Abstract
Seizures in the pediatric population commonly occur, and when proper rescue medication is not administered quickly, the risk of neurologic compromise emerges. For many years, rectal diazepam has been the standard of care, but recent interest in a more cost-effective, safe alternative has led to the investigation of intranasal midazolam for this indication. Although midazolam and diazepam are both members of the benzodiazepine class, the kinetic properties of these 2 anticonvulsants vary. This paper will review available data pertaining to the efficacy, safety, cost, and pharmacokinetics of intranasal midazolam versus rectal diazepam as treatment for acute seizures for children in the prehospital, home, and emergency department settings.
INDEX TERMS: benzodiazepines, pediatrics, prehospital, seizures, therapeutics
INTRODUCTION
Seizures in children account for up to 25% of all pediatric emergency medical service calls in the United States and may account for up to 15% of pediatric air transports.1 Most seizures self-terminate within 5 minutes, but those lasting longer warrant medication administration for seizure cessation and status epilepticus avoidance.2 Although the time period for diagnosing status epilepticus is controversial, traditional classification is defined as either one continuous seizure lasting 30 minutes, or a series of seizures that recurs in which the patient did not regain full consciousness between seizures.3 When seizures are prolonged or seizure activity is recurrent, the patient is at risk for neurologic compromise, as well as increased morbidity and mortality.3 In addition to a drug's efficacy in terminating status epilepticus, its pharmacokinetics, route, and ease of administration are key factors to consider in drug selection. Although seizures and anticonvulsants have been studied for many years, the optimal mode for rapid cessation of seizure activity outside the hospital setting has yet to be identified. Benzodiazepines are the first-line agents for treatment of acute seizures, with diazepam, clonazepam, lorazepam, and midazolam being the most widely used of the class.4 Diazepam and midazolam offer unique routes of delivery that will be compared and contrasted in this review.
PHARMACOKINETICS
Rapid penetration into the central nervous system (CNS) is a critical factor for the efficacy of benzodiazepines for status epilepticus treatment. Benzodiazepines work by allosterically modifying gamma-aminobutyric acid receptors to enhance inhibition in the CNS.4 The rate and extent of entry into the brain and cerebrospinal fluid are determined by chemical properties that are unique for each agent within the benzodiazepine class. Generally speaking, benzodiazepines are highly lipophilic, allowing rapid penetration into the CNS, but the duration of action varies.4 Because the oral route is not ideal and intravenous access is not always available, there is a medical necessity for alternative modes of providing medication to seizing patients in the acute setting. During the past several years, the number of studies investigating intranasal and rectal routes of administration of benzodiazepines has grown exponentially. These routes are very attractive because they are both rich in venous circulation and offer the benefit of avoiding intravenous line placement.5
Rapidity of drug delivery to the CNS is easily achieved when using the transmucosal, richly vascular tissue of the nares and rectum (Figure).5 The nasal cavity is covered with a thin mucus layer, a monolayer ciliated epithelium, and is innervated by an abundant underlying blood supply.6 Under ideal conditions, most medication is absorbed from the nasal mucosa and reaches the cerebral cortex within 2 to 5 minutes, thus avoiding first-pass metabolism.7 Medications suitable for intranasal use must be water-soluble, small enough to permeate nasal mucosa, and potent enough to be effective in small doses. It is important, however, to keep in mind that physiologic changes of the nasal passages can occur, for example, during allergic and vasomotor rhinitis, after physical trauma, and during times of increased mucus production.6 One distinct disadvantage of using intranasal midazolam is the increased nasal mucus production that is sometimes observed during seizure activity. Increases in mucus production and changes in mucociliary clearance rates could affect midazolam's bioavailability.6,8
Figure.
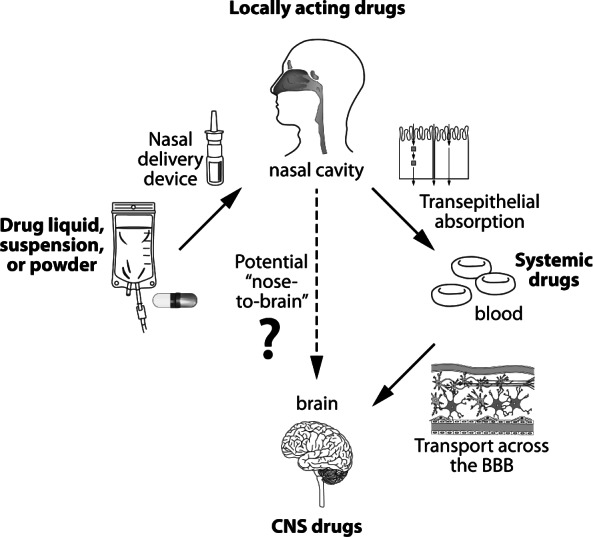
Drug delivery to the CNS from nasal formulations.
CNS, Central Nervous System; BBB, Blood Brain Barrier
Midazolam's small molecular weight of 325.8 Da allows for easy permeation of nasal mucosa.6 Midazolam dissolves in water at a low pH, but at physiologic pH it becomes lipophilic, allowing the medication to cross the blood-brain barrier.4,7 Rey and colleagues9 studied the pharmacokinetics of intranasal midazolam in 6 children (ages 1–5 years) and found the time to maximum concentration to be 12 minutes with a half-life of 2.2 hours when a dose of 0.2 mg/kg was administered. The maximum concentration was determined to be 104 ± 32 mg/L, and the mean absolute bioavailability of the intranasal route was 55%. The plasma clearance and volume of distribution was twice as high as the same dose administered intravenously.9
Rectal mucosa is also a very viable tissue, but unlike nasal mucosa, it does not offer the advantage of bypassing hepatic first-pass metabolism.10,11 Since its appearance more than 20 years ago, rectal diazepam gel has provided a safe, effective alternative to intravenous diazepam in urgent situations. Although well accepted, rectal diazepam gel does have a few disadvantages. After repeated doses, diazepam can accumulate and place the patient at risk for serious adverse drug reactions, such as respiratory depression.10 The pharmacokinetics of the rectal gel formulation has not been specifically studied in the pediatric population, but it is known that the rectal absorption of a diazepam solution is rapid.4 Studies found a maximum concentration of 337 mcg/L was reached in 20 minutes in 5 children ages 11 to 15 years with the solution administered rectally.4 Infants were found to have effective anticonvulsant concentrations in an average of 4 minutes after a 0.7 mg/kg rectal dose of a solution.4 Yet, the rectal bioavailability is highly variable, ranging from 50% to 100%. Additionally, some studies have shown erratic absorption of diazepam suppositories from rectal tissue.4
In most clinical situations, duration of action is an important factor to consider when choosing one agent over another within the same medication class. Although it is helpful to be aware that diazepam and midazolam have durations of action of less than 2 hours and of 3 to 4 hours, respectively, clinicians must be mindful that the duration of action of benzodiazepines does not correlate directly with plasma concentration.4 Bhattacharyya and colleagues12 observed that 6.25% of patients had seizure recurrence within 60 minutes of rectal diazepam administration, which was true of only 3.26% of patients in the intranasal midazolam group.
TIME TO SEIZURE CESSATION
The main goals of rapid seizure cessation include prevention of status epilepticus, avoidance of anoxic brain injury, and maintenance of neurologic function. Prospective and retrospective evidence shows that intranasal midazolam terminates seizures at least as fast as rectal diazepam.1,5,8,12–15 Most clinical studies have used a 5 mg/mL midazolam solution dosed at 0.2 mg/kg (divided into each nostril) administered via an intranasal mucosal atomization (IN-MADD) device or nasal dropper as suggested by Kyrkou and colleagues.16
Three open-labeled studies prospectively evaluated the time to seizure cessation with a dose of nasal midazolam (Table).5,14,15 Lahat and colleagues5 assessed intranasal midazolam in the treatment of acute childhood seizures in the emergency department (ED). Children experiencing a generalized seizure lasting longer than 10 minutes were eligible for inclusion in the study. Of the 20 children participating in the study, intranasal midazolam successfully treated all but 1 patient, whose seizure was also refractory to diazepam and required phenytoin for seizure cessation. The mean time to seizure control with the midazolam was 3.5 minutes (range, 2.5–5 minutes). There were no repeat seizures within 60 minutes of midazolam use.
Table.
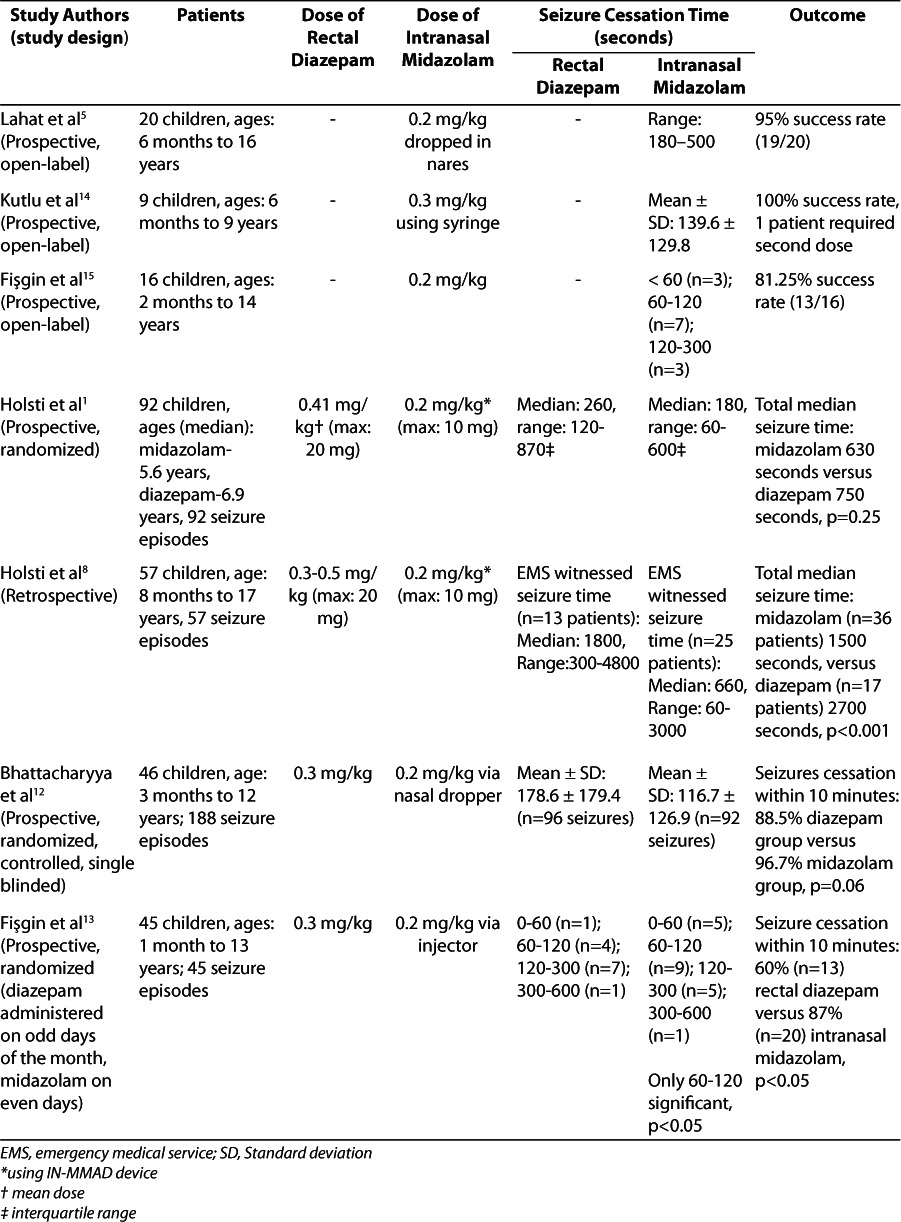
Kutlu et al14 administered midazolam to 9 patients who were seen at an inpatient pediatric clinic or in the ED with a seizure lasting more than 10 minutes but less than 30 minutes. Seizures, both focal and generalized, were controlled within a mean time of 139.6 seconds. Only 1 patient required a second dose of midazolam, and all 9 patients experienced cessation. Two patients had seizure recurrence 3 to 4 hours after the dose but were successfully treated with a repeat dose of midazolam.
Fişgin and colleagues15 administered intranasal midazolam by injector to 16 children with an acute seizure lasting longer than 10 minutes. Two patients were diagnosed with status epilepticus, defined by the authors as a seizure lasting longer than 30 minutes. Most of the patients (68.7%) experienced generalized tonic-clonic seizures, whereas 4 patients had simple partial seizures and 2 had febrile seizures. A single dose of midazolam administered by hospital personnel provided a positive response in 13 patients (81.8%), all of whom stopped seizing in less than 5 minutes and experienced no reoccurrence. Of those 13 patients, 3 (18.7%) stopped seizing within 1 minute. Those who continued to seize received rescue rectal diazepam, but this intervention also proved ineffective in all 3 patients. One patient with generalized tonic-clonic seizures was diagnosed with a brain abscess and required a continuous infusion of midazolam after failing intranasal midazolam, rectal diazepam, and intravenous phenobarbital. Another patient with generalized tonic-clonic seizures, Alport syndrome, and uremic encephalopathy required phenobarbital for seizure cessation. The third patient, who had no prior seizure history but arrived in status epilepticus (secondary generalization after a focal seizure), required phenytoin to terminate the seizure. Because of the positive response in >80% of patients, the authors concluded that intranasal midazolam is effective for treatment of acute seizures in the hospital setting.
Four studies have compared intranasal midazolam to rectal diazepam (Table).1,8,12,13 Bhattacharyya and colleagues12 compared intranasal midazolam to rectal diazepam in 46 children who presented to the ED with febrile or afebrile seizures. Baseline characteristics were similar between the 2 groups. Cessation of seizure was defined as visible stopping of the convulsions or a return of “purposeful response to external stimuli.” They determined a mean time to seizure cessation of 2.97 minutes and 1.95 minutes in the rectal diazepam and intranasal midazolam groups, respectively (p=0.005). Seizures stopped within 10 minutes of drug administration in 88.5% of the diazepam group and 96.7% in the midazolam group (p=0.06). More than 6% of the diazepam group experienced a seizure within 1 hour of drug administration, but only 3% of patients in the midazolam group experienced such recurrence. The authors found intranasal midazolam to be an efficacious route as an anti-convulsant and superior to diazepam for quickness of response and ease of administration when administered by ED personnel.
Fişgin and colleagues13 evaluated 45 children who presented to the ED with an acute seizure. Patients were randomized to rectal diazepam or intranasal midazolam. Most patients had a history of tonic-clonic seizures, but patients with febrile seizures were also included. No difference was found between groups related to seizure type or patient characteristics. Midazolam had a higher rate of seizure termination than diazepam at 1 to 2 minutes (p<0.05). Seizure cessation within 10 minutes occurred in 60% (n=13) of those receiving rectal diazepam versus 87% (n=20) of patients administered intranasal midazolam (p<0.05). This study did not address efficacy based on seizure type or state if medication preparation time was included in the time to cessation. A total of 9 patients in the diazepam group and 3 patients in the midazolam group failed to respond to initial therapy (p<0.05). These patients received the other study drug at the 10-minute evaluation. A total of 5 patients responded to midazolam and 2 patients responded to diazepam. The authors concluded that intranasal midazolam is a more effective anticonvulsant than rectal diazepam.
Holsti and colleagues8 retrospectively studied the effectiveness and complications of intranasal midazolam compared with rectal diazepam for treatment of seizures in the prehospital setting. Fifty-seven children with a variety of seizure disorders were evaluated and treated with either intranasal midazolam or rectal diazepam by the paramedics prior to arrival at the ED. Data show substantially better results for the intranasal midazolam group compared with the rectal diazepam group. The median seizure time witnessed by emergency medical services for the intranasal midazolam group was 11 minutes compared with 30 minutes for the rectal diazepam group (p=0.003). Status epilepticus, defined as seizure greater than 30 minutes, was described in 25% of the midazolam group and 50% of the diazepam group, which could be a reason for the longer median times to cessation compared with other studies. However, there was no difference found in seizure types between the 2 groups. The need for emergent intubation (11% vs. 42%), requirement for hospital admission (40% vs. 89%), and intensive care unit admission (16% vs. 59%) all statistically favor intranasal midazolam administration at a dose of 0.2 mg/kg divided into each nare using a mucosal atomization device. The authors determined that intranasal midazolam was more effective than rectal diazepam for controlling seizures when administered by emergency medical personnel prior to arrival at the ED.
Given the satisfactory results of their 2007 study, Holsti et al1 later set out to evaluate 92 seizure episodes where caretakers administered medications at home prior to calling emergency medical personnel. Caretakers were randomized to use either intranasal midazolam via the IN-MADD device, or rectal diazepam, and were instructed to administer the drug if the patient's seizure lasted more than 5 minutes. Patients with all seizure types were included, and patient characteristics were similar for both study groups. There were 50 children who received intranasal midazolam and 42 who received rectal diazepam. The median time for administration was the same for both groups: 5 minutes. The intranasal midazolam group reported a median time to seizure cessation of 3 minutes. The rectal diazepam group was 1.3 minutes slower, with a median time to seizure cessation of 4.3 minutes (95% confidence interval [95% CI], 0.0–3.5 minutes; p=0.09). There was a 2-minute time frame difference in the median total seizure time between groups, which was not statistically significant. Although significance was not reached in this study, the authors emphasize the trend in faster seizure control with intranasal midazolam administered by caretakers as a home rescue medication.
Overall, these studies conclude that intranasal midazolam is effective and consistently faster at aborting seizure activity than rectal diazepam. Although time to seizure cessation varied from study to study, intranasal midazolam proved efficacious when administered not only by ED personnel, but also by paramedics and caregivers in the prehospital and home settings.
SAFETY
Common adverse drug reactions associated with benzodiazepines include oxygen desaturation leading to respiratory distress, oxygen desaturation, and bradycardia. No one trial has focused solely on comparing the adverse drug reactions of intranasal midazolam to rectal diazepam, but useful information can be extrapolated from several past studies.1,8,12,16,17 A prospective, randomized trial by Holsti and colleagues1 found no detectable difference in the number of adverse drug reactions among the intranasal midazolam and rectal diazepam groups. Occurrence of repeated seizure within 12 hours, need for ED services, and respiratory depression rates were similar. One child in each group had a repeat seizure within 12 hours (odds ratio [OR], 0.8; 95% CI, 0–67.3), and 21% in the midazolam group versus 17% in the diazepam group required care in the ED (OR, 1.1; 95% CI, 0.4–2.7). One patient in the midazolam group required intubation (OR, 0.8; 95% CI, 0 to infinity), but no comment or detail regarding this patient's outcome or specific demographic information was provided.
Bhattacharyya and colleagues12 extensively studied adverse drug reactions of intranasal midazolam compared with rectal diazepam. They found that mean oxygen saturation (SaO2) after 5, 10, and 30 minutes of intranasal midazolam did not vary, whereas SaO2 in the rectal diazepam group decreased at 5 and 30 minutes from predrug mean value (p<0.05). One child developed hypoxia and required 7 hours of oxygen inhalation therapy after receiving rectal diazepam. Other adverse drug reactions, such as vomiting and excessive drowsiness, were seen in 10 of 96 patients (10.4%) in the rectal diazepam group, whereas no such adverse reactions were observed in the intranasal midazolam group, even on repeated use (p=0.009). The children who experienced vomiting and excessive drowsiness were those who required multiple doses of rectal diazepam. These adverse drug reactions are thought to have occurred because of drug accumulation following repeated administration. The authors concluded that midazolam has a good safety profile when given via the nares.
A comparative study by Fişgin and colleagues13 evaluated 45 children receiving either intranasal midazolam or rectal diazepam in the emergency room. Only 2 of 45 patients were adversely affected, both with atypical effects of tachypnea and tachycardia after midazolam administration. One patient had tachypnea at 5 minutes after intranasal midazolam administration, and another experienced tachycardia at 10 minutes. These effects were believed to be due to pain associated with nasal mucosal irritation. Neither of these events was statistically different from the diazepam group (p>0.05).
To dissolve and therefore become suitable for intranasal use, midazolam must be buffered to a pH of 3, which is irritating to the nares.6 One method of preventing nasal irritation is to administer intranasal lidocaine 60 seconds before midazolam using a mucosal atomization device. Chiaretti and colleagues18 found a single 10-mg dose of atomized lidocaine prior to a 0.2 to 0.5 mg/kg dose of intranasal midazolam to be effective at reducing nasal irritation, but the children in this study were given midazolam for conscious sedation rather than for seizures. This may be a viable option in the future if development of a premixed lidocaine/midazolam solution is feasible, but the delay in midazolam administration that would occur from giving lidocaine first is not ideal and could lead to patient harm.
COST
There are several methods of measuring cost-effectiveness. As far as direct medication cost per dose, intranasal midazolam is much less expensive. A single dose of Diastat Acu-Dial (Valeant Pharmaceuticals, Bridgewater, NJ) diazepam rectal gel is approximately $371.19 Generic diazepam rectal gel is $334, whereas a dose of intranasal midazolam is a mere $12.1,19 One study determined that at about 10% of the cost of rectal diazepam, intranasal midazolam is a cost-effective and likely more convenient alternative medication for rapid seizure termination.16 A limitation of intranasal midazolam use is the lack of availability from retail pharmacies. Patients must purchase needleless syringes along with the prescribed vials of midazolam or order the IN-MADD online. Intranasal mucosal atomizer devices may also be available for purchase through medical supply companies. Midazolam has been studied using a nasal dropper for administration as well as the IN-MADD. The IN-MADD is an applicator placed on top of a syringe that distributes midazolam in a 30-μm particle size, coating the nasal mucosa. The cost of this device is $2.45 and has been proven to be as effective as a nasal dropper for midazolam administration.1,8 Total cost for a dose of intranasal midazolam plus IN-MADD applicator is around $16. Prefilled midazolam syringes for nasal use are unable to be recommended at this time due to lack of stability studies.
Holsti et al8 have assessed cost-effectiveness measured by total hospital charges. The median total hospital charge for patients receiving intranasal midazolam was $1459 compared with $6980 for the rectal diazepam group (p<0.0001). Potential confounders, such as level and extent of care provided, were not assessed. Long-term cost analysis, including indirect costs accrued from recurrent hospitalizations and the number of doses required per seizure episode, needs to be further evaluated. Many variables of cost exist, and one may argue that one medication is better than the next because it is less expensive. However, the only guarantee is that potential patients who use their prescribed anticonvulsants as directed could potentially avoid the ED or intensive care unit visits, which would make a substantial difference in health care–related spending.
EASE AND CONVENIENCE OF USE
Medications need to be simple and convenient to use for patients and their caregivers to remain compliant with anticonvulsant rescue therapies. As contemporary as it may seem, the topic of drug formulation design is one of high importance. Nasal or rectal administration is a viable option when in a home or non-hospital setting, because intravenous administration is not possible without an intravenous line and intramuscular injection is not an option. Depending on the age of the child, using a medication rectally, such as diazepam, may be socially awkward, difficult to administer, and unacceptable in some settings. The rectal formulation is difficult to administer to actively seizing patients and should be avoided in those with rectal or colon deformities. Although it may seem difficult to administer an intranasal medication during a seizure, one must realize that removing garments to administer a rectal medication is challenging and time consuming. The quicker an anticonvulsant medication can be administered, the less likely the need for multiple doses. In addition, if there is less hesitation to administer the medication by other personnel, such as a schoolteacher, improvement in patient care and outcomes may be seen.
Harbord and colleagues2 evaluated the acceptance of intranasal midazolam for acute seizure management when given by parents, caregivers, teachers, and first aiders. Once trained to administer midazolam directly into the nares from the original plastic ampule, 100% of parents, 79% of teachers, and 58% of school assistants preferred its use to waiting anxiously for an ambulance to arrive. Of the 27 completed questionnaires, 90% reported no difficulty in administering the medication. The responders commented that the intranasal midazolam was less intrusive, allowed greater privacy, and was more suitable for use in the community compared with rectal diazepam. Surveys show that, although not yet clinically accepted, intranasal midazolam is socially acceptable to caregivers and patients alike. Holsti and colleagues1 also surveyed caregivers and found they were satisfied with ease of administration and overall satisfaction of the IN-MADD device versus rectal diazepam. On a scale of 0 to 10, with 10 being greatly satisfied, the interquartile range for the 21 caregivers providing a response regarding IN-MADD was 6, whereas the inter-quartile range for the 13 caregivers providing a response regarding rectal diazepam was 8. A statistically significant difference was not found. In the Bhattacharyya and colleagues12 evaluation, drug administration time was shorter with midazolam than diazepam (p=0.002). However, Holsti and colleagues1 did not find a statistical difference in administration time.
CONCLUSION
Intranasal midazolam was found to be efficacious and reasonably safe for treatment of acute seizures in the pediatric population. Various studies have demonstrated a shorter time to seizure cessation with intranasal midazolam versus rectal diazepam in children in the community, prehospital, and ED settings. Many first responders, including caregivers, prefer intra-nasal midazolam and deem it less invasive for patients. A good safety profile also supports the use of intranasal midazolam, with fewer patients experiencing respiratory depression and oxygen desaturation compared with rectal diazepam. Although intranasal midazolam is less expensive on a direct cost basis, future cost analysis that considers dose, recurrence of seizures, emergency medical technician calls, ED admissions, and treatment of complications should be performed. Optimal dosing of intranasal midazolam for all patients, including those with rhinitis and other nasal abnormalities, needs to be defined, although the studies presented primarily used 0.2 mg/kg per dose. Intranasal midazolam should be considered as an anticonvulsant agent for community, prehospital, and ED use in children when intravenous access is not available and the rectal route is not desirable.
ABBREVIATIONS
- CNS
central nervous system
- ED
emergency department;
- IN-MADD
intranasal mucosal atomization device
- SaO2
oxygen saturation
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Holsti M, Dudley N, Schunk J. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med. 2010;164(8):747–753. doi: 10.1001/archpediatrics.2010.130. et al. [DOI] [PubMed] [Google Scholar]
- 2.Harbord MG, Kyrkou MR, Kay D. Use of intranasal midazolam to treat acute seizures in paediatric community settings. J Paediatr Child Health. 2004;40(9–10):556–558. doi: 10.1111/j.1440-1754.2004.00463.x. et al. [DOI] [PubMed] [Google Scholar]
- 3.ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med. 2004;43(5):605–625. doi: 10.1016/j.annemergmed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Rey E, Tréluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures: focus on delivery routes. Clin Pharmacokinet. 1999;36(6):409–424. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lahat E, Goldman M, Barr J. Intra-nasal midazolam for childhood seizures. Lancet. 1998;352(9128):620. doi: 10.1016/S0140-6736(05)79574-X. et al. [DOI] [PubMed] [Google Scholar]
- 6.Wermeling DP. Intranasal delivery of anticonvulsant medications for treatment of seizures. Neurotherapeutics. 2009;6(2):352–358. doi: 10.1016/j.nurt.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan HB, Miller GS. Home management of breakthrough seizures. In: Maria BL, editor. Current Management in Child Neurology. 3rd ed. Shelton, CT: People's Medical Publishing House; 2005. pp. 129–133. [Google Scholar]
- 8.Holsti M, Sill BL, Firth SD. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23(3):148–153. doi: 10.1097/PEC.0b013e3180328c92. et al. [DOI] [PubMed] [Google Scholar]
- 9.Rey E, Delaunay L, Pons G. Pharmacokinetics of midazolam in children: comparative study of intranasal and intravenous administration. Eur J Clin Pharmacol. 1991;41(4):355–357. doi: 10.1007/BF00314967. et al. [DOI] [PubMed] [Google Scholar]
- 10.O'Regan ME, Brown JK, Clarke M. Nasal rather than rectal benzodiazepines in the management of acute childhood seizures. Dev Med Child Neurol. 1996;38(11):1037–1045. doi: 10.1111/j.1469-8749.1996.tb15064.x. [DOI] [PubMed] [Google Scholar]
- 11.O'Dell C, Shinnar S, Ballaban-Gil KR. Rectal diazepam gel in the home management of seizures in children. Pediatr Neurol. 2005;33(3):166–172. doi: 10.1016/j.pediatrneurol.2005.03.005. et al. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya M, Kalra V, Gulati S. Intra-nasal midazolam vs rectal diazepam in acute childhood seizures. Pediatr Neurol. 2006;34(5):355–359. doi: 10.1016/j.pediatrneurol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Fişgin T, Gurer Y, Tezic T. Effects of intranasal midazolam and rectal diazepam on acute convulsions in children: prospective randomized study. J Child Neurol. 2002;17(2):123–126. doi: 10.1177/088307380201700206. et al. [DOI] [PubMed] [Google Scholar]
- 14.Kutlu NO, Yakinci C, Dogrul M. Intra-nasal midazolam for prolonged convulsive seizures. Brain Dev. 2000;22(6):300–361. doi: 10.1016/s0387-7604(00)00155-8. et al. [DOI] [PubMed] [Google Scholar]
- 15.Fişgin T, Gurer Y, Senbil N. Nasal midazolam effects on childhood acute seizure. J Child Neurol. 2000;15(12):833–835. doi: 10.1177/088307380001501219. et al. [DOI] [PubMed] [Google Scholar]
- 16.Kyrkou M, Harbord M, Kyrkou N. Community use of intranasal midazolam for managing prolonged seizures. J Intellect Dev Disabil. 2006;31(3):131–138. doi: 10.1080/13668250600847021. et al. [DOI] [PubMed] [Google Scholar]
- 17.Jeannet PY, Roulet E, Maeder-Ingvar M. Home and hospital treatment of acute seizures in children with nasal midazolam. Eur J Paediatr Neurol. 1999;3(2):73–77. doi: 10.1053/ejpn.1999.0185. et al. [DOI] [PubMed] [Google Scholar]
- 18.Chiaretti A, Barone G, Rigante D. Intranasal lidocaine and midazolam for procedural sedation in children. Arch Dis Child. 2011;96(2):160–163. doi: 10.1136/adc.2010.188433. et al. [DOI] [PubMed] [Google Scholar]
- 19.Diazepam. Hudson, OH: LexiComp Online. LexiComp. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6728. March 30, 2013. [Google Scholar]


