Abstract
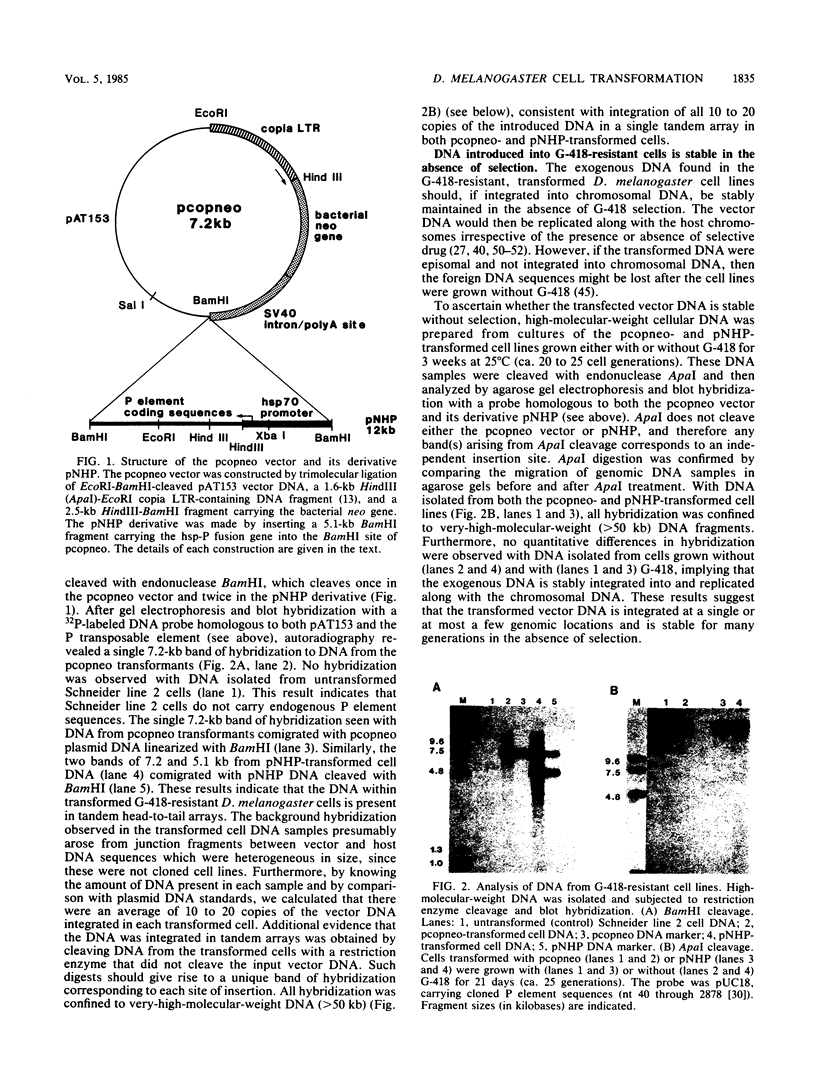
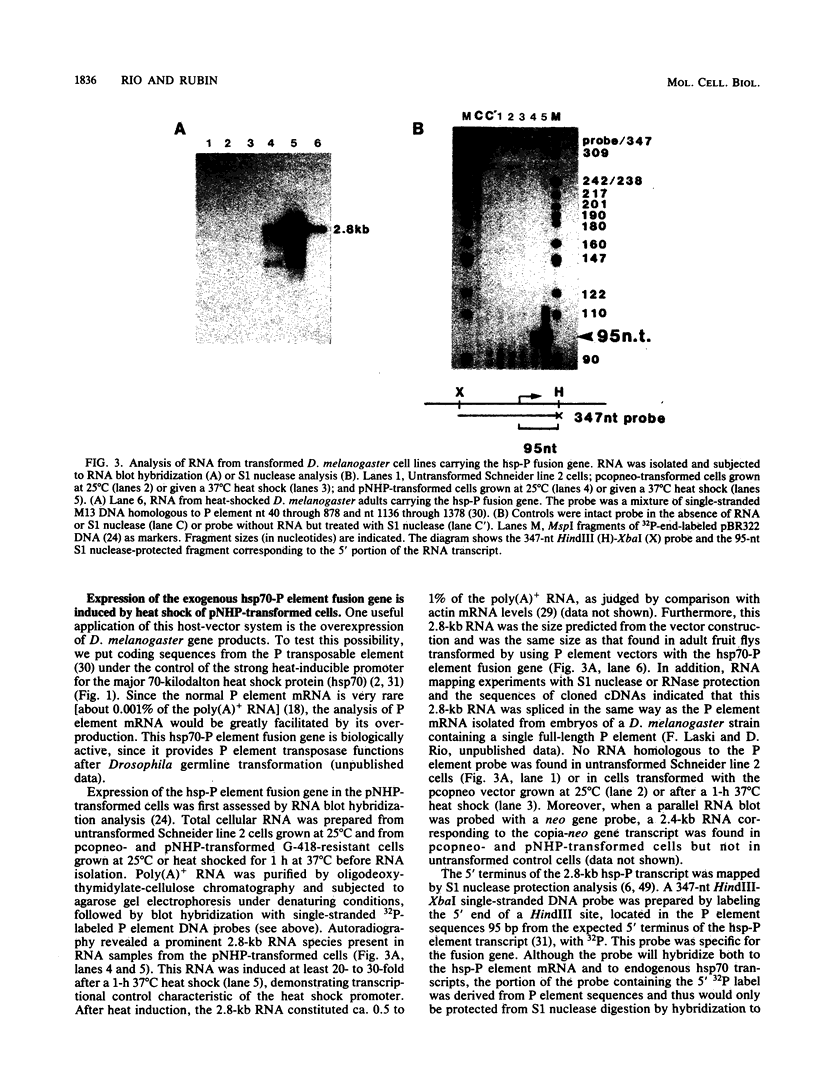
We have developed a method for the stable and efficient introduction of foreign DNA into Drosophila melanogaster tissue culture cells. A plasmid vector was constructed that carries the bacterial neomycin resistance gene under the transcriptional control of the copia transposable element long terminal repeat promoter. After calcium phosphate-DNA transfection, this vector rendered D. melanogaster cells resistant to the aminoglycoside G-418, a derivative of gentamicin. The vector DNA appeared to be integrated in long tandem arrays of 10 to 20 copies per cell and was stable for many generations in the absence of selection. To test the usefulness of this system for introducing nonselected DNA into D. melanogaster cells, a gene fusion between the P transposable element and the hsp70 promoter was inserted into the copia-neomycin resistance plasmid. After transfection and establishment of a G-418-resistant cell line, the hsp-P fusion gene was found to be efficiently transcribed after heat shock.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Nüsslein-Volhard C. Information for the dorsal--ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature. 1984 Sep 20;311(5983):223–227. doi: 10.1038/311223a0. [DOI] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti S., Graham F. L. Transfer of the gene for thymidine kinase to thymidine kinase-deficient human cells by purified herpes simplex viral DNA. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1590–1594. doi: 10.1073/pnas.74.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyajati C., Dray J. F. Cloned Drosophila alcohol dehydrogenase genes are correctly expressed after transfection into Drosophila cells in culture. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1701–1705. doi: 10.1073/pnas.81.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bonner J. J., Parks C., Parker-Thornburg J., Mortin M. A., Pelham H. R. The use of promoter fusions in Drosophila genetics: isolation of mutations affecting the heat shock response. Cell. 1984 Jul;37(3):979–991. doi: 10.1016/0092-8674(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Bourouis M., Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. doi: 10.1002/j.1460-2075.1983.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Sinclair J. H., Sang J. H., Ish-Horowicz D. An assay for transient gene expression in transfected Drosophila cells, using [3H]guanine incorporation. EMBO J. 1984 Nov;3(11):2549–2554. doi: 10.1002/j.1460-2075.1984.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Levis R., Simon M. A., Rubin G. M. The 5' termini of RNAs encoded by the transposable element copia. Nucleic Acids Res. 1981 Dec 11;9(23):6279–6291. doi: 10.1093/nar/9.23.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A., Posakony J. W., Maniatis T. Correct developmental expression of a cloned alcohol dehydrogenase gene transduced into the Drosophila germ line. Cell. 1983 Aug;34(1):59–73. doi: 10.1016/0092-8674(83)90136-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T., Levis R., Rubin G. M. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984 Feb;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Kaufman T. C., Lewis R., Wakimoto B. Cytogenetic Analysis of Chromosome 3 in DROSOPHILA MELANOGASTER: The Homoeotic Gene Complex in Polytene Chromosome Interval 84a-B. Genetics. 1980 Jan;94(1):115–133. doi: 10.1093/genetics/94.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Kaufman T. C., Denell R. E., Tallerico P. Genetic Analysis of the Antennapedia Gene Complex (Ant-C) and Adjacent Chromosomal Regions of DROSOPHILA MELANOGASTER. I. Polytene Chromosome Segments 84b-D. Genetics. 1980 Jun;95(2):367–381. doi: 10.1093/genetics/95.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Wakimoto B. T., Denell R. E., Kaufman T. C. Genetic Analysis of the Antennapedia Gene Complex (Ant-C) and Adjacent Chromosomal Regions of DROSOPHILA MELANOGASTER. II. Polytene Chromosome Segments 84A-84B1,2. Genetics. 1980 Jun;95(2):383–397. doi: 10.1093/genetics/95.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., McDougall J. K. Biochemical transformation of mouse cells by fragments of herpes simplex virus DNA. Cell. 1977 May;11(1):233–241. doi: 10.1016/0092-8674(77)90334-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- O'hare K., Levis R., Rubin G. M. Transcription of the white locus in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6917–6921. doi: 10.1073/pnas.80.22.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Pollack Y., Stein R., Razin A., Cedar H. Methylation of foreign DNA sequences in eukaryotic cells. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6463–6467. doi: 10.1073/pnas.77.11.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C., Clark S. G., Tjian R. A mammalian host-vector system that regulates expression and amplification of transfected genes by temperature induction. Science. 1985 Jan 4;227(4682):23–28. doi: 10.1126/science.2981116. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schaffner W. Direct transfer of cloned genes from bacteria to mammalian cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2163–2167. doi: 10.1073/pnas.77.4.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholnick S. B., Morgan B. A., Hirsh J. The cloned dopa decarboxylase gene is developmentally regulated when reintegrated into the Drosophila genome. Cell. 1983 Aug;34(1):37–45. doi: 10.1016/0092-8674(83)90134-4. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell. 1983 Aug;34(1):47–57. doi: 10.1016/0092-8674(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Struhl G. A gene product required for correct initiation of segmental determination in Drosophila. Nature. 1981 Sep 3;293(5827):36–41. doi: 10.1038/293036a0. [DOI] [PubMed] [Google Scholar]
- Struhl G., Brower D. Early role of the esc+ gene product in the determination of segments in Drosophila. Cell. 1982 Nov;31(1):285–292. doi: 10.1016/0092-8674(82)90428-7. [DOI] [PubMed] [Google Scholar]
- Tsui L. C., Breitman M. L., Siminovitch L., Buchwald M. Persistence of freely replicating SV40 recombinant molecules carrying a selectable marker in permissive simian cells. Cell. 1982 Sep;30(2):499–508. doi: 10.1016/0092-8674(82)90247-1. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wakimoto B. T., Kaufman T. C. Analysis of larval segmentation in lethal genotypes associated with the antennapedia gene complex in Drosophila melanogaster. Dev Biol. 1981 Jan 15;81(1):51–64. doi: 10.1016/0012-1606(81)90347-x. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]





