Abstract
Genetically modified mesenchymal stem cells (MSCs) have great potential in the application of regenerative medicine and molecular therapy. In the present manuscript, we introduce a nanopolymer, polyethylenimine600-β-cyclodextrin (PEI600-β-CyD), as an efficient polyplex-forming plasmid delivery agent with low toxicity and ideal transfection efficiency on primary MSCs. PEI600-β-CyD causes significantly less cytotoxicity and apoptosis on MSCs than 25 kDa high-molecular-weight PEI (PEI25kDa). PEI600-β-CyD also exhibits similar transfection efficiency as PEI25kDa on MSCs, which is higher than that of PEI600Da. Quantum dot-labeled plasmids show that PEI600-β-CyD or PEI25kDa delivers the plasmids in a more scattered manner than PEI600Da does. Further study shows that PEI600-β-CyD and PEI25kDa are more capable of delivering plasmids into the cell lysosome and nucleus than PEI600Da, which correlates well with the results of their transfection-efficiency assay. Moreover, among the three vectors, PEI600-β-CyD has the most capacity of enhancing the alkaline phosphatase activity of MSCs by transfecting bone morphogenetic protein 2, 7, or special AT-rich sequence-binding protein 2. These results clearly indicate that PEI600-β-CyD is a safe and effective candidate for the nonviral gene delivery of MSCs because of its ideal inclusion ability and proton sponge effect, and the application of this nanopolymer warrants further investigation.
Keywords: polyethylenimine, cyclodextrin, gene delivery, mesenchymal stem cells
Introduction
Mesenchymal stem cells (MSCs) refer to a heterogeneous population of cells that are usually isolated from the bone marrow and have a large capacity for self-renewal while maintaining their multipotency to differentiate into osteoblasts, chondrocytes, adipocytes, and tendon cells. Hence, MSCs are widely used in tissue engineering to repair or regenerate mesenchymal tissues, such as bone, cartilage, muscle, or tendon.1
The capacity of genetically modified MSCs to generate tissues and release therapeutic factors such as growth factors for accelerating regeneration has drawn increasing research interest. Under this condition, genetically modified MSCs serve as a base for cell-mediated gene therapy or even as a therapeutic drug-delivery system.2,3 MSCs also exhibit vital characteristics, including tumor tropism, nonimmunogenicity, convenience of isolation, and efficient ex vivo processing that may be useful in treating cancer.4 However, safety issues such as mutagenesis, toxicity, and immunogenicity caused by viral vectors in these cases remain an important concern. Hence, the development of a safe and efficient nonviral gene-delivery system has drawn increasing attention. Such a system is expected to overcome the limitations associated with the viral approach.5
Cationic liposomal and polymeric vectors are two main types of nonviral vectors that are intensively investigated. Cationic liposomes and cationic polymers are made of positively charged lipids and polymers, respectively. Both possess characteristics that favor interaction with negatively charged DNA and cell membranes. Among cationic polymers, polyethylenimine (PEI) has emerged as a promising delivery reagent. The cytotoxicity and efficiency of PEI is dependent on its molecular weight (MW). High-MW PEIs exhibit relatively high transfection efficiency and higher cytotoxicity6 PEI with MW 25 kDa (PEI25kDa), one of the most effective gene-delivery cationic polymers studied to date, has been used as a gene-delivery vector since 1995. However, PEI25kDa is considered suboptimal for gene delivery because of its high cytotoxicity, which is attributed to the large numbers of PEI25kDa protonable nitrogens.7 Low-MW PEIs (<2000 Da) exhibit significantly low toxicity but have almost no transfection efficiency.8 Thus, recent studies have focused on the reconstruction of low-MW PEIs, such as poly(L-lysine) and poly(ethylene glycol),9 as well as chitosan modifications to enhance the efficiency of low-MW PEI without altering its low cytotoxicity.10 Cyclodextrin (CyD) is among the preferred methods, with high potential owing to its relatively low cytotoxicity and high inclusion capacity.11,12 CyDs are cup-shaped and nature-resourced molecules comprising six, seven, or eight glucose units (called α-, β-, and γ-CyD, respectively). CyDs can disrupt biological membranes by complexation with phospholipids and cholesterols. Combined with either viral or nonviral gene carriers, CyDs can enhance the gene-transfer efficiencies of these carriers.13–15 We synthesized PEI600-β-CyD, a cationic polymer, in which PEI polymers with an MW of 600 Da are linked by β-CyD with low toxicity and high efficiency in neurons.16
Bone morphogenetic proteins (BMPs) are vital growth factors in the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) during osteogenesis and fracture repair.17 Recombinant human BMP2 and BMP7 are clinically used in the revision of posterolateral spine fusion and long-bone nonunions.18–20 Special AT-rich sequence-binding protein 2 (SATB2) is another factor responsible for osteoblast differentiation of BMSCs.21 Overexpression of SATB2 promotes osteogenesis and improves bone mass in mice.22 Genetically modified BMSCs are widely used in bone-tissue engineering.23
We examined the cytotoxicity, apoptosis, and transfection efficiency of PEI600-β-CyD on primary rat MSCs and investigated the distribution of plasmids at 4 hours posttransfection by PEI600-β-CyD. The purpose was to elucidate the mechanism through which PEI600-β-CyD delivers the exogenous plasmids into MSCs. Alkaline phosphatase (ALP) activity was measured 2 and 7 days posttransfection by BMP2, BMP7, and SATB2 plasmids by using PEI600-β-CyD. PEI25kDa and PEI600Da were used as positive control and negative control, respectively.
Materials and methods
Materials
PEI600 (#408719; Sigma-Aldrich, St Louis, MO, USA), PEI25kDa (#408727; Sigma-Aldrich), and β-CyD (#C4767; Sigma-Aldrich) were purchased commercially. PEI600-β-CyD was synthesized using the method described previously16 Briefly, β-CyD (0.42 g, 0.37 mmol) and 1,1-carbonyldiimidazole (CDI) (0.80 g, 5.2 mmol, #115533; Sigma-Aldrich) were dissolved in 6 mL N, N-dimethyl formamide (#D4551; Sigma-Aldrich). The mixture was stirred at room temperature for 1 hour under nitrogen and precipitated in cold diethyl ether (#296082; Sigma-Aldrich). The resulting CyD-CDI was filtered using a 0.22 μm hydrophilic filter (#SLGP033RS; EMD Millipore, Billerica, MA, USA) dissolved in 5 mL dimethyl sulfoxide (DMSO) (#94563; Sigma-Aldrich), and stored at 4°C. PEI600Da (1.5 g) was dissolved in 3 mL DMSO. The described CyD-CDI in 5 mL DMSO and 0.3 mL triethylamine (Et3N) (#471283; Sigma-Aldrich) were added drop-wise to the PEI solution after 1.5 hours with stirring, followed by reaction for 5 hours. After the reaction, the mixture was dialyzed in water for 2 days and then freeze-dried for another 2 days. PEI600Da, PEI600-β-CyD, and PEI25kDa were dissolved in distilled water and sterilized using a 0.22 μm filter (#SLGP033RS; EMD Millipore) before use.
Harvest and culture of rat MSCs
Eight-week-old Wistar rats weighing 180–200 g used in the study were obtained from the Laboratory Animal Center of Shanghai Institute for Biological Sciences. The animals were handled in accordance with the policies of the Shanghai Jiao Tong University School of Medicine and the National Institute of Health. To summarize, bone marrow from the femurs of each rat was aspirated and collected in a 10 cm dish containing 10 mL modified Eagle’s medium (MEM) (#51412C; Sigma-Aldrich) with 10% fetal bovine serum (#A15–204; PAA, Pasching, Austria), 100 U/mL penicillin G, and 100 mg/L streptomycin (#15070–063; Life Technologies, Carlsbad CA, USA), and then cultured at 37°C with 5% CO. After culture for 48 hours, nonadherent cells were removed by washing with phosphate-buffered saline (PBS), and adherent cells were cultured in a complete medium that was replaced every 2 days. After achieving a confluence of 80%, the cultures were digested using 0.25% trypsin/ethylenediaminetetraacetic acid (#25200-056; Life Technologies). The cells at passages 2–6 were used in the following experiments.24
Lineage identification of rat MSCs
BMSCs (5 × 105 cells) at passage 3 were incubated with 1 μg of phycoerythrin-conjugated or fluorescein isothiocyanate-conjugated mouse anti-rat monoclonal antibodies (R&D Systems, Minneapolis, MN, USA) for 45 minutes at room temperature. After washing with fluorescence-activated cell-sorting (FACS) buffer (PBS with 10% bovine serum albumin and 1% sodium azide) at 2000 rpm for 5 minutes, the stained cells were resuspended in 300 mL of ice-cold FACS buffer and subjected to FACS analysis (BD Biosciences, San Jose, CA, USA). A total of 104 events were counted for each sample. The percentage of cells with positive signal was calculated using the FACScan program (BD Biosciences). Antibodies used included anti-cluster of differentiation (CD)-45 (#557015; BD Biosciences), anti-CD34 (#sc-7324; Santa Cruz Biotechnology, Dallas, TX, USA), anti-CD31 (#555027; BD Biosciences), anti-CD44 (#ab33900; Abcam, Cambridge, MA, USA), and anti-CD90.1 (#551401; BD Biosciences). They were replaced with phycoerythrin- or fluorescein isothiocyanate-conjugated isotype-matched immunoglobulin G1 in the negative controls (#IC002P or #IC002F; R&D Systems). Triplicates of cells from three rats were examined.
Multilineage differentiation potential
For osteogenic differentiation assays, BMSCs seeded at 1 × 104 cells/cm2 were cultured in complete culture medium until confluent. They were then incubated in basal medium or osteogenic medium (basal medium supplemented with 1 nM dexamethasone (#D4902; Sigma-Aldrich), 50 mM ascorbic acid (#A4403; Sigma-Aldrich), and 20 mM beta-glycerolphosphate (#G9891; Sigma-Aldrich) for 21 days) for the assessment of Alizarin Red S (#A5533; Sigma-Aldrich) staining. For Alizarin Red S staining, the cultured cells were rinsed with PBS three times and fixed with 4% paraformaldehyde for 10 minutes at room temperature. The fixed cells were then soaked in 0.5% Alizarin Red S for 30 minutes at room temperature, washed with PBS, and then observed under a digital camera (IXUS 115 HS; Canon, Tokyo, Japan).
For chondrogenic differentiation assays, BMSCs were harvested and resuspended at a density of 1 × 107 cells/mL. Twenty microliters’ cell suspension was carefully placed in the interior of a twelve-well plate. The cells were allowed to adhere at 37°C, 5% CO2 for 2 hours, followed by the addition of 500 μL chondrogenic medium that contained basal medium, supplemented with 10 ng/mL recombinant human transforming growth factor beta 1 (#7666-MB-005; R&D Systems) and 50 ng/mL recombinant human insulin-like growth factor 1 (#6630-GR-025; R&D Systems). The cell droplets coalesced and became spherical after 24 hours. The culture medium was changed every 3 days. The micromass was fixed for Alcian blue (#A3157; Sigma-Aldrich) staining after 14 days.
For adipogenic differentiation assays, BMSCs (1 × 104 cells/cm2) were cultured in complete culture medium. Two days later, the medium was replaced with basal or adipogenic medium, which was basal medium supplemented with 500 nM dexamethasone, 0.5 mM isobutylmethylxanthine (#I7018; Sigma-Aldrich), 50 mM indomethacin (#I7378; Sigma-Aldrich), and 10 mg/mL insulin (#I3536; Sigma-Aldrich). After another 2 days, the medium was replaced with basal medium containing only insulin. The medium was changed at 2-day intervals. The cells were cultured for 14 days for Oil Red O (#O0625; Sigma-Aldrich) staining. The presence of oil droplets was confirmed by staining the cells with 0.3% fresh Oil Red O solution for 30 minutes after fixation with 70% ethanol for 10 minutes.
MTT assay
To determine the cytotoxicity of PEI600-β-CyD on MSCs, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (#M5655; Sigma-Aldrich) assay was applied to the previously described protocol.25 To summarize, MSCs were seeded at 1 × 104 cells/well in 96-well plates and incubated for 24 hours. The culture medium was replaced with 200 μL serial dilutions of polymer solutions. The concentrations of polymers ranged from 0.001953 μg/μL to 32 μg/μL dissolved in complete culture medium, and then the cells were incubated for 4 hours at 37°C under 5% CO2. The polymer solutions were replaced with 200 μL of 0.5 mg/mL filtered MTT reagent prepared in a fresh complete culture medium, and the cells were incubated for another 4 hours at 37°C under 5% CO2. The unreacted MTT was removed and violet crystals in each well were dissolved in 200 μL DMSO. Optical density (OD) was measured using an enzyme-linked immunosorbent assay reader (M200 Pro Safire; Tecan, Männedorf, Switzerland) at wavelengths of 570 and 630 nm. Cell viability (%) was calculated as follows: ([OD570 – OD630] test/[OD570 – OD630] control) × 100%.
Apoptosis assay
Annexin V and propidium iodide (PI) double-staining were conducted to determine the apoptosis level caused by the polymer solutions. MSCs were seeded at 2 × 105 cells/well in six-well plates and incubated for 24 hours. The polymer solutions were added to the culture medium at a final concentration of 0.125 μg/μL. After incubation for 4 hours, a portion of the cells were fixed in 4% paraformaldehyde (#158127; Sigma-Aldrich) and stained with Hoechst 33342 (1 μM, #H3570; Life Technologies). The images were recorded using a fluorescent inverted microscope. The remainder of the cells were collected and resuspended in 1 × binding buffer to a proper concentration of about 1 × 106 cells/mL. The cell suspension added with 5 μL annexin V and 1 μL PI (#V23200; Life Technologies) was incubated for 15 minutes in the dark at room temperature. Thereafter, 400 μL 1 × binding buffer was added to the suspension, and the samples were examined by flow cytometry (FACSAria; BD Biosciences) at a wavelength of 488 nm.
In vitro transfection-efficiency assay
MSCs were seeded at 1 × 105 cells/well in a twelve-well plate at 24 hours pretransfection. The polyplexes of PEI600-β-CyD/pEGFP, which had a pDNA concentration of 2 μg/well, were prepared at varying N/P (where N is the amount of nitrogen in PEI, and P is the amount of phosphate in 1 μg DNA) ratios and incubated for 30 minutes at room temperature. The original culture medium was replaced with the polyplex solutions added with 1 mL Opti-MEM (#31985-088; Life Technologies).
After incubation for 4 hours at 37°C under 5% CO2, the transfection medium was replaced with a fresh complete culture medium, and then the cells were incubated for 48 hours. To visualize the expression of EGFP in MSCs transfected by polyplexes, a microscopic evaluation of the EGFP expression was performed at 48 hours posttransfection. The transfection efficiency was then quantified using flow cytometry at a wavelength of 488 nm.
Intracellular tracking assay
To determine the intracellular distribution of plasmids transfected by polyplexes in living MSCs, the polyplexes with pEGFP labeled with streptavidin quantum dots (#A10196; Life Technologies) were transfected into MSCs.26 The transfection medium was discarded after the cells were incubated for 4 hours. Subsequently, the cells were either not stained or just stained with Hoechst 33342. To test the influence of lysosomes to complexes, polyplexes with EGFP labeled with Cy5 (#MIR7025; Mirus, Madison, WI, USA) were transfected into MSCs. After 4 hours’ incubation, the transfection medium was discarded and the cells were stained by Hoechst 33342 and LysoTracker Red DND-99 dye (#I34202; Life Technologies) to distinguish the nucleus and lysosome, respectively. The samples were observed using a laser scanning confocal microscope (TCS SP5; Leica Microsystems, Wetzlar, Germany).
ALP activity analysis
MSCs were seeded at 2 × 105 cell/well in a six-well plate 24 hours pretransfection. The polyplexes of PEI600-β-CyD/pDNA were prepared at N/P = 30. The polyplexes of PEI25kDa/pDNA and PEI600Da/pDNA were prepared at N/P = 10. The negative control group contained only the naked pDNA. Three kinds of pDNA (BMP2, BMP7, and SATB2) were transfected into MSCs. The concentration of pDNA was 4 μg/well. The polyplexes were incubated for 30 minutes at room temperature. The original culture medium was replaced with the polyplex solutions added with 1 mL Opti-MEM. The transfection medium was replaced with a fresh complete culture medium after incubation for 4 hours at 37°C under 5% CO2. The ALP activity was determined by measuring the conversion of p-nitrophenyl phosphate (#P4744; Sigma-Aldrich) to p-nitrophenol after incubation for 2 days and 7 days. The substrate solution was prepared by dissolving 4-nitrophenyl phosphate disodium salt hexahydrate into a substrate buffer containing 50 mmol/L glycine (#50046; Sigma-Aldrich) and 1 mmol/L MgCl2 (#M8266; Sigma-Aldrich) at pH 10.5. The cells were collected and washed twice with PBS. After addition of 80 μL deionized water, the tube was subjected to three repeated cycles of freezing and thawing in a −80°C refrigerator and at room temperature. The cell lysates were centrifuged for 10 minutes at 12,000 g, and the supernate was used for ALP activity analysis. Up to 50 μL supernate was added into a 150 μL substrate solution in each well. After incubation for 15 minutes, the reaction was stopped by 200 μL 3 M sodium hydroxide (#S8045; Sigma-Aldrich). The absorbance was measured at 405 nm by a microplate reader. The ALP activity was normalized to protein concentration.
Statistical analysis
Analysis of variance and Student’s t-test were used for statistical analysis, and a P-value lower than 0.05 was considered significant.
Results
Identification and characterization of rat MSCs
The isolated BMSCs positively expressed MSC markers CD44 and CD90.1, but negatively expressed hematopoietic marker CD34, leukocyte marker CD45, and endothelial cell marker CD31 (Figure 1A). Osteogenic differentiation assays indicated that most cells formed mineralized calcium deposits, which was confirmed by Alizarin red S staining after 21 days of induction (Figure 1B). The chondrogenic differentiation of rat MSCs was verified by positive Alcian blue staining after 14 days of micromass culture and induction. The micromass after chondrogenic induction in the chondrogenic medium showed higher Alcian blue staining compared with the basal medium (Figure 1C). The adipogenic differentiation capacity of BMSCs was verified by Oil Red O staining. Lipid droplets were formed in the BMSCs after 14 days of adipogenic induction, which were not observed in the BMSCs cultured in the basal medium (Figure 1D).
Figure 1.
Lineage identification and characterization of rat MSCs. (A) Expression of rat MSC markers (CD44 and CD90.1), endothelial cell marker (CD31), leukocyte marker (CD45), and hematopoietic stem cell marker (CD34) on rat BMSCs. (B) Alizarin Red S staining of rat BMSCs detected after culture for 21 days in osteogenic medium or basal medium. (C) Chondrogenic differentiation potential of the micromass of rat BMSCs in vitro. Alcian blue staining of cells was detected after culture for 14 days in chondrogenic medium or basal medium. (D) Adipogenic differentiation potential of rat BMSCs in vitro.
Note: Oil Red O staining of cells was detected after culture for 14 days in adipogenic medium or basal medium.
Abbreviations: MSC, mesenchymal stem cell; BMSCs, bone marrow mesenchymal stem cells.
Cytotoxicity of PEI600-β-CyD
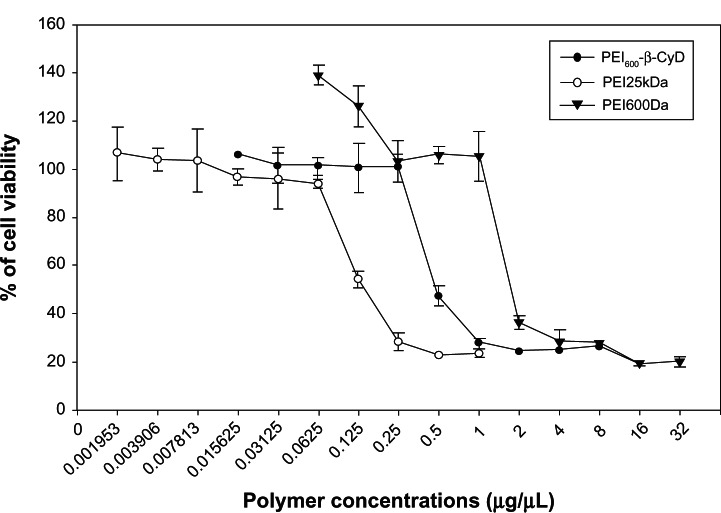
MTT assay was performed to evaluate the cytotoxicity of PEI600-β-CyD on MSCs after incubation for 4 hours with various concentrations of PEI600Da, PEI600-β-CyD, and PEI25kDa. The results showed that PEI25kDa was more toxic than both PEI600-β-CyD and PEI600Da, and PEI600-β-CyD was more toxic than PEI600Da (Figure 2). The median lethal dose of PEI25kDa was approximately 0.125 μg/μL, whereas the median lethal dose values of PEI600-β-CyD and PEI600Da on MSCs were approximately 0.5 and 1.75 μg/μL, respectively (Figure 2).
Figure 2.
Cytotoxicity of PEI600-β-CyD on MSCs using MTT assay.
Notes: The cell-viability percentages of PEI600Da, PEI600-β-CyD, and PEI25kDa on MSCs are plotted as polymer concentrations (μg/μL). Each group has ten plots.
Abbreviation: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin.
Apoptosis induced by PEI600-β-CyD
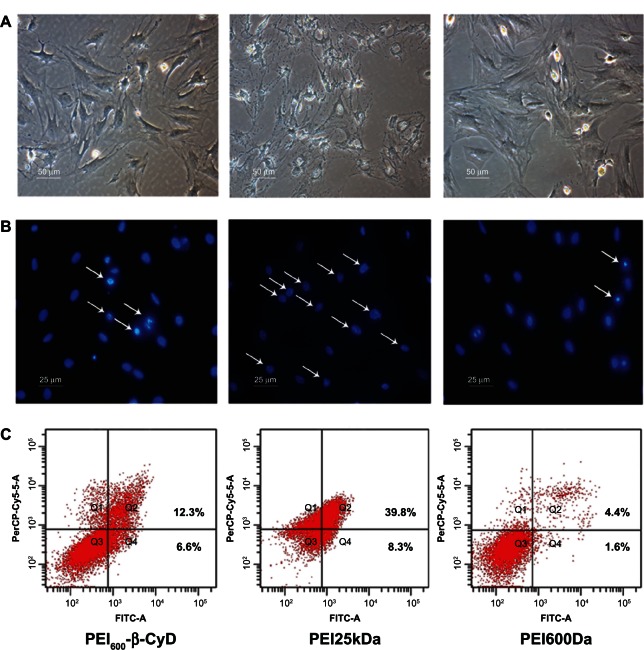
We measured the apoptosis of MSCs induced by PEI600-β-CyD. The shapes of MSCs in the PEI600Da and PEI600-β-CyD groups were apparently the same when viewed under an optical microscope (Figure 3A). However, the shape of MSCs in the PEI25kDa group was abnormal because of serious shrinkage of the cytoplasm and cell membrane. We then tested the ratios of the cells with highly condensed chromatin in each group. The ratio was determined to be markedly higher in the PEI25kDa group than in the other two groups (Figure 3B). These results indicated that unlike PEI600-β-CyD, PEI25kDa can significantly increase the apoptosis level in MSCs compared with the other two polymers. Then, the double-staining annexin V and PI assay were applied to quantify the apoptosis level in MSCs induced by these vectors. The total percentage of cells in early apoptosis (Q4 zone) and dead (Q2 zone) stages was shown to be markedly higher in the PEI25kDa group (48.1%) than in both the PEI600-β-CyD (18.9%) and PEI600Da (6.0%) groups (Figure 3C).
Figure 3.
Apoptosis of MSCs induced by PEI600-β-CyD. (A) Photos taken using an optical microscope after incubation for 4 hours of each polymer at a concentration of 0.125 μg/μL. (B) Photos taken using a fluorescent microscope after Hoechst staining following incubation for 4 hours of each polymer at a concentration of 0.125 μg/μL. White arrows indicate cells with highly condensed chromatin. (C) Percentages of cells in early apoptosis (Q4 zone) or dead (Q2 zone) stage induced by each polymer.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells.
Transfection efficiency of PEI600-β-CyD
The relationship between the N/P ratio and the transfection efficiency of PEI600-β-CyD on MSCs was examined by microscopic and flow cytometry. The transfection efficiency was enhanced when the N/P ratio increased from 10 to 30. However, the cytotoxicity and apoptosis induced by PEI600-β-CyD became evident when the N/P ratio was at least 40 (data not shown). Therefore, peak transfection efficiency appeared at the N/P ratio of 30 (Figure 4A and B).
Figure 4.
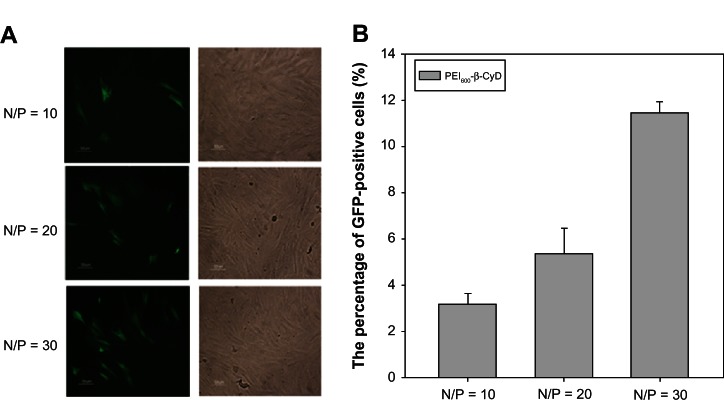
Relationship between the N/P ratio and the transfection efficiency of PEI600-β-CyD. (A) Microscopic evaluation of the EGFP expression of MSCs transfected by PEI600-β-CyD at different N/P ratios ranging from 10 to 30. (B) Quantification of the transfection efficiencies of PEI600-β-CyD on MSCs at different N/P ratios by flow cytometry.
Note: Data are shown as means ± standard deviation of three independent experiments. N, amount of nitrogen in PEI; P, amount of phosphate in 1 μg DNA.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells; EGFP, enhanced green fluorescent protein.
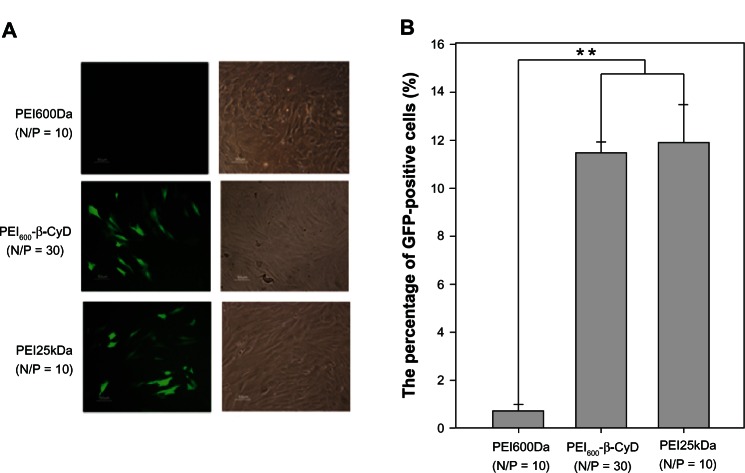
The same methods were applied to compare the transfection efficiency of PEI600-β-CyD on MSCs with the other nonviral vectors PEI600Da and PEI25kDa. Compared with the top transfection efficiency of PEI25kDa on MSCs at N/P = 10, no significant differences were observed on the top transfection efficiencies between PEI600-β-CyD and PEI25kDa. The transfection efficiency of PEI600Da on MSCs was almost 0, and the transfection efficiencies of PEI600-β-CyD and PEI25kDa were significantly higher than that of PEI600Da (Figure 5A and B).
Figure 5.
Transfection efficiencies of PEI600-β-CyD and other vectors. (A) Microscopic evaluation of the EGFP expression of MSCs transfected using PEI600Da (N/P = 10), PEI600-β-CyD (N/P = 30), and PEI25kDa (N/P = 10). (B) Quantification of the transfection efficiencies of the four nonviral vectors by flow cytometry.
Notes: Data are shown as means ± standard deviation of three independent experiments. **P < 0.05. N, amount of nitrogen in PEI; P, amount of phosphate in 1 μg DNA.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells; EGFP, enhanced green fluorescent protein.
Intracellular distribution of plasmids transfected by PEI600-β-CyD
After further investigation, we compared the intracellular distribution of plasmids in MSCs at 4 hours posttransfection caused by each nonviral gene vector. The plasmids in the PEI600Da group were likely to agglomerate into big dots, which cannot easily permeate the cell nucleus. The intensities of plasmids in the PEI600-β-CyD and PEI25kDa groups were markedly stronger than those in the PEI600Da group (Figure 6).
Figure 6.
Intracellular distribution of plasmids in MSCs 4 hours posttransfection using quantum-dot labeling. MSCs are transfected by complexes of PEI600Da/pEGFP (N/P = 10), PEI600-β-CyD/pEGFP (N/P = 30), and PEI25kDa/pEGFP (N/P = 10). The samples are collected 4 hours posttransfection. Panel (A) shows the merged photos (63 × 2.5) of nuclei stained with Hoechst 33342 (blue) and quantum dot-labeled plasmids (red). Panel (B) is the 2.5-fold magnification of the part illustrated in panel (A) from each group. N, amount of nitrogen in PEI; P, amount of phosphate in 1 μg DNA.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells; EGFP, enhanced green fluorescent protein.
To study the influence of lysosomes on the complexes, another method was applied to stain simultaneously the lysosomes and nuclei without influencing the discrimination of plasmids. The results showed that most of the plasmids remained outside the lysosome for 4 hours posttransfection in the PEI600Da group, and only one-sixth of the cells had the plasmids in their lysosomes and nuclei.
The intensities of plasmids in the cytoplasm or nucleus of MSCs in the PEI25kDa and PEI600-β-CyD groups were markedly stronger than that in the PEI600Da group (Figure 7A). The percentages of cells with plasmids in their nuclei were also significantly higher in the PEI25kDa (9/9) and PEI600-β-CyD (7/7) groups than in the PEI600Da (1/6) group (Figure 7B).
Figure 7.
Intracellular distribution of plasmids in MSCs 4 hours posttransfection using Cy5 labeling. A MSCs were transfected by complexes of PEI600Da/pEGFP (N/P = 10), PEI600-β-CyD/pEGFP (N/P = 30), and PEI25kDa/pEGFP (N/P = 10). The upper panel shows the merged photos (63) of plasmids labeled by Cy5 (green) at 649 nm excitation, lysosomes stained with LysoTracker red DND-99 dye (red), and nuclei stained with Hoechst 33342 (blue). The lower panel shows merged photos (63 × 3) taken by the same method as the upper panel. (B) Quantification of the percentage of cells with green plasmid signals in their lysosomes or nuclei in each group in the upper panel of (A). N, amount of nitrogen in PEI; P, amount of phosphate in 1 μg DNA.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells; EGFP, enhanced green fluorescent protein.
ALP activity of MSCs transfected by PEI600-β-CyD
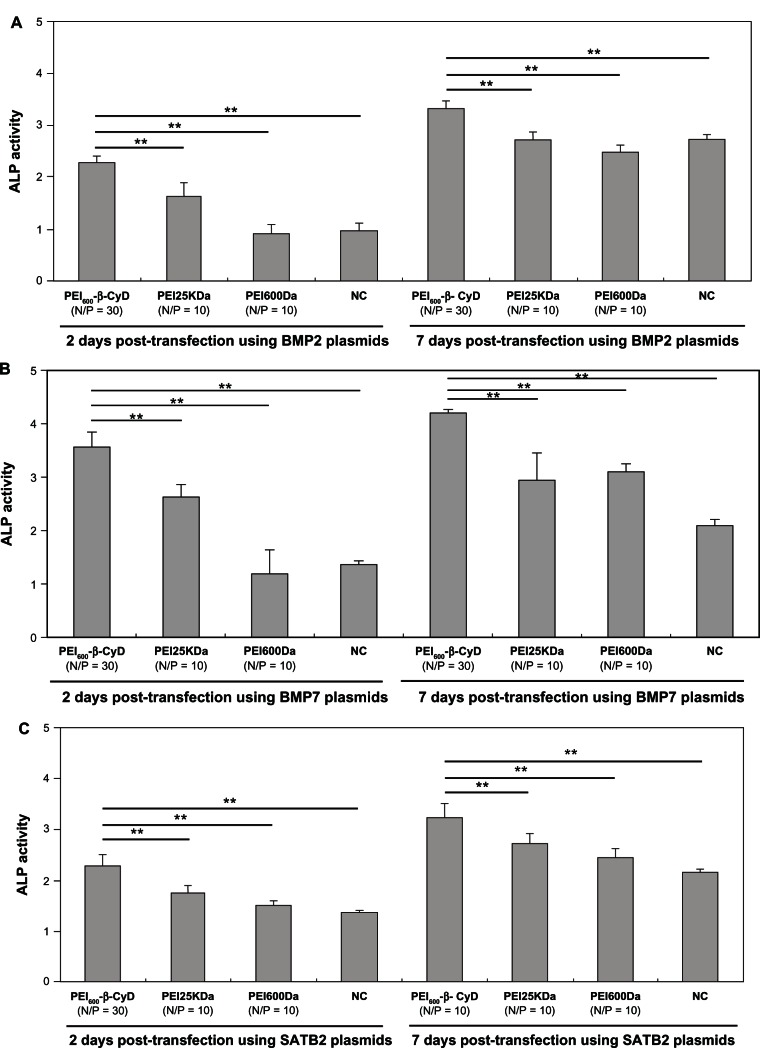
ALP activity is an important indicator of osteogenic differentiation of MSCs. BMP2, BMP7, and SATB2 can promote the osteogenic differentiation of MSCs by improving ALP activity. In this study, ALP activity was shown to be more significantly enhanced when BMP2 was transfected into MSCs by PEI600-β-CyD than by PEI25kDa, PEI600Da, or naked plasmid BMP2 (pBMP2) at 2 and 7 days posttransfection (Figure 8A). The MSCs transfected by BMP7 or SATB2 using PEI600-β-CyD also had significantly higher ALP activities than those using PEI25kDa, PEI600Da, and naked pBMP2 at 2 and 7 days posttransfection (Figure 8B and C).
Figure 8.
ALP activity of MSCs transfected by PEI600-β-CyD. ALP analysis 2 and 7 days after transfection of MSCs by complexes of (A) PEI600-β-CyD/pBMP2 (N/P = 30), PEI25kDa/pBMP2 (N/P = 10), PEI600Da/pBMP2 (N/P = 10), and naked pBMP2, (B) PEI600-β-CyD/pBMP7 (N/P = 30), PEI25kDa/pBMP7 (N/P = 10), PEI600Da/pBMP7 (N/P = 10), and naked pBMP7, and (C) PEI600-β-CyD/pSATB2 (N/P = 30), PEI25kDa/pSATB2 (N/P = 10), PEI600Da/pSATB2 (N/P = 10), and naked pSATB2.
Notes: Data are shown as means ± standard deviation of three independent experiments. **P < 0.05.
Abbreviations: PEI600-β-CyD, polyethylenimine600-β-cyclodextrin; MSCs, mesenchymal stem cells; ALP, alkaline phosphatase; BMP, bone morphogenetic protein; SATB2, Special AT-rich sequence-binding protein 2.
Discussion
The safety of a gene-delivery vector is the primary consideration for its clinical application. Despite the high efficiency exhibited by PEI25kDa in terms of gene delivery both in vitro and in vivo,27 the high cytotoxicity and serious tissue damage caused by this high-MW PEI still need to be examined.28,29 Recent studies have focused on enhancing the efficiency of low-MW PEI without altering its cytotoxicity. This objective can be achieved in two ways. One technique involves the assembly of low-MW PEI into high-MW PEI by using low-toxicity cross-linkers to enhance the transfection efficiency of the former without altering its cytotoxicity. This method allows the degradation of high-MW PEI into low-MW PEI with low toxicity after transfection. The other method involves the modification of low-MW PEI to enhance its transfection efficiency. CyD, a safe and nature-resourced material, is approved by the FDA for use in humans and proven to have high inclusion capacity with phospholipids and cholesterol. Hence, we modified CyD on low-MW PEI to develop PEI600-β-CyD, a novel nonviral gene vector.
The complex of PEI600-β-CyD with plasmid has been verified in previous studies to possess favorable physicochemical properties for transfection. For example, this complex has an average particle size of approximately 200 nm and a zeta potential of approximately 40 mV.16 PEI600-β-CyD also has an ideal transfection efficiency on numerous cancer cell lines.25
This study showed that the cytotoxicity and apoptosis level of MSCs induced by PEI600-β-CyD are significantly lower than those induced by PEI25kDa, whereas the cytotoxicity and apoptosis levels induced by PEI600-β-CyD are slightly higher than those induced by PEI600Da. These phenomena may be attributed to the inclusion of cholesterol on the cell membrane by CyD.30,31
The relationship between the transfection efficiency and the N/P ratio of PEI600-β-CyD on MSCs is proportional when the N/P ratio ranges from 10 to 30. The highest transfection efficiency is achieved when the N/P ratio is 30. The increased PEI600-β-CyD when the N/P ratio is increased can improve the stabilization of nanoparticles. However, PEI600-β-CyD in excessive quantities can result in serious cytotoxicity through its inclusion ability because of the free PEI600-β-CyD.
The highest transfection efficiency of PEI600-β-CyD on MSCs is almost similar to the transfection efficiency of PEI25kDa, and is remarkably higher than that of PEI600Da. This phenomenon may also be attributed to the inclusion ability of CyD, which can enhance the endocytosis of the plasmid.
To elucidate further the mechanism through which PEI600-β-CyD delivers the exogenous plasmids into MSCs, we transfected the labeled plasmids using PEI600-β-CyD, and observed their intracellular distributions for 4 hours posttransfection. The plasmids transfected by PEI600Da are more likely to agglomerate as large dots than those transfected by either PEI600-β-CyD or PEI25kDa. This phenomenon may be attributed to the increased positive-charge density in the polymers of PEI600-β-CyD and PEI25kDa. A higher zeta potential of complexes can reduce aggregation.5
CyD modification on PEI600Da can effectively enhance the endocytosis efficiency of the polymer, because the intensity of plasmids in the lysosomes in the PEI600-β-CyD group is remarkably higher than that in the PEI600Da group. The low-MW PEI – PEI600Da – on PEI600-β-CyD retained its proton sponge effect and exhibited nearly the same buffering ability as PEI25kDa to overcome the influence of lysosomes in MSCs. Hence, the ratios of the cells with plasmids in lysosomes in the PEI600-β-CyD and PEI25kDa groups are almost similar. Similarly, the ratios of the cells with plasmids in nuclei in the PEI600-β-CyD and PEI25kDa groups are nearly equal, which indicates that the plasmids transfected by PEI600-β-CyD and PEI25kDa have similar kinetics in MSCs.
Although the transfection efficiency of PEI600-β-CyD is not higher than that of PEI25kDa, its ALP activity is significantly higher than that of PEI25kDa when BMP2, BMP7, and SATB2 plasmids are transfected into MSCs. The higher cytotoxicity of PEI25kDa inhibits the osteogenic differentiation of MSCs. Hence, PEI600-β-CyD possesses an enhanced clinical value in tissue engineering.
Conclusion
PEI600-β-CyD, a novel nonviral gene vector, is designed by combining the inclusion ability of CyD and the proton sponge effect of PEI in one single vector. This combination can enhance transfection efficiency without seriously altering the low cytotoxicity of low-MW PEI. This study is the first to test the cytotoxicity and transfection efficiency of PEI600-β-CyD on MSCs for potential applications in tissue engineering and cancer therapy. The results prove that PEI600-β-CyD exhibits lower cytotoxicity and almost the same level of efficiency compared with PEI25kDa on MSCs in vitro. Further investigation is needed to broaden the application of PEI600-β-CyD in in vivo tissue engineering or cancer therapy of genetically modified MSCs.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology of China (2011DFA30790, 2010CB945600)-CIHR(CCM 104888), National Natural Science Foundation of China (81190133), Chinese Academy of Sciences (XDA01030404, KSCX2-EW-Q-1-07), Science and Technology Commission of Shanghai Municipality (12411951100, 11QH1401600), and the Shanghai Municipal Education Commission (J50206, 10SG22).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Chanda D, Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008;15(10):711–715. doi: 10.1038/gt.2008.35. [DOI] [PubMed] [Google Scholar]
- 3.Menon LG, Shi VJ, Carroll RS. StemBook [Internet] Cambridge (MA): Harvard Stem Cell Institute; Jan 15, 2009. Mesenchymal stromal cells as a drug delivery system. [PubMed] [Google Scholar]
- 4.Ling X, Marini F, Konopleva M, et al. Mesenchymal stem cells overexpressing IFN-beta inhibit breast cancer growth and metastases through Stat3 Signaling in a syngeneic tumor model. Cancer Microenviron. 2010;3(1):83–95. doi: 10.1007/s12307-010-0041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40(3):159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45(3):268–275. doi: 10.1002/(sici)1097-4636(19990605)45:3<268::aid-jbm15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 7.Florea BI, Meaney C, Junqinger HE, et al. Transfection efficiency and toxicity of polyethylenimine in differentiated Calu-3 and nondifferentiated COS-1 cell cultures. AAPS PharmSci. 2002;4(3):E12. doi: 10.1208/ps040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer D, Bieber T, Li Y, et al. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16(8):1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 9.Dai J, Zou S, Pei Y, Cheng D, Ai H, Shuai X. Polyethylenimine-grafted copolymer of poly(l-lysine) and poly(ethylene glycol) for gene delivery. Biomaterials. 2011;32(6):1694–1705. doi: 10.1016/j.biomaterials.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 10.Jere D, Jiang HL, Kim YK, et al. Chitosan-graft-polyethylenimine for Akt1 siRNA delivery to lung cancer cells. Int J Pharm. 2009;378(1–2):194–200. doi: 10.1016/j.ijpharm.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 12.Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3(12):1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- 13.Croyle MA, Roessler BJ, Hsu CP, Sun R, Amidon GL. Beta cyclodextrins enhance adenoviral-mediated gene delivery to the intestine. Pharm Res. 1998;15(9):1348–1355. doi: 10.1023/a:1011985101580. [DOI] [PubMed] [Google Scholar]
- 14.Arima H, Kihara F, Hirayama F, Uekama K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with alpha-, beta-, and gamma-cyclodextrins. Bioconjug Chem. 2001;12(4):476–484. doi: 10.1021/bc000111n. [DOI] [PubMed] [Google Scholar]
- 15.Lawrencia C, Mahendran R, Esuvaranathan K. Transfection of urothelial cells using methyl-beta-cyclodextrin solubilized cholesterol and Dotap. Gene Ther. 2001;8(10):760–768. doi: 10.1038/sj.gt.3301462. [DOI] [PubMed] [Google Scholar]
- 16.Tang GP, Guo HY, Alexis F, et al. Low molecular weight polyethylenimines linked by beta-cyclodextrin for gene transfer into the nervous system. J Gene Med. 2006;8(6):736–744. doi: 10.1002/jgm.874. [DOI] [PubMed] [Google Scholar]
- 17.Satija NK, Gurudutta GU, Sharma S, et al. Mesenchymal stem cells: molecular targets for tissue engineering. Stem Cells Dev. 2007;16(1):7–23. doi: 10.1089/scd.2006.9998. [DOI] [PubMed] [Google Scholar]
- 18.Abd-El-Barr MM, Cox JB, Antonucci MU, Bennett J, Murad GJ, Pincus DW. Recombinant human bone morphogenetic protein-2 as an adjunct for spine fusion in a pediatric population. Pediatr Neurosurg. 2011;47(4):266–271. doi: 10.1159/000335424. [DOI] [PubMed] [Google Scholar]
- 19.Bright C, Park YS, Sieber AN, Kostuik JP, Leong KW. In vivo evaluation of plasmid DNA encoding OP-1 protein for spine fusion. Spine. 2006;31(19):2163–2172. doi: 10.1097/01.brs.0000232721.59901.45. [DOI] [PubMed] [Google Scholar]
- 20.Tressler MA, Richards JE, Sofianos D, Comrie FK, Kregor PJ, Obremskey WT. Bone morphogenetic protein-2 compared to autologous iliac crest bone graft in the treatment of long bone nonunion. Orthopedics. 2011;34(12):877–884. doi: 10.3928/01477447-20111021-09. [DOI] [PubMed] [Google Scholar]
- 21.Zhang P, Men J, Fu Y, et al. Contribution of SATB2 to the stronger osteogenic potential of bone marrow stromal cells from craniofacial bones. Cell Tissue Res. 2012;350(3):425–437. doi: 10.1007/s00441-012-1487-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Tu Q, Grosschedl R, et al. Roles of SATB2 in osteogenic differentiation and bone regeneration. Tissue Eng Part A. 2011;17(13–14):1767–1776. doi: 10.1089/ten.tea.2010.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tai K, Pelled G, Sheyn D, et al. Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng Part A. 2008;14(10):1709–1720. doi: 10.1089/ten.tea.2007.0241. [DOI] [PubMed] [Google Scholar]
- 24.Tu J, Wang H, Li H, Dai K, Wang J, Zhang X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials. 2009;30(26):4369–4376. doi: 10.1016/j.biomaterials.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 25.Yao H, Ng SS, Tucker WO, et al. The gene transfection efficiency of a folate-PEI600-cyclodextrin nanopolymer. Biomaterials. 2009;30(29):5793–5803. doi: 10.1016/j.biomaterials.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Ho YP, Chen HH, Leong KW, Wang TH. Evaluating the intracellular stability and unpacking of DNA nanocomplexes by quantum dots-FRET. J Control Release. 2006;116(1):83–89. doi: 10.1016/j.jconrel.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Intra J, Salem AK. Characterization of the transgene expression generated by branched and linear polyethylenimine-plasmid DNA nanoparticles in vitro and after intraperitoneal injection in vivo. J Control Release. 2008;130(2):129–138. doi: 10.1016/j.jconrel.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11(6):990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Xiong MP, Forrest ML, Ton G, Zhao A, Davies NM, Kwon GS. Poly(aspartate-g-PEI800), a polyethylenimine analogue of low toxicity and high transfection efficiency for gene delivery. Biomaterials. 2007;28(32):4889–4900. doi: 10.1016/j.biomaterials.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 30.Kiss T, Fenyvesi F, Bácskay I, et al. Evaluation of the cytotoxicity of beta-cyclodextrin derivatives: evidence for the role of cholesterol extraction. Eur J Pharm Sci. 2010;40(4):376–380. doi: 10.1016/j.ejps.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 31.Matilainen L, Toropainen T, Vihola H, et al. In vitro toxicity and permeation of cyclodextrins in Calu-3 cells. J Control Release. 2008;126(1):10–16. doi: 10.1016/j.jconrel.2007.11.003. [DOI] [PubMed] [Google Scholar]