Abstract
In this work, microparticles were prepared by spray-drying using albumin, chondroitin sulfate, and hyaluronic acid as excipients to create a controlled-release methylprednisolone system for use in inflammatory disorders such as arthritis. Scanning electron microscopy demonstrated that these microparticles were almost spherical, with development of surface wrinkling as the methylprednisolone load in the formulation was increased. The methylprednisolone load also had a direct influence on the mean diameter and zeta potential of the microparticles. Interactions between formulation excipients and the active drug were evaluated by x-ray diffraction, differential scanning calorimetry, and thermal gravimetric analysis, showing limited amounts of methylprednisolone in a crystalline state in the loaded microparticles. The encapsulation efficiency of methylprednisolone was approximately 89% in all formulations. The rate of methylprednisolone release from the microparticles depended on the initial drug load in the formulation. In vitro cytotoxic evaluation using THP-1 cells showed that none of the formulations prepared triggered an inflammatory response on release of interleukin-1β, nor did they affect cellular viability, except for the 9.1% methylprednisolone formulation, which was the maximum test concentration used. The microparticles developed in this study have characteristics amenable to a therapeutic role in inflammatory pathology, such as arthritis.
Keywords: spray-drying, microparticles, methylprednisolone, chondroitin sulfate, hyaluronic acid, cytotoxic assays
Introduction
Arthritis is a disease that features joint swelling and/or cartilage erosion, which eventually leads to loss of mobility and deterioration of quality of life.1 Currently, there is no curative treatment for the disease,2 and the medications available, ie, analgesics or non-steroidal anti-inflammatory drugs,3 disease-modifying antirheumatic drugs, biological therapy,4 and glucocorticoids, only mitigate its symptomatology.5 Glucocorticoids are the most powerful anti-inflammatory drugs known, and inhibit migration of leukocytes to sites of inflammation by decreasing or inhibiting the action of some of the chemical mediators of inflammation.6 Methylprednisolone is often contained in formulations given by intra-articular injection to treat certain types of arthritis,7 and there is good evidence to support appropriate use of intra-articular corticosteroids in patients with osteoarthritis of the knee. However, because of the need for multiple intra-articular injections, damage to the cartilage is accelerated.8 Therefore, it is essential to develop new formulations of established drugs used in the treatment of inflammatory diseases, allowing sustained drug release to avoid degradation of cartilage and reduce the risk of joint sepsis as a result of repeated injections.
Development of pharmaceutical microtechnology incorporating drugs and using biodegradable, biocompatible, and nontoxic materials has shown great promise.9 Microparticles are a novel therapy in arthritis, because they can act as small reservoir systems for drugs such as methylprednisolone.10 The drug release profile depends on various parameters, including size, morphology, porosity, degradability, polymer permeability, and surface features of the active compound.11 Another feature of microparticles is their ability to target drugs, in particular those with high toxicity and those having a low therapeutic index, to specific tissues12,13 Drug targeting can reduce the number of doses required while increasing the drug concentration at the desired site, thus contributing to a decrease or inhibition of toxic effects.14 One of the most original techniques used in the preparation of microparticles is spray-drying,15 which has the advantages of being able to be adapted for production on an industrial scale as well as an ability to encapsulate a range of hydrosoluble16 and liposoluble (either sensitive or thermoresistant) substances.17 The features of the final product, including moisture, particle size, and production yield, are determined by the physicochemical characteristics of the microparticles, as well as by factors such as feed rate, temperature, air flow, and aspirator.17,18 Moreover, use of ultrasound in the spray-drying technique helps to streamline the manufacturing process by reducing energy consumption, increasing the yield from the procedure, and avoiding problems such as condensation in the drying chamber.19–21
Previous research has established that use of excipients derived from components occurring naturally in the human body improves drug biocompatibility and transport through the usual metabolic pathways.22 Albumin has been widely used in the manufacture of microparticles, acting as a vehicle for drug delivery to specific sites.23 Hyaluronic acid and chondroitin sulfate are polysaccharides found in the synovial fluid, extracellular matrix, and connective tissue, and have an important role in articular homeostasis.24,25 In addition, they have anti-inflammatory and anticatabolic properties that attenuate production of inflammatory mediators by chondrocytes,26 as well as having an analgesic action in arthritic joints.27 It has also been demonstrated that hyaluronic acid and chondroitin sulfate reduce friction and strain on the articular cartilage.28 The pharmacological features of particular excipients have been exploited when devising a matrix for encapsulation of corticosteroids29 and disease-modifying agents in the case of arthritic disorders.30
The toxicological profile of any microparticle formulation requires evaluation before it can be used safely in human medicine. In vitro cytotoxicity assays compared with animal models enable evaluation of toxicity in a more simple, rapid, and cost-effective way.31 Cell viability is the most commonly researched parameter in cytotoxicity tests, and is determined from various cellular processes.31 Among the most widely used are the MTT assay which determines mitochondrial function in the cell32 and the lactate dehydrogenase release assay which identifies cell membrane damage33 or death.34,35 Some researchers have shown that microparticles are able to induce an inflammatory response (demonstrated by release of interleukin-1β) because of the presence of inflammatory precursors, eg, bacteria or endotoxins acquired during the manufacturing process, certain components of the microparticle formulation (eg, polymers, excipients), and wastes produced by the manufacturing process (eg, organic solvents).31 In vitro quantification of interleukin-1β is done using the enzyme-linked immunosorbent assay, which has become the most frequently used method for quantification of interleukin-1β in supernatants of cell cultures via antibody and enzymatic recognition reactions.36
This work describes the manufacture of methylprednisolone microparticles using a spray-drying technique. Both hyaluronic acid and chondroitin sulfate, which are found naturally in the joint cavity, have been used to improve the physical features of spray-dried powders and been found to have the potential to modulate drug release.37,38 The aim of this study was to encapsulate methylprednisolone into microparticles, to assess the influence of excipients on in vitro drug release under sink conditions, and to provide a preliminary evaluation of the toxicity of this drug delivery system, given the prospect of its eventual application in the clinical treatment of inflammatory conditions.
Materials and methods
Materials
Bovine serum albumin, hyaluronic acid sodium salt from Streptococcus equi species, chondroitin sulfate sodium salt from bovine trachea, and N-(2-hydroxyethyl) piperazine-N′-(2-ethanesulfonic acid) (HEPES) were obtained from Sigma-Aldrich (St Louis, MO, USA). Methylprednisolone was provided by Spectrum Laboratories Inc (Rancho Dominguez, CA, USA). Ethanol of analytical grade and acetonitrile of high-performance liquid chromatography grade were purchased from Merck (Darmstadt, Germany). Water was purified using an Elga® Purelab Classic UVF system (High Wycombe, UK). The human monocytic cell line THP-1 used in this study was purchased from ATCC (American Type Cell Culture, Manassas, VA, USA).
Preparation of microparticles
Biodegradable microparticles containing methylprednisolone were prepared using a spray-drying technique. Briefly 60 mg albumin, 200 mg of hyaluronic acid and 340 mg of chondroitin sulfate were dissolved into 140 mL of water, and methylprednisolone (4.4, 10, and 60 mg, corresponding to 0.73%, 1.64%, and 9.1% by weight) was dissolved into 60 mL of ethanol. Both solutions were mixed and placed in a mini spray-dryer (B-290, Büchi, Flawil, Switzerland) equipped with a 0.7 mm two-fluid nozzle. We optimized the process parameters to prevent condensation during spraying, including those that directly affected the final state of the product (eg, spray gas flow and the peristaltic pump, see Table 1). The powdered samples were stored at room temperature under vacuum conditions in a desiccator immediately after spray-drying to limit uptake of moisture in the time interval between production and testing. The yield was computed as a percentage of the mass of powder collected divided by the initial solid mass in the solution prior to spray-drying.
Table 1.
Optimized parameters for the spray-drying process
| Parameters | Range | Optimal value |
|---|---|---|
| Inlet temperature (°C) | 100–140 | 115 |
| Air flow (L/h) | 283–1052 | 538* |
| Peristaltic pump (mL/min) | 6–12 | 6 |
| Proportions of solvents water: ethanol (v/v) | 70:30 50:50 30:70 |
70:30 |
Note:
100 psi of pressure.
Encapsulation efficiency of methylprednisolone
First, 11 mg of microparticles containing methylprednisolone 0.73%, 1.64%, or 9.1% were prepared weighed and then dissolved in 10 mL of water by stirring in a vortex mixer (Vortex-Genie®, Scientific Industries Inc, Bohemia, NY, USA) at 3200 rpm. Next, 10 mL of acetonitrile containing prednisone as the internal standard was added then stirred in a vortex mixer at 3200 rpm and sonicated (Sonics and Materials Inc, Newtown, CT, USA) immediately at 80% amplitude for 2 minutes. One milliliter of the solution obtained was filtered through a 0.22 μm polyvinylidene difluoride membrane. The amount of methylprednisolone in the microparticles was determined by injecting 20 μL of the filtered solution into a liquid chromatographic column (Merck Hitachi LaChrom Elite L-2130, Merck) equipped with a Rheodyne 7725i injection valve (Sigma-Aldrich) and a programmable ultraviolet absorbance detector (Merck Hitachi LaChrom Elite L-240, Merck). The analysis was carried out at 244 nm using a reverse phase LiChrosper® C18 column (5 μm, 250 × 4.0 mm, Merck) with a solvent mixture of 40% acetonitrile and 60% water delivered isocratically at a flow rate of 1 mL per minute. All analyses were performed at ambient temperature (approximately 23°C). The method developed showed suitable linearity between 0.2 μg/mL and 624 μg/mL (y = 0.0523x + 0.0107; with r2 = 1). Encapsulation efficiency for the methylprednisolone-loaded particles (MP-EE) was expressed as w/w %:
| (1) |
Where, MPmicro = Total amount of methylprednisolone in microparticles. MP0 = Initial amount of methylprednisolone.
Scanning electron microscopy
Scanning electron microscopy was performed using a JEOL-JSM 6380 LV instrument (JEOL Techniques Ltd Tokyo, Japan) furnished with an Inca X-Sight 7582 (Oxford Instruments, Abingdon, UK) operating at a 20 kV accelerating voltage. The microparticles were coated with a gold layer of about 150 Å thickness, using an Edwards S 150 sputter coater (Agar Scientific, Standsted UK).
Zeta potential and particle size
The zeta potential was measured using a Malvern Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) based on quasi-elastic light scattering at 25°C following 1/50 (v/v) dilution in a 1 mM NaCl solution. The values reported represent the average of three measurements. The size distribution of the powder was measured by light diffraction using a Mastersizer 2000 equipped with a Scirocco dry dispenser (Malvern Instruments, Orsay Cedex, France) at a dispersing pressure of 2 bar. Each sample was measured in triplicate. Data are expressed in terms of the particle diameter, at 10%, 50%, and 90% of volume distribution (D10, D50, and D90, respectively). The volume distribution span, which is the distribution width measurement relative to the median diameter, was calculated as follows:
| (2) |
X-ray diffraction
X-ray powder diffraction patterns were measured using a D4 Endeavor® diffractometer (Bruker, Billerica, MA. USA) with Ni-filtered Cu Kα1 radiation while being operated at 40 kV and 20 mA. The step width was 0.02°, with a one second counting time per step. Samples were run between 3° and 50° 2θ.
Differential scanning calorimetry
The microparticles were analyzed in aluminum pans using a differential scanning calorimeter (GmbH 822e, Mettler Toledo, Columbus, OH, USA) furnished with a EK90/MT type Thermo Haake intracooler (Sigma-Aldrich, St Gallen, Switzerland). Differential scanning calorimetry runs were conducted under a nitrogen atmosphere flow rate of 20 mL per minute, while temperature ranged from 25°C to 300°C, at a heating rate of 5°C per minute.
Thermogravimetric analysis
Thermogravimetric analysis was carried out using an STA 449 F3 Jupiter® device (Netzsch-Gerätebau GmbH, Selb, Germany). Sample weights ranged from 10 mg to 15 mg. The sample pan was placed in the balance system while the temperature was increased from 25°C to 100°C at a heating rate of 5°C per minute. The sample pan weight was recorded continuously.
In vitro drug release study
In vitro release of methylprednisolone from the microparticles was studied under sink conditions, whereby the drug concentration in the total medium was kept 10 times lower than the solubility of saturated methylprednisolone in HEPES buffer (120 μg/mL). A weighed amount of microparticles equivalent to about 3 mg of methylprednisolone was resuspended in 10 mL of 10 mM HEPES buffered saline (150 mM NaCl, pH 7.4) by means of vortexing, then put into a dialysis tube (molecular weight cutoff 12,000 Da, Sigma Chemical Corporation, St Louis, MO, USA). The dialysis tube was then placed inside a 300 mL glass bottle containing 240 mL of HEPES buffered saline. A release test was performed at 37°C, with a 100 rpm stirring rate on a Multistirred 6® (Velp Scientifica, Milan, Italy). At predetermined time intervals, 1.0 mL aliquots were withdrawn from the outer aqueous solution and replaced by a similar volume of fresh medium. The methylprednisolone released was quantified by high-performance liquid chromatography. Each in vitro release experiment was repeated three times.
In vitro cytotoxic assays
Cell viability assay
The cell growth rate was assessed using a commercially available Vybrant® MTT cell proliferation assay Kit (Invitrogen, Carlsbad CA, USA). Briefly, THP-1 cells were suspended at a final concentration of 1 × 106 cells/mL in growth medium consisting of RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum, 1% L-glutamine, 1% of 100 mM sodium pyruvate, 1% of 1 M HEPES, 1% of 1.4 M glucose, 0.1% of 0.05 mM 2-mercaptoethanol and 1% penicillin-streptomycin.
| (3) |
Membrane damage assay
Lactate dehydrogenase release was measured to quantify membrane damage using a cytotoxicity detection kit (LDH12 Cobas CIII, Roche, Germany). Briefly, THP-1 cells were seeded into 24-well plates (1 × 106 cells/well). After exposure to increasing microparticle concentrations (0.02–5 mg/mL), the culture supernatants were removed and centrifuged at 5000 rpm for 5 minutes to remove cell debris and microparticles. Next, 1 × 106 cells were subjected to sonication for one minute at 130 Watts as a control for total lysis. The lactate dehydrogenase released was measured (ie, total lysis control) and then used as an index of cellular membrane integrity, as follows:
| (4) |
where “LDH in sample” is the lactate dehydrogenase released into the external medium, “LDH 0%” is the amount of lactate dehydrogenase present in the culture medium, and “LDH 100%” is the lactate dehydrogenase released after sonication, corresponding to 100% cell lysis.
Enzyme-linked immunosorbent assay for interleukin-1β quantification
An in vitro study of interleukin-1β quantification was performed on cells exposed to suspensions containing different microparticle concentrations (0.02–5 mg/mL), either unloaded and loaded with 1.64% or 9.1% microparticles, using a commercially available human interleukin-1β enzyme-linked immunosorbent assay kit (Ready-SET-Go!®, eBioscience Inc, San Diego, CA, USA), following the manufacturer’s instructions. The optical density from the individual wells was measured using a spectrophotometer at 450 nm (Bio-Tek Instruments Inc). The assay quantification limit was 7.8 pg/mL.
Results and discussion
Microparticles containing methylprednisolone were formulated by spray-drying and evaluated to determine the influence of excipients on in vitro drug release under sink conditions and to assess the toxicity of this method of drug delivery in THP-1 cells, considering their potential for use in the clinical treatment of inflammatory disorders.
Optimization of formulation parameters
The formulation parameters were optimized by modifying the ratios and concentrations of the components (ie, albumin, chondroitin sulfate, and hyaluronic acid) in the spraying solution (200 mL water:ethanol 70:30 v:v mixture). The morphology of the microparticles was assessed by scanning electron microscopy (Figure 1). When the microparticles were produced only with albumin, they were found to be polydispersed and oval with a rough surface, especially those that were of the smallest sizes (Figure 1A). From this formulation, microparticles containing 67% albumin and 33% chondroitin sulfate were produced, which showed markedly superior morphological characteristics, becoming less rough and being semispherical (Figure 1B). A similar morphology was achieved by adding 33% hyaluronic acid to the 67% albumin-based formulation. A marked increase in diameter was found in these microparticles, demonstrating that the presence of albumin contributes to their oval shape and surface texture (Figure 1C). Therefore, the decision was made to produce microparticles containing smaller amounts of albumin and an increased chondroitin sulfate content (10% albumin, 33% hyaluronic acid, 57% chondroitin sulfate). The final microparticle preparation had improved morphological features, with a spherical shape and an almost smooth surface (Figure 1D).
Figure 1.
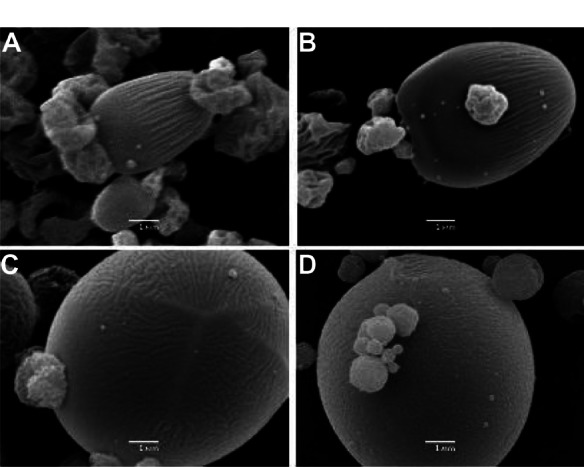
Microparticles obtained by varying the components of the formulation. (A) Albumin 6 g/L, (B) albumin at 67% and chondroitin sulfate at 33%, 3 g/L, (C) albumin at 67% and hyaluronic acid at 33%, 3 g/L, and (D) albumin at 10%, hyaluronic acid at 33%, and chondroitin sulfate at 57%, 3 g/L.
Optimization of process parameters
Once the composition and ratio of the excipients composing the microparticle matrix were established, optimization of the manufacturing equipment parameters was carried out (Table 1). These parameters were adjusted to prevent condensation in the drying chamber, and to optimize particle characteristics, such as particle size, which directly affects the status of the final product. It was observed that when the feed rate was increased by means of a peristaltic pump, condensation took place within the equipment drying chamber. Therefore, a value of 6 mL per minute was set.39 In addition, inlet temperatures lower than 115°C and an ethanol ratio in aqueous solution lower than 30% generated a decrease in outlet temperature and, consequently, condensation inside the drying chamber.40
Recent research has shown that submicron-sized microparticles are taken up rapidly by phagocytic cells in the synovium, whereas microspheres ranging in size from 3 μm to 60 μm remain dispersed in the joint cavity or adherent to the surface of the synovial membrane, and are well tolerated. However, microspheres in the smaller size range trigger a marked inflammatory response, causing joint swelling and loss of proteoglycans.41 In order to produce particles with an optimum mean diameter (3–60 μm) it was necessary to reduce the spraying air flow rate, setting 538 L per hour as the lower limit, given that lower flow rates led to condensation inside the drying chamber.
Once the process parameters were optimized, different amounts of methylprednisolone were included with the aim of producing microparticles loaded with different concentrations of active drug (0.73%, 1.64%, and 9.1%), along with the excipients sprayed in the 70:30 water:ethanol mixture. Figure 2 shows the influence of methylprednisolone load on the morphology and size of the microparticles. All the microparticles maintained their spherical shape, but their surface roughness increased in direct proportion to the concentration of methylprednisolone in the formulation. Previous research has reported that the surface characteristics of microparticles do not affect cell attachment, proliferation, or uniform distribution in cells.42
Figure 2.
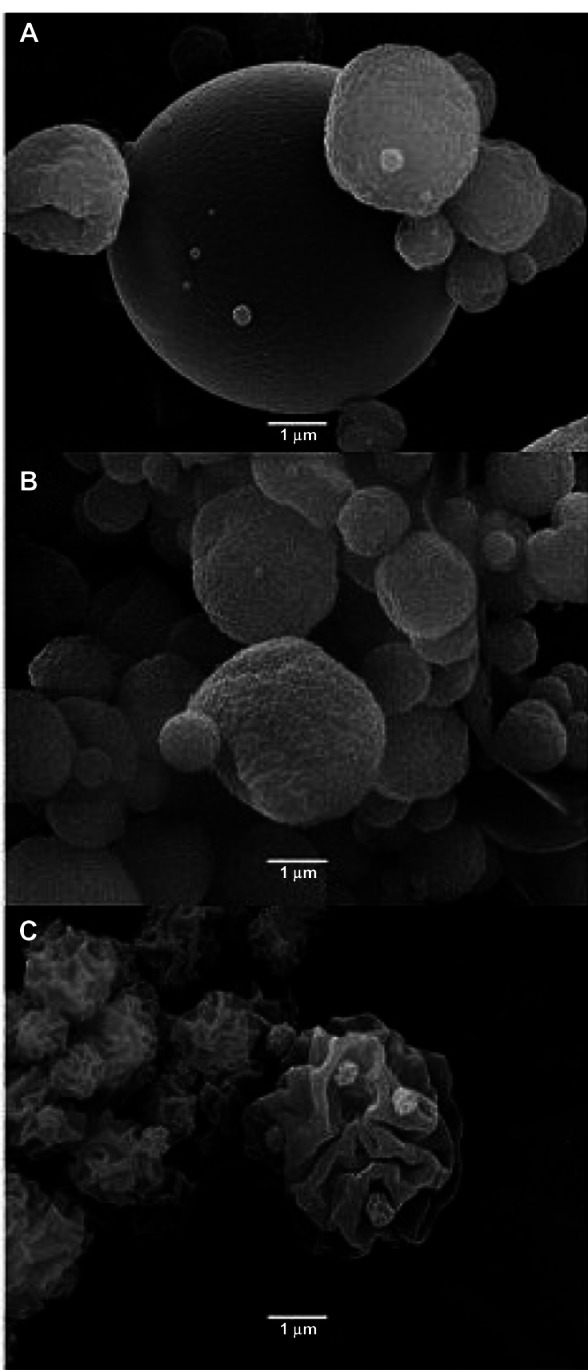
Methylprednisolone-loaded microparticles at concentrations of (A) 0.73%, (B) 1.64%, and (C) 9.1%.
Encapsulation efficiency for methylprednisolone
The encapsulation efficiency for methylprednisolone was rather high, ranging from 89.20% to 95.43% for all the microparticles produced (Table 2), with a statistically significant difference (P < 0.05) in the highest values for drug entrapment achieved when working with a 1.64% methylprednisolone concentration. Nevertheless, it was noted that there was no statistically significant difference in encapsulation efficiency for the process yield according to methylprednisolone concentration (P > 0.999). Indeed, previous studies have reported that the spray-drying process provides a fairly high encapsulation efficiency compared with other methods, such as solvent emulsion/evaporation.43
Table 2.
Encapsulation efficiency of methylprednisolone in the microparticles (n = 8) and percentage yield of the process (n = 3)
| Microparticles (MP) | Encapsulation efficiency of methylprednisolone (%) | Yield of the process (%) |
|---|---|---|
| Unloaded | – | 37.1 ± 6.7 |
| MP at 0.73% | 91.48 ± 26.35 | 37.7 ± 2.4 |
| MP at 1.64% | 95.43 ± 10.70 | 38.0 ± 6.5 |
| MP at 9.10% | 89.20 ± 6.46 | 33.7 ± 5.0 |
Particle size and zeta potential
Table 3 shows the particle sizes for the unloaded and loaded microparticles. Statistical analysis showed a meaningful difference (P < 0.05) between the mean diameters ([D50]). All microparticles showed a bimodal size distribution, thus having a huge population, most likely caused by aggregation of particles as a result of fusion or melting during the final stages of the drying process.44 The agglomerates were not completely broken down, in spite of the dispersion pressure (2 bar) used in the laser diffraction equipment. However, aggregation of microparticles loaded with methylprednisolone 9.1% was found to be decreased, with a mean diameter (D50) of 16.73 μm, similar to what was observed by scanning electron microscopy (Figure 2). This decrease in aggregation can be explained by the surface roughness, which decreases the forces existing between the particles.16,45 Earlier studies of dexamethasone encapsulation showed that changes in particle size distribution can be due to particle aggregation, such that both the mean and median sizes increased steeply as more dexamethasone was incorporated into the formulation, with the size increase being greater at higher dexamethasone concentrations.29
Table 3.
Particle size and zeta potential of microparticles (n = 3)
| Microparticles | Diameters (μm) ± SD
|
Span | Zeta potential (mV) ± DS | ||
|---|---|---|---|---|---|
| D10 | D50 | D90 | |||
| Unloaded | 2.34 ± 0.08 | 23.29 ± 0.96 | 59.77 ± 1.99 | 2.47 | −48.33 ± 3.28 |
| MP at 0.73% | 2.11 ± 0.06 | 31.43 ± 0.18 | 81.24 ± 0.06 | 2.52 | −46.70 ± 3.97 |
| MP at 1.64% | 2.09 ± 0.00 | 32.72 ± 0.32 | 86.01 ± 1.36 | 2.56 | −36.37 ± 4.56 |
| MP at 9.10% | 1.59 ± 0.02 | 16.73 ± 0.12 | 53.08 ± 0.14 | 3.08 | −35.60 ± 7.64 |
Abbreviation: SD, standard deviation.
The zeta potential for all formulations was found to be negative, from −48 to −35 mV, with the zeta potential becoming less negative as the concentration of methylprednisolone increased, possibly because of the contribution to neutrality provided by the active principle. In spite of this, it is believed that such an order of magnitude promotes repulsion among particles, keeping them as individual entities when dispersed in water. This suggests formation of flocculated and stable suspensions in aqueous medium, which, in turn, build up noncompacted aggregates that are easy to resuspend by the time they settle.46
Structural and thermal analysis
The physical state of the drug contained in the microparticles was established by crystallographic analysis using x-ray diffraction. Figure 3 shows the x-ray diffraction patterns for the individual components of the formulations, physical mixtures, and microparticles. Typical peaks are observed in the methylprednisolone diffraction pattern, indicating their crystalline nature, while albumin, hyaluronic acid, and chondroitin sulfate show a mainly shapeless structure with limited crystallinity (Figure 3A). With respect to the physical mixtures of the components of the formulation prepared using the same ratios as in the manufactured microparticles, methylprednisolone was observed to be present in a crystalline form, for which the peaks in intensity were proportional to the concentration of the active principle found in each mixture (Figure 3B). However, the characteristic peaks of methylprednisolone were not seen in the manufactured microparticles. In spite of this, the intensity of the signal increased proportionally to the methylprednisolone concentration loaded into the microparticles, which was not seen in the empty microparticles (Figure 3C). Such a signal may be attributable to the structural rearrangement undergone by components of the microparticles during the manufacturing process, indicating some modifications to their crystalline configuration. Nevertheless, the intensity of the peak generated is linked to the presence of methylprednisolone in a crystalline state.
Figure 3.
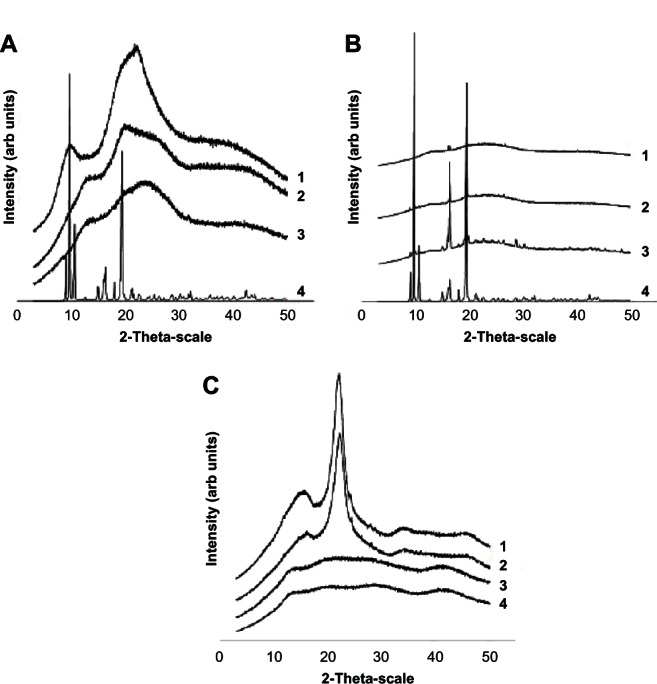
(A) X-ray diffraction patterns for the formulation components including (1) albumin, (2) hyaluronic acid, (3) chondroitin sulfate, and (4) methylprednisolone. (B) Physical mixtures of the formulation components, including (1) methylprednisolone 0.73%, (2) methylprednisolone 1.64%, (3) methylprednisolone 9.1%, and (4) compared with methylprednisolone. (C) Methylprednisolone-loaded microparticles at (1) 9.1%, (2) 1.64%, (3) 0.73%, and (4) comparison with unloaded microparticles.
The physical state of the drug when encapsulated in microparticles was further established by differential scanning calorimetry. Figure 4 shows the thermograms obtained for the components of the formulation. Chondroitin sulfate and hyaluronic acid have endothermic peaks within the temperature range of 50°C–150°C, which may reflect loss of the volatile components.47 Further, the thermogram for chondroitin sulfate shows an exothermic peak at 231°C, while hyaluronic acid shows two exothermic peaks at 227°C and 243°C, respectively, which are attributable to degradation of these polysaccharides.24,47 The methylprednisolone thermogram shows an endothermic transition at 220°C and an endothermic melting peak at 238°C (Figure 4A).48 However, differential scanning calorimetry curves for all the microparticle preparations show an endothermic peak typical of albumin denaturation, as well as an exothermic peak at 221°C possibly due to degradation or decomposition of the component resulting from the formulation excipients molecular crossing-over (Figure 4B). This same exothermic peak was seen for the physical mixtures of the separate components in proportions similar to those used in the microparticles (Figure 4C).
Figure 4.
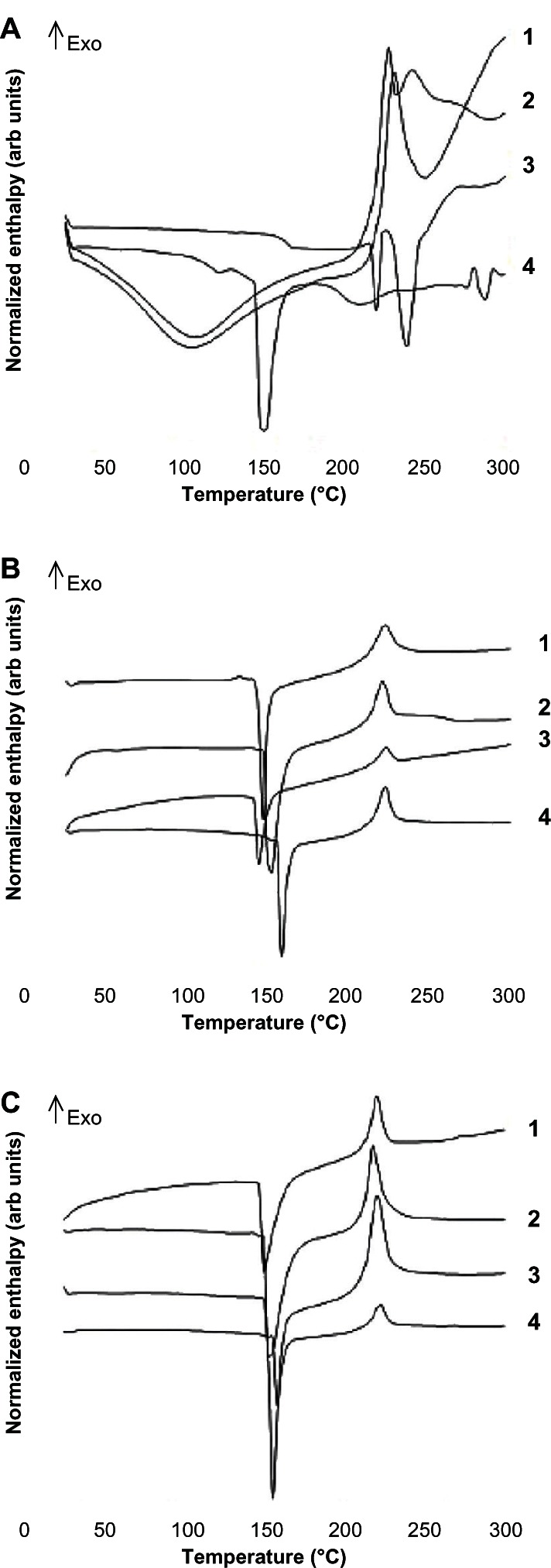
(A) Differential scanning calorimetry curves for the formulation components, ie, (1) chondroitin sulfate, (2) hyaluronic acid, (3) methylprednisolone, and (4) albumin. (B) Methylprednisolone-loaded microparticles at concentrations of (1) 9.1%. (2) 1.64%, (3) 0.73%, and (4) unloaded microparticles. (C) Physical mixtures of the formulation components, including (1) methylprednisolone 9.1%, (2) methylprednisolone 1.64%, (3) methylprednisolone 0.73%, and (4) comparison with unloaded microparticles.
Given that the behavior found in the differential scanning calorimetry patterns generated by all microparticles and physical mixtures of the formulation components was the same, a thermogravimetric analysis was undertaken for microparticles prepared with a methylprednisolone concentration of 9.1%, together with an assessment of that process due to the exothermic peak seen at 221°C. The thermogravimetric pattern showed a loss of mass from the initial weight of the settled microparticles, with a point of inflection in the curve generated at the same temperature as that which had been observed in the exothermic peak at 221°C which became obvious in the differential scanning calorimetry thermogram. All these findings point to this peak being generated via a degradation or decomposition process.49 Structural characterization of the microparticles clearly shows an interaction between the components of the formulation as well as adequate molecular dispersion of the drug within the microparticle matrix.
In vitro drug release from microparticles
The amount of methylprednisolone released from the microparticles was calculated on the basis of the actual amount of methylprednisolone encapsulated inside the microparticles. Figure 5 shows the release of methylprednisolone from the microparticles under sink conditions. As expected, diffusion of free methylprednisolone from the dialysis bag was faster and complete after 8 hours, as compared with the methylprednisolone-loaded microparticles, all of which showed a biphasic pattern that consisted of a burst release within the first 4 hours, followed by a slower and more steady release phase (Figure 5A). This burst release may be due to the fact that the active principle is linked to the surface or embedded in the external layers of the microparticles.50 The maximum drug release from the microparticles loaded with methylprednisolone 0.73% was 94% after 4 days of incubation. However, for the same incubation period, the maximum drug release for methylprednisolone encapsulated at 1.64% was 90%, while that for methylprednisolone encapsulated at 9.1% was just 65% (Figure 5B). When the incubation time was extended to 25 days, 80% drug release was observed for the latter formulation (Figure 5C).
Figure 5.
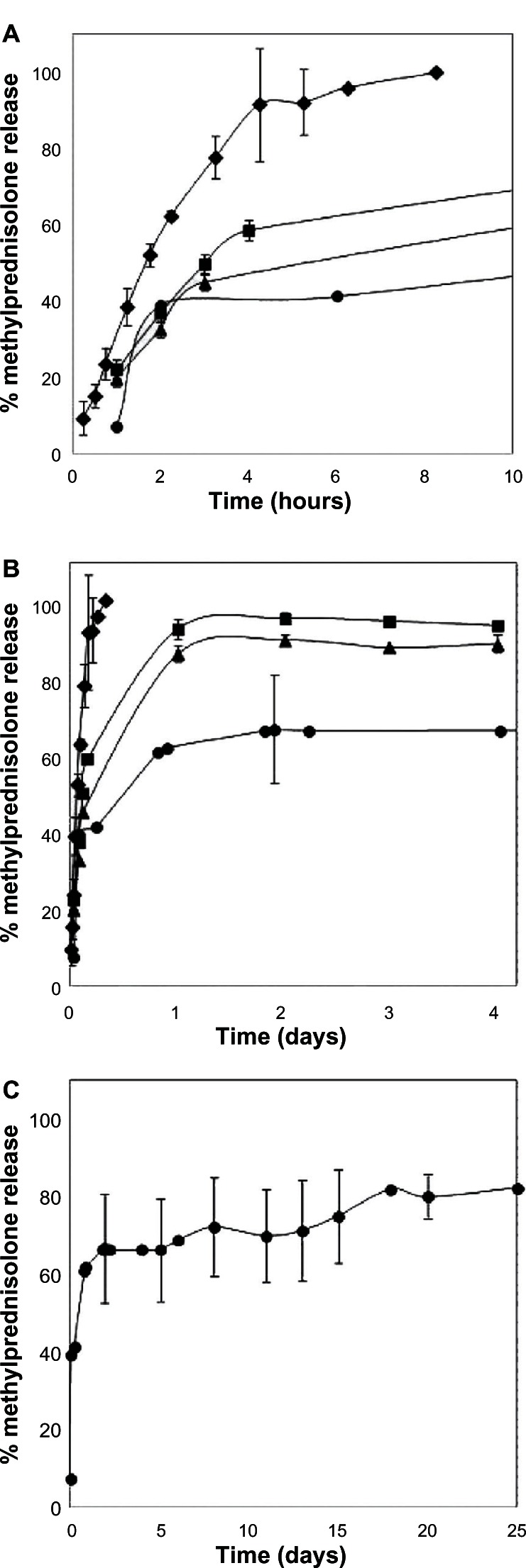
Kinetics of methylprednisolone diffusion in HEPES buffer (pH 7.4) under sink conditions (♦) and in vitro release profile of methylprednisolone from microparticles, in turn including (■) methylprednisolone 0.73%, (▲) methylprednisolone 1.64%, and (•) methylprednisolone 9.1% kept in release medium for (A) 10 hours, (B) 4 days and (C) 25 days. Data represent the mean ± standard deviation (n = 2).
Our release kinetics study demonstrated that microparticles act as reservoirs, maintaining constant release of the active principle according to incubation time, despite the fact that the components of the microparticle formulation are water-soluble.10 The ability of hyaluronic acid and chondroitin sulfate to thicken enables formation of a gelled structure with which the drug interacts and in which it remains trapped, thus delaying its release.25 After 25 days of incubation, the microparticles remaining in the dialysis membrane were observed by scanning electron microscopy (Figure 6). It can be seen that they maintained their spherical shape after the components were hydrated, with the presence of pores or channels in the surfaces of the microparticle. Such pores or channels may develop when methylprednisolone is being released.50
Figure 6.
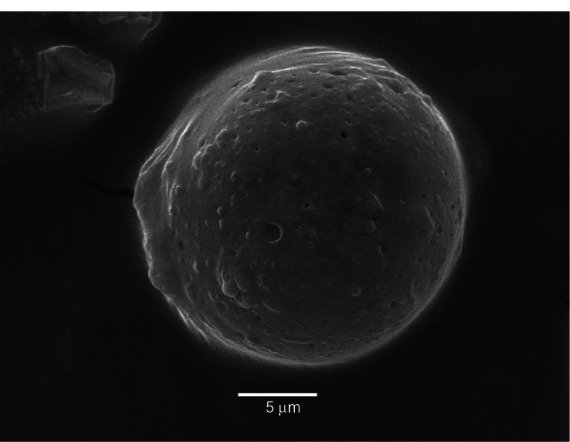
Scanning electron microscopic image of a typical microparticle loaded with methylprednisolone 9.1% after an in vitro release study (25 days).
It is known that drug release from microparticles in vivo depends on the quantity and composition of the fluid, and its rate of exchange or replenishment. However, the results of our study provide information about the behavior of such a system in a biological fluid environment for a specific period of time. In fact, when compared with the control, it was demonstrated that, even under sink conditions, microparticles may retard the release of methylprednisolone which probably has biological transcendence in a future in vivo release study.
In vitro cytotoxic assays
After the microparticles were prepared and characterized, those with the highest methylprednisolone loads were chosen (1.64% and 9.1%) for the in vitro cytotoxicity assays conducted in THP-1 cell cultures, with 24 hours and 48 hours of exposure time, and at different concentrations (0.02–5 mg/mL) of microparticle suspension. The same procedure was followed for the empty microparticles.
Cell viability assay
According to our MTT assay (Figure 7), the THP-1 cells maintained 100% viability after 24 hours of exposure to all concentrations of the empty control microparticles, except for the 5 mg/mL concentration, where 66% viability was recorded. On the other hand, THP-1 cells that had been exposed to different microparticle concentrations containing methylprednisolone 1.64% also showed a marked decrease in cell viability of 75% with the 5 mg/mL concentration. When microparticles containing methylprednisolone 9.1% were used at a 5 mg/mL concentration, cell viability showed a statistically significant decrease of about 40% (P < 0.05). Similar results for THP-1 cell viability were obtained after 48 hours of culture, with 0% cellular viability reached at a microparticle concentration of 5 mg/mL.
Figure 7.
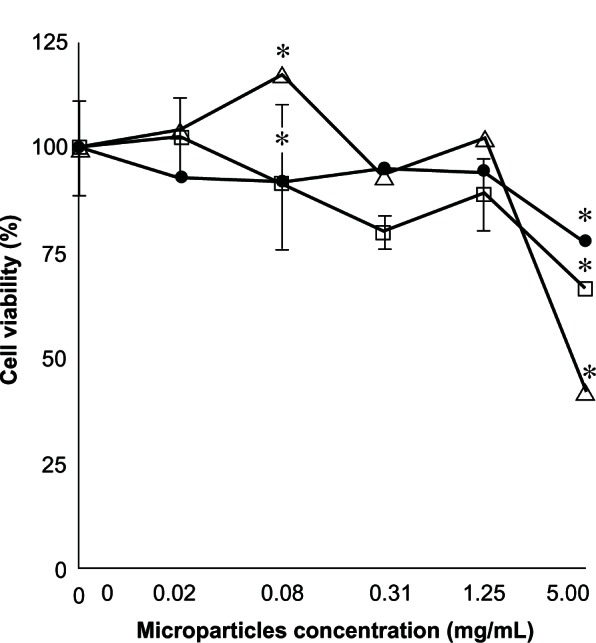
Percentage cell viability found by MTT assay of a THP-1 cell exposed to different concentrations over 24 hours. (□) Unloaded microparticles, (•) microparticles loaded with methylprednisolone 1.64%, (∆) and methylprednisolone 9.1% (n = 2) ± standard deviation.
Note: Significant difference, P < 0.05.
Membrane damage assay
The potential of the microparticle formulations to be toxic to the cell membrane was evaluated using the lactate dehydrogenase assay (Figure 8). THP-1 cell cultures exposed to different concentrations (0.02–5 mg/mL) of unloaded microparticles did not show any lactate dehydrogenase release after a 24-hour exposure period, indicating no membrane damage. However, cultures exposed to different concentrations of microparticles containing methylprednisolone 1.64%, showed increased release of lactate dehydrogenase, reaching 20% with the 5 mg/mL concentration. When using microparticles containing methylprednisolone 9.1%, release of lactate dehydrogenase also showed a statistically significant increase, reaching 47% for the 5 mg/mL concentration (P < 0.05). Similar trends were observed after 48 hours of culture, with lactate dehydrogenase release reaching 100% for microparticles containing methylprednisolone 9.1% at a microparticle concentration of 5 mg/mL.
Figure 8.
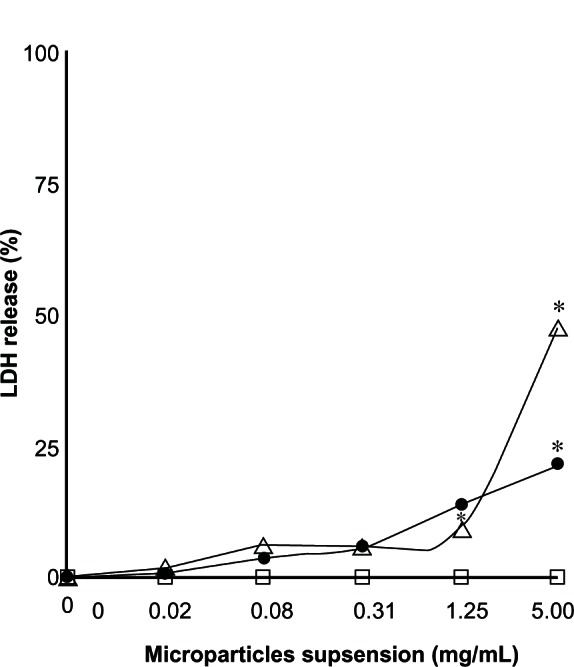
Percentage of lactate dehydrogenase release found by lactate dehydrogenase assay for a THP-1 cell exposed to different concentrations over 24 hours. (□) Unloaded microparticles, (•) microparticles loaded with methylprednisolone 1.64%, and (∆) and methylprednisolone 9.1% (n = 2) ± standard deviation.
Note: Significant difference, P < 0.05.
Previous studies evaluating the effect of methylprednisolone on the cell cycle showed that exposure to high corticosteroid concentrations exerts a negative effect on osteoblasts by hindering their proliferation.51 Likewise, similar studies have shown that high corticosteroid doses inhibit synthesis of proteoglycans and also negatively affect the structural arrangement of collagen within cartilage.52 These studies provided the basis for the effect we observed, ie, a decrease in proliferation capacity and viability when THP-1 cells were exposed to high concentrations (5 mg/mL) of the methyl-prednisolone 9.1% formulation, in turn linked to damage to the functional organelles of the cell or to destabilization or disruption of the cell membrane. However, some studies have shown that materials that lead to a viability greater than 80% are considered as biocompatible,53 so it can be concluded that microparticles manufactured to contain only the matrix, as well as those ones loaded with methylprednisolone 16.4% and 9.1%, do not exert toxic effects on THP-1 cells at microparticle concentrations below 1.25 mg/mL.
Inflammatory response
The inflammatory response was determined by quantification of the amount of interleukin-1β present in the supernatant of THP-1 cell cultures exposed to different microparticle concentrations (0.02–5 mg/mL), none of which was able to induce release of interleukin-1β from THP-1 cells at concentrations similar to or higher than 7.8 pg/mL (minimum concentration for quantification). A literature review has shown that interleukin-1β levels in normal serum are below 15 pg/mL,54 whereas levels associated with a number of infectious diseases and noninfectious inflammatory diseases, such as Crohn’s disease, are higher than this level.55 Increased interleukin-1β levels have also been found in the synovial fluid of patients with rheumatoid arthritis.56 Other studies have confirmed these findings, indicating that one of the mechanisms of action explaining the anti-inflammatory effect of corticosteroids is inhibition of interleukin-1β production.57,58
Conclusion
Microparticles loaded with methylprednisolone were successfully formulated by spray-drying, and were then characterized and assessed in vitro. Microparticles were produced using biodegradable excipients, including albumin, chondroitin sulfate, and hyaluronic acid, thereby mimicking the properties of the extracellular structure of cartilage matrix. These microparticles may have applications in cartilage tissue engineering if developed for intra-articular injection in inflammatory pathologies like arthritis. The best outcomes were obtained with microparticles containing methylprednisolone 9.1%, which had an encapsulation efficiency higher than 89% and showed controlled-release behavior, achieving drug release of 80% in vitro after 25 days. Toxicological assessment shows that these microparticles are biocompatible and do not generate an inflammatory response, and enable cell viability and cell membrane integrity at concentrations lower than 5 mg/mL. This research suggests that methylprednisolone microparticles may become a pharmacological option for the treatment of inflammatory diseases such as arthritis in the future.
Acknowledgments
The authors would like to thank the Chilean Council for Science and Technology, (CONICYT-FONDECYT) (Project 11080101). B Tobar-Grande is supported by the International Cooperation Agency of Chile (AGCI). Institut Galien ParisSud is a member of the Laboratory of Excellence LERMIT supported by a grant from ANR (ANR-10-LABX-33). We would also like to thank Margarita González and Mario Rodríguez for their fruitful technical discussions concerning this work.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Khanna D, Arnold EL, Pencharz JN, et al. Measuring process of arthritis care: the Arthritis Foundation’s quality indicator set for rheumatoid arthritis. Semin Arthritis Rheum. 2006;35(4):211–237. doi: 10.1016/j.semarthrit.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Butoescu N, Jordan O, Petri-Fink A, Hofmann H, Doelker E. Co-encapsulation of dexamethasone 21-acetate and SPIONs into biodegradable polymeric microparticles designed for intra-articular delivery. J Microencapsul. 2008;25(5):339–350. doi: 10.1080/02652040801999551. [DOI] [PubMed] [Google Scholar]
- 3.Murakami N, Takase H, Saito T, Iwata K, Miura H, Naruse T. Effects of a novel non-steroidal anti-inflammatory drug (M-5011) on bone metabolism in rats with collagen-induced arthritis. Eur J Pharmacol. 1998;352(1):81–90. doi: 10.1016/s0014-2999(98)00342-2. [DOI] [PubMed] [Google Scholar]
- 4.Yen JH. Treatment of early rheumatoid arthritis in developing countries. Biologics or disease-modifying anti-rheumatic drugs? Biomed Pharmacother. 2006;60(10):688–692. doi: 10.1016/j.biopha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Lundberg IE, Grundtman C, Larsson E, Klareskog L. Corticosteroids – from an idea to clinical use. Best Pract Res Clin Rheumatol. 2004;18(1):7–19. doi: 10.1016/j.berh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Molecular mechanisms and cellular effects of glucocorti-costeroids. Immunol Allergy Clin North Am. 2005;25(3):451–468. doi: 10.1016/j.iac.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher HR, Chen LX. Injectable corticosteroids in treatment of arthritis of the knee. Am J Med. 2005;118(11):1208–1214. doi: 10.1016/j.amjmed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Walker-Bone K, Javaid K, Arden N, Cooper C. Regular review: medical management of osteoarthritis. BMJ. 2000;321(7266):936–940. doi: 10.1136/bmj.321.7266.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson CL, Santini JT, Jr, Langer R. Reservoir-based drug delivery systems utilizing microtechnology. Adv Drug Deliv Rev. 2012;64(14):1590–1560. doi: 10.1016/j.addr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeidat WM. Recent patents review in microencapsulation of pharmaceuticals using the emulsion solvent removal methods. Recent Pat Drug Deliv Formul. 2009;3(3):178–192. doi: 10.2174/187221109789105612. [DOI] [PubMed] [Google Scholar]
- 11.Butoescu N, Jordan O, Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: a review of the factors influencing their performance. Eur J Pharm Biopharm. 2009;73(2):205–218. doi: 10.1016/j.ejpb.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Cerroni B, Chiessi E, Margheritelli S, Oddo L, Paradossi G. Polymer shelled microparticles for a targeted doxorubicin delivery in cancer therapy. Biomacromolecules. 2011;12(3):593–601. doi: 10.1021/bm101207k. [DOI] [PubMed] [Google Scholar]
- 13.Pranzatelli MR. Innovations in drug delivery to the central nervous system. Drugs Today (Barc) 1999;35(6):435–448. doi: 10.1358/dot.1999.35.6.544930. [DOI] [PubMed] [Google Scholar]
- 14.Butoescu N, Seemayer CA, Foti M, Jordan O, Doelker E. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials. 2009;30(9):1772–1780. doi: 10.1016/j.biomaterials.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Patel RP, Patel MP, Suthar AM. Spray drying technology: an overview. Indian J Sci Technol. 2009;2(10):44–47. [Google Scholar]
- 16.Cruz L, Fattal E, Tasso L, et al. Formulation and in vivo evaluation of sodium alendronate spray-dried microparticles intended for lung delivery. J Control Release. 2011;152(3):370–375. doi: 10.1016/j.jconrel.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 17.Rattes ALR, Oliveira WP. Spray drying conditions and encapsulating composition effects on formation and properties of sodium diclofenac microparticles. Powder Technology. 2007;171:7–14. [Google Scholar]
- 18.Wang AJ, Lu YP, Zhu RF, Li ST, Ma XL. Effect of process parameters on the performance of spray dried hydroxyapatite microspheres. Powder Technology. 2009;191:1–6. [Google Scholar]
- 19.Barba AA, D’amore M, Cascone S, Lamberti A, Titomanlio G. Intensification of biopolymeric microparticles production by ultrasonic assisted atomization. Chem Eng Process. 2010;48:1475–1481. [Google Scholar]
- 20.Cascone S, Lamberti G, Titomanlio G, Barba AA, d’Amore M. Microencapsulation effectiveness of small active molecules in biopolymer by ultrasonic atomization technique. Drug Dev Ind Pharm. 2012;38(12):1486–1493. doi: 10.3109/03639045.2011.653814. [DOI] [PubMed] [Google Scholar]
- 21.Dalmoro A, Barba AA, Lamberti G, d’Amore M. Intensifying the microencapsulation process: ultrasonic atomization as an innovative approach. Eur J Pharm Biopharm. 2012;80(3):471–477. doi: 10.1016/j.ejpb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Mansour HM, Sohn M, Al-Ghananeem A, Deluca PP. Materials for pharmaceutical dosage forms: molecular pharmaceutics and controlled release drug delivery aspects. Int J Mol Sci. 2010;11(9):3298–3322. doi: 10.3390/ijms11093298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urs AVR, Kavitha K, Sockan GN. Albumin microspheres: an unique system as drug delivery carriers for non steroidal anti-inflammatory drugs (NASIDs) Int J Pharm Sci Rev Res. 2010;5(2):10–17. [Google Scholar]
- 24.Kafedjiiski K, Jetti RK, Foger F, et al. Synthesis and in vitro evaluation of thiolated hyaluronic acid for mucoadhesive drug delivery. Int J Pharm. 2007;343(1–2):48–58. doi: 10.1016/j.ijpharm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Sui W, Huang L, Wang J, Bo Q. Preparation and properties of chitosan chondroitin sulfate complex microcapsules. Colloids Surf B Biointerfaces. 2008;65(1):69–73. doi: 10.1016/j.colsurfb.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Pecchi E, Priam S, Mladenovic Z, et al. A potential role of chondroitin sulfate on bone in osteoarthritis: inhibition of prostaglandin E and matrix metalloproteinases synthesis in interleukin-1beta-stimulated osteoblasts. Osteoarthritis Cartilage. 2012;20(2):127–135. doi: 10.1016/j.joca.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Oerther S, Payan E, Lapicque F, et al. Hyaluronate-alginate combination for the preparation of new biomaterials: investigation of the behaviour in aqueous solutions. Biochim Biophys Acta. 1999;1426(1):185–194. doi: 10.1016/s0304-4165(98)00155-x. [DOI] [PubMed] [Google Scholar]
- 28.Katta J, Jin Z, Ingham E, Fisher J. Chondroitin sulphate: an effective joint lubricant? Osteoarthritis Cartilage. 2009;17(8):1001–1008. doi: 10.1016/j.joca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Gaete C, Tsapis N, Silva L, et al. Supramolecular organization and release properties of phospholipid-hyaluronan microparticles encapsulating dexamethasone. Eur J Pharm Biopharm. 2008;70(1):116–126. doi: 10.1016/j.ejpb.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Park Y, Kemp MM, et al. Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern. Anal Biochem. 2009;385(1):57–64. doi: 10.1016/j.ab.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur J Pharm Biopharm. 2009;72(2):370–377. doi: 10.1016/j.ejpb.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Kim DH, Lee SH, Kim KN, Kim WM, Shim IB, Lee YK. Cytotoxicity of ferrite particles by MTT and agar diffusion methods for hyperthermic application. J Magn Magn Mater. 2005;293:287–292. [Google Scholar]
- 33.Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. 2006;160(2):171–177. doi: 10.1016/j.toxlet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Biggs DL, Lengsfeld CS, Hybertson BM, Ng KY, Manning MC, Randolph TW. In vitro and in vivo evaluation of the effects of PLA microparticle crystallinity on cellular response. J Control Release. 2003;92(1–2):147–161. doi: 10.1016/s0168-3659(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Gelein R, Corson N, et al. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology. 2011;287(1–3):99–104. doi: 10.1016/j.tox.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones CF, Grainger DW. In vitro assessments of nanomaterial toxicity. Adv Drug Deliv Rev. 2009;61(6):438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Surendrakumar K, Martyn GP, Hodgers EC, Jansen M, Blair JA. Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Release. 2003;91(3):385–394. doi: 10.1016/s0168-3659(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 38.Kim SJ, Hahn SK, Kim MJ, Kim DH, Lee YP. Development of a novel sustained release formulation of recombinant human growth hormone using sodium hyaluronate microparticles. J Control Release. 2005;104(2):323–335. doi: 10.1016/j.jconrel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Walton DE, Mumford CJ. The morphology of spray-dried particles: the effect of process variables upon the morphology of spray-dried particles. Chem Eng Res Des. 1999;77(5):442–460. [Google Scholar]
- 40.Baldinger A, Clerdent L, Rantanen J, Yang M, Grohganz H. Quality by design approach in the optimization of the spray-drying process. Pharm Dev Technol. 2012;17(4):389–397. doi: 10.3109/10837450.2010.550623. [DOI] [PubMed] [Google Scholar]
- 41.Cilurzo F, Selmin F, Minghetti P, Montanari L. Design of methylprednisolone biodegradable microspheres intended for intra-articular administration. AAPS Pharm Sci Tech. 2008;9(4):1136–1142. doi: 10.1208/s12249-008-9158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S, Fu X. Cell behavior on microparticles with different surface morphology. J Alloys Compd. 2010;493(1–2):246–251. [Google Scholar]
- 43.Vehring R. Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tewa-Tagne P, Briancon S, Fessi H. Spray-dried microparticles containing polymeric nanocapsules: formulation aspects, liquid phase interactions and particles characteristics. Int J Pharm. 2006;325(1–2):63–74. doi: 10.1016/j.ijpharm.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Gervelas C, Serandour AL, Geiger S, et al. Direct lung delivery of a dry powder formulation of DTPA with improved aerosolization properties: effect on lung and systemic decorporation of plutonium. J Control Release. 2007;118(1):78–86. doi: 10.1016/j.jconrel.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 46.Simonoska-Crcarevska M, Glavas-Dodov M, Goracinova K. Chitosan coated Ca-alginate microparticles loaded with budesonide for delivery to the inflamed colonic mucosa. Eur J Pharm Biopharm. 2008;68(3):565–578. doi: 10.1016/j.ejpb.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Fajardo AR, Piai JF, Rubira AF, Muniz EC. Time- and pH-dependent self-rearrangement of a swollen polymer network based on polyelectrolytes complexes of chitosan/chondroitin sulfate. Carbohydr Polym. 2010;80:934–943. [Google Scholar]
- 48.Guillory JK. Heats of transition of methylprednisolone and sulfathiazole by a differential thermal analysis method. J Pharm Sci. 1967;56(1):72–76. doi: 10.1002/jps.2600560115. [DOI] [PubMed] [Google Scholar]
- 49.Sui X, Wei W, Yang L, et al. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int J Pharm. 2012;423(2):471–479. doi: 10.1016/j.ijpharm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Yesil-Celiktas O, Cetin-Uyanikgil EO. In vitro release kinetics of polycaprolactone encapsulated plant extract fabricated by supercritical antisolvent process and solvent evaporation method. J Supercrit Fluids. 2012;62:219–225. [Google Scholar]
- 51.Garcia-Martinez O, Diaz-Rodriguez L, Rodriguez-Perez L, De Luna-Bertos E, Reyes Botella C, Ruiz CC. Effect of acetaminophen, ibuprofen and methylprednisolone on different parameters of human osteoblast-like cells. Arch Oral Biol. 2011;56(4):317–323. doi: 10.1016/j.archoralbio.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Caron JP. Intra-articular injections for joint disease in horses. Vet Clin North Am Equine Pract. 2005;21(3):559–573. doi: 10.1016/j.cveq.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Campos E, Cordeiro R, Santos AC, Matos C, Gil MH. Design and characterization of bi-soft segmented polyurethane microparticles for biomedical application. Colloids Surf B Biointerfaces. 2011;88(1):477–482. doi: 10.1016/j.colsurfb.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Marti L, Moreno A, Filella X, et al. Cytokines value as a sepsis and mortality predictor in elderly patients with fever. Med Clin (Barc) 2003;121(10:):361–366. doi: 10.1016/s0025-7753(03)73952-9. Spanish. [DOI] [PubMed] [Google Scholar]
- 55.Jose Leon A, Garrote JA, Arranz E. Cytokines in the pathogenesis of inflammatory bowel diseases. Med Clin (Barc) 2006;127(4:):145–152. doi: 10.1157/13090382. Spanish. [DOI] [PubMed] [Google Scholar]
- 56.Campo GM, Avenoso A, D’Ascola A, et al. Hyaluronan in part mediates IL-1beta-induced inflammation in mouse chondrocytes by up-regulating CD44 receptors. Gene. 2012;494(1):24–35. doi: 10.1016/j.gene.2011.11.064. [DOI] [PubMed] [Google Scholar]
- 57.Torkildsen O, Vedeler CA, Ulvestad E, Aarseth JH, Nyland HI, Myhr KM. High dose methylprednisolone induces FcyRI on granulocytes in MS-patients. J Neuroimmunol. 2005;167(1–2):138–142. doi: 10.1016/j.jneuroim.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida N, Yoshikawa T, Nakamura Y, et al. Methylprednisolone inhibits neutrophil-endothelial cell interactions induced by interleukin-1 beta under flow conditions. Life Sci. 1997;60(25):2341–2347. doi: 10.1016/s0024-3205(97)00290-7. [DOI] [PubMed] [Google Scholar]


