Abstract
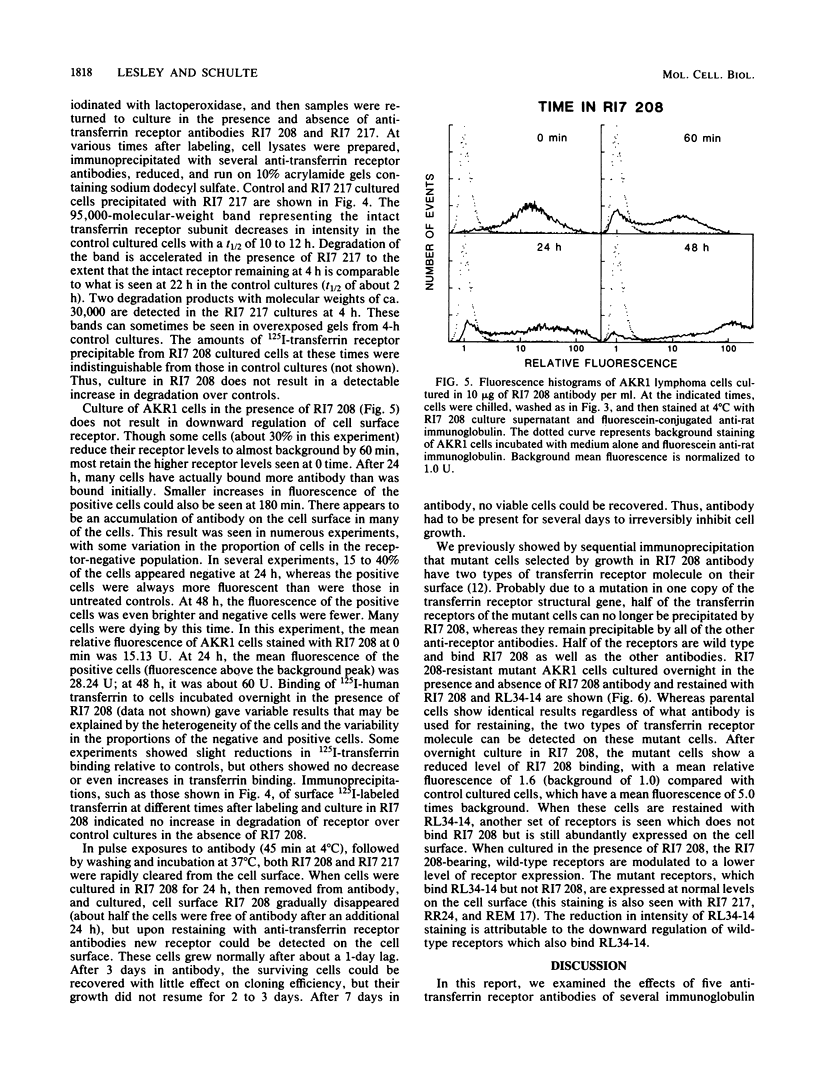
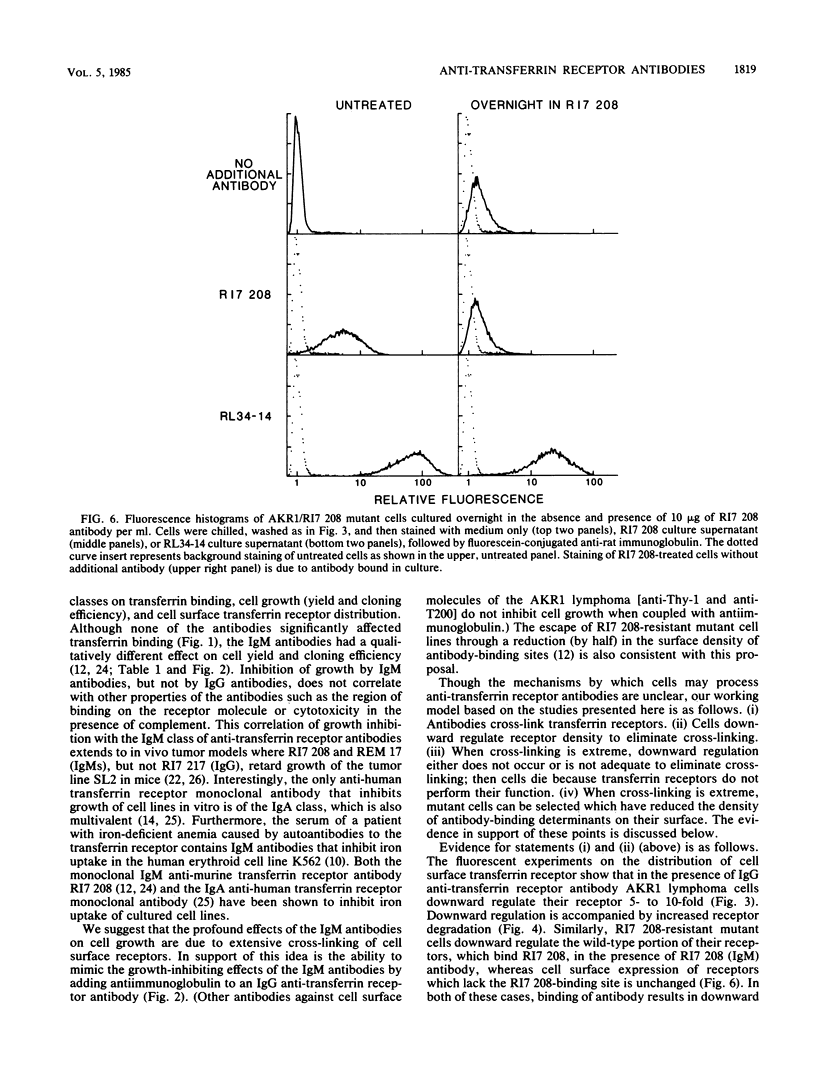
Five anti-murine transferrin receptor monoclonal antibodies have been characterized with respect to immunoglobulin class, effects on binding of transferrin, and effects on AKR1 lymphoma cell growth in vitro. The immunoglobulin M (IgM) antibodies, but not the IgG antibodies, prevent cell growth. We suggest that the profound effects of the IgM antibodies on cell growth are probably due to extensive cross-linking of cell surface receptors. In support of this, we are able to mimic the growth-inhibiting effects of the IgM antibodies by adding antiimmunoglobulin to an IgG antibody. By flow microfluorimetry, we show that an IgG antibody by itself induces up to a 10-fold downward regulation in the cell surface transferrin receptor, which is accompanied by accelerated receptor degradation. A similar downward regulation is seen in mutant cells resistant to growth inhibition by an IgM antibody, when grown in the selecting antibody. Wild-type cells grown in the presence of IgM antibody do not show receptor downward regulation. Inhibitory effects of antibody plus antiimmuoglobulin on mutant cells are also consistent with extensive cross-linking causing inhibition of growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Bretscher M. S. Transferrin receptor and its recycling in HeLa cells. EMBO J. 1982;1(3):351–355. doi: 10.1002/j.1460-2075.1982.tb01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges K. R., Cudkowicz A. Effect of iron chelators on the transferrin receptor in K562 cells. J Biol Chem. 1984 Nov 10;259(21):12970–12977. [PubMed] [Google Scholar]
- Enns C. A., Larrick J. W., Suomalainen H., Schroder J., Sussman H. H. Co-migration and internalization of transferrin and its receptor on K562 cells. J Cell Biol. 1983 Aug;97(2):579–585. doi: 10.1083/jcb.97.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Hyman E. S. Acquired iron-deficiency anemia caused by an antibody against the transferrin receptor. N Engl J Med. 1984 Jul 26;311(4):214–218. doi: 10.1056/NEJM198407263110402. [DOI] [PubMed] [Google Scholar]
- Lesley J. F., Schulte R. J. Selection of cell lines resistant to anti-transferrin receptor antibody: evidence for a mutation in transferrin receptor. Mol Cell Biol. 1984 Sep;4(9):1675–1681. doi: 10.1128/mcb.4.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Schulte R., Trotter J. Expression of transferrin receptor on murine hematopoietic progenitors. Cell Immunol. 1984 Jan;83(1):14–25. doi: 10.1016/0008-8749(84)90220-x. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mendelsohn J., Trowbridge I., Castagnola J. Inhibition of human lymphocyte proliferation by monoclonal antibody to transferrin receptor. Blood. 1983 Oct;62(4):821–826. [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. M., Yenokida G., James S. P. The role of the transferrin receptor in human B lymphocyte activation. J Immunol. 1984 Nov;133(5):2437–2441. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Disposition of T200 glycoprotein in the plasma membrane of a murine lymphoma cell line. J Biol Chem. 1980 Feb 25;255(4):1662–1669. [PubMed] [Google Scholar]
- Ralph P. Retention of lymphocyte characteristics by myelomas and theta + -lymphomas: sensitivity to cortisol and phytohemagglutinin. J Immunol. 1973 Jun;110(6):1470–1475. [PubMed] [Google Scholar]
- Shindelman J. E., Ortmeyer A. E., Sussman H. H. Demonstration of the transferrin receptor in human breast cancer tissue. Potential marker for identifying dividing cells. Int J Cancer. 1981 Mar 15;27(3):329–334. doi: 10.1002/ijc.2910270311. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R., Honeysett J. M., Trowbridge I. Effects of anti-transferrin receptor antibodies on growth of normal and malignant myeloid cells. Int J Cancer. 1983 Sep 15;32(3):343–349. doi: 10.1002/ijc.2910320314. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Hyman R. Thy-1 variants of mouse lymphomas: biochemical characterization of the genetic defect. Cell. 1975 Nov;6(3):279–287. doi: 10.1016/0092-8674(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Schulte R. Murine cell surface transferrin receptor: studies with an anti-receptor monoclonal antibody. J Cell Physiol. 1982 Sep;112(3):403–410. doi: 10.1002/jcp.1041120314. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Newman R. A., Domingo D. L., Sauvage C. Transferrin receptors: structure and function. Biochem Pharmacol. 1984 Mar 15;33(6):925–932. doi: 10.1016/0006-2952(84)90447-7. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]