell surface HSPGs (26, 62). However, the importance of these lipases in HSPG-mediated clearance in vivo has been difficult to assess, because knockout models of the lipases are confounded by lipolysis defects and other effects on lipoprotein metabolism. We were unable to detect LPL or HL on freshly isolated TRLs from Ndst1f/fAlbCre– or Ndst1f/fAlbCre+ mice and human TRLs. Furthermore, anti-human LPL mAb has no effect on binding of human TRLs to HSPG in vitro (J. Gonzales and J.D. Esko, unpublished observations). Enrichment of TRLs and other lipoproteins with LPL or HL can clearly provide additional capacity for HSPG interaction, but the physiologic relevance of these observations with respect to hepatic clearance remains questionable, based on the findings presented here.
Based on the ability of free heparan sulfate chains to bind to human VLDL particles, we believe that binding of TRLs to SDC1 most likely depends on multivalent interactions. Interestingly, gel filtration of the heparan sulfate chains from hepatoma cells and human liver indicate an average molecular mass of approximately 16 kDa, corresponding to approximately 60 disaccharides per chain (63). Most heparin-binding proteins require only 4–6 disaccharides for binding (64); thus, the chains can easily bind multiple protein ligands if they possess the appropriate arrangement of sulfated sugars and epimers of uronic acids. We therefore propose a model in which a TRL particle binds to 1 or more heparan sulfate chains on SDC1 via multiple contacts with ApoE or combinations of ApoE and ApoAV.
Hypertriglyceridemia is an independent risk factor for cardiovascular disease, and there is increasing evidence that remnant TRLs contribute to the development of atherosclerosis (1, 2). Our findings support this conclusion and provide the first evidence that TRL clearance mediated by ApoE and ApoAV binding to HSPGs is atheroprotective. Intriguingly, Apoe–/–Ndst1f/fAlbCre+ mice had elevated plasma triglycerides, but plasma cholesterol was not affected, which suggests that the impaired clearance of TRLs drives the more extensive development of atherosclerosis in this model. This conclusion is in agreement with prior studies showing that transgenic overexpression of human ApoAV, which reduces plasma triglycerides, provides atheroprotection in mouse models of dyslipidemias (65, 66).
Type III hyperlipidemia is characterized by the accumulation of plasma triglycerides and cholesterol, related in part to homozygous expression of ApoE2 (49). For reasons not fully understood, only approximately 10% of individuals with ApoE2 homozygosity develop hyperlipidemia and cardiovascular disease, while the majority of the individuals are normo- and even hypocholesterolemic, with no increase in cardiovascular risk. The development of overt hyperlipidemia might therefore require a secondary genetic or environmental factor in addition to ApoE2 expression. Genetic variation in ApoAV is obviously one factor that could determine whether ApoE2 homozygous individuals develop dysbetalipoproteinemia. Another factor that should be considered is the composition of heparan sulfate, which varies among different individuals (67). Thus, further analysis of hepatic heparan sulfate and allelic variants of genes involved in heparan sulfate metabolism and SDC1 expression are warranted in order to determine whether HSPG alterations contribute to hypertriglyceridemia and atherosclerosis in humans.
In conclusion, our results provide insights into the mechanism of HSPG-mediated clearance of TRLs as well as the first genetic evidence of atheroprotection by hepatic HSPGs. We identified ApoE and ApoAV as the dominant ligands on TRLs that bind to hepatocyte HSPGs and demonstrated that disruption of this multivalent interaction resulted in increased atherosclerosis in mice.
Methods
Further information can be found in Supplemental Methods.
Mice and animal husbandry.
Ndst1f/fAlbCre+ mice were described previously (12). Apoe–/– mice were from Jackson Laboratory (43), and Apob100/100 and Apob48/48 mice were provided by S. Young (UCLA, Los Angeles, California, USA; ref. 68). Ldlr–/–Lrp1f/fAlbCre+ mice were generated by crossbreeding Lrp1f/f, Ldlr–/–, and AlbCre+ mice purchased from Jackson Laboratory. Mice were weaned at 3 weeks, maintained on a 12-hour light/12-hour dark cycle, and fed water and standard rodent chow (Harlan Tekland) ad libitum. Genotyping was performed as described previously (12, 43, 68, 69).
Cell culture.
The human hepatocarcinoma cell line Hep3B was obtained from ATCC (HB-8064) and cultured in MEM (Invitrogen) supplemented with 10% fetal bovine serum (Gemini Bio-Products), nonessential amino acids, sodium pyruvate, penicillin, and streptomycin.
TRL isolation.
Murine TRLs were isolated from blood samples drawn by cardiac puncture into BD Microtainer tubes with EDTA. Samples (200 μl/tube) were centrifuged for 4 hours at 175,000 g in a Beckman 42.2Ti rotor. The top fraction (50 μl; δ < 1.006 g/ml) was collected and used for subsequent studies. Human TRLs (δ < 1.006 g/ml) were isolated from plasma by centrifugation for 16 hours at 135,000 g in a Beckman 50.3Ti rotor. Donors were healthy, normally fed volunteers (3–4 hours after meal). Isolated TRLs were quantified by BCA protein assay (Pierce). Plasma lipids and lipoproteins were analyzed as previously described (see Supplemental Methods and ref. 13).
Statistics.
Statistical analyses were performed using Prism software (version 5; GraphPad Software). Data were analyzed by 2-tailed Student’s t test or 1-way ANOVA and presented as mean ± SEM. P values less than 0.05 were considered significant.
Study approval.
All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care–approved vivaria in the School of Medicine, UCSD, following standards and procedures approved by the local Institutional Animal Care and Use Committee (La Jolla, California, USA). Human TRL donors (healthy, normally fed volunteers) provided informed consent according to a protocol approved by the University of California San Diego Human Subjects Program.
Supplementary Material
Acknowledgments
We thank Joseph Witztum for his many helpful discussions and critical reading of this manuscript. We thank Steve Young for the ApoB-deficient mice and Linda Curtiss for the ApoB Abs and helpful discussion. This work was supported by Ruth L. Kirschstein NRSA award F31HL977212 (to J.C. Gonzales), European Community FP7 Award PIOF-GA-2010-273994 (to P.L.S.M. Gordts), and NIH grant GM33063 (to J.D. Esko).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2013;123(6):2742–2751. doi:10.1172/JCI67398.
References
- 1.Kolovou GD, et al. Assessment and clinical relevance of non-fasting and postprandial triglycerides: an expert panel statement. Curr Vasc Pharmacol. 2011;9(3):258–270. doi: 10.2174/157016111795495549. [DOI] [PubMed] [Google Scholar]
- 2.Nordestgaard BG, Freiberg JJ. Clinical relevance of non-fasting and postprandial hypertriglyceridemia and remnant cholesterol. Curr Vasc Pharmacol. 2011;9(3):281–286. doi: 10.2174/157016111795495585. [DOI] [PubMed] [Google Scholar]
- 3.Havel RJ, Kane JP. The Metabolic and Molecular Bases of Inherited Disease. 2001. Structure and metabolism of plasma lipoproteins. In: Scriver CR, eds. pp. 2705–2716. New York, New York, USA: McGraw-Hill; [Google Scholar]
- 4.Beigneux AP, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5(4):279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies BS, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12(1):42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams KJ, Chen K. Recent insights into factors affecting remnant lipoprotein uptake. Curr Opin Lipidol. 2010;21(3):218–228. doi: 10.1097/MOL.0b013e328338cabc. [DOI] [PubMed] [Google Scholar]
- 7.Foley EM, Esko JD. Hepatic heparan sulfate proteoglycans and endocytic clearance of triglyceride-rich lipoproteins. Prog Mol Biol Transl Sci. 2010;93:213–233. doi: 10.1016/S1877-1173(10)93010-X. [DOI] [PubMed] [Google Scholar]
- 8.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 9.Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A. 1994;91(10):4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101(3):689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacArthur JM, et al. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J Clin Invest. 2007;117(1):153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanford KI, et al. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J Clin Invest. 2009;119(11):3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford KI, et al. Heparan sulfate 2-O-sulfotransferase is required for triglyceride-rich lipoprotein clearance. J Biol Chem. 2010;285(1):286–294. doi: 10.1074/jbc.M109.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodoglugil U, Williamson DW, Yu Y, Farrer LA, Mahley RW. Glucuronic acid epimerase is associated with plasma triglyceride and high-density lipoprotein cholesterol levels in Turks. Ann Hum Genet. 2011;75(3):398–417. doi: 10.1111/j.1469-1809.2011.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, et al. Type 2 diabetes in mice induces hepatic overexpression of sulfatase 2, a novel factor that suppresses uptake of remnant lipoproteins. Hepatology. 2010;52(6):1957–1967. doi: 10.1002/hep.23916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassing HC, et al. Inhibition of hepatic sulfatase-2 in vivo: A novel strategy to correct diabetic dyslipidemia. Hepatology. 2012;55(6):1746–1753. doi: 10.1002/hep.25580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisgraber KH, Rall SC. Human apolipoprotein B-100 heparin-binding sites. J Biol Chem. 1987;262(23):11097–11103. [PubMed] [Google Scholar]
- 19.Flood C, Gustafsson M, Richardson PE, Harvey SC, Segrest JP, Boren J. Identification of the proteoglycan binding site in apolipoprotein B48. J Biol Chem. 2002;277(35):32228–32233. doi: 10.1074/jbc.M204053200. [DOI] [PubMed] [Google Scholar]
- 20.Weisgraber KH, Rall SC, Jr, Mahley RW, Milne RW, Marcel YL, Sparrow JT. Human apolipoprotein E. Determination of the heparin binding sites of apolipoprotein E3. J Biol Chem. 1986;261(5):2068–2076. [PubMed] [Google Scholar]
- 21.Fielding PE, Ishikawa Y, Fielding CJ. Apolipoprotein E mediates binding of normal very low density lipoprotein to heparin but is not required for high affinity receptor binding. J Biol Chem. 1989;264(21):12462–12466. [PubMed] [Google Scholar]
- 22.Saito H, et al. Characterization of the heparin binding sites in human apolipoprotein E. J Biol Chem. 2003;278(17):14782–14787. doi: 10.1074/jbc.M213207200. [DOI] [PubMed] [Google Scholar]
- 23.Lookene A, Beckstead JA, Nilsson S, Olivecrona G, Ryan RO. Apolipoprotein A-V-heparin interactions: implications for plasma lipoprotein metabolism. J Biol Chem. 2005;280(27):25383–25387. doi: 10.1074/jbc.M501589200. [DOI] [PubMed] [Google Scholar]
- 24.Hill JS, Yang D, Nikazy J, Curtiss LK, Sparrow JT, Wong H. Subdomain chimeras of hepatic lipase and lipoprotein lipase. Localization of heparin and cofactor binding. J Biol Chem. 1998;273(47):30979–30984. doi: 10.1074/jbc.273.47.30979. [DOI] [PubMed] [Google Scholar]
- 25.Sendak RA, Berryman DE, Gellman G, Melford K, Bensadoun A. Binding of hepatic lipase to heparin: identification of specific heparin-binding residues in two distinct positive charge clusters. J Lipid Res. 2000;41(2):260–268. [PubMed] [Google Scholar]
- 26.Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. Lipoprotein lipase enhances binding of lipoproteins to heparan sulfate on cell surfaces and extracellular matrix. J Clin Invest. 1992;90(5):2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams KJ, Fless GM, Petrie KA, Snyder ML, Brocia RW, Swenson TL. Mechanisms by which lipoprotein lipase alters cellular metabolism of lipoprotein(a), low density lipoprotein, and nascent lipoproteins. Roles for low density lipoprotein receptors and heparan sulfate proteoglycans. J Biol Chem. 1992;267(19):13284–13292. [PubMed] [Google Scholar]
- 28.Ji ZS, Brecht WJ, Miranda RD, Hussain MM, Innerarity TL, Mahley RW. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268(14):10160–10167. [PubMed] [Google Scholar]
- 29.Ji ZS, Dichek HL, Miranda RD, Mahley RW. Heparan sulfate proteoglycans participate in hepatic lipase- and apolipoprotein E-mediated binding and uptake of plasma lipoproteins, including high density lipoproteins. J Biol Chem. 1997;272(50):31285–31292. doi: 10.1074/jbc.272.50.31285. [DOI] [PubMed] [Google Scholar]
- 30.Fuki IV, et al. The syndecan family of proteoglycans. Novel receptors mediating internalization of atherogenic lipoproteins in vitro. J Clin Invest. 1997;100(6):1611–1622. doi: 10.1172/JCI119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skottova N, Savonen R, Lookene A, Hultin M, Olivecrona G. Lipoprotein lipase enhances removal of chylomicrons and chylomicron remnants by the perfused rat liver. J Lipid Res. 1995;36(6):1334–1344. [PubMed] [Google Scholar]
- 32.Merkel M, et al. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc Natl Acad Sci U S A. 1998;95(23):13841–13846. doi: 10.1073/pnas.95.23.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahley RW, Huang Y. Atherogenic remnant lipoproteins: role for proteoglycans in trapping, transferring, and internalizing. J Clin Invest. 2007;117(1):94–98. doi: 10.1172/JCI30889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng Y, Foley EM, Gonzales JC, Gordts PL, Li Y, Esko JD. Shedding of syndecan-1 from human hepatocytes alters very low density lipoprotein clearance. Hepatology. 2012;55(1):277–286. doi: 10.1002/hep.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havel RJ, Kane JP, Kashyap ML. Interchange of apolipoproteins between chylomicrons and high density lipoproteins during alimentary lipemia in man. J Clin Invest. 1973;52(1):32–38. doi: 10.1172/JCI107171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young SG, Koduri RK, Austin RK, Bonnet DJ, Smith RS, Curtiss LK. Definition of a nonlinear conformational epitope for the apolipoprotein B-100-specific monoclonal antibody, MB47. J Lipid Res. 1994;35(3):399–407. [PubMed] [Google Scholar]
- 37.Young SG, Smith RS, Hogle DM, Curtiss LK, Witztum JL. Two new monoclonal antibody-based enzyme-linked assays of apolipoprotein B. Clin Chem. 1986;32(8):1484–1490. [PubMed] [Google Scholar]
- 38.Milne R, et al. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989;264(33):19754–19760. [PubMed] [Google Scholar]
- 39.Curtiss LK, Edgington TS. Immunochemical heterogeneity of human plasma apolipoprotein B. I. Apolipoprotein B binding of mouse hybridoma antibodies. J Biol Chem. 1982;257(24):15213–15221. [PubMed] [Google Scholar]
- 40.Heeren J, Niemeier A, Merkel M, Beisiegel U. Endothelial-derived lipoprotein lipase is bound to postprandial triglyceride-rich lipoproteins and mediates their hepatic clearance in vivo. J Mol Med. 2002;80(9):576–584. doi: 10.1007/s00109-002-0351-5. [DOI] [PubMed] [Google Scholar]
- 41.Grosskopf I, et al. Apolipoprotein A-V deficiency results in marked hypertriglyceridemia attributable to decreased lipolysis of triglyceride-rich lipoproteins and removal of their remnants. Arterioscler Thromb Vasc Biol. 2005;25(12):2573–2579. doi: 10.1161/01.ATV.0000186189.26141.12. [DOI] [PubMed] [Google Scholar]
- 42.Wasteson A. A method for the determination of the molecular weight and molecular-weight distribution of chondroitin sulphate. J Chromatogr. 1971;59(1):87–97. doi: 10.1016/s0021-9673(01)80009-1. [DOI] [PubMed] [Google Scholar]
- 43.Plump AS, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-G. [DOI] [PubMed] [Google Scholar]
- 44.Wilsie LC, Gonzales AM, Orlando RA. Syndecan-1 mediates internalization of apoE-VLDL through a low density lipoprotein receptor-related protein (LRP)-independent, non-clathrin-mediated pathway. Lipids Health Dis. 2006;5:23. doi: 10.1186/1476-511X-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamilton RL, Wong JS, Guo LS, Krisans S, Havel RJ. Apolipoprotein E localization in rat hepatocytes by immunogold labeling of cryothin sections. J Lipid Res. 1990;31(9):1589–1603. [PubMed] [Google Scholar]
- 46.Ji ZS, Fazio S, Lee YL, Mahley RW. Secretion-capture role for apolipoprotein E in remnant lipoprotein metabolism involving cell surface heparan sulfate proteoglycans. J Biol Chem. 1994;269(4):2764–2772. [PubMed] [Google Scholar]
- 47.Horie Y, Fazio S, Westerlund JR, Weisgraber KH, Rall SC. The functional characteristics of a human apolipoprotein E variant (cysteine at residue 142) may explain its association with dominant expression of type III hyperlipoproteinemia. J Biol Chem. 1992;267(3):1962–1968. [PubMed] [Google Scholar]
- 48.Ji ZS, Fazio S, Mahley RW. Variable heparan sulfate proteoglycan binding of apolipoprotein E variants may modulate the expression of type III hyperlipoproteinemia. J Biol Chem. 1994;269(18):13421–13428. [PubMed] [Google Scholar]
- 49.Mahley RW, Huang Y, Rall SC. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia). Questions, quandaries, and paradoxes. J Lipid Res. 1999;40:1933–1949. [PubMed] [Google Scholar]
- 50.Pennacchio LA, et al. An apolipoprotein influencing triglycerides in humans and mice revealed by comparative sequencing. Science. 2001;294(5540):169–173. doi: 10.1126/science.1064852. [DOI] [PubMed] [Google Scholar]
- 51.Merkel M, et al. Apolipoprotein AV accelerates plasma hydrolysis of triglyceride-rich lipoproteins by interaction with proteoglycan-bound lipoprotein lipase. J Biol Chem. 2005;280(22):21553–21560. doi: 10.1074/jbc.M411412200. [DOI] [PubMed] [Google Scholar]
- 52.Shu X, et al. Intravenous injection of apolipoprotein A-V reconstituted high-density lipoprotein decreases hypertriglyceridemia in apoav–/– mice and requires glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1. . Arterioscler Thromb Vasc Biol. 2010;30(12):2504–2509. doi: 10.1161/ATVBAHA.110.210815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilsson SK, Heeren J, Olivecrona G, Merkel M. Apolipoprotein A-V; a potent triglyceride reducer. Atherosclerosis. 2011;219(1):15–21. doi: 10.1016/j.atherosclerosis.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Talmud PJ, Cooper JA, Hattori H, Miller IP, Miller GJ, Humphries SE. The apolipoprotein A-V genotype and plasma apolipoprotein A-V and triglyceride levels: prospective risk of type 2 diabetes. Results from the Northwick Park Heart Study II. Diabetologia. 2006;49(10):2337–2340. doi: 10.1007/s00125-006-0387-0. [DOI] [PubMed] [Google Scholar]
- 55.O’Brien PJ, et al. The novel apolipoprotein A5 is present in human serum, is associated with VLDL, HDL, and chylomicrons, and circulates at very low concentrations compared with other apolipoproteins. Clin Chem. 2005;51(2):351–359. doi: 10.1373/clinchem.2004.040824. [DOI] [PubMed] [Google Scholar]
- 56.Mahley RW, Ji ZS. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40(1):1–16. [PubMed] [Google Scholar]
- 57.Camejo G, Hurt-Camejo E, Wiklund O, Bondjers G. Association of apo B lipoproteins with arterial proteoglycans: Pathological significance and molecular basis. Atherosclerosis. 1998;139(2):205–222. doi: 10.1016/S0021-9150(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 58.Diard P, Malewiak MI, Lagrange D, Griglio S. Hepatic lipase may act as a ligand in the uptake of artificial chylomicron remnant-like particles by isolated rat hepatocytes. Biochem J. 1994;299(pt 3):889–894. doi: 10.1042/bj2990889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dichek HL, et al. Overexpression of hepatic lipase in transgenic mice decreases apolipoprotein B-containing and high density lipoproteins. Evidence that hepatic lipase acts as a ligand for lipoprotein uptake. J Biol Chem. 1998;273(4):1896–1903. doi: 10.1074/jbc.273.4.1896. [DOI] [PubMed] [Google Scholar]
- 60.Dichek HL, et al. Hepatic lipase overexpression lowers remnant and LDL levels by a noncatalytic mechanism in LDL receptor-deficient mice. J Lipid Res. 2001;42(2):201–210. [PubMed] [Google Scholar]
- 61.Dichek HL, Qian K, Agrawal N. The bridging function of hepatic lipase clears plasma cholesterol in LDL receptor-deficient “apoB-48-only” and “apoB-100-only” mice. J Lipid Res. 2004;45(3):551–560. doi: 10.1194/jlr.M300459-JLR200. [DOI] [PubMed] [Google Scholar]
- 62.Beisiegel U, Weber W, Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 1991;88(19):8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyon M, Deakin JA, Gallagher JT. Liver heparan sulfate structure. A novel molecular design. J Biol Chem. 1994;269(15):11208–11215. [PubMed] [Google Scholar]
- 64.Conrad HE. Heparin-Binding Proteins. San Diego, California, USA: Academic Press; 1998. [Google Scholar]
- 65.Mansouri RM, et al. Atheroprotective effect of human apolipoprotein A5 in a mouse model of mixed dyslipidemia. Circ Res. 2008;103(5):450–453. doi: 10.1161/CIRCRESAHA.108.179861. [DOI] [PubMed] [Google Scholar]
- 66.Grosskopf I, Shaish A, Afek A, Shemesh S, Harats D, Kamari Y. Apolipoprotein A-V modulates multiple atherogenic mechanisms in a mouse model of disturbed clearance of triglyceride-rich lipoproteins. Atherosclerosis. 2012;224(1):75–83. doi: 10.1016/j.atherosclerosis.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 67.Wei W, Ninonuevo MR, Sharma A, Danan-Leon LM, Leary JA. A comprehensive compositional analysis of heparin/heparan sulfate-derived disaccharides from human serum. Anal Chem. 2011;83(10):3703–3708. doi: 10.1021/ac2001077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farese RV, et al. Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc Natl Acad Sci U S A. 1996;93(13):6393–6398. doi: 10.1073/pnas.93.13.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rohlmann A, Gotthardt M, Willnow TE, Hammer RE, Herz J. Sustained somatic gene inactivation by viral transfer of Cre recombinase. Biotechnology. 1996;14(11):1562–1565. doi: 10.1038/nbt1196-1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.