Abstract
Objective:
Cross-sectional studies suggest that sleep fragmentation is associated with cognitive performance in older adults. We tested the hypothesis that sleep fragmentation is associated with incident Alzheimer's disease (AD) and the rate of cognitive decline in older adults.
Design:
Prospective cohort study.
Setting:
Community-based
Participants:
737 community dwelling older adults without dementia.
Measurements and Results:
Sleep fragmentation was quantified from up to 10 consecutive days of actigraphy. Subjects underwent annual evaluation for AD with 19 neuropsychological tests. Over a follow-up period of up to 6 years (mean 3.3 years), 97 individuals developed AD. In a Cox proportional hazards model controlling for age, sex, and education, a higher level of sleep fragmentation was associated with an increased risk of AD (HR = 1.22, 95%CI 1.03-1.44, P = 0.02 per 1SD increase in sleep fragmentation). An individual with high sleep fragmentation (90th percentile) had a 1.5-fold risk of developing AD as compared with someone with low sleep fragmentation (10th percentile). The association of sleep fragmentation with incident AD did not vary along demographic lines and was unchanged after controlling for potential confounders including total daily rest time, chronic medical conditions, and the use of common medications which can affect sleep. In a linear mixed effect analysis, a 0.01 unit increase in sleep fragmentation was associated with a 22% increase in the annual rate of cognitive decline relative to the average rate of decline in the cohort (Estimate = -0.016, SE = 0.007, P = 0.03).
Conclusions:
Sleep fragmentation in older adults is associated with incident AD and the rate of cognitive decline.
Citation:
Lim ASP; Kowgier M; Yu L; Buchman AS; Bennett DA. Sleep fragmentation and the risk of incident alzheimer's disease and cognitive decline in older persons. SLEEP 2013;36(7):1027-1032.
Keywords: Actigraphy, Alzheimer's disease, cognitive decline, epidemiology, sleep fragmentation
INTRODUCTION
Cognitive impairment and dementia together constitute a growing public health concern. The prevalence of dementia among Americans over the age of 71 has been estimated at 14%,1 and a further 22% have cognitive impairment without dementia.2 Moreover, the number of Americans older than 65 years old will double to about 80 million by the year 2030, with the most rapid growth in those 80 years or older.3 Both animal and human studies suggest that sleep disruption may contribute to cognitive impairment and neurodegeneration.4–9 While several cross-sectional studies have reported associations between sleep and cognitive function in older adults,10–12 longitudinal studies linking objectively measured sleep function in older adults with the risk of developing AD or the rate of cognitive decline are lacking. This is in part because standard sleep measurement approaches such as polysomnography perturb natural sleep behavior, usually are limited to a single night of testing, and by virtue of their expense limit the number of individuals that can be assessed.
Small portable devices such as actigraphs have been used in several recent studies to obtain objective measures of sleep-wake behavior in older adults in community-based settings.
These small devices, which can be worn on the wrist, are nonintrusive and do not perturb natural sleep. Actigraphs measure rest and activity continuously 24 hours/day for days to weeks, capturing total daily sleep and providing investigators with objective measures that circumvent recall bias and sleep misperception, which can affect traditional self-report measures. Analytical approaches applied to polysomnographic data have also been applied to actigraphic data, such as state transition based analyses for the quantification of sleep fragmentation.13–17
We used data from 737 older adults without dementia participating in in the Rush Memory and Aging Project (MAP) to test the hypothesis that sleep fragmentation in community-dwelling older adults is associated with the risk of incident AD and the rate of cognitive decline. Sleep fragmentation was quantified from up to 10 days of continuous actigraphic recordings as previously described.13 Subjects also underwent structured annual evaluation which included a battery of 19 neuropsychological tests for up to 6 years to identify the development of AD and to assess the rate of cognitive decline.
METHODS
Subjects
The Rush Memory and Aging Project (MAP) is an ongoing community-based cohort study of aging which began in 1997.18 Actigraphy was added in 2005, so only a subset of MAP participants has undergone actigraphy. Inclusion criteria for these analyses required valid baseline actigraphy, cognitive testing without dementia at the time of actigraphy, and at least 1 follow-up cognitive assessment to allow a determination of incident AD and cognitive decline. At the time of these analyses, 958 subjects had undergone actigraphy and completed their baseline evaluation. Of 958 subjects with valid actigraphy and cognitive testing, 64 were excluded due to clinical dementia (see below) at the time of actigraphy testing, and a further 157 did not have a 2nd follow-up cognitive assessment, either because they died before follow-up testing (18 participants) or had not been in the study long enough (139 participants). This left 737 participants included in these analyses.
Statement of Ethics Approval
The study was conducted in accordance with the latest version of the Declaration of Helsinki and was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all subjects.
Assessment of Sleep Fragmentation
The actigraph used in this study was the Actical (Phillips Respironics, Bend, OR). The Actical is a wristwatch-like accelerometer that continuously measures acceleration primarily in an axis parallel to the face of the device, rectifies this signal, summates the resultant signal across time, and then digitally samples and records this sum as an “activity count” for each 15-sec period—referred to hereafter as one “epoch.” The higher the activity count, the higher the level of activity. The actigraphs were placed on subjects' non-dominant wrists by study staff. Participants were instructed to leave the device on their wrist until study staff returned to remove the device after 10 days. In order to decrease participant burden and to improve reliability of data collection among persons who develop dementia, participants were not asked to complete sleep diaries.
After the recording period, actigraphic data was downloaded onto a PC and analyses were performed using algorithms implemented in the MATLAB language (Mathworks, Natick, MA).
We quantified sleep fragmentation using a recently developed probabilistic state transition approach which produces a single metric kRA.13,14 Briefly, kRA represents the probability per 15-sec epoch of having an arousal, as indicated by movement (i.e., a non-zero activity count), after a long (∼5 min) period of rest (i.e., sleep). The higher the kRA the more quickly bouts of sleep/rest end in arousals and hence the greater the degree of sleep fragmentation. In a previous study, we examined the effect of using non-zero activity count thresholds (ranging from 0 to the 10th percentile of counts per epoch) to define activity vs. rest and found that the calculation of kRA was relatively insensitive to the specific choice of threshold.13 The transition metric kRA has a theoretical advantage over standard summary measures of fragmentation such as sleep efficiency (SE) and wake time after sleep onset (WASO) because these traditional metrics do not explicitly consider the temporal distribution of wake and sleep across the night—only their amounts. Whereas an individual with a single 2-h awakening and another individual with 240 30-sec awakenings per 8 h of sleep would have identical SE and WASO, the former would have a relatively low kRA while the latter would have a relatively high kRA. This is an important distinction because there is increasing appreciation that the frequency of arousal can have deleterious effects independent of the total time spent awake.9,19 As described previously,13,14 kRA is calculated on the basis of the entire actigraphic record and considers sleep fragmentation irrespective of when it occurs (i.e., both daytime naps and nighttime sleep), although as we showed previously, the value of kRA is most heavily influenced by the period of the day in which the greatest amount of sleep occurs, which for most individuals is the night. We excluded from analysis any day with more than 4 straight hours of complete inactivity, which we considered suspicious for device removal. However, because device removal diaries were not required of the participants (in order to decrease participant burden) it is possible that briefer periods of device removal may have been inadvertently included in our analysis. Figure 1 shows 24 h of actigraphic data from representative individuals with low (Figure 1A, 10th percentile) and high (Figure 1B, 90th percentile) sleep fragmentation. In a separate study of 105 individuals from whom we obtained simultaneous actigraphy and polysomnography, the fragmentation metric kRA correlated with several standard polysomnographic metrics of sleep fragmentation including arousal index (R = +0.6, P < 0.0001; Figure S1A), SE (R = -0.6, P < 0.0001; Figure S1B), and WASO (R = +0.5, P < 0.0001; Figure S1C). SE and WASO could not be directly determined from the actigraphs in our study because algorithms for inferring sleep/wake state from actigraphic data are actigraph-specific, and such algorithms have not been developed for the Actical. Moreover, the determination of SE and WASO depends on knowing the time in bed as determined by a sleep diary, which, to decrease participant burden, was not obtained in the present study.
Figure 1.
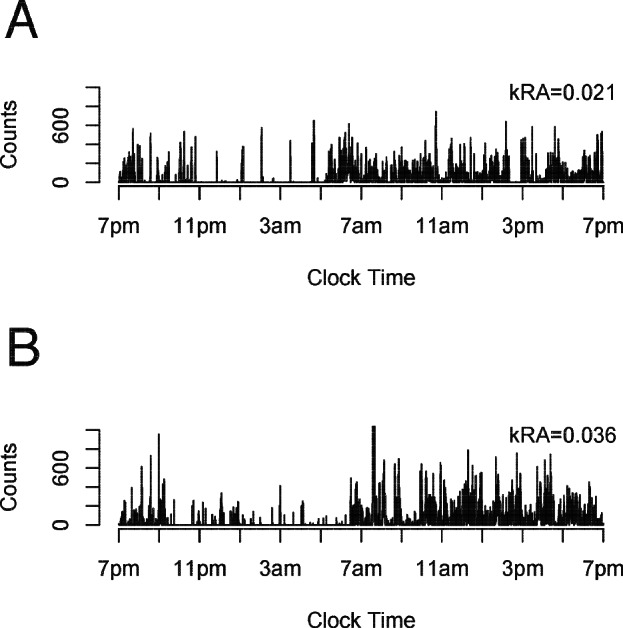
Illustrative actigraphic recordings. 24 h of actigraphy from two representative participants at the 10th (Panel A; kRA 0.021) and 90th (Panel B; kRA 0.036) percentiles of sleep fragmentation.
Assessment of Cognitive Function and Clinical Diagnoses
Trained technicians administered 19 cognitive tests (Word List Recall, Word List Delay, Word List Recognition, Immediate Story Recall, Delayed Story Recall, Logical Memory Ia, Logical Memory IIa, Boston Naming, Reading Test, Verbal Fluency, Digit Span Forward, Digit Span Backward, Digit Ordering, Symbol Digit, Number Comparison, Stroop Color Naming, Stroop Word Naming, Line Orientation, and Progressive Matrices) spanning 5 cognitive domains (episodic memory, working memory, semantic memory, perceptual speed, and visuospatial abilities) which were used to create a composite measure of global cognitive function as described in detail in prior publications.20 This measure was constructed such that 0 represents the mean score of all MAP participants at study baseline, positive scores indicate better performance,21 and 1 unit represents approximately 1 standard deviation of performance.
Individuals were classified as having AD as previously described.21 Briefly, the results of cognitive tests were reviewed by a neuropsychologist to determine the presence or absence of cognitive impairment. A clinician then combined all available cognitive and clinical data to determine whether the subject had AD or not according to the NINDS-ADRDA criteria.22
Subjects were classified by a clinician as having Parkinson disease using CAPIT criteria.23 The presence/absence of a history of stroke was determined by a clinician considering all available clinical information including physical examination.
Assessment of Other Covariates
Age was computed from the self-reported date of birth and the date of actigraphy. Years of education was recorded at the time of the baseline interview. Sex was recorded at the time of the baseline interview. Medications were inspected and coded using the Medi-Span system (Medi-Span, Inc.). Presence/absence of hypertension, coronary artery disease, congestive heart failure, cancer, and thyroid disease were based on annual interview. Participants were considered to have diabetes if they were taking diabetes medications or endorsed a diagnosis of diabetes on interview. Depressive symptoms were assessed with a 10-item version of the Center for Epidemiologic Studies-Depression Scale.18
Mean total daily activity, expressed in counts per day, was calculated from each actigraphic record by taking the total sum of all activity counts in each recording and dividing by the total number of days. Mean total daily rest, expressed in hours, was calculated from each actigraphic record by taking the total duration in hours of all 15-sec epochs in each record with no recorded movement, and dividing by the total number of days.
Statistical Analysis
We tested for associations between the sleep fragmentation metric kRA and the outcome of incident AD using a series of Cox-proportional hazards models using the Efron approximation to account for ties resulting from the grouped-survival data. First we examined the association of kRA with incident AD in a core model adjusted for age, sex, and education. Interaction terms between kRA and age, sex, and education were initially considered, but were excluded from all subsequent models because they were nonsignificant (P > 0.05 for all). Next, to exclude the possibility that any observed associations may be driven by differences in the hours of rest per day, we augmented this model to include a term for hours of rest per day. Moreover, to explore the effect of potential clinical confounders, we further augmented our model to adjust for a number of clinical covariates including number of depressive symptoms, presence/absence of Parkinson disease, presence/absence of stroke, presence/absence of hypertension, presence/absence of diabetes, presence/absence of coronary artery disease, presence/absence of congestive heart failure, presence/absence of cancer, presence/absence of thyroid disease, and use of antidepressant, sedative-hypnotic, and anxiolytic medications. For all Cox models, examination of Martingale residual plots ensured the correct functional form for all covariates, examination of delta-beta plots did not identify unduly influential points, and examination of scaled Schoenfeld residual plots confirmed the proportional hazards assumption.
We tested for associations between the sleep fragmentation metric kRA and subsequent change in global cognition using a linear mixed-effect model adjusted for age, sex, education, and including terms for their interaction with time. This model included subject-specific random terms for slope and intercept. An autoregressive covariance structure was used as it yielded the lowest Akaike Information Criterion value among several covariance structures considered. Examination of residual plots confirmed model assumptions concerning the independence and distribution of within-group errors and random errors.
All statistical analyses were carried out in the R programming language.24
RESULTS
Clinical and Actigraphic Characteristics of Participants
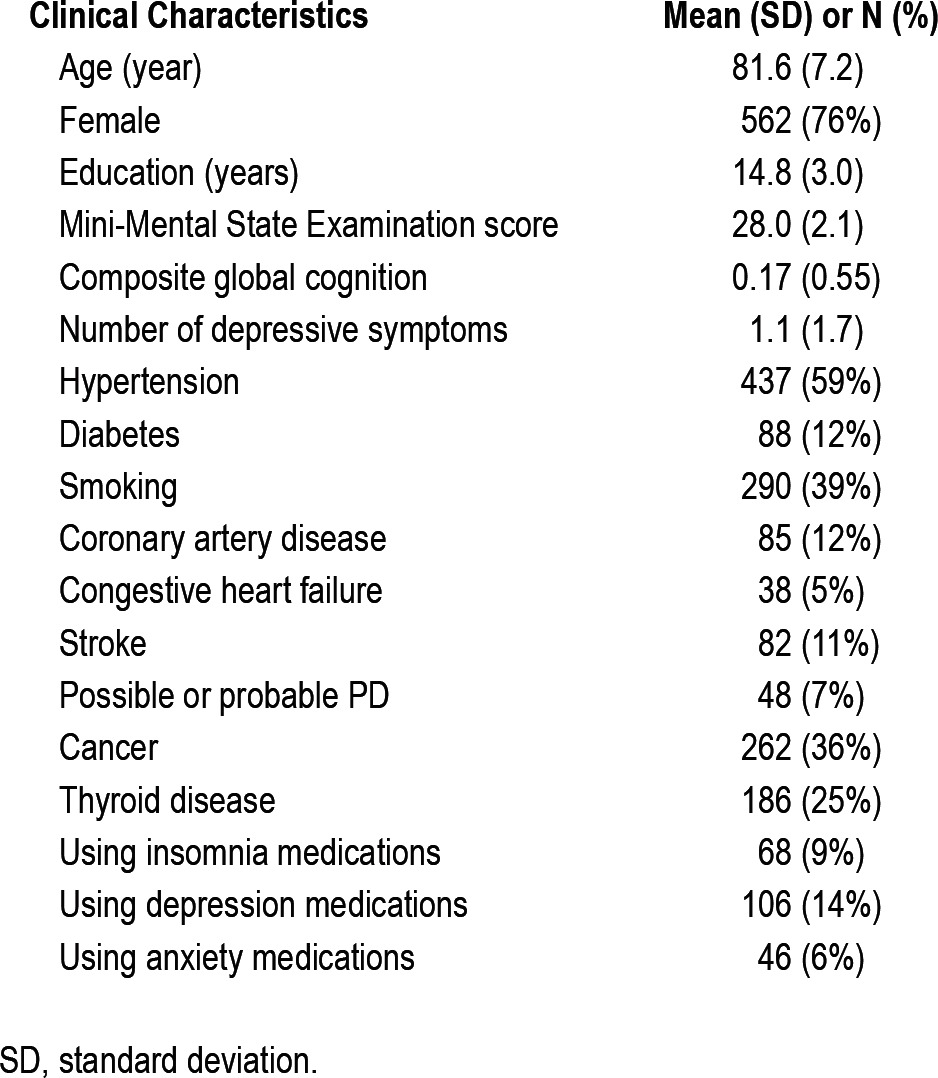
Clinical characteristics of the 737 participants in this study at the time actigraphy was recorded are included in Table 1. On average, actigraphy was recorded for 9.3 days (SD 0.9). The mean value of the sleep fragmentation metric kRA was 0.029 (range 0.0156-0.0823, SD 0.0074). kRA represents the probability per 15-sec epoch of having an arousal, as indicated by movement (i.e., a non-zero activity count), after a long period of rest (i.e., sleep). The higher the kRA the more quickly bouts of sleep/ rest end in arousals and hence the greater the degree of sleep fragmentation. kRA was weakly correlated with age (r = -0.09, P = 0.02) and education (r = -0.10, P = 0.01) and was higher in men than in women (effect = +0.003, P < 0.001).
Table 1.
Clinical Characteristics of Study Subjects (N = 737)
Sleep Fragmentation and Incident AD
Over a mean follow-up of 3.3 years (SD 1.7), 97 (13% of 737) persons developed AD. Using a Cox proportional hazards model adjusted for age, sex, and education, the sleep fragmentation metric kRA was associated with the risk of developing AD, with a 1 standard deviation (SD) increase in kRA associated with a hazard ratio (HR) of 1.22 (95% CI 1.03-1.44, P = 0.02) for incident AD. The effect of kRA on the risk of AD did not vary by age, sex, or level of education (P > 0.05 for all interaction terms). In separate analyses of men (n = 174) and women (n = 563), the estimates of the effect of a 1 SD difference in kRA on the risk of AD were similar, although the confidence intervals on the effect estimates were larger because of the smaller sample size (for males HR = 1.33, 95% CI 0.90-1.96, P = 0.15; for females HR = 1.29, 95% CI 0.99-1.69, P = 0.06).
To illustrate these results, we compare model predictions for incident AD for 2 average individuals with high and low sleep fragmentation (whose recordings shown in Figure 1). An individual with high (90th percentile; solid line) sleep fragmentation would be expected to have a 1.5-fold increased risk for developing AD as compared to an individual with low (10th percentile; dotted line) sleep fragmentation (Figure 2).
Figure 2.
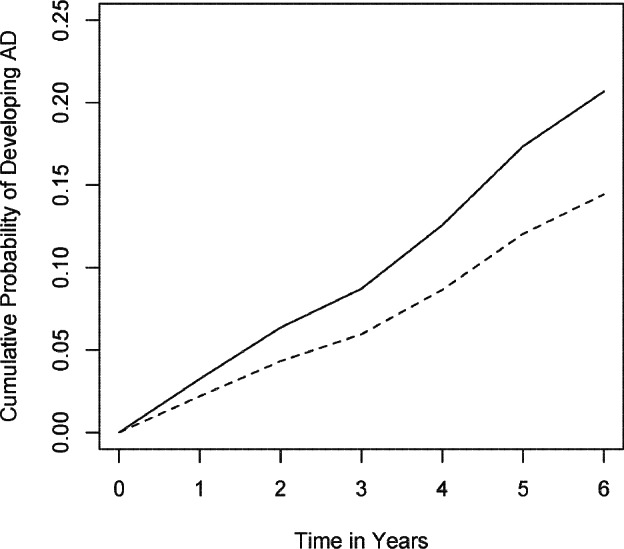
Expected risk of AD. The model predicted risk of AD based on the entire cohort is illustrated for two hypothetical average participants with high (Solid line: 90th percentile; kRA = 0.036) and low (Dotted line: 10th percentile; kRA = 0.021) levels of sleep fragmentation.
It may be conceptually expected that a higher degree of sleep fragmentation may affect overall daily rest. Inclusion of a term for total daily rest in our model did not substantially change the association between kRA and the risk of incident AD (HR = 1.24, 95% CI 1.05-1.45, P = 0.01 for a 1 SD increase in kRA).
Sleep Fragmentation, Other Covariates and Incident AD
We further augmented our model to examine whether several potential confounders would attenuate the associations between sleep fragmentation and incident AD.
We recently demonstrated that total daily activity is associated with the risk of incident AD in older individuals.25 To examine whether the association between sleep fragmentation and the risk of incident AD may be mediated by differences in total daily activity, we augmented our core model to include a term for total daily activity. In this augmented model, the association between kRA and the risk of incident AD was relatively unchanged (HR = 1.22, 95% CI 1.04-1.43, P = 0.01 for a 1SD increase in kRA). Increased total daily activity was associated with a decreased risk of incident AD (HR = 0.61, 95% CI 0.47-
0.79, P = 0.0002 for a 1SD increase in total daily activity). Chronic health problems such as depression, Parkinson disease, hypertension, diabetes, coronary artery disease, congestive heart failure, cancer, thyroid disease, and stroke may affect sleep fragmentation, risk of dementia, or both. In a linear regression model, of these comorbidities, only presence/absence of PD was associated with kRA (effect estimate = +0.004, SE = 0.001, P = 0.0003). We augmented our core model to include terms for the number of depressive symptoms, presence/absence of stroke, presence/absence of PD, presence/absence of hyper-tension, presence/absence of diabetes, presence/absence of coronary artery disease, presence/absence of congestive heart failure, presence/absence of thyroid disease, and presence/absence of cancer. In this augmented model, of these additional comorbidities, only the number of depressive symptoms was associated with an increased risk of incident Alzheimer's disease (HR = 1.23; 95% CI 1.09-1.38; P = 0.0004). The association between sleep fragmentation and the risk of AD was not significantly attenuated by adjustment for these comorbidities (HR 1.27, 95% CI 1.01-1.59, P = 0.04 for a 1SD increase in kRA).
Several classes of medication including antidepressants, sedative-hypnotics, and anxiolytics may potentially alter sleep architecture. The use of these medications did not appreciably change the association between sleep fragmentation and the risk of AD (HR = 1.25, 95% CI 1.06-1.48, P = 0.01 for a 1SD increase in kRA).
Sleep Fragmentation and Cognitive Decline
A diagnosis of AD can be thought of as a point on a continuum of cognitive decline. To ensure that our results were not influenced by diagnostic misclassification or individuals with lower cognition, we examined the association between kRA and rate of cognitive decline using a composite measure of global cognition.18
In a linear mixed model examining the level and rate of change of composite global cognitive performance as a function of sleep fragmentation at baseline, adjusted for age, sex, and education and their interactions with time, increased kRA was associated with lower baseline cognitive performance (Estimate -0.07, SE 0.023, P = 0.007 per 0.01 unit increase in kRA) as well as with a more rapid rate of global cognitive decline (Estimate = -0.016, SE = 0.007, P = 0.03 per 0.01 unit increase in kRA × time; Figure 3), which represents a 22% increase compared to the average rate of cognitive decline in the cohort (Estimate = -0.072, SE = 0.005, P < 0.0001) for each 0.01 unit increase in kRA.
Figure 3.
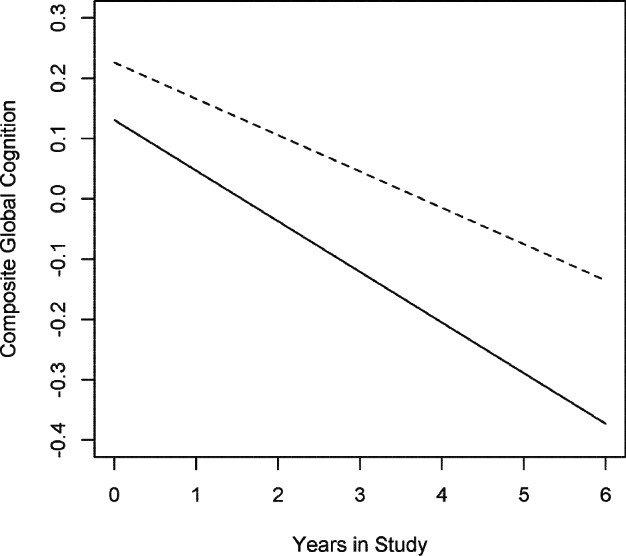
Expected cognitive decline. The model predicted rate of global cognitive decline based on the entire cohort is illustrated for 2 hypothetical average participants with high (Solid line: 90th percentile; kRA = 0.036) and low (Dotted line: 10th percentile; kRA = 0.021) levels of sleep fragmentation.
DISCUSSION
In this group of more than 700 older individuals without dementia, an objective measure of increased sleep fragmentation was associated with a higher risk for the subsequent development of AD. These findings were not accounted for by variation in total daily rest time, total physical activity, the presence of chronic medical conditions, or the use of common medications which can affect sleep. Furthermore, in a separate analysis, a higher level of sleep fragmentation at baseline was associated with a faster decline in cognition, making it unlikely that these findings are due to diagnostic misclassification. These data support a link between sleep fragmentation and the risk of developing AD in older persons, and raise the possibility that interventions to improve sleep continuity may offer a potentially useful strategy for reducing the burden of cognitive impairment and dementia in old age.
Cognitive impairment and dementia are common and are anticipated to become even more so as the global population ages. In 2001, there were 24.3 million people with dementia in the world, and this is anticipated to increase to 81.1 million by 2040.26 As many as 50% of older adults report sleep problems,27 many of which may be amenable to medical treatments or behavioral modification. Given the magnitude of both cognitive impairment and sleep disturbances in older adults, determining whether sleep fragmentation is associated with incident AD and cognitive decline in old age has important public health implications.
Rapid advances in technology have led to the development of devices which can provide objective sleep measures in large numbers of older adults in the community setting which complement traditional laboratory based polysomnographic assessments of sleep. Previous studies have demonstrated associations between actigraphically inferred sleep measures, including nocturnal wake/sleep time11,12 and global metrics of rest-activity variability,10 and cognitive performance in a number of domains. Building on these studies, we developed kRA as an actigraphic metric of sleep fragmentation13 and demonstrated that it is associated with cross-sectional cognitive performance.14 The current study extends this prior cross-sectional work in several important ways. First, a higher level of sleep fragmentation is associated with an increased risk of developing incident AD. This association was robust and was not attenuated by demographic factors, medical comorbidities, or the use of medication known to affect sleep. Furthermore, a higher level of sleep fragmentation is also associated with a more rapid rate of cognitive decline.
Experimental work points to several potential biological links between sleep disruption, cognitive decline, and neurodegeneration. In Drosophila melanogaster, chronic sleep restriction is associated with alterations in presynaptic bouton density.28 In rodent models, sleep disruption has been associated with abnormalities of myelin-related gene expression,29 hippocampal neurogenesis,30 hippocampal synaptic plasticity,7,8,31 susceptibility to Ca2+-mediated excitotoxicity,32 and neuronal susceptibility to ischemic damage.32 Moreover, in a rodent model of AD, chronic pharmacological or environmental sleep disruption was associated with a more rapid accumulation of amyloid beta pathology.5 Further neuroimaging and clinical-autopsy studies may be helpful in identifying the specific neuroanatomical circuits and processes affected by sleep fragmentation and designing mechanistically targeted approaches to enhance their resilience to the deleterious effects of sleep disturbance.
There are several limitations to the current study. First, it was fundamentally an observational study, precluding certainty about the causal direction of any associations seen. A second limitation of our study is that we cannot exclude the possibility that the association between sleep fragmentation and incident AD that we observed may have been mediated by unmeasured medical comorbidities, including sleep apnea which was not formally assessed in the present study. A third limitation of our study is that we studied an all-volunteer cohort that had a high proportion of women, which may limit generalizability to the general population. Fourth, although there is a partial concordance between rest-activity and sleep-wake, this is not complete. While runs of movement are likely to represent wakefulness, and long runs of rest are likely to represent sleep, short runs of rest may represent either quiet wakefulness or light sleep. Notwithstanding the theoretical ambiguity, others have shown good concordance between actigraphic and polysomnographic metrics of sleep time.33 Moreover, in the supplemental material, we provide data obtained from a sleep clinic population that support a relationship between kRA derived from actigraphic data and sleep fragmentation quantified by conventional polysomno-graphic metrics. Further studies in non-sleep clinic populations with simultaneous recording of actigraphy and polysomnography will be helpful in further comparing kRA to conventional polysomnographic metrics of sleep fragmentation.
This study also has several strengths. Objective non-intrusive measurements of sleep fragmentation were obtained over relatively long periods of time (up to 10 days) in participants' usual environments, avoiding confounding by poor recall or misperception (which can be a problem with self-report sleep function) while also avoiding perturbation of subjects' natural sleep behavior by the recording apparatus (a problem with polysomnography) and averaging out the effects of day-to-day variability. Moreover, a large number of older non-demented subjects were assessed annually for up to 6 years with a rigorous, standardized, well characterized, and well-validated cognitive test battery, allowing a high degree of certainty regarding measurement of cognitive status and diagnosis of AD.
This work demonstrates an association between sleep fragmentation, cognitive decline, and the risk of subsequent AD. This raises the possibility that interventions to decrease sleep fragmentation may offer a potentially useful strategy for reducing the risk of AD and slowing cognitive decline in older individuals. Meanwhile, further neuroimaging and neuropatho-logical work may be helpful in identifying the neuroanatomical circuits and processes affected by sleep fragmentation and designing mechanistically targeted approaches to enhance their resilience to the deleterious effects of sleep disturbance.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Lim has served as a consultant for UCB Pharma Inc. Dr. Bennett has received honoraria for non-industry sponsored lectures and has served as a consultant to Danone, Inc., Wilmar Schwabe GmbH & Co., Eli Lilly, Inc., Schlesinger Associates, Geson Lehrman Group. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to the participants and the staff of the Rush Memory and Aging Project and the Rush Alzheimer's Disease Center for this work. The authors also acknowledge the staff in the sleep laboratory at Sunnybrook Health Sciences Centre. The study was supported by NIA grants R01AG17917, R01AG24480, R01NS078009 and R01AG34374, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund. The funding sources had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
SUPPLEMENTAL MATERIAL
METHODS
We examined the relationship between kRA and sleep fragmentation determined by polysomnography in 105 individuals who underwent simultaneous diagnostic polysomnography and actigraphy.
Subjects were recruited from consecutive patients undergoing diagnostic polysomnography in the clinical sleep laboratory at Sunnybrook Health Sciences Centre in Toronto, Canada. Clinical, actigraphic, and polysomnographic characteristics of the subjects are shown in Table S1.
Clinical, Actigraphic, and Polysomnographic Characteristics of the Study Subjects (n = 105)
Actigraphy was recorded using the Actical (Phillip-Respironics, Bend, OR) as described in the main text. The actigraphs were placed on subjects' non-dominant wrists at the commencement of overnight polysomnography and removed at the end of overnight polysomnography. A mean of 7.0 hours (SD 0.6) of recording per subject were obtained. kRA was then calculated for each subject as described in the main text.
Polysomnography was performed and scored in accordance with American Academy of Sleep Medicine guidelines.1 Arousal index, sleep efficiency, and wake time after sleep onset were calculated using standard methods.2
We compared kRA to arousal index, sleep efficiency, wake time after sleep onset, and proportion of total sleep time spent in stage N1 sleep by calculating bivariate Pearson correlation coefficients.
RESULTS
kRA calculated from actigraphic data was strongly correlated with arousal index (R = +0.6, P < 0.0001; Figure S1A) and sleep efficiency (R = -0.6, P < 0.0001; Figure S1B) and moderately correlated with wake time after sleep onset (R = +0.4, P < 0.0001; Figure S1C).
Association between actigraphic and polysomnographic metrics of sleep fragmentation.
REFERENCES
- 1.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–32. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–34. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dramatic Changes in U.S. Aging. 2006. (Accessed at http://www.census.gov/newsroom/releases/archives/aging_population/cv06_36.html.)
- 4.Pallier PN, Maywood ES, Zheng Z, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington's disease. J Neurosci. 2007;27:7869–78. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet MH. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging. 1989;10:21–5. doi: 10.1016/s0197-4580(89)80006-5. [DOI] [PubMed] [Google Scholar]
- 7.Tartar JL, Ward CP, McKenna JT, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tartar JL, McKenna JT, Ward CP, McCarley RW, Strecker RE, Brown RE. Sleep fragmentation reduces hippocampal CA1 pyramidal cell excitability and response to adenosine. Neurosci Lett. 2010;469:1–5. doi: 10.1016/j.neulet.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnet MH. Performance and sleepiness as a function of frequency and placement of sleep disruption. Psychophysiology. 1986;23:263–71. doi: 10.1111/j.1469-8986.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 10.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–35. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim AS, Yu L, Costa MD, et al. Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep. 2011;34:1569–81. doi: 10.5665/sleep.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim AS, Yu L, Costa MD, et al. Increased fragmentation of rest-activity patterns is associated with a characteristic pattern of cognitive impairment in older individuals. Sleep. 2012;35:633–40. doi: 10.5665/sleep.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norman RG, Scott MA, Ayappa I, Walsleben JA, Rapoport DM. Sleep continuity measured by survival curve analysis. Sleep. 2006;29:1625–31. doi: 10.1093/sleep/29.12.1625. [DOI] [PubMed] [Google Scholar]
- 16.Swihart BJ, Caffo B, Bandeen-Roche K, Punjabi NM. Characterizing sleep structure using the hypnogram. J Clin Sleep Med. 2008;4:349–55. [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchi MT, Cash SS, Mietus J, Peng CK, Thomas R. Obstructive sleep apnea alters sleep stage transition dynamics. PLoS One. 2010;5:e11356. doi: 10.1371/journal.pone.0011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bien-ias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 19.Rolls A, Colas D, Adamantidis A, et al. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci U S A. 2011;108:13305–10. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc. 2005;11:400–7. [PubMed] [Google Scholar]
- 21.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–76. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 24.R Development Core Team. R: A language and environment for statistical computing. Vienne, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 25.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–9. doi: 10.1212/WNL.0b013e3182535d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 28.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science. 2011;332:1576–81. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98:1632–45. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- 30.Roman V, Van der Borght K, Leemburg SA, Van der Zee EA, Meerlo P. Sleep restriction by forced activity reduces hippocampal cell proliferation. Brain Res. 2005;1065:53–9. doi: 10.1016/j.brainres.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Arrigoni E, Lu J, Vetrivelan R, Saper CB. Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus. Eur J Neurosci. 2009;30:2112–20. doi: 10.1111/j.1460-9568.2009.07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Souza L, Smaili SS, Ureshino RP, et al. Effect of chronic sleep restriction and aging on calcium signaling and apoptosis in the hippocampus of young and aged animals. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:23–30. doi: 10.1016/j.pnpbp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/ wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Kushida CA, Littner MR, Morgenthaler T, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan SF. 1 ed. Westchester, IL: American Academy of sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical, Actigraphic, and Polysomnographic Characteristics of the Study Subjects (n = 105)
Association between actigraphic and polysomnographic metrics of sleep fragmentation.


