Abstract
Introduction
The potential neurochemical toxicity associated with methamphetamine (MA) or marijuana (MJ) use on the developing adolescent brain is unclear, particularly with regard to individuals with concomitant use of MA and MJ (MA+MJ). In this study, proton magnetic resonance spectroscopy (MRS) was utilized to measure in vivo brain N-acetylaspartate plus N-acetylaspartyl glutamate (tNAA, an indicator of intact neuronal integrity) levels.
Methods
Three adolescent groups from Cape Town, South Africa completed MRS scans as well as clinical measures including a drug use history. Subjects included (1) nine MA (age = 15.7 ± 1.37), (2) eight MA+MJ (age = 16.2 ± 1.16) using adolescents and (3) ten healthy controls (age = 16.8 ± 0.62). Single voxel spectra were acquired from midfrontal gray matter using a point-resolved spectroscopy sequence (PRESS). The MRS data were post-processed in the fully automated approach for quantitation of metabolite ratios to phosphocreatine plus creatine (PCr+Cr).
Results
A significant reduction in frontal tNAA/PCr+Cr ratios was seen in the MA+MJ group compared to the healthy controls (p = 0.01, by 7.2 %) and to the MA group (p = 0.04, by 6.9 %). Significant relationships were also observed between decreased tNAA/PCr+Cr ratios and drug use history of MA or MJ (total cumulative lifetime dose, age of onset, and duration of MA and MJ exposure) only in the MA+MJ group (all p < 0.05).
Conclusions
These findings suggest that in adolescents, concomitant heavy MA+MJ use may contribute to altered brain metabolites in frontal gray matter. The significant associations between the abnormal tNAA/PCr+Cr ratios and the drug use history suggest that MA+MJ abuse may induce neurotoxicity in a dose-responsive manner in adolescent brain.
Keywords: methamphetamine, marijuana, adolescent, imaging, magnetic resonance spectroscopy
Introduction
There is widespread use of methamphetamine (MA) and marijuana (MJ) among adolescents [1]. However, the neurobiological and neurochemical impact of heavy MA and MJ use is not well understood in developing adolescents, particularly with regard to the concomitant use of methamphetamine and marijuana (MA+MJ). MA use is strongly linked to brain toxicity, but MJ use has been associated with both neurotoxic and neuroprotective effects in adults [2–5]. It is not known what the combined effects of MA+MJ use would be on the developing adolescent brain. Simultaneous use of MA+MJ by adolescents may accentuate or attenuate central nervous system (CNS) neurotoxicity and may be associated with psychiatric symptoms during the CNS maturation period that takes place during adolescence.
MA is a powerful addictive stimulant that has high potential for abuse in adolescents as it can be manufactured inexpensively and is readily available [6–8]. Preclinical studies have shown that effects following MA exposure include alterations in neurotransmitters such as dopamine and serotonin [9], as well as changes in oxygen-based free radicals, oxidative stress, lipid oxidation, DNA damage, mitochondrial dysfunction, apoptosis, hyperthermia, excitotoxicity, and neuroinflammation [10, 11]. MA using adolescents have significant neuropsychological deficits such as slowed performance on the Stroop Interference task as well as reduced scores on the Wechsler Intelligence Scales-III and Peg Board task [12, 13]. These cognitive changes may be related to MA induced alterations of the frontal cortex as a range of neuroimaging techniques in adult studies have reported significant correlations between several imaging markers and total amount of MA use in a dose responsive manner. For instance, total MA use has been significantly correlated to decreased frontal glucose metabolism [14], compromised neuronal viability [15], and the presence of severe deep white matter hyperintensities [16].
MJ is the most widely used illicit drug in the world and continues to be the most popular illicit drug among adolescents [17]. The main active ingredient in MJ is Δ9-tetrahydrocannabinol (THC) which binds to the cannabinoid receptor 1 that is found predominantly in the CNS [3, 18–20]. Previous preclinical and clinical research on MJ abuse has produced mixed results regarding potential neurotoxic or neuroprotective effects [2–5]. For example, the endogenous cannabinoid system has been associated with neuroprotection due to vasodilatory effects, inhibition of cytokines, as well as reduction in oxidative damage and glutamatergic overstimulation [5, 21]. Also, a possible therapeutic potential of cannabinoids has been suggested for the treatment of neurodegenerative disorders such as Huntington’s disease [22] and multiple sclerosis [23]. On the other hand, CNS toxicity of MJ has been reported in animal and preclinical studies which have reported neurotransmitter alterations [24] and Δ9-THC induced cell death and DNA fragmentation of cultured rat neurons in the hippocampus [25, 26]. Further, an association between MJ use and psychosis has been reported in subjects who have a vulnerability to develop schizophrenia [27, 28]. Of relevance, a recent meta-analysis revealed that cannabis use is associated with earlier onset of psychosis [29]. Findings on the effects of cognitive function are inconsistent. One study with MJ using adolescents reported a normal range of working and associative memory [30] however there is significant evidence that MJ use is associated with impairments of both basic and complex executive function [31]. Moreover, dose-responsive impairments of episodic memory and learning ability have been demonstrated in adult MJ users [32].
Brain magnetic resonance spectroscopy (MRS) has enabled us to directly measure in vivo human brain chemical levels. This MRS technique has increasingly been used to study the effects of drug abuse on the human brain. Proton (1H) MRS involves the acquisition of spectra with multiple discernible peaks to identify alterations in cerebral concentrations of N-acetylaspartate plus N-acetylaspartyl glutamate (tNAA), phosphocreatine plus creatine (PCr+Cr), glycerophosphocholine plus phosphocholine (GPC+PC), glutamate plus glutamine (Glu+Gln), and myo-inositol (mI). It has been reported that abstinent adult MA users have significantly lower tNAA, primarily a marker of neuronal viability or integrity, in the frontal lobe, anterior cingulate and basal ganglia, but not in the occipital visual cortex [15, 33–36]. However, the neurotoxic insults caused by drugs may differ between adolescents and adults because early exposure to MA and MJ may alter the ongoing course of brain maturation in adolescents who will be experiencing rapid brain changes. For example, it has been proposed that early onset of drug use may lead to more rapid development of drug dependence [37]. Compared to adult studies neurochemical data from MA using adolescents is limited except for one 1H MRS study of peri-adolescents (13 to 23 years) [38], which found no significant differences in metabolite levels in MA using adolescents compared to healthy controls. Two adult studies examining the effects of MJ on brain metabolites reported that smokers had reduced prefrontal NAA levels [4] and reduced basal ganglia GPC+PC, mI, and Glu+Gln levels [39]. Most recently, an adolescent 1H MRS study revealed that anterior cingulate in marijuana users (average age 17.8 years) had significantly decreased NAA, PCr+Cr, mI and glutamate levels [40]. However, the extent to which MJ impacts concurrent MA using adolescent brain is not known.
The present investigation hypothesizes that concurrent adolescent users of MA and MJ would have significantly decreased frontal tNAA/PCr+Cr ratios compared to healthy controls. We also hypothesized that tNAA/PCr+Cr ratios will be significantly associated with lifetime exposure, duration, and age of onset of MA and MJ use.
1. Material and Methods
1.1 Subjects
This study was approved by the Institutional Review Board of the University of Stellenbosch. A total 27 subjects participated in the MRS study and received single voxel MRS and drug use assessment. Study participants consisted of nine MA abusing adolescents (age = 15.7 ± 1.37, 6 female and 3 male), eight MA+MJ abusing adolescents (age = 16.2 ± 1.16, 5 female and 3 male), and ten healthy controls (age = 16.8 ± 0.62, 7 female and 3 male). Drug abusing adolescents were enrolled with the inclusion criteria as follows (1) age range: 13 to 17 years old, (2) both females and males, and (3) diagnostic criteria for current MA or MA+MJ dependence as preferred drug of abuse. Adolescents were evaluated with the Schedule for Affective Disorders and Schizophrenia for School Aged Children (6–18 years) Lifetime Version (K-SADS) [41], and the Multidimensional Anxiety Scale for Children (MASC) [42]. Self-reported substance use data were collected during the K-SADS diagnostic interview. While substance misusing adolescents and non using control adolescents did endorse ADHD symptoms, PTSD symptoms and anxiety symptoms, no individual met diagnostic criteria for an Axis I disorder. Moreover, no significant difference was found between groups in terms of the prevalence of symptoms. Exclusion criteria were (1) major medical conditions including renal, pulmonary, and cardiac disease, (2) major psychiatric disorders including schizophrenia, bipolar disorder, and other drug dependence such as alcohol, opioid and cocaine as preferred drug of abuse, (3) inability to give informed consent or assent, or (4) contraindications to magnetic resonance imaging such as metal implants.
Healthy subjects were recruited with the inclusion criteria (1) age range: 13 to 17 years old, (2) No clinically significant medical and psychiatric disorders. Exclusion criteria for the healthy comparison subjects were the same as for the drug abuse groups. No participants were HIV-seropositive. For the screening and safety assessments in all subjects, a complete medical history, vital signs, full physical examination and clinical assessments were obtained. Detailed demographic and clinical characteristics for abuse patients and healthy subjects are presented in Tables 1 and 2.
Table 1.
Clinical characteristics and demographics
| Subject demographics | MA (n = 9) | MA+MJ (n = 8) | HC (n = 10) | Significance |
|---|---|---|---|---|
|
| ||||
| mean (SD) | mean (SD) | mean (SD) | ||
| Age (years) | 15.7 (1.37) | 16.2 (1.16) | 16.8 (.62) | F(2,24) = 2.82, p = .08 |
| Female (%) | 70 % | 67 % | 63 % | FET, p = .56 |
| Education (years) | 8.0 (1.50) | 8.6 (.92) | 9.1 (.57) | F(2,24) = 2.57, p = .10 |
| Repeated grades | .33 (.50) | .88 (.83) | .40 (.52) | F(2,24) = 1.90, p = .17 |
| Annual income (ZAR)† | 59,545 (14,287) | 54,551 (18,176) | 55,153 (10,938) | F(2,24) = .31, p = .73 |
| Language (English/Afrikaans) | 1/8 | 1/7 | 3/7 | FET, p = .38 |
Annual income was estimated from the parents of the enrolled adolescents.
Abbreviations: MA, methamphetamine; MA+MJ, methamphetamine and marijuana; HC, healthy controls; FET, Fisher’s Exact Test.
Table 2.
Profiles of drug use history
| MA | MA + MJ | HC | |||
|---|---|---|---|---|---|
|
| |||||
| Substance Use characteristics | (n = 9) | (n = 8) | ANOVA† | (n = 10) | |
|
| |||||
| Mean (SD) | Mean (SD) | F(1,15) | p | Mean (SD) | |
| MA (lifetime ‘hits’) | 579 (542) | 837 (531) | .97 | .34 | - |
| MA, age of first regular use (years) | 14.2 (1.37) | 14.1 (1.40) | .02 | .89 | - |
| MA, duration (months) | 21.7 (12.5) | 22.0 (10.3) | .003 | .95 | - |
| MJ (lifetime ‘joints’) | 6 (5) | 1099 (866) | 14.46 | .002* | - |
| MJ, age of first regular use (years) | - | 14.0 (1.37) | - | - | - |
| MJ, duration (months) | - | 26.5 (10.2) | - | - | - |
| Alcohol, total lifetime use (standard units) | 13.1 (19.9) | 24.5 (31.6) | .81 | .38 | 5.4 (9.6) |
| Alcohol, age of first regular use (years) | - | - | - | - | - |
| Alcohol, duration (months) | - | - | - | - | - |
| Nicotine, total lifetime use (cigarettes) | 5020 (7687) | 5126 (4144) | .001 | .97 | 552 (1280) |
| Nicotine, age of first regular use (years)‡ | 13.0 (1.9) | 14.0 (1.0) | 1.52 | .24 | 15.1 (1.5) |
| Nicotine, duration (months)‡ | 30.9 (12.2) | 30.7 (11.3) | .001 | .98 | 19 (21.2) |
ANOVA, Analysis of variance between MA and MA+MJ;
Two subjects in the MA group, one subject in the MA+MJ group, and eight subjects in the HC group did not smoke.
Statistical significance;
Abbreviations: MA, methamphetamine; MJ, marijuana
1.2 Magnetic Resonance Data Acquisition
1.2.1 Structural images
All MR scans were performed using a 3 Tesla Siemens scanner (Trio, Siemens AG, Germany) using a volume coil for transmission and reception. High resolution T1-weighted anatomical Magnetization-Prepared Rapid Acquisition with Gradient echo (MPRAGE) images were used for positioning spectra voxels. The parameters for MPRAGE were as follows; 3D, TR/TE/TI = 2300/3.93/1100 ms, slice thickness = 1.0 mm, average number = 1, flip angle = 12 °, bandwidth = 130 Hz/pixel, FOV = 270, matrix = 256 × 179 × 157 and no time gap.
1.2.2 1H Magnetic Resonance Spectroscopy
Single voxel MR spectra were acquired from the midfrontal anterior cingulate area, which corresponds to Brodmann area 32. The cubic voxel (8 cm3) was positioned just above an imaginary line connecting the anterior commissure and posterior commissure, and just anterior to corpus callosum (see Figure 1). The anterior and posterior commissures were used as references points for voxel placement. We selected midfrontal gray matter as our volume of interest in the present study to investigate metabolite abnormalities in MA and MJ using adolescents since neurocognitive studies suggest that frontally mediated executive functions are impacted by MJ [43–45] and MA [12, 46]. Also, cannabinoid receptors are present with the highest density in the frontal lobe [47] and the cannabinoid receptor density is reported to be nearly twice as high in the frontal lobe compared to the occipital lobe [48].
Figure 1.
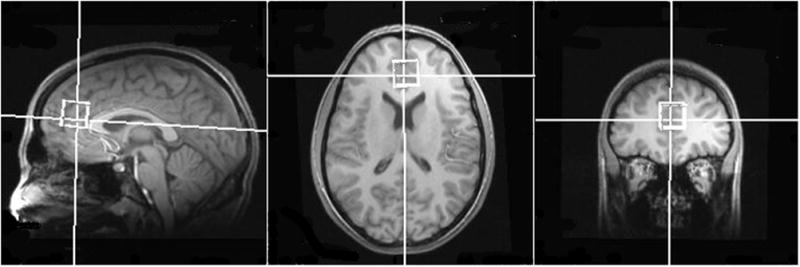
Representative single voxel placement at midfrontal lobe in a methamphetamine and marijuana using adolescent.
MRS data was acquired using point-resolved spectroscopy (PRESS) with the following parameters: TR/TE = 1500/30 ms, voxel size = 2 × 2 × 2 cm, dwell time = 500 ms, flip angle = 90 ° and average number = 128. Water suppression was achieved using a chemical shift selective (CHESS) sequence. To achieve appropriate linewidths, high order manual adjustment was performed using the first and second-order shim coils (x, y, z, z2, xy, yz, xz, x2, y2) following the automated shimming routine that is provided by the Siemens system. During the shimming procedure, typical unsuppressed water line widths were less than 10 Hz. Spectra with line widths greater than 15 Hz were re-shimmed.
For the spectral analysis, Linear Combination of Model Spectra (LCModel) was used [49]. The macromolecule and lipid basis spectra were included in the LCModel fitting to correctly fit the complete spectral signal. This model is fully automatic and user-independent. A nearly model-free constrained regularization method is used for convolution and baseline estimation. The signal-to-noise ratio and a full width at half maximum of each spectrum was checked for quality to ensure they were adequate for reliable peak fitting for the metabolites of interest. Only metabolite measurements having Cramer–Rao lower bounds < 20 % were reported [49].
Using the structural MPRAGE image, tissue segmentation was performed using FSL (FMRIB’s Software Library) software and the gray matter tissue fraction in the voxel was employed as a covariate to adjust for metabolite differences in gray matter and white matter. Spectroscopic analysis measured metabolite levels of tNAA, GPC+PC, mI and Glu+Gln. Metabolite ratios to PCr+Cr were reported in order to cancel out variable coil loading factors associated with the MRS measurement. PCr and Cr play a significant role in oxidative cellular energy metabolism in the brain, providing a temporal and spatial energy buffer to maintain constant high energy levels [50]. Prior adult MRS studies have not reported altered frontal PCr+Cr levels in MJ users [4, 39], but one adolescent MRS study reported reduced PCr+Cr levels in the frontal lobe of MJ using adolescents [40]. However, the potential decreases of PCr+Cr levels, if any, would be biased against our hypothesized reduction in ratios of tNAA/PCr+Cr.
1.3 Statistical Data Analysis
Analysis of variance (ANOVA) was used for between-group comparisons involving continuous metabolite levels and demographic data. Fisher’s exact tests (FET) compared groups on categorical variables. Age, sex, and gray matter tissue fraction in the voxel were used as covariates. The correlations between metabolite levels and drug use variables were examined using linear regression analysis. The regression analyses allowed us to examine the relationship between metabolite alteration and drug use profiles such as total amount of drug use, duration of drug use, and age of onset of drug use. Models with all terms and pairwise interactions between covariates were first considered, and interactions not significant were removed from the models. The Sidak multiple comparison procedure was employed to adjust for experimentwise error rate [51]. Statistical significance was defined at an alpha level of p = 0.05, two-tailed. Stata for Unix, version 12 (StataCorp, College Station, TX) was used for all computations.
2. Results
2.1 Demographic characteristics
ANOVA revealed that the three groups (MA, MA+MJ, and controls) did not differ on demographic variables including age, years of successfully completed education, and repeated grades (all p>0.05). The estimation of annual income of parents of study participants was similar among three groups (Table 1). Females outnumbered males and English speakers were in the minority (vs. Afrikaans-speakers) but subject groups showed no significant between-group differences in gender or language use (all p > 0.05) (Table 1). 96 % of the sample was right-handed, compared to 4 % left-handed, not being statistically different among groups (FET, p = 0.30). There were no significant differences in lifetime alcohol dosage between MA groups and the control group (F(1,25) = 2.32, p = 0.14). MA groups smoked more cigarettes than controls (F(1,25) = 5.29, p = 0.03) but there was no difference between the MA groups in lifetime nicotine dosage (F(1,15) = 0.001, p = 0.97). In many respects the profiles of drug use history were similar between the MA and MA+MJ groups (see Table 2).
2.2 Brain metabolite levels
ANOVA revealed a significant difference of tNAA/PCr+Cr ratios among the groups (F(2,24) = 4.54, p = 0.02). The significant difference in tNAA/PCr+Cr ratios was maintained, after controlling for age and education (F(2,22) = 4.23, p = 0.03). Post hoc comparisons, Sidak tests, revealed that the MA+MJ group had a statistically significant reduction in midfrontal gray matter tNAA/PCr+Cr ratios compared to healthy controls by 7.2 % (p = 0.03) and to MA-only users by 6.9 % (p = 0.04) (Table 3). The decrease in tNAA/PCr+Cr levels in the MA-only group was not significantly different from values observed in controls (p = 0.88). Metabolite ratios such as mI/PCr+Cr, GPC+PC/PCr+Cr, and Glu+Gln/PCr+Cr did not show any significant between-group differences (all p>0.05) (Table 3). When we combined MA+MJ and MA as one group and compared the combined group to the healthy group, we found no between group differences for the metabolite ratios.
Table 3.
Proton metabolite ratios in the frontal lobe
| Metabolites† | MA (n = 9) | MA+MJ (n = 8) | HC (n = 10) | ANOVA | |
|---|---|---|---|---|---|
|
| |||||
| mean (SD) | mean (SD) | mean (SD) | F(2,24) | p | |
| tNAA/PCr+Cr | 1.295 (.078)* | 1.205 (.084)*† | 1.299 (.056)† | 4.54 | .02** |
| GPC+PC/PCr+Cr | .224 (.018) | .216 (.021) | .229 (.020) | 1.03 | .37 |
| mI/PCr+Cr | .962 (.090) | .963 (.066) | .923 (.131) | .47 | .63 |
| Glu+Gln/PCr+Cr | 1.805 (.133) | 1.801 (.216) | 1.841 (.167) | .15 | .86 |
| Gray matter tissue fraction (%) | .75 (.04) | .74 (.07) | .70 (.11) | .94 | .40 |
Statistical significance among groups.
Sidak post hoc tests (* p=.04, † p=.03).
Abbreviations: tNAA, N-acetylaspartate + N-acetylaspartyl glutamate; GPC+PC, glycerophosphocholine + phosphocholine; Glu+Gln, glutamate+glutamine; PCr+Cr, phosphocreatine + creatine. ANOVA, Analysis of variance; MA, methamphetamine; MJ, marijuana; HC, healthy controls
No significant differences were found for alcohol and nicotine use between MA+MJ and MA groups (Table 2). To ensure that our results did not arise from the confounding effects of other drugs such as alcohol and nicotine, we checked the interaction of other drug use on tNAA/PCr+Cr ratios. There were no significant between-group interactions with alcohol (p = 0.47) or with nicotine (p = 0.85). Furthermore, between-group differences in tNAA/PCr+Cr ratios were maintained after controlling for alcohol (p = 0.004) and nicotine (p = 0.036) use.
2.3 Regression analysis with drug use variables
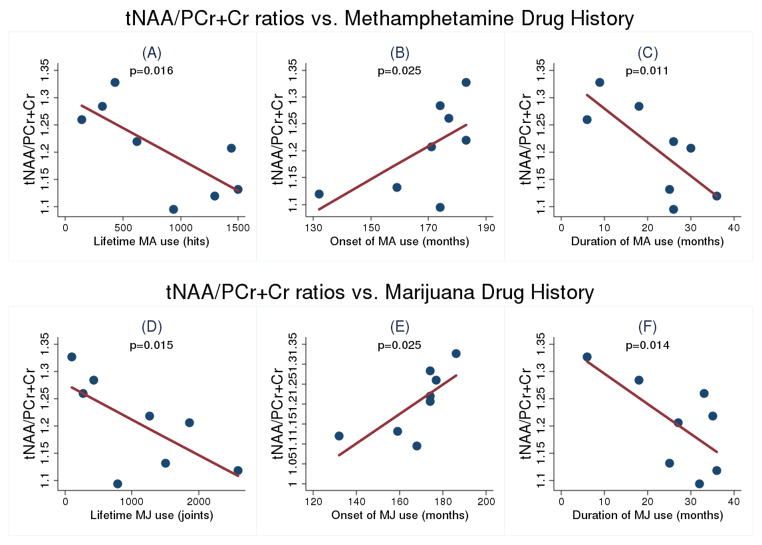
There were significant correlations between brain tNAA/PCr+Cr and clinical indicators of drug use severity only in the MA+MJ group (Figure 4). Specifically, in the MA+MJ group, we found significant negative correlations between tNAA/PCr+Cr and total cumulative lifetime dose of MA (F(1,6) = 11.13, p = 0.02) and of MJ (F(1,6) = 11.44, p = 0.02). Significant positive correlations were seen between tNAA/PCr+Cr and age in months at which participants first began using MA (F(1,6) = 8.84, p = 0.03) and MJ (F(1,6) = 8.86, p = 0.03). Further, significant negative correlations were observed between tNAA/PCr+Cr and duration of MA (F(1,6) = 13.44, p = 0.01) and MJ (F(1,6) = 11.68, p = 0.01) use, respectively. In contrast, there were no statistically significant associations for clinical indicators of drug use with other metabolites. Additionally, to examine a possible clinical association of tNAA/PCr+Cr with MA use, the MA+MJ and MA groups were combined into a single group and correlation analyses with drug use profiles were performed. We found no significant association between the combined group and MA amount, MA onset and MA duration with tNAA/PCr+Cr ratios.
Figure 4.
Significant correlations between tNAA/PCr+Cr ratios and clinical variables of MA and MJ use history in concurrent MA+MJ group: (A) Total MA use amount; (B) Age of first MA use; (C) MA use duration; (D) Total MJ use amount; (E) Age of first MJ use; and (F) MJ use duration
3. Discussion
In this study, we report that combined MA+MJ exposure in adolescents was significantly associated with altered brain neurochemistry, as evidenced by decreased tNAA/PCr+Cr ratios. This finding is in line with previous studies reporting that MJ use contributed to abnormal neuronal integrity in polydrug abusers [52] and that MA+MJ using adults had significant frontal, temporal, and striatal metabolic abnormalities, compared to subjects solely using MA [53]. Moreover, there were significant associations between drug use severity indices and decreased tNAA/PCr+Cr ratios in MA+MJ users. These findings suggest that in developing adolescents, combined exposure to MA and MJ may be related to increased risk of neurotoxicity in a dose-responsive manner, possibly due to an alteration in neurochemical development. These results are significant since the results emphasize the potential added harm associated with combined MA+MJ use to the health of adolescents. This is the first study to examine frontal lobe metabolite abnormalities in concurrent users of MA+MJ in adolescents without comorbid psychiatric disorders.
In adult MRS studies, decreased NAA levels in frontal lobe have been previously reported in relation to the use of MA [15, 33, 35] and MJ [4], which are in line with present findings of frontal metabolic abnormalities in MA and MJ using adolescents. However, in the present study, the MA+MJ group and not the MA-only group, was observed to have significantly decreased tNAA/PCr+Cr ratios compared to healthy controls. Moreover, significant correlations of tNAA/PCr+Cr ratios with drug use profiles were present only in the MA+MJ group, which may denote drug-related “neurotoxicity”, rather than “neuroprotection”, induced by MJ use in MA using adolescents. Either independent MJ induced neurotoxicity or the effects of combined MA+MJ use may have produced these study results, producing a larger effect size in the MA+MJ group than the MA group. Thus, studies including additional MJ-only group will be needed to clarify these findings.
Several factors may account for the inconsistencies between the published adult MRS studies and our adolescent study. First, the developing adolescent brain experiences significant neuronal changes associated with brain maturation processes such as arborization (i.e., neuritic sprouting), myelination by oligodendrocytes, and pruning, which may contribute vulnerability or resistance to the toxicity of substance use during this stage. Second, age-dependent pharmacokinetic factors may result in different responses to MA exposure. For example, in animal studies, young rodents have been reported to have less toxicity to MA exposure [54, 55]. Third, there was a relatively smaller amount of lifetime MA use in the MA-only group than in the MA+MJ group. However, this difference in lifetime MA use did not reach statistical significance, and it is unclear whether this reduction could account for the lack of effect. Fourth, the gender difference (high female/male ratio) might have influenced our observed metabolite differences between groups.
While it is tempting to speculate that the reduced tNAA/PCr+Cr ratios observed only in the MA+MJ group might arise from synergistic neurotoxicity of MA and MJ, the present results are not sufficient to support this argument without an MJ-only group. The absence of an MJ-only group in our study, prevents us from making a direct comparison of MJ-only effects versus MA+MJ or MA effects in metabolite alterations of the adolescent subjects. Therefore, the decreased tNAA/PCr+Cr ratios in the MA+MJ group may reflect either synergistic toxicity or the effects of MJ alone. Interestingly, a recent adolescent MRS study that investigated MJ effects in the frontal brain region reported that NAA levels were reduced in adolescent MJ users compared to healthy adolescents [40]. Although the cannabinoid system has been reported to have neuroprotective properties in degenerative disorders in humans such as Alzheimer’s disease [56] and multiple sclerosis [21, 57] and in rodent models of an acute neurodegenerative condition [58], such studies were undertaken in older study groups with lower amounts/potency of MJ and other comorbid drug use.
The present study findings indicate a dose-response association between drug use severity and neuronal viability/function, showing that heavier MA+MJ use is particularly associated with neurochemical dysfunction. We also found that decreased tNAA/PCr+Cr ratios had a significant association with earlier initiation of first MA and MJ use, a higher cumulative lifetime dose of MA and MJ, and greater daily amounts of MA and MJ.
Neurochemical toxicity of the cannabinoid system in MA using adolescents is clearly a complicated phenomenon as the cannabinoid system itself has a complex relationship to the reward system of the brain. Although interactions between cannabinoid receptors and responsiveness to MA have been reported [59], the exact neurobiological mechanisms associated with concomitant use of MA+MJ are uncertain and will be a subject for further investigation. For example, MA may initiate a cascade of interacting neuroinflammatory, toxic, vascular and hypoxic factors [60], superimposed by insults of MJ use, finally resulting in neurochemical disturbances within the specific CNS network as well as changing the neural structures related to the sensation of pleasure. In this regard, a twin study has reported that MJ using twins were more than four times more likely to become dependent on psychostimulants than co-twin controls [61]. Therefore, chronic MJ exposure in MA-abusing adolescents may interrupt endogenous cannabinoid and immune systems in the brain [3] so that MJ and MJ metabolites may directly impair the resilience in developing brain.
When considering our findings, several limitations should be in kept mind and the results cautiously interpreted. First, the main findings in our 1H MRS data are based on an assessment of metabolite ratios to total creatine levels, and not absolute values. Although this is commonly accepted in the MRS literature [62], especially in clinical settings [63–66], decreased tNAA/PCr+Cr values may have originated from an increased denominator (i.e., PCr+Cr levels). For instance, several 1H MRS studies in human brain development have reported that total creatine levels in the brain increase during early life [69–71], although one study reported a constant level of total creatine after the first year [72]. However, in studies of drug abuse, the opposite direction of alteration in PCr+Cr levels has been noted in adult MA users who were found to have decreased PCr+Cr levels in basal ganglia [33, 35, 67] and decreased PCr levels in frontal lobe [68]. One study on MJ using adolescents reported decreased PCr+Cr levels in the frontal lobe [40]. Therefore, to address potential confounds of age or maturation, we carried out additional analyses. First, regression analysis between PCr+Cr/GPC+PC and age showed no significant relationship in any group (p = 0.90). Second, healthy adolescents (mean age = 16.8 ± 0.62) were slightly older than participants in the MA+MJ and MA groups (mean age = 15.7 ± 1.37 and 16.2 ± 1.16, respectively). Therefore, it is less likely that increased PCr+Cr levels with age might result in lower tNAA/PCr+Cr ratios in our MA+MJ group.
Although we used a 3 Tesla scanner, the complex resonances of Glu and Gln could not be separated in the spectral data analysis as the analysis requires special sequences such as 2D echo time averaging [74]. We observed no group differences in Gln+Glu/PCr+Cr ratios but this does not preclude the possibility that individual Glu and Gln levels are altered in our MA or MA+MJ users.
This investigation included a small sample size with a focus on female adolescents. However, considering the modest sample size, gender effects should not be decisively considered. We used self-reported data to generate substance use history of the adolescents. Thus, both potential misclassification and selection bias may have introduced some degree of error.
The purity, composition and effective dose of available MA and MJ are also highly variable. Further, MA and MJ use are often combined with alcohol and tobacco, which involve uncontrolled factors in our study. However, because alcohol drinking and smoking were similar between MA and MA+MJ groups (Table 2), and covarying these variables maintained statistical significance for between-group tNAA/PCr+Cr ratios (all p < 0.05), it seems unlikely that those factors biased the current findings.
Due to the small sample size, corrections for multiple comparisons were not performed during the correlation analyses. Caution is advised when reviewing the correlation results [75], where a total of six multiple comparisons are included, expanding the chance of Type I statistical errors. Given the a priori hypothesis for our investigation, however, we believe that our findings represent true effects, and are not the result of Type I statistical errors.
The relationship between prodromal symptoms of psychosis and brain metabolites was not assessed in the present study, despite the potential association of MJ use in adolescents for the development of psychosis [29]. Future studies investigating both brain metabolite levels and prodromal symptoms in MA and MJ using adolescents may shed light on the specific neurochemical profiles associated with psychotic symptoms in adolescents.
The MRS scans and clinical analyses performed are cross-sectional and do not shed light on the longitudinal patterns of MA and MJ use. For example, we cannot ascertain from our study design whether the associated neurotoxicity from the MA and MJ use occurred at specific stages of drug use [76] since different stages of the addiction process may have different levels of vulnerability. Future investigations warrant longitudinal study designs to fully characterize within-subject changes in aspects of the neurotoxicity and neurobiology associated with MA and MJ. In addition, follow-up studies are needed to examine whether abstinence from MA and MJ use in adolescents will improve neurochemical profiles and to better understand the developmental changes related to possible consequences of concurrent MA and MJ use.
Finally, the present data are based on interviews and MRS scans carried out on MA and MJ abusing adolescents from Cape Town, South Africa. Because of the uniqueness of our study population, including Afrikaans and English language speakers, low education and poverty, our findings may not generalize to all adolescent populations. Yet, our findings do suggest that the toxic effects of MA abuse may be worsened with concomitant use of MJ in adolescents.
4. Conclusion
The study findings suggest that there is a degree of interplay between MA and MJ use on brain metabolites as the concomitant use of MA+MJ in the developing brain significantly decreased midfrontal tNAA/PCr+Cr levels. More importantly, the study finding that decreased tNAA/PCr+Cr ratios are significantly correlated with drug use severity indices, showing a dose-responsive relationship between metabolite alteration and drug exposure, supports our hypothesis that early exposure of MA+MJ may result in neurochemical alteration. The neurochemical evidence supports the importance of early intervention in adolescent MA+MJ abuse.
Figure 2.
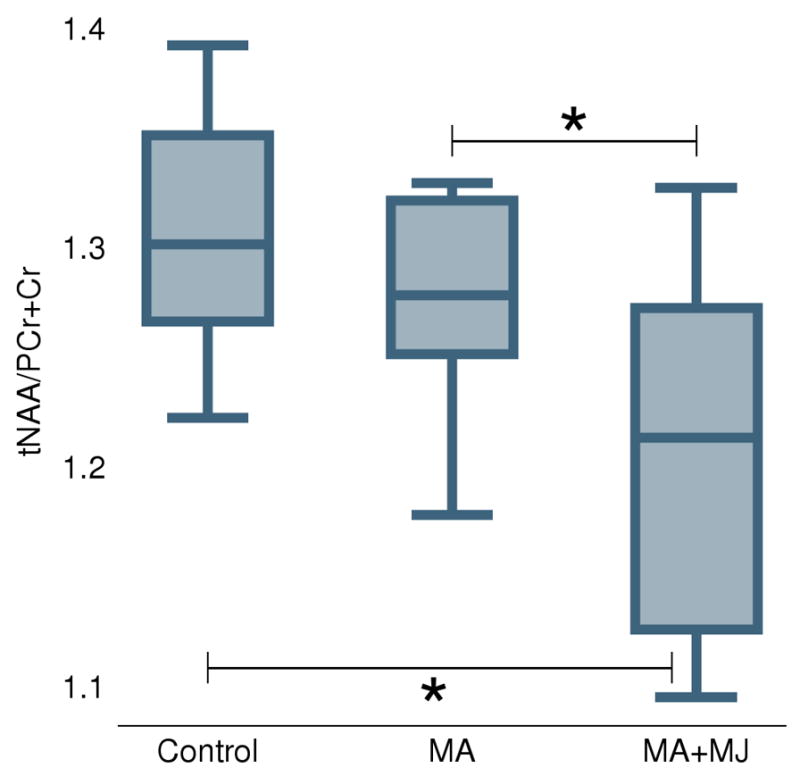
Boxplots for the three groups of control subjects, MA-only, and concomitant MA and MJ users. Abbreviations: MA, methamphetamine; MA+MJ, methamphetamine and marijuana; tNAA/PCr+Cr, N-acetylaspartate/creatine ratio. * Statistically significant difference at the 0.05 level.
Figure 3.
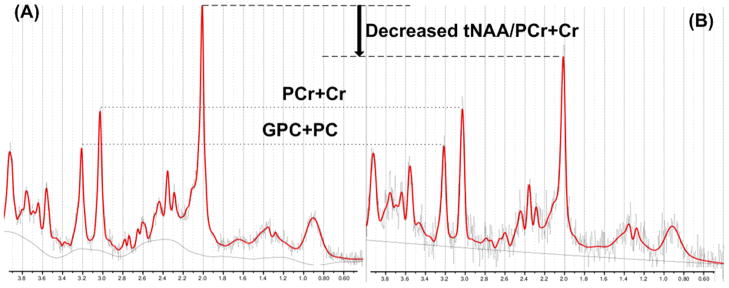
Comparison of tNAA/PCr+Cr ratios between a healthy subject and a MA+MJ user: (A) normal proton spectra in a healthy subject, and (B) a MA+MJ user had decreased tNAA/PCr+Cr ratios compared to the healthy volunteer. Abbreviations: tNAA; N-acetylaspartate + N-acetylaspartyl glutamate; PCr+Cr, phosphocreatine + creatine; and GPC+PC, glycerophosphocholine and phosphocholine.
Research Highlights.
Frontal gray matter tNAA/PCr+Cr ratios were reduced in adolescents using MA+MJ.
Total lifetime amount of MA+MJ had a significant negative correlation with tNAA/PCr+Cr.
Duration of MA+MJ use showed a significant negative correlation with tNAA/PCr+Cr.
Earlier age of onset of MA+MJ use had negative impacts on tNAA/PCr+Cr in adolescents.
Acknowledgments
This study was supported by funding from NIH R21 DA021422 (DYT) and 1R01 DA020269 (DYT). Special thanks are extended to Namkug Kim and Andrew Prescot, who provided technical support for brain tissue segmentation.
Abbreviations
- MA
methamphetamine
- MJ
marijuana
- MA+MJ
methamphetamine + marijuana
- CNS
central nervous system
- HIV
human immunodeficiency virus
- THC
Δ9-tetrahydrocannabinol
- MRS
magnetic resonance spectroscopy
- tNAA
N-acetylaspartate + N-acetylaspartyl glutamate
- PCr
phosphocreatine
- PCr+Cr
phosphocreatine + creatine
- GPC+PC
glycerophosphocholine + phosphocholine
- Glu+Gln
glutamate + glutamine
- mI
myo-inositol
- PRESS
point-resolved spectroscopy
- LCModel
linear combination of model spectra
Footnotes
Disclosure
Dr. Yurgelun-Todd is a consultant for Eli Lilly, Novartis, and has research support from Kyowa Hakko. Dr. Renshaw is a consultant for Kyowa Hakko and Ridge Diagnostics. He has research grant support from Eli Lilly, Hoffman-La Roche, and GlaxoSmithKline. Dr. Stein has received research grants and/or consultancy honoraria from Abbott, Astrazeneca, Eli-Lilly, GlaxoSmithKline, Jazz Pharmaceuticals, Johnson & Johnson, Lundbeck, Orion, Pfizer, Pharmacia, Roche, Servier, Solvay, Sumitomo, Takeda, Tikvah, and Wyeth.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smart RG, Ogborne AC. Drug use and drinking among students in 36 countries. Addictive behaviors. 2000;25:455–60. doi: 10.1016/s0306-4603(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 2.Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95:175–84. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 3.Grotenhermen F. Cannabinoids. Curr Drug Targets CNS Neurol Disord. 2005;4:507–30. doi: 10.2174/156800705774322111. [DOI] [PubMed] [Google Scholar]
- 4.Hermann D, Sartorius A, Welzel H, Walter S, Skopp G, Ende G, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61:1281–9. doi: 10.1016/j.biopsych.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Orgado J, Fernandez-Lopez D, Lizasoain I, Romero J. The seek of neuroprotection: introducing cannabinoids. Recent Pat CNS Drug Discov. 2007;2:131–9. doi: 10.2174/157488907780832724. [DOI] [PubMed] [Google Scholar]
- 6.von MC, Brecht ML, Anglin MD. Use ecology and drug use motivations of methamphetamine users admitted to substance abuse treatment facilities in Los Angeles: an emerging profile. J Addict Dis. 2002;21:45–60. doi: 10.1300/j069v21n01_05. [DOI] [PubMed] [Google Scholar]
- 7.Stoops WW, Tindall MS, Mateyoke-Scrivner A, Leukefeld C. Methamphetamine use in nonurban and urban drug court clients. Int J Offender Ther Comp Criminol. 2005;49:260–76. doi: 10.1177/0306624X04273438. [DOI] [PubMed] [Google Scholar]
- 8.Hadland SE, Marshall BD, Kerr T, Lai C, Montaner JS, Wood E. Ready Access to Illicit Drugs among Youth and Adult Users. Am J Addict. 2012;21:488–90. doi: 10.1111/j.1521-0391.2012.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–60. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- 10.Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. Int Rev Neurobiol. 2009;88:101–19. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King G, Alicata D, Cloak C, Chang L. Neuropsychological deficits in adolescent methamphetamine abusers. Psychopharmacology (Berl) 2010;212:243–9. doi: 10.1007/s00213-010-1949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–38. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 14.Kim YT, Lee SW, Kwon DH, Seo JH, Ahn BC, Lee J. Dose-dependent frontal hypometabolism on FDG-PET in methamphetamine abusers. J Psychiatr Res. 2009;43:1166–70. doi: 10.1016/j.jpsychires.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, et al. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug and alcohol dependence. 2007;88:28–35. doi: 10.1016/j.drugalcdep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Bae SC, Lyoo IK, Sung YH, Yoo J, Chung A, Yoon SJ, et al. Increased white matter hyperintensities in male methamphetamine abusers. Drug and alcohol dependence. 2006;81:83–8. doi: 10.1016/j.drugalcdep.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975–2007. Volume I: Secondary School Students (NIH Publication No. 08–6418A) Annual Volumes on Trends in Drug Use and Related Factors. 2008;I:707. [Google Scholar]
- 18.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–80. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 19.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–48. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 20.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 21.Croxford JL, Pryce G, Jackson SJ, Ledent C, Giovannoni G, Pertwee RG, et al. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J Neuroimmunol. 2008;193:120–9. doi: 10.1016/j.jneuroim.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Glass M, Dragunow M, Faull RL. The pattern of neurodegeneration in Huntington’s disease: a comparative study of cannabinoid, dopamine, adenosine and GABA(A) receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience. 2000;97:505–19. doi: 10.1016/s0306-4522(00)00008-7. [DOI] [PubMed] [Google Scholar]
- 23.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, et al. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–7. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- 24.Ali SF, Newport GD, Scallet AC, Gee KW, Paule MG, Brown RM, et al. Effects of chronic delta-9-tetrahydrocannabinol (THC) administration on neurotransmitter concentrations and receptor binding in the rat brain. Neurotoxicology. 1989;10:491–500. [PubMed] [Google Scholar]
- 25.Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci. 1998;18:5322–32. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzman M, Sanchez C, Galve-Roperh I. Control of the cell survival/death decision by cannabinoids. J Mol Med. 2001;78:613–25. doi: 10.1007/s001090000177. [DOI] [PubMed] [Google Scholar]
- 27.Hall W, Degenhardt L, Teesson M. Cannabis use and psychotic disorders: an update. Drug Alcohol Rev. 2004;23:433–43. doi: 10.1080/09595230412331324554. [DOI] [PubMed] [Google Scholar]
- 28.Drewe M, Drewe J, Riecher-Rossler A. Cannabis and risk of psychosis. Swiss Med Wkly. 2004;134:659–63. doi: 10.4414/smw.2004.10802. [DOI] [PubMed] [Google Scholar]
- 29.Large M, Sharma S, Compton MT, Slade T, Nielssen O. Cannabis Use and Earlier Onset of Psychosis: A Systematic Meta-analysis. Archives of general psychiatry. 2011;68:555–61. doi: 10.1001/archgenpsychiatry.2011.5. [DOI] [PubMed] [Google Scholar]
- 30.Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:561–72. 72 e1–3. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- 33.Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–9. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- 34.Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry research. 2002;116:43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Ernst T, Speck O, Grob CS. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. The American journal of psychiatry. 2005;162:361–9. doi: 10.1176/appi.ajp.162.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, et al. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol. 2007;13:150–9. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- 37.Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addictive behaviors. 2009;34:319–22. doi: 10.1016/j.addbeh.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cloak CC, Alicata D, Chang L, Andrews-Shigaki B, Ernst T. Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug and alcohol dependence. 2011;119:207–15. doi: 10.1016/j.drugalcdep.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: A proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 42.March JS, Sullivan K. Test-retest reliability of the Multidimensional Anxiety Scale for Children. J Anxiety Disord. 1999;13:349–58. doi: 10.1016/s0887-6185(99)00009-2. [DOI] [PubMed] [Google Scholar]
- 43.Fontes MA, Bolla KI, Cunha PJ, Almeida PP, Jungerman F, Laranjeira RR, et al. Cannabis use before age 15 and subsequent executive functioning. Br J Psychiatry. 2011;198:442–7. doi: 10.1192/bjp.bp.110.077479. [DOI] [PubMed] [Google Scholar]
- 44.Crean RD, Tapert SF, Minassian A, Macdonald K, Crane NA, Mason BJ. Effects of chronic, heavy cannabis use on executive functions. J Addict Med. 2011;5:9–15. doi: 10.1097/ADM.0b013e31820cdd57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and alcohol dependence. 2012;121:159–62. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henry BL, Minassian A, Perry W. Effect of methamphetamine dependence on everyday functional ability. Addictive behaviors. 2010;35:593–8. doi: 10.1016/j.addbeh.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 48.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 50.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–43. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 51.Ludbrook J. Multiple comparison procedures updated. Clin Exp Pharmacol Physiol. 1998;25:1032–7. doi: 10.1111/j.1440-1681.1998.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 52.Cowan RL, Joers JM, Dietrich MS. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3 T magnetic resonance spectroscopy study. Pharmacol Biochem Behav. 2009;92:105–10. doi: 10.1016/j.pbb.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Voytek B, Berman SM, Hassid BD, Simon SL, Mandelkern MA, Brody AL, et al. Differences in regional brain metabolism associated with marijuana abuse in methamphetamine abusers. Synapse. 2005;57:113–5. doi: 10.1002/syn.20155. [DOI] [PubMed] [Google Scholar]
- 54.Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- 55.Riddle EL, Kokoshka JM, Wilkins DG, Hanson GR, Fleckenstein AE. Tolerance to the neurotoxic effects of methamphetamine in young rats. Eur J Pharmacol. 2002;435:181–5. doi: 10.1016/s0014-2999(01)01592-8. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez BG, Blazquez C, Gomez dPT, Guzman M, de CML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1904–13. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pope C, Mechoulam R, Parsons L. Endocannabinoid signaling in neurotoxicity and neuroprotection. Neurotoxicology. 2010;31:562–71. doi: 10.1016/j.neuro.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van dSM, Veldhuis WB, Bar PR, Veldink GA, Vliegenthart JF, Nicolay K. Neuroprotection by Delta9-tetrahydrocannabinol, the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6475–9. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landa L, Sulcova A, Slais K. Involvement of cannabinoid CB1 and CB2 receptor activity in the development of behavioural sensitization to methamphetamine effects in mice. Neuro Endocrinol Lett. 2006;27:63–9. [PubMed] [Google Scholar]
- 60.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Annals of the New York Academy of Sciences. 2010;1187:101–21. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lynskey MT, Heath AC, Bucholz KK, Slutske WS, Madden PA, Nelson EC, et al. Escalation of drug use in early-onset cannabis users vs co-twin controls. Jama. 2003;289:427–33. doi: 10.1001/jama.289.4.427. [DOI] [PubMed] [Google Scholar]
- 62.Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240:318–32. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- 63.Chu WC, Chik KW, Chan YL, Yeung DK, Roebuck DJ, Howard RG, et al. White matter and cerebral metabolite changes in children undergoing treatment for acute lymphoblastic leukemia: longitudinal study with MR imaging and 1H MR spectroscopy. Radiology. 2003;229:659–69. doi: 10.1148/radiol.2293021550. [DOI] [PubMed] [Google Scholar]
- 64.Vermathen P, Laxer KD, Schuff N, Matson GB, Weiner MW. Evidence of neuronal injury outside the medial temporal lobe in temporal lobe epilepsy: N-acetylaspartate concentration reductions detected with multisection proton MR spectroscopic imaging--initial experience. Radiology. 2003;226:195–202. doi: 10.1148/radiol.2261011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rai GS, McConnell JR, Waldman A, Grant D, Chaudry M. Brain proton spectroscopy in dementia: an aid to clinical diagnosis. Lancet. 1999;353:1063–4. doi: 10.1016/s0140-6736(98)03759-3. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz-Pena JL, Pinero P, Sellers G, Argente J, Casado A, Foronda J, et al. Magnetic resonance spectroscopy of normal appearing white matter in early relapsing-remitting multiple sclerosis: correlations between disability and spectroscopy. BMC Neurol. 2004;4:8. doi: 10.1186/1471-2377-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekine Y, Minabe Y, Kawai M, Suzuki K, Iyo M, Isoda H, et al. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27:453–61. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 68.Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, et al. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug and alcohol dependence. 2012 doi: 10.1016/j.drugalcdep.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van der Knaap MS, van der Grond J, van Rijen PC, Faber JA, Valk J, Willemse K. Age-dependent changes in localized proton and phosphorus MR spectroscopy of the brain. Radiology. 1990;176:509–15. doi: 10.1148/radiology.176.2.2164237. [DOI] [PubMed] [Google Scholar]
- 70.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1993;30:424–37. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto T, Tayama M, Miyazaki M, Fujii E, Harada M, Miyoshi H, et al. Developmental brain changes investigated with proton magnetic resonance spectroscopy. Developmental medicine and child neurology. 1995;37:398–405. doi: 10.1111/j.1469-8749.1995.tb12023.x. [DOI] [PubMed] [Google Scholar]
- 72.Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, et al. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric research. 1999;46:474–85. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- 73.Bluml S, Wisnowski JL, Nelson MD, Jr, Paquette L, Gilles FH, Kinney HC, et al. Metabolic Maturation of the Human Brain From Birth Through Adolescence: Insights From In Vivo Magnetic Resonance Spectroscopy. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: Assessment of metabolite discrimination and general reproducibility. Journal of magnetic resonance imaging : JMRI. 2012 doi: 10.1002/jmri.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saville DJ. Multiple Comparison Procedures: The Practical Solution. The American Statistician. 1990;44:174–80. [Google Scholar]
- 76.Koob GF, Le MM. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]