Abstract
We characterized the sources of variability for polybrominated diphenyl ethers (PBDEs) in residential dust and provided guidance for investigators who plan to use residential dust to assess exposure to PBDEs. We collected repeat dust samples from 292 households in the Northern California Childhood Leukemia Study during two sampling rounds (from 2001–2007 and during 2010) using household vacuum cleaners and measured 22 PBDEs using high resolution gas chromatography-high resolution mass spectrometry. Median concentrations for individual PBDEs ranged from <0.1–2,500 ng per g of dust. For each of eight representative PBDEs, we used a random-effects model to apportion total variance into regional variability (0–11%), intra-regional between-household variability (17–50%), within-household variability over time (38–74%), and within-sample variability (0–23%) and we used a mixed-effects model to identify determinants of PBDE levels. Regional differences in PBDE dust levels were associated with residential characteristics that differed by region, including the presence of furniture with exposed or crumbling foam and the recent installation of carpets in the residence. Intra-regional differences between households were associated with neighborhood urban density, racial and ethnic characteristics, and to a lesser extent, income. For some PBDEs, a decreasing time trend explained a modest fraction of the within-household variability; however, most of the within-household variability was unaccounted for by our mixed-effects models. Our findings indicate that it may be feasible to use residential dust for retrospective assessment of PBDE exposures in studies of children’s health (e.g., the Northern California Childhood Leukemia Study).
Keywords: Environmental exposures, house dust, polybrominated diphenyl ethers
1. INTRODUCTION
Polybrominated diphenyl ethers (PBDEs) have been used worldwide as chemical flame retardants to treat plastics and textiles in consumer products (U.S. Environmental Protection Agency 2010). Three commercial PBDE mixtures, Penta-BDE (composed primarily of BDEs 99, 47, 100, 153, and 154), Octa-BDE (composed primarily of BDEs 183, 197, 207, 196, and 153), and Deca-BDE (composed primarily of BDE-209), have been manufactured (La Guardia et al. 2006). In the U.S., Penta- and Octa-BDE are no longer used and Deca-BDE is being phased out (U.S. Environmental Protection Agency 2012); however, consumer goods that have been treated with any of the three PBDE commercial mixtures can still be found in U.S. homes. Because PBDEs are not chemically bound to the polymers they treat, these additives can migrate into the environment. Indeed, PBDEs have been found in residential dust at high concentrations – with several studies of U.S. homes reporting median concentrations for major PBDE congeners of at least one part per million (Whitehead et al. 2011). Due to the State of California’s unique flammability standards, dust samples from California homes have been reported to have exceptionally high levels of PBDEs (Dodson et al. 2012; Zota et al. 2008). Investigators have demonstrated that PBDE levels in paired samples of human serum and residential dust are significantly correlated, suggesting that dust ingestion is an important route of exposure to PBDEs in U.S. homes (Johnson et al. 2010; Stapleton et al. 2012).
While many researchers have measured PBDEs in dust, most estimate human exposure to PBDEs using a single dust sample and only a few have sampled dust repeatedly in the same households and characterized the variability of dust measurements within households over time (Allen et al. 2008a; Batterman et al. 2009; Dodson et al. 2012; Harrad et al. 2008; Muenhor and Harrad 2012; Vorkamp et al. 2011). The magnitude of temporal variability that exists in residential-dust measurements over years or decades has not been estimated and may be important for accurate assessments of long-term exposure. Moreover, previous investigations have not compared the magnitude of within-household temporal variability to the magnitude of between-household variability. When estimating the health effects related to a chemical exposure, it is this variance ratio that is related to the degree of exposure measurement error and predictive of the underestimation of risk estimates (Armstrong 1998).
To characterize the long-term temporal variability of PBDE concentrations in residential dust, we analyzed 22 PBDEs, in dust samples collected in two rounds separated by 3–8 years. Because exposures to PBDEs have been associated with endocrine disruption (Chevrier et al. 2010; Meeker et al. 2009; Stapleton et al. 2011; Turyk et al. 2008), adverse birth outcomes (Chao et al. 2007; Harley et al. 2010; Harley et al. 2011), and adverse neurological development (Eskenazi et al. 2013; Herbstman et al. 2010); we also identified determinants of residential-dust PBDE levels and discuss strategies to limit human exposures to PBDEs. Finally, because Penta-BDE and Octa-BDE were banned for distribution in commerce on and after June 1, 2006 in California (Chan 2004), we evaluate the long-term trends in residential-dust PBDE levels from 2001 to 2010.
2. METHODS
2.1. Study population
Residential dust samples for our PBDE analysis were collected as part of the Northern California Childhood Leukemia Study, a case–control study conducted in the San Francisco Bay area and California Central Valley. Residential dust samples were originally collected from study homes as one strategy for identifying possible environmental risk factors for childhood leukemia and various persistent environmental contaminants including pesticides, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons have been measured in the samples. Homes of children with leukemia and homes of healthy children were eligible for initial dust collection (from 2001–2007) if the children were 0–7 years-old at study enrollment. Subsequently, in 2010, a subset of the households that participated in the initial dust collection was eligible for repeated dust collection if the family was still living in the same home. Among 629 households who participated in the initial dust collection, 225 were eligible for a second dust collection and 203 households had two dust samples analyzed for PBDEs. For an additional 89 households who participated in the initial dust collection, but who were ineligible for repeated dust collection, we also analyzed their original dust sample for PBDEs. We obtained written informed consent from participating subjects in accordance with the institutional review boards’ requirements at the University of California, Berkeley.
2.2. Collection of residential dust
We collected dust samples from subjects’ household vacuum cleaners during two sampling rounds; from 2001–2007 and again during 2010. The median interval between repeated sample collections was 4.8 years (range of 2.6 – 8.6 years). During the first round of dust sampling, we collected vacuum cleaner dust and administered a questionnaire during an in-home visit. For the second round of dust sampling, we interviewed subjects via telephone and instructed them to mail the contents of their vacuum cleaners to the study center in prepaid parcels. We stored dust samples away from heat (4°C or colder) and light prior to chemical analysis.
2.3. Laboratory analysis of PBDEs
The analytical protocol for PBDE analysis has been previously described (Whitehead 2011). Briefly, we homogenized and fractionated the dust samples using a mechanical sieve shaker equipped with a 100-mesh sieve to obtain dust particles smaller than 150 μm. Portions of fine dust (0.2 g) were spiked with nine 13C-labeled internal standards, extracted via accelerated solvent extraction, purified by silica-gel column chromatography and gel permeation chromatography, concentrated to 250 μL, solvent exchanged into tetradecane, and spiked with two 13C-labeled recovery standards. Finally, we analyzed 22 PBDEs [BDEs 28, 32, 47, 66, 71, 99, 100, 153, 154, 155, 179, 183, 190, 196, 197, 201, 202, 203, 206, 207, 208, and 209] using isotope dilution/high resolution gas chromatography-high resolution mass spectrometry (HRGC-HRMS, DFS, Thermo-Finnigan, Bremen, Germany) equipped with a DB-5 column (15m × 0.25mm i.d., 0.1μm film thickness, J&W Scientific, USA) and operated in electron impact ionization-selective ion monitoring (EI-SIM) mode (for a list of ions used to quantify PBDE concentrations refer to the Appendix, Table A1; for details regarding the thermal stability of BDE-209 during analysis refer to the Appendix, Table A2). Because our findings were similar for PBDE congeners within each homologue group, we present results for eight representative PBDEs, one from each homologue group from tri- to deca-BDE.
2.4. Household characteristics
Parents initially participated in structured in-home interviews designed to ascertain information relevant to childhood leukemia. Among other things, this questionnaire established general demographic information such as the annual household income, the educational attainment of the parents, and the ethnicity and race of the parents (mothers were categorized into three groups: Hispanic; non-Hispanic, White or Asian; and non-Hispanic, non-White, non-Asian, i.e., non-Hispanic mothers of other races). Subsequently, households participating in the repeated dust collection (N = 203) completed an additional telephonic questionnaire designed to ascertain information about sources of residential chemical exposures. The latter questionnaire covered topics related to sources of PBDEs (See Appendix, Table A3 for details), including the quantity and daily use of televisions and computers; the presence of upholstered furniture (quantity as well as quality, i.e., the presence of crumbling or exposed foam, and age, i.e., purchased before or after 2006); and the quantity and installation history (since move-in date) of carpeting. Moreover, this questionnaire obtained information about residential characteristics including, the square footage, construction date, construction material, and type of residence (e.g., single family home, apartment) as well as resident activities including, occupations, window and air conditioner use, and shoe removal habits. Finally, we gathered information about the characteristics of the vacuum cleaner including the type of vacuum and the frequency of its use.
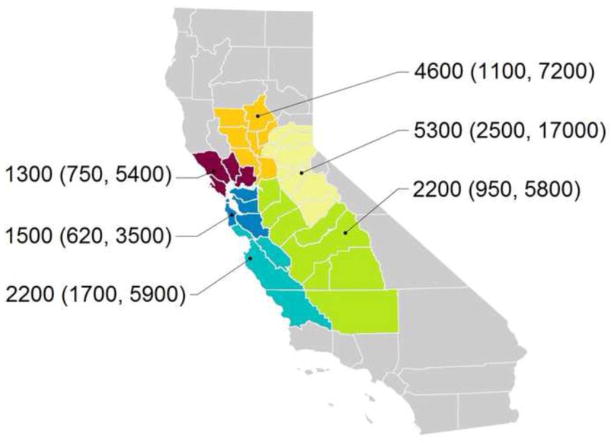
We used a global positioning device to determine the latitude and longitude for each residence and classified each residence as belonging to one of six geographic regions as shown in Figure 1. We linked each location to the corresponding U.S. Census block and identified each residence as urban, suburban, or rural based on the Census Bureau’s delineations (U.S. Census Bureau).
Figure 1. Regional variability in BDE-99 concentrations. Shown are median (interquartile range) BDE-99 concentrations (in ng/g) in dust samples collected from 202 residences in the Northern California Childhood Leukemia Study during 2010.
BLUE = the metropolitan San Francisco Bay area (Alameda, Contra Costa, Santa Clara, San Francisco, and San Mateo counties);
RED = the northern San Francisco Bay area (Marin, Napa, Solano, and Sonoma counties);
ORANGE = the Sacramento Valley (Butte, Colusa, Glenn, Sacramento, Sutter, Yolo, and Yuba counties);
YELLOW = the Sierra Mountains (Amador, Calaveras, El Dorado, Mariposa, Nevada, Placer, and Tuolumne counties);
GREEN = the San Joaquin Valley (Fresno, Kern, Kings, Madera, Merced, San Joaquin, Stanislaus, Tulare counties);
TEAL = the California central coast (Monterey, San Benito, San Luis Obispo, and Santa Cruz counties).
2.5. Differences between duplicate, replicate, and repeat samples
We analyzed samples in batches of 12, with each batch consisting of 8 samples, 1 method blank, 1 duplicate sample pair (i.e., two 200-mg portions of fine dust taken from the same vacuum cleaner), and 1 inter-batch quality control sample (i.e., a 200-mg portion of fine dust taken from the quality control vacuum cleaner dust). Because we prepared and analyzed an inter-batch quality control replicate alongside each successive sample batch, the inter-batch quality control results illustrate the reproducibility of the dust preparation and analytical methods over the course of the study. Likewise, the duplicate samples illustrate the reproducibility of the dust preparation and analytical methods within each sample batch. For some batches, we replaced the inter-batch quality control sample with a National Institute of Standards and Technology Standard Reference Material 2585 (NIST SRM 2585) dust sample, which contained certified concentrations of eleven of the 22 PBDEs analyzed in this study.
NIST has homogenized the SRM 2585 dust with a rigorous protocol, so results obtained from any 200-mg replicate should be highly reproducible. To compare the magnitude of variability observed in quality control samples to the magnitude of variability observed in repeat samples collected at intervals of 3–8 years we calculated the relative percent difference (RPD) [i.e., RPD=200*abs(sample1 − sample2)/(sample1 + sample2)]. For the 40 inter-batch quality control replicates and 16 NIST SRM 2585 replicates it was necessary to compare multiple combinations of matched sample pairs, so RPDs were calculated using a random sampling routine with replacement (100,000 iterations). We also calculated the ratio between repeat samples (i.e., round 2 sample/round 1 sample).
2.6. Data imputation
We determined method reporting limits (MRL) for each PBDE based on the precision of the results from the method blanks (i.e., MRL = 3 * standard deviation of the signal of each PBDE in the method blanks from 57 sample runs). We assigned all values below the MRL (see Table 1) a concentration equal to the MRL divided by the square root of two (Hornung and Reed 1990). Because, some participants were unable or unwilling to complete all aspects of the questionnaires, in regression analyses, missing questionnaire responses were replaced by the population average from non-missing households (e.g., eight respondents did not know their residence’s construction date and we used the population average, i.e., 1972; see Appendix, Table A4 for details on data imputation). Including data from households with incomplete questionnaires in our multivariable regression models maximized sample size and statistical power. We were also unable to pinpoint six residences using the global positioning system, so we approximated their location using postal codes.
Table 1.
Summary statistics for PBDE measurements in dust collected on two occasions from 292 residences in the Northern California Childhood Leukemia Study, 2001–2007 and 2010.
| PBDE | Number of bromine atoms, homologue group prefix | Method reporting limit, ng/g | Dust Collection Round 1, 2001–2007, N = 292
|
Dust Collection Round 2, 2010, N = 203
|
Maximum Concentration, ng/g | Spearman Rank Correlation Coefficient Between Rounds, rs | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| Values Above Method Reporting Limit, N (%) | Median concentration, ng/g | Values Above Method Reporting Limit, N (%) | Median concentration, ng/g | ||||||
| BDE-28 | 3, tri | 0.5 | 290(100) | 24 | 202(100) | 20 | 470 | 0.51 | <0.0001 |
| BDE-32 | 3, tri | 0.1 | 31(11) | <MRL | 0(0) | <MRL | 44 | NA | |
| BDE-47 | 4, tetra | 7 | 292(100) | 1,500 | 202(100) | 1,300 | 50,000 | 0.51 | <0.0001 |
| BDE-66 | 4, tetra | 0.9 | 291(100) | 26 | 202(100) | 25 | 610 | 0.50 | <0.0001 |
| BDE-71 | 4, tetra | 0.4 | 269(92) | 41 | 195(97) | 38 | 4,400 | 0.31 | <0.0001 |
| BDE-99 | 5, penta | 10 | 292(100) | 2,400 | 202(100) | 2,100 | 89,000 | 0.56 | <0.0001 |
| BDE-100 | 5, penta | 3 | 292(100) | 400 | 202(100) | 330 | 13,000 | 0.48 | <0.0001 |
| BDE-153 | 6, hexa | 2 | 292(100) | 310 | 203(100) | 290 | 12,000 | 0.55 | <0.0001 |
| BDE-154 | 6, hexa | 1 | 292(100) | 180 | 203(100) | 150 | 8,500 | 0.54 | <0.0001 |
| BDE-155 | 6, hexa | 0.2 | 292(100) | 10 | 203(100) | 9.0 | 510 | 0.55 | <0.0001 |
| BDE-179 | 7, hepta | 0.2 | 95(33) | <MRL | 39(19) | <MRL | 340 | NA | |
| BDE-183 | 7, hepta | 0.4 | 292(100) | 28 | 201(100) | 17 | 2,500 | 0.41 | <0.0001 |
| BDE-190 | 7, hepta | 0.2 | 235(85) | 1.6 | 100(71) | 1.1 | 190 | 0.41 | <0.0001 |
| BDE-196 | 8, octa | 0.6 | 292(100) | 12 | 201(99) | 8.2 | 870 | 0.34 | <0.0001 |
| BDE-197 | 8, octa | 0.4 | 292(100) | 12 | 202(100) | 7.6 | 1,200 | 0.35 | <0.0001 |
| BDE-201 | 8, octa | 0.5 | 292(100) | 4.5 | 203(100) | 3.6 | 730 | 0.22 | 0.002 |
| BDE-202 | 8, octa | 0.4 | 274(94) | 1.3 | 191(94) | 1.4 | 92 | 0.22 | 0.002 |
| BDE-203 | 8, octa | 0.6 | 291(100) | 12 | 202(100) | 8.3 | 870 | 0.26 | 0.0001 |
| BDE-206 | 9, nona | 3 | 292(100) | 71 | 201(99) | 75 | 6,200 | 0.19 | 0.007 |
| BDE-207 | 9, nona | 2 | 292(100) | 57 | 203(100) | 54 | 5,000 | 0.19 | 0.005 |
| BDE-208 | 9, nona | 2 | 292(100) | 36 | 203(100) | 33 | 1,600 | 0.16 | 0.02 |
| BDE-209 | 10, deca | 60 | 292(100) | 2,300 | 202(100) | 2,500 | 220,000 | 0.18 | 0.01 |
MRL = Method reporting limit
2.7. Random-effects models
To apportion the observed variance in PBDE concentrations into four components describing regional variability, intra-regional between-household variability, within-household variability over time, and within-sample variability we used a hierarchical random-effects model,
| (1) |
for h = 1,2,…,6 regions; i = 1,2,…,293 households (i.e., 292 study residences and the inter-batch quality control residence); j = 1 or 2 repeat sample collections; and k = 1,2…,40 replicate samples from the same vacuum bag (see Appendix for additional details regarding Models 1–6).
We assume bh, bhi, bhij, and ehijk are mutually independent and normally distributed random variables, with means of zero and variances of , and , representing the between-region variability, the intra-regional between-household variability, the within-household variability over time, and the within-sample variability, respectively. Using Proc Mixed (SAS v.9.1, Cary, NC) we fit the model described in Equation 1 and estimated variance components ( ) and variance ratios . For each PBDE, we used the magnitude of the variance ratio to estimate the potential impact of measurement error on an odds ratio (ORTrue = 2.0) for a hypothetical case-control study that employs a single dust sample to assess long-term average exposure to PBDEs , as previously described (Whitehead et al. 2012).
2.8. Mixed-effects models
We used mixed-effects models to identify determinants of PBDE concentrations at each hierarchical level. We considered explanatory variables based on each question listed in Table A3 (see appendix) for inclusion in mixed-effects models and retained factors that explained variability in PBDE levels. In addition to the Model 1 random effects, we included five fixed effects that explained regional differences in PBDE levels in Model 2; namely, the presence of upholstered furniture with crumbling or exposed foam, carpet installation since move-in date, residence less than 25% carpeted, residence square footage, and residence construction date.
Likewise, in addition to the Model 1 random effects, we included four fixed effects that explained differences in PBDE levels between households within a region in Model 3; namely residence in a rural location; mother is Hispanic; mother is not Hispanic, White, or Asian; and household annual income is at least $75,000.
Similarly, in addition to the Model 1 random effects, we included two fixed effects that explained changes in PBDE levels within households over time in Model 4; namely the trend in PBDE levels over time and the change in PBDE levels from first to second sampling round associated with carpet installation (any time after residents moved in).
In the fully saturated Model 5, we included the random effects from Model 1 as well as each explanatory variable (hereafter referred to as covariates) from Models 2–4. We fit each of the above mixed-effects models (Models 2–5) for 451 observations with covariate data (i.e., 406 samples collected from 203 homes during repeat sample collections and 45 duplicate samples) and excluded the 139 observations without covariate data (i.e., 40 inter-batch quality control replicates and 89 samples with 10 duplicates collected during Round 1). For comparison, we re-ran the random-effects model (Model 1) using this set of 451 observations.
2.9. Factors that increased within-household variability
To identify factors that resulted in large within-household changes in PBDE concentrations between sampling rounds, we used a multivariable linear regression model of RPD magnitude (Model 6). These factors were not necessarily associated with systematic increases or decreases in PBDE levels; rather they were associated with increased variability within a home. We considered each fixed effect from Model 5 as well as case-control status for inclusion as explanatory variables in the RPD model.
3. RESULTS
Table 1 shows summary statistics for PBDE measurements made in 292 California households. Common PBDEs found in commercial mixtures (e.g., BDE-47, BDE-99, and BDE-209) were detected in 100% of dust samples. Median PBDE concentrations ranged from <0.1–2,400 ng/g for dust samples collected in Round 1 and from <0.1–2,500 ng/g for dust samples collected in Round 2. Spearman rank correlation coefficients for inter-round comparisons of dust concentrations of PBDEs ranged from 0.16–0.56 (p-value < 0.02 for all PBDEs). Inter-round correlations were generally stronger for households with repeat samples collected 3–6 years apart than for households with repeat samples collected 7–8 years apart (see Appendix, Table A5). With the exception of BDE-71, we observed an inverse relationship between the number of bromine atoms in each PBDE congener and the strength of the inter-round correlation (i.e., rs for tri- to hexa-BDEs > rs for hepta-BDEs > rs for octa-BDEs > rs for nona- to deca-BDEs).
3.1. Variability in samples compared to quality control samples
Table 2 shows the median and maximum RPDs between concentrations of eight representative PBDEs in matched pairs of various quality control dust samples and in matched pairs of dust samples from two sample collections separated by 3–8 years. For 55 pairs of duplicate samples analyzed in the same batch, the RPDs between matched PBDE concentrations were generally modest for tri- to hexa-BDEs (median RPD range: 3–6%) and slightly larger for hepta- to deca-BDEs (median RPD range: 12–19%). Likewise, when 16 replicate NIST SRM 2585 dust samples were analyzed over the course of the study, the RPDs between randomly selected pairs of PBDE concentrations were generally modest, with tri- to hexa-BDEs having smaller RPDs (median RPD range: 6–13%) than hepta- to deca-BDEs (median RPD range: 11–16%). In 40 replicate quality control samples analyzed alongside successive sample batches over the course of the study, RPDs between randomly selected pairs of PBDE concentrations were generally modest for tri- to hexa-BDEs (median RPD range: 9–11%), but substantially larger for the hepta- to deca-BDEs (median RPD range: 30–39%). In comparison to the quality control samples, the 203 matched pairs of dust samples from repeat sample collections had the highest RPDs (median RPD range: 58–68%). Expressed as a ratio, PBDE concentrations in repeat dust samples typically differed by approximately 2-fold (e.g., interquartile ratio range: 0.5–1.9; 0.5–1.8; 0.5–1.8, for BDEs 47, 99, and 209 respectively). Levels of tri- to hexa-BDEs increased in roughly the same number of homes as they decreased (i.e., median ratio ~ 1), but more than half of the homes experienced a decrease in levels of hepta- to deca-BDEs (i.e., median ratio < 0.9). Some households had widely differing PBDE concentrations in dust from the two collection rounds (e.g., concentrations of BDEs 47, 99, and 209 increased within a home by as much as 36-, 33-, and 520-fold between rounds, respectively and concentrations of BDEs 47, 99, and 209 decreased within a home by as much as 53-, 87-, and 79-fold between rounds, respectively).
Table 2.
Relative percent difference and ratios between concentrations of PBDEs in matched samples.
| PBDE | Relative Percent Difference between Matched Samples
|
Ratio between Repeat Samples (Round 2/Round 1)a | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Batch Quality Control, Duplicate Samples | Inter-batch Quality Control, NIST SRM 2585 | Inter-batch Quality Control, Replicate Samples | Repeat Samples, Round 1 & Round 2 | ||||||||||||
|
|
|
|
|
|
|||||||||||
| Median | Max | Median | Max | Median | Max | Median | Max | Min | 5th | 25th | Median | 75th | 95th | Max | |
| BDE-28 | 3.4 | 180 | 6.1 | 27 | 11 | 51 | 58 | 190 | 0.03 | 0.15 | 0.54 | 1.02 | 1.8 | 4.1 | 46 |
| BDE-47 | 4.8 | 30 | 7.8 | 27 | 9.1 | 36 | 64 | 190 | 0.02 | 0.12 | 0.51 | 0.97 | 1.9 | 5.2 | 36 |
| BDE-99 | 5.7 | 130 | 13 | 46 | 11 | 54 | 61 | 200 | 0.01 | 0.13 | 0.51 | 0.99 | 1.8 | 7.4 | 33 |
| BDE-153 | 3.3 | 23 | 7.3 | 25 | 9.0 | 59 | 65 | 190 | 0.02 | 0.15 | 0.47 | 0.92 | 1.7 | 5.2 | 31 |
| BDE-183 | 12 | 200 | 13 | 41 | 30 | 200 | 66 | 200 | 0.001 | 0.13 | 0.38 | 0.68 | 1.4 | 5.6 | 36 |
| BDE-196 | 19 | 130 | 15 | 68 | 39 | 140 | 68 | 190 | 0.02 | 0.12 | 0.37 | 0.68 | 1.3 | 7.5 | 66 |
| BDE-206 | 12 | 91 | 11 | 52 | 35 | 130 | 64 | 200 | 0.02 | 0.09 | 0.47 | 0.82 | 1.7 | 8.9 | 270 |
| BDE-209 | 17 | 110 | 16 | 55 | 32 | 140 | 66 | 200 | 0.01 | 0.13 | 0.45 | 0.88 | 1.8 | 8.6 | 520 |
Max = Maximum
NIST SRM 2585 = National Institute of Standards and Technology Standard Reference Material No. 2585
Relative percent difference, RPD = 200*abs(sample 1 − sample 2)/(sample 1 + sample 2)
Ratio < 1 indicates a decrease in PBDE concentration from Round 1 to Round 2
3.2. Random-effects modeling
For each of eight representative PBDEs, Table 3 shows estimated variance components from the hierarchical random-effects model (Model 1) with corresponding variance ratios. Between-region variability accounted for 0–11% of the total variability in PBDE concentrations and regional variability was only evident for tri- to hexa-BDEs. For example, Figure 1 shows the regional variability of BDE-99 concentrations. Intra-regional between-household variability accounted for 17–50% of the total variability in PBDE concentrations and between-household variability was less evident for BDE-206 and BDE-209. Within-household variability over time accounted for 38–74% of the total variability in PBDE concentrations and within-household variability was greatest for BDE-206 and BDE-209. Within-sample analytical variability accounted for 0.5–23% of the total variability in PBDE concentrations and analytical variability was more evident for hepta- to deca-BDEs. The variance ratio ranged from 0.8–4.5. Based on these variance ratios, we would expect a hypothetical ORTrue of 2.0 to be attenuated to a value as low as 1.1 (range 1.1–1.5) in a case-control study that employs a single dust sample to assess long-term average exposure to PBDEs.
Table 3.
Estimated variance components for selected PBDEs from the random-effects model (Model 1a) with corresponding variance ratios.
| PBDE | Variance Component Estimate (95% Confidence Interval)
|
λb |
|
Percent of Total Variance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Between-region, | Intra-regional between-household, | Within-household over time, | Within-sample, |
|
|
|
|
||||
| BDE-28 | 0.12 (0.04, 2.1) | 0.43 (0.32, 0.61) | 0.49 (0.40, 0.62) | 0.06 (0.05, 0.09) | 1.0 | 1.4 | 11 | 39 | 44 | 6 | |
| BDE-47 | 0.09 (0.02, 3.0) | 0.48 (0.36, 0.69) | 0.62 (0.52, 0.76) | 0.01 (0.00, 0.01) | 1.1 | 1.4 | 7 | 40 | 52 | 0.5 | |
| BDE-99 | 0.10 (0.03, 3.0) | 0.66 (0.50, 0.90) | 0.68 (0.57, 0.84) | 0.02 (0.01, 0.03) | 0.9 | 1.4 | 7 | 45 | 47 | 1 | |
| BDE-153 | 0.07 (0.02, 7.2) | 0.72 (0.56, 0.96) | 0.64 (0.53, 0.78) | 0.01 (0.01, 0.01) | 0.8 | 1.5 | 5 | 50 | 45 | 0.5 | |
| BDE-183 | 0 | 0.55 (0.40, 0.81) | 0.54 (0.39, 0.78) | 0.32 (0.25, 0.44) | 1.6 | 1.3 | 0 | 39 | 38 | 23 | |
| BDE-196 | 0 | 0.40 (0.28, 0.63) | 0.66 (0.54, 0.83) | 0.10 (0.08, 0.14) | 1.9 | 1.3 | 0 | 34 | 57 | 9 | |
| BDE-206 | 0.01 (0.002, 430) | 0.19 (0.10, 0.51) | 0.82 (0.67, 1.02) | 0.09 (0.07, 0.12) | 4.5 | 1.1 | 0.9 | 17 | 74 | 8 | |
| BDE-209 | 0.02 (0.003, 51) | 0.19 (0.10, 0.52) | 0.79 (0.64, 1.00) | 0.12 (0.09, 0.16) | 4.4 | 1.1 | 1 | 17 | 71 | 10 | |
In Table 3, Model 1 was fit for 590 observations including 406 samples collected from 203 homes during repeat sample collections, 89 samples from homes that were sampled once, 55 duplicate samples, and 40 inter-batch quality control replicates.
Estimated variance ratio,
Expected odds ratio,
3.3. Mixed-effects modeling
For each of eight representative PBDEs, Table 4 compares estimated variance components from the hierarchical random-effects model without covariates (Model 1) and the mixed-effects models that included explanatory variables (Models 2–5). Model 2 included the presence of exposed foam, carpet coverage, carpet installation, residential square footage, and residence construction date and explained 17–100% of the regional variability in PBDE concentrations. As illustrated in Figure 1, we observed the highest tri- to hexa-BDE concentrations in homes from the Sierra Mountain region. Households in this region were more likely to be recently constructed (median construction date was 1992), more likely to have furniture with crumbling or exposed foam (22%), more likely to have carpeted floors (92% were at least 25% carpeted), and less likely to have installed new carpeting since moving in (23%) than households from any other region. Model 3 included the urban density of the residence, the mother’s race and ethnicity, and household annual income and explained 0–28% of the intra-regional between-household variability in PBDE concentrations. Model 4 included the trend in PBDE levels over time and the change in PBDE levels associated with carpet installation and explained 0–8% of the within-household variability in PBDE concentrations over time. Model 5 included each of the covariates from Models 2–4 and explained 0–52% of the regional variability, 0–31% of the intra-regional between-household variability, and 0–8% of the within-household variability in PBDE concentrations over time.
Table 4.
Changes in estimated variance components for selected PBDEs from the random-effects modela to the mixed-effects models.
| PBDE | Model 1, Random effects, No Covariates
|
Model 2, Mixed effectsb
|
Model 3, Mixed effectsc
|
Model 4, Mixed effectsd
|
Model 5, Mixed effects, All Covariates
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| σ2BR | σ2BH | σ2WH | σ2BR | %BR | σ2BH | %BH | σ2WH | %WH | σ2BR | %BR | σ2BH | %BH | σ2WH | %WH | |
| BDE-28 | 0.10 | 0.47 | 0.47 | 0.05 | 55 | 0.37 | 22 | 0.45 | 4 | 0.07 | 31 | 0.3 | 25 | 0.46 | 3 |
| BDE-47 | 0.06 | 0.53 | 0.64 | 0 | 100 | 0.38 | 28 | 0.62 | 3 | 0.04 | 32 | 0.3 | 31 | 0.62 | 3 |
| BDE-99 | 0.08 | 0.73 | 0.69 | 0.01 | 81 | 0.54 | 26 | 0.69 | 0 | 0.04 | 50 | 0.5 | 31 | 0.69 | 0 |
| BDE-153 | 0.05 | 0.76 | 0.65 | 0 | 100 | 0.58 | 24 | 0.63 | 3 | 0.02 | 52 | 0.5 | 26 | 0.63 | 3 |
| BDE-183 | 0.02 | 0.57 | 0.57 | 0.02 | 17 | 0.52 | 8 | 0.53 | 8 | 0.02 | −12 | 0.5 | 2 | 0.53 | 8 |
| BDE-196 | 0 | 0.42 | 0.70 | 0 | 0 | 0.43 | −2 | 0.67 | 5 | 0 | 0 | 0.4 | −8 | 0.67 | 5 |
| BDE-206 | 0 | 0.19 | 0.88 | 0 | 0 | 0.20 | −4 | 0.88 | 0 | 0 | 0 | 0.2 | −6 | 0.88 | 0 |
| BDE-209 | 0 | 0.19 | 0.85 | 0 | 0 | 0.19 | −2 | 0.85 | 0 | 0 | 0 | 0.1 | 1 | 0.86 | −1 |
σ2BR = Between-region variance
σ2BH = Intra-regional between-household variance
σ2WH = Within-household variance over time
%BR = Percent of between-region variance from the random-effects model (Model 1) explained by the covariates included in the mixed-effects model (Model 2 or 5)
%BH = Percent of intra-regional between-household variance from the random-effects model (Model 1) explained by the covariates included in the mixed-effects model (Model 3 or 5)
%WH = Percent of within-household variance over time from the random-effects model (Model 1) explained by the covariates included in the mixed-effects model (Model 4 or 5)
In Table 4, Models 1–5 were fit for 451 observations including 406 samples collected from 203 homes during repeat sample collections and 45 duplicate samples; excluding 139 observations without covariate data (40 inter-batch quality control replicates and 89 samples with 10 duplicates from homes that were sampled during Round 1 only).
Mixed effects included in model 2 are presence of upholstered furniture with crumbling or exposed foam, carpet installation since move-in date, residence less than 25% carpeted, residence square footage, and residence construction date
Mixed effects included in model 3 are rural location, mother’s race/ethnicity, household annual income is at least $75,000
Mixed effects included in model 4 are time trend in PBDE levels over time and the change in PBDE levels from first to second sampling round associated with carpet installation (any time after residents moved in)
For each of eight representative PBDEs, the percent change in PBDE concentrations associated with a unit increase in each of the fixed effects included in Model 5 is shown in Table 5. Five covariates significantly (p-value < 0.05) affected concentrations of multiple tri- to hexa-BDEs using Model 5. Rural residences had significantly lower tri- to hexa-BDE concentrations in their dust than households in more urban areas (37–44% lower). Compared to households with non-Hispanic, White or Asian mothers, households with Hispanic mothers had significantly higher BDE-47 and BDE-99 concentrations in their dust (45 and 46% higher, respectively) and households with non-Hispanic mothers of other races had significantly higher tri- to hexa-BDE concentrations (160–270% higher). Residences containing upholstered furniture with exposed or crumbling foam had significantly higher tri- to hexa-BDE concentrations than homes without crumbling foam (52–62% higher). Concentrations of BDEs 28, 47, and 153 decreased significantly from Round 1 to Round 2 when new carpets were installed any time after move-in date (34, 35, and 30% decrease, respectively).
Table 5.
Percent change in concentrations of selected PBDEs associated with a unit increase in each of the fixed effects included in the saturated hierarchical mixed-effects model (Model 5a).
| PBDE | Crumbling or exposed foam present | Carpet coverage <25% | Installed carpet since move-in | Installed carpet since move-in, Round 2 v. Round 1 | Residential square footage, per 1000 ft2 | Residence construction date, per year | Residence in rural location | Mother is Hispanic | Mother is not Hispanic, White, or Asian | Household annual income ≥$75,000 | Time trend, per year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-28 | 52** | −19 | −0.4 | −34*** | 2 | 0.2 | −37** | 35* | 160*** | −21* | 2 |
| BDE-47 | 55** | −13 | 2 | −35*** | −12 | 0.2 | −38*** | 45** | 220*** | −16 | 2 |
| BDE-99 | 60** | −16 | −14 | −24 | −14 | 0.3 | −42*** | 46** | 270*** | −22 | 1 |
| BDE-153 | 62** | −10 | −3 | −30** | −14 | 0.5 | −44*** | 36 | 250*** | −25* | 0.04 |
| BDE-183 | 6 | −0.2 | 21 | −7 | −8 | 0.6 | −42** | −20 | 75 | −22 | −5** |
| BDE-196 | −10 | −6 | −7 | −1 | −7 | 0.5 | −12 | −20 | −24 | −5 | −5** |
| BDE-206 | −10 | −18 | −15 | 17 | −4 | 0.1 | 3 | −5 | −31 | 12 | −3 |
| BDE-209 | −5 | −18 | −17 | 10 | −10 | 0.2 | 10 | 1 | −36 | 16 | −1 |
Regression coefficient for fixed effect from Model 5 is significantly different from 0, p-value < 0.1
Regression coefficient for fixed effect from Model 5 is significantly different from 0, p-value < 0.05
Regression coefficient for fixed effect from Model 5 is significantly different from 0, p-value < 0.01
In Table 5, Model 5 was fit for 451 observations including 406 samples collected from 203 homes during repeat sample collections and 45 duplicate samples; excluding 139 observations without covariate data (40 inter-batch quality control replicates and 89 samples with 10 duplicates from homes that were sampled during Round 1 only).
Of the covariates used in Model 5, few were associated with significant changes in concentrations of hepta- to deca-BDEs. We observed a significant decreasing trend in BDE-183 and BDE-196 over the course of the study (5% decrease per year for both). We also noted that rural residences had significantly lower concentrations of BDE-183 than more urban areas (42% lower). No covariates used in Model 5 were associated with a significant change in concentrations of nona- to deca-BDEs.
Several other covariates were associated with marginal changes in PBDE concentrations in Model 5. Households with an annual income of at least $75,000 had lower concentrations of tri- to hepta-BDEs than households with an annual income less than $75,000 (16–25% lower), but the effect was not significant. Residences with little or no carpet had lower concentrations of each PBDE than residences with at least 25% of their floors carpeted (0.2–19% lower), but the effect was not significant. In addition, recently built homes had higher PBDE concentrations than older homes (0.1–0.6% increase per year increment in construction date), but the effect was not significant. Finally, a 1,000 ft2 increase in residential square footage was associated with a decrease in concentrations of tetra- to deca-BDEs (4–14% decrease), but the effect was not significant.
3.4. Factors that increased within-household variability
As shown in Table A6 (See Appendix), three variables were found to be significantly associated with RPD magnitude in repeat samples using Model 6; the time interval between repeated dust sample collections, carpet installation since the move-in date, and the quantity of upholstered furniture items that were purchased by the residents between 2007–2010 (categorical response: 0, 1–2, 3–5, or >5 items). The length of the time interval between sample collections was positively associated with increased variability in concentrations of BDE-47 and BDE-99 in repeat samples (p-value = 0.09 and 0.03, respectively). Residents who had carpet installed in their home (any time since move-in) had increased variability in levels of BDEs 99, 153, and 183 in repeat samples (p-value = 0.08, 0.05, and 0.02, respectively). Finally, the purchase of new upholstered furniture (from 2007–2010) was associated with increased variability in levels of BDEs 28, 47, 99, and 153 in repeat samples (p-value = 0.09, 0.08, 0.11, and 0.01, respectively). For the eight representative PBDEs, RPDs between repeat samples were similar for case and control populations.
4. DISCUSSION
4.1. Variability in quality control samples
To use residential-dust measurements to assess exposures to PBDEs, we must first characterize the reliability of these measurements. We observed modest variability in concentrations of tri- to hexa-BDEs measured in replicate quality control samples compared to the more variable hepta- to deca-BDEs concentrations. Using a scanning electron microscope coupled with X-ray energy dispersive spectrometry, Webster et al. (2009) showed that non-volatile BDE-209 molecules are extremely unevenly distributed in dust samples -- occurring in widely scattered and highly contaminated “hot-spots”. In contrast, the more volatile tri- to hexa-BDEs may be more homogeneously distributed throughout dust samples, resulting in the observed improvement in analytical reproducibility. While our dust preparation protocol used a mechanical sieve shaker to homogenize household vacuum cleaner dust, the NIST SRM 2585 dust preparation protocol included additional homogenization using a modified food processor, a compressed air jet, and a cone blender. Additional dust homogenization improved analytical reproducibility for hepta- to deca-BDEs and we recommend that future investigators homogenize each residential-dust sample using a commercial blender. To avoid cross-contamination we recommend the use of a stainless steel blender container that is thoroughly washed with soapy water and solvent rinsed between samples.
4.2. Variability in repeat dust samples
Previous investigators have demonstrated that within-household PBDE variability exceeds the expected magnitude of analytical variability (Harrad et al. 2008; Muenhor and Harrad 2012; Vorkamp et al. 011). Likewise, we observed that the variability in matched pairs of dust samples from repeat sample collections exceeded the analytical variability observed in matched pairs of quality control samples.
Compared to the modest inter-round correlation observed in our study (rs range: 0.16–0.56), Allen et al. (2008a) reported higher inter-round correlations for PBDE congeners from the Penta-BDE and Deca-BDE mixtures (rp range: 0.49–0.92). In particular, Allen et al. reported much stronger inter-round correlations for concentrations of BDE-209 in repeat vacuum dust samples (rp = 0.91) compared to our findings (rs = 0.18). In both studies, inter-round correlations for PBDE congeners from the Octa-BDE mixture (i.e., BDEs 183, 196, 197, and 203) were modest. Whereas we collected dust during two sampling rounds separated by 3–8 years, Allen et al. (2008a) collected dust during two sampling rounds separated by eight months. The high inter-round correlation observed by Allen et al. was attributed to the lack of changes in home furnishings over the short sampling interval (i.e., the PBDE sources did not vary between sampling rounds). In contrast, we suspect that many residents in our study did change home furnishings between sampling rounds (e.g., 57% purchased upholstered furniture after 2006 and 45% had new carpets installed in their home at any time) and we found that the introduction of new carpeting and new upholstered furniture in a home tended to increase the magnitude of within-household changes in levels of tri- to hexa-BDEs.
To some extent, the observed range of inter-round correlation for different PBDE congeners in our study may be explained by the lifetime of the products in which the different PBDE congeners are used. Because our questionnaire was not sufficiently detailed to estimate the rate of replacement for various household items, we cannot evaluate this hypothesis.
Alternatively, we hypothesize that the higher inter-round correlations observed for low molecular weight PBDEs (i.e., tri- to hexa-BDEs) in our study were due to the tendency for these more volatile compounds to partition between indoor dust, air, and household surfaces (Watkins et al. 2011; Weschler and Nazaroff 2008). We suggest that, in the presence of such partitioning, when items treated with the Penta-BDE mixture were removed from a home and dust contaminated with tri- to hexa-BDEs was removed from the surface of carpets during typical household cleaning, other indoor surfaces and particles deep within carpets were still contaminated with tri to hexa-BDEs and acted as secondary sources for newly settled dust that was later sampled for analysis. In this way, changes in Penta-BDE sources in a home would have resulted in only subtle changes in tri- to hexa-BDE concentrations over time. In contrast, less volatile, high molecular weight PBDEs (i.e., hepta- to deca-BDEs) partition less readily between dust and indoor surfaces (Weschler and Nazaroff 2010). Thus, we suspect that when items treated with the Octa-BDE and Deca-BDE mixtures were added or removed from a home and dust contaminated with hepta- to deca-BDEs was removed from the surface of carpets during typical household cleaning, the result was relatively large changes in concentrations of these high molecular weight PBDEs over time.
Investigators have reported large temporal variability in concentrations of components of the Penta-BDE mixture in repeat dust samples collected in 2006 and 2011, with as much as a 20-fold decrease reported for one household (Dodson et al. 2012). Investigators have also reported large temporal variability in major PBDE congener concentrations in dust samples collected at shorter intervals (Harrad et al. 2008; Muenhor and Harrad 2012). Muenhor and Harrad (2012) collected eight dust samples at monthly intervals from each of fourteen spot locations in two homes and reported that BDE-47 and BDE-99 concentrations at a single location varied by as much as 11- and 14-fold, respectively. Similarly, Harrad et al. (2008) collected nine or ten dust samples at monthly intervals from each of three rooms and reported that BDEs 47, 99, and 209 concentrations from a single room varied by as much as 135-, 30-, and 400-fold. In each of these studies variability in PBDE concentrations over time were attributed to changes in home furnishings between sampling rounds. Likewise, we observed that over the 3–8 year interval between sampling rounds, concentrations of major BDEs 47, 99, and 209 changed by as much as 53-, 87-, and 520-fold, respectively.
To design effective assessments of chemical exposure, we must consider the ratio of the variance (over time) within a household to the variance between households, as large variance ratios translate to imprecise exposure classification and tend to result in the underestimation of risk estimates (Armstrong 1998). For example, we observed that the variance in BDE-209 concentrations measured in repeat dust samples collected from the same household over a period of several years was four times greater than the variance in mean BDE-209 concentrations from different households across the study population. With a corresponding variance ratio of λ= 4.4 and a true effect size of ORTrue = 2.0, if an investigator estimated long-term average exposures to BDE-209 using a single dust sample, he/she would be expected to observe an ORExp = 1.1. In contrast, for tri- to hexa-BDEs we would expect the variance in PBDE concentrations measured in repeat dust samples collected from the same household over a period of several years to be roughly equal to the variance in mean PBDE concentrations in different households across the study population and the attenuation of risk estimates would be expected to be less extreme.
In case-control studies, if past levels of chemical exposures are of interest and sample collection must be carried out after disease diagnosis, large unexplained within-household variability over time is problematic. We found that the magnitude of within-household changes in levels of PBDEs (as measured by RPD) were, on average, similar for homes of children with leukemia and for homes of healthy children, suggesting that measures of PBDE concentrations in dust samples can be used as unbiased markers of exposures for children with leukemia. However, we found that concentrations of tri- to hexa-BDEs changed more between sampling rounds when the time interval from the first to second dust collection was longer. Thus, in a case-control study the best strategy may be to limit the time interval between the critical window of exposure and sample collection. As such, we suggest that investigators who plan to use residential dust to estimate past levels of PBDE contamination in case-control studies should start sampling as soon as possible after subject enrollment to reduce the time from diagnosis to residential exposure assessment.
If long-term average chemical exposures are of interest and prospective sample collection is feasible (e.g., cohort studies), investigators can improve the precision of their exposure estimates and limit the attenuation of observed risk estimates by making repeated exposure measurements on each study subject (Whitehead et al. 2012). Because analytical variability was generally small compared to the variability within households over time, this strategy of analyzing repeat dust samples would increase precision more efficiently than analyzing several replicates for each dust sample. To maximize the precision of exposure estimates it would be necessary to use the same dust collection and dust preparation method for each repeated dust sample.
4.3. Impact of California flammability standards
Round 1 median PBDE concentrations were similar to median concentrations reported for dust collected during the same time period from other California residences. For example, Zota et al. (2008) reported median BDE-99 concentrations of 3,830 ng/g and 1,160 ng/g for Richmond and Bolinas, California, respectively; Hwang et al. (2008) reported median BDE-99 concentrations of 4,375 ng/g for Davis, California; Quiros-Alcala et al. (2011) reported median BDE-99 concentrations of 5,450 ng/g and 4,450 ng/g for Salinas and Oakland, California, respectively; Dodson et al. (2012) reported median BDE-99 concentrations of 2,200 ng/g for the San Francisco Bay Area in 2006; compared to the median BDE-99 concentration from sampling round 1 of 2,400 ng/g in our study. Zota et al. (2008) hypothesized that the relatively high levels of BDE-99 (as well as BDE-47 and BDE-100) observed in residential dust from California homes was the result of the state’s unique flammability standard, Technical Bulletin 117. The standard requires that resilient filling materials used in upholstered furniture (e.g., polyurethane foam) must resist a 12-second exposure to an open flame (Department of Consumer Affairs, Bureau of Home Furnishings and Thermal Insulation 2000) and, to meet the requirements, many upholstered furniture items sold in California before June 1, 2006 were treated with the Penta-BDE mixture. Our results provide further support for the hypothesis that one unintended consequence of California’s unique flammability standard is higher levels of tri- to hexa-BDEs in residential dust from California homes.
4.4. Determinants of PBDE concentrations in residential dust
PBDE-treated consumer products are the primary sources of PBDE contamination in the residential environment. However, it can be difficult to relate PBDE concentrations in dust to counts of furniture or electronics, because items that appear similar may have widely varying PBDE content (Allen et al. 2008b). Even before PBDE regulation began, a variety of other flame retardants had been used to treat consumer items (Stapleton et al. 2012). Not surprisingly then, we did not observe a correlation between the number or use of televisions or computers and the concentrations of hepta- to deca-BDEs in dust. Moreover, we did not observe a correlation between the number of upholstered pieces of furniture and the concentrations of tri- to hexa-BDEs in dust. However, we did find that having upholstered furniture with crumbling or exposed foam resulted in higher concentrations of tri- to hexa-BDEs (i.e., those PBDEs found in the Penta-BDE mixture). Thus, we suggest that an individual could reduce their exposure to PBDEs by removing any furniture with exposed foam from their home. Subtle differences in furniture quality (e.g., leather vs. fabric upholstery) may also impact the efficiency with which PBDEs are transferred from furniture foam to the residential environment, but we did not collect this information in our questionnaire.
We found that residents who had carpets installed in their homes tended to experience reductions in tri- to hexa-BDE levels from the first to second dust sampling round. One explanation for this observation is that when new carpets were installed, carpet pads containing high levels of PBDEs were removed and replaced with carpet pads that contained lower levels of PBDEs. The vast majority of U.S. carpet pads consist of bonded scraps of polyurethane foam recycled from industrial and consumer sources (Oler 2005). After the Penta-BDE mixture was banned, foam scrap from industrial sources no longer contained PBDEs and there was likely a reduction in PBDE content in new carpet pads (despite the continued use of PBDE-contaminated foam scraps from recycled carpet pads). Unfortunately, we did not ascertain the year of carpet installation (merely whether installation occurred any time since the family’s move-in date), so we cannot identify households that installed new carpet pads after the Penta-BDE mixture was banned.
Several covariates, including maternal ethnicity, household income, and urban density, explained some of the variability in PBDE levels between households within the same region. Investigators have suggested that socioeconomic factors such as income and education may be determinants of PBDE exposure, as individuals in low-income households (Zota et al. 2008) and children whose mothers (Rose et al. 2010) or caregivers (Windham et al. 2010) are less educated have been shown to have elevated PBDE body burdens. Similarly, in our population, lower income households had marginally higher tri- to hexa-BDE dust concentrations than higher income households. Windham et al. (2010) noted that among a cohort of girls 6–8 years-old, serum PBDE levels were highest for African American girls, followed by Hispanic girls, and white girls had the lowest levels. Likewise, in our study population, households with non-Hispanic White or Asian mothers had the lowest tri- to hexa-BDE dust concentrations, whereas households with Hispanic mothers had higher levels, and households with non-Hispanic, mothers of other races (including households with African American mothers) had the highest levels. In our study population, the lowest income households were often Hispanic households (i.e., 68% of households with income <$30,000 per year had a Hispanic mother), making it difficult to resolve the independent effects of income and ethnicity on PBDE levels in Model 5. Our findings suggest that Hispanic families and low-income families may be disproportionally exposed to PBDEs via contaminated residential dust.
Previous investigators have suggested that the age or quality of upholstered furniture in low-income homes may explain the observed discrepancy in PBDE body burdens by income status (Zota et al. 2008). We did not observe a relationship between household income or ethnicity and the presence of crumbling or exposed foam. The age of upholstered furniture was also unrelated to the income status and ethnicity of the residents.
Harrad and Hunter (2006) have shown that in air and soil samples collected from a transect of the West Midlands, UK, concentrations of tri- to hexa-BDEs decreased with increasing distance from the city. The authors hypothesized that urban areas have more contaminated air and soil, because these areas have a higher density of indoor environments containing products treated with PBDEs. They suggested that PBDEs volatilize indoors, ventilate outdoors, and deposit and accumulate in soil. It follows that PBDE-contaminated soil in urban areas could be tracked inside urban homes and we suggest that this external PBDE contamination was the source of the elevated levels of tri- to hepta-BDEs observed in dust from urban homes in our analysis.
4.5. Time trends in PBDE concentrations
Dust collection began in 2001 prior to the phase-out of the Penta-BDE and Octa-BDE mixtures and the second dust collection was in 2010 after the phase-out. Thus, we hypothesized that concentrations of the tri- to octa-BDEs would be reduced from the first sampling round to the second. Indeed, previous investigators have reported trends toward lower levels of PBDEs in repeat dust samples collected from 16 California homes in 2006 and 2011 (Dodson et al. 2012). Likewise, we observed significant reductions in several congeners included in the Octa-BDE mixture (BDEs 183, 196, 197, and 203). Moreover, we observed slightly lower median concentrations of the PBDE congeners included in the Penta-BDE mixture (BDEs 47, 99, 100, 153, and 154) in the second round of dust sampling compared to the first and, in Model 5, reductions in these PBDEs were evident for residents who reported carpet installation in their homes. Residential contamination from these PBDEs will likely persist at least until furniture items treated with the Penta-BDE mixture are removed. Moreover, because the semi-volatile PBDEs included in the Penta-BDE mixture partition between dust and other household surfaces (Watkins et al. 2011; Weschler and Nazaroff 2008), complete removal of PBDEs may prove challenging.
4.6. Limitations
The questionnaire information used in this analysis was collected largely during the second sampling round and we did not evaluate changes in most explanatory variables between sampling rounds. For example, we did not identify pieces of furniture that were replaced between the first and second sampling rounds. As a result, we were able to explain very little of the within-household variability observed in PBDE levels.
In some instances, our assessment of PBDE sources lacked specificity. For example, we did not identify pieces of furniture that were likely to contain PBDEs using X-ray fluorescent detection of bromine or by examination of manufacturer’s labels. Moreover, we did not ascertain information about the type or age of insulation present in the home, the type of floor present in homes without carpet, or the presence or quantity of electrical or electronic devices other than televisions and computers – each of these factors may have explained additional variability in PBDE levels between homes.
Although we collected repeated residential dust samples over long time intervals (up to eight years between sample collections) our sampling strategy was limited to a maximum of two samples per household and we did not collect repeat samples from homes over short intervals of time. Consequently, we were unable to estimate the short-term temporal variability of PBDEs in residential dust for this study population (i.e., month-to-month variability).
We obtained dust samples from vacuum cleaners that were used for typical household cleaning. From one home to the next and from one sampling round to the next, each vacuum cleaner may have been used in a different combination of rooms and at different proximity to PBDE sources. Differences in vacuum cleaning practices between and within homes could be responsible for some of the unexplained variability in PBDE levels. Moreover, it is possible that some vacuum cleaners may have been treated with PBDEs, resulting in an overestimation of the PBDE content of a household’s dust. In contrast, other vacuum cleaners may have heated the dust they collect, resulting in an underestimation of the PBDE content of a household’s dust. Differences in vacuum cleaners used to collect dust may have resulted in additional unexplained variability in PBDE levels. Finally, our sampling strategy was not designed to evaluate the temporal variability of PBDEs in a specific room using repeated measurements from the same location and we were unable to investigate the spatial variability of PBDE concentrations in dust collected from different rooms in the same house.
4.7. Conclusions
In summary, we identified several potential determinants of PBDE concentrations in dust, including furniture condition, urban density, resident ethnicity, and, to a lesser extent, household income. Our findings suggest that Hispanic families and low-income families may be disproportionally exposed to PBDEs via contaminated residential dust. For some PBDEs (i.e., BDE-183 and BDE-196), long-term trends towards lower concentrations were observed; however, most of the changes in PBDE levels within-households were unaccounted for by our mixed-effects models. Despite the substantial unexplained within-household variability, we found that PBDE concentrations were correlated within households between two sampling rounds separated by 3–8 years, suggesting that it may be feasible to use residential dust for retrospective assessment of PBDE exposures in studies of children’s health.
HIGHLIGHTS.
PBDE concentrations were correlated between sampling rounds separated by 3–8 years
PBDE concentrations were higher in Hispanic and low-income households
PBDE sources included upholstered furniture with crumbling or exposed foam
Levels of Penta-BDE constituents remained elevated in dust from California homes, despite the mixture’s recent ban
Regional differences in PBDE concentrations were observed across California
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences (NIEHS, grant numbers R01ES009137, R01ES015899, P42ES0470518, and P01ES018172); by the Intramural Research Program of the National Cancer Institute (NCI), National Institute of Health (Subcontracts 7590-S-04, 7590-S-01); by the NCI (Contract N02-CP-11015); and by the Environmental Protection Agency (EPA, grant number RD83451101). Funding sources were not involved with the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication. We thank the families for their participation. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Drs. Mignon Loh and Katherine Matthay), Children’s Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children’s Hospital (Dr. Gary Dahl), Children’s Hospital Oakland (Dr. James Feusner), Kaiser Permanente Oakland (Drs. Daniel Kronish and Stacy Month), Kaiser Permanente Roseville (Drs. Kent Jolly and Vincent Kiley), Kaiser Permanente Santa Clara (Drs. Carolyn Russo, Denah Taggart, and Alan Wong), and Kaiser Permanente San Francisco (Dr. Kenneth Leung). Finally, we acknowledge the entire Northern California Childhood Leukemia Study staff for their effort and dedication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Todd P. Whitehead, Email: toddpwhitehead@berkeley.edu.
F. Reber Brown, Email: FRBrown@dtsc.ca.gov.
Catherine Metayer, Email: cmetayer@berkeley.edu.
June-Soo Park, Email: jpark@dtsc.ca.gov.
Monique Does, Email: mobuff@berkeley.edu.
Myrto X. Petreas, Email: mpetreas@dtsc.ca.gov.
Patricia A. Buffler, Email: pab@berkeley.edu.
Stephen M. Rappaport, Email: srappaport@berkeley.edu.
References
- Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ Int. 2008a;34:1085–91. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Linking PBDEs in house dust to consumer products using X-ray fluorescence. Environ Sci Technol. 2008b;42:4222–8. doi: 10.1021/es702964a. [DOI] [PubMed] [Google Scholar]
- Armstrong BG. Effect of measurement error on epidemiological studies of environmental and occupational exposures. Occup Environ Med. 1998;55:651–6. doi: 10.1136/oem.55.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterman SA, Chernyak S, Jia C, Godwin C, Charles S. Concentrations and emissions of polybrominated diphenyl ethers from U.S. houses and garages. Environ Sci Technol. 2009;43:2693–700. doi: 10.1021/es8029957. [DOI] [PubMed] [Google Scholar]
- Chan. California Assembly Bill No 2587 Hazardous Chemicals. 2004. [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int. 2007;33:239–45. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environ Health Perspect. 2010;118:1444–9. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Consumer Affairs, Bureau of Home Furnishings and Thermal Insulation. Technical Bulletin 117: Requirements, Test Procedure, and Apparatus for Testing the Flame Retardance of Resilient Filling Materials Used in Upholstered Furniture. 2000. [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environmental science & technology. 2012;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, et al. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environ Health Perspect. 2013;121(2):257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am J Epidemiol. 2011;174:885–92. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S, Hunter S. Concentrations of polybrominated diphenyl ethers in air and soil on a rural-urban transect across a major UK conurbation. Environ Sci Technol. 2006;40:4548–53. doi: 10.1021/es0606879. [DOI] [PubMed] [Google Scholar]
- Harrad S, Ibarra C, Abdallah MA, Boon R, Neels H, Covaci A. Concentrations of brominated flame retardants in dust from United Kingdom cars, homes, and offices: causes of variability and implications for human exposure. Environ Int. 2008;34:1170–5. doi: 10.1016/j.envint.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:48–51. [Google Scholar]
- Hwang HM, Park EK, Young TM, Hammock BD. Occurrence of endocrine-disrupting chemicals in indoor dust. Sci Total Environ. 2008;404:26–35. doi: 10.1016/j.scitotenv.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environ Sci Technol. 2010;44(14):5627–32. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Guardia MJ, Hale RC, Harvey E. Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures. Environmental science & technology. 2006;40:6246–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ. 2009;407:3425–9. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muenhor D, Harrad S. Within-room and within-building temporal and spatial variations in concentrations of polybrominated diphenyl ethers (PBDEs) in indoor dust. Environ Int. 2012;47:23–7. doi: 10.1016/j.envint.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Oler WH. Statement of the carpet cushion council on legislative initiatives to ban products containing polybrominated diphenyl ethers (PBDEs) Bryn Mawr, PA: Carpet Cushion Council; 2005. [Google Scholar]
- Quiros-Alcala L, Bradman A, Nishioka M, Harnly ME, Hubbard A, McKone TE, et al. Concentrations and loadings of polybrominated diphenyl ethers in dust from low-income households in California. Environ Int. 2011;37(3):592–6. doi: 10.1016/j.envint.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IN, Hertz-Picciotto I. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ Sci Technol. 2010;44:2648–53. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120:1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environ Health Perspect. 2011;119:1454–9. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ Sci Technol. 2012;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environ Health Perspect. 2008;116:1635–41. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000 census of population and housing. U.S. Department of Commerce, Economics and Statistics Administration; [Google Scholar]
- U.S. Environmental Protection Agency. Polybrominated Diphenyl Ethers (PBDEs) Washington, D.C: [Accessed in 2010]. Updated 2010. Available from: http://www.epa.gov/oppt/pbde/ [Google Scholar]
- U.S. Environmental Protection Agency. [Accessed in 2013];Polybrominated Diphenyl Ethers (PBDEs) Action Plan Summary. Updated 2012. Available from: http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/pbde.html.
- Vorkamp K, Thomsen M, Frederiksen M, Pedersen M, Knudsen LE. Polybrominated diphenyl ethers (PBDEs) in the indoor environment and associations with prenatal exposure. Environ Int. 2011;37:1–10. doi: 10.1016/j.envint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Stapleton HM, Webster TF. Relationships between PBDEs in office air, dust, and surfaces wipes. 12th Workshop on Brominated and other Flame Retardants; Boston, MA. 2011. [Google Scholar]
- Webster TF, Harrad S, Millette JR, Holbrook RD, Davis JM, Stapleton HM, et al. Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ Sci Technol. 2009;43:3067–72. doi: 10.1021/es803139w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ, Nazaroff WW. SVOC partitioning between the gas phase and settled dust indoors. Atmospheric Environment. 2010;44:3609–3620. [Google Scholar]
- Weschler CJ, Nazaroff WW. Semivolatile organic compounds in indoor environments. Atmospheric Environment. 2008;42:9018–9040. [Google Scholar]
- Whitehead T, Metayer C, Buffler P, Rappaport SM. Estimating exposures to indoor contaminants using residential dust. J Expo Sci Environ Epidemiol. 2011;21:549–64. doi: 10.1038/jes.2011.11. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Nuckols JR, Ward MH, Rappaport SM. Carpet-dust chemicals as measures of exposure: Implications of variability. Emerg Themes Epidemiol. 2012;9:2. doi: 10.1186/1742-7622-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP. ProQuest Dissertations and Theses. 2011. Estimating Environmental Exposures to Indoor Contaminants using Residential-Dust Samples. [DOI] [PubMed] [Google Scholar]
- Windham GC, Pinney SM, Sjodin A, Lum R, Jones RS, Needham LL, et al. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ Res. 2010;110:251–7. doi: 10.1016/j.envres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42:8158–64. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]