Abstract
Study Objective:
To investigate the structural changes in patients with chronic primary insomnia and the relationships with clinical features of insomnia.
Design:
Statistical parametric mapping 8-based voxel-based morphometry was used to identify differences in regional gray and white matter between patients with chronic primary insomnia and normal controls.
Setting:
University hospital.
Patients and Participants:
Twenty-seven patients and 27 age/sex-matched controls.
Interventions:
Regional differences were compared using two-sample t-tests with age, sex, and intracranial volume as covariates.
Measurements and Results:
The patients were a mean age of 52.3 y and had a mean history of insomnia of 7.6 y. Patients displayed cognitive deficits in attention, frontal/executive function, and nonverbal memory. Patients also displayed significantly reduced gray matter concentrations (GMCs) in dorsolateral prefrontal and pericentral cortices, superior temporal gyrus, and cerebellum and decreased gray matter volumes in medial frontal and middle temporal gyri compared with control patients with the cluster threshold ≥ 50 voxels at the level of uncorrected P < 0.001. Negative correlations were found between GMC of the prefrontal cortex and insomnia severity and the wakefulness after sleep onset, and between GMC of pericentral cortex and sleep latencies. None of the findings continued to be significant after correction for multiple comparisons.
Conclusions:
We found gray matter deficits in multiple brain regions including bilateral frontal lobes in patients with psychophysiologic insomnia. Gray matter deficit of the pericentral and lateral temporal areas may be associated with the difficulties in sleep initiation and maintenance. It is still unclear whether gray matter reductions are a preexisting abnormality or a consequence of insomnia.
Citation:
Joo EY; Noh HJ; Kim JS; Koo DL; Kim D; Hwang KJ; Kim JY; Kim ST; Kim MR; Hong SB. Brain gray matter deficits in patients with chronic primary insomnia. SLEEP 2013;36(7):999-1007.
Keywords: Brain, gray matter concentration, insomnia, voxel-based morphometry
INTRODUCTION
Chronic primary insomnia (PI) is a disorder whereby patients experience chronically disturbed sleep and sleep loss, non-refreshing sleep, and heightened arousal in bed; these conditions cannot be attributed to a comorbid medical or psychiatric disorder.1 PI may accompany neurocognitive problems, and deficits in attention and working memory.2–6
Many neuroimaging studies have been performed to investigate the structural or functional derangement in brains of patients with chronic insomnia. Patients suffering from PI displayed a relative increase of global cerebral metabolic rate for glucose utilization during sleep and while awake, compared with normal controls (NCs) in a study that used positron emission tomography.7 These findings were consistent with the suggestion that chronic insomnia is associated with enhanced brain metabolism and that a hyperarousal state during sleep might contribute to the occurrence of chronic insomnia.8 However, another study that used single-photon emission computed tomography yielded contrary findings, with patients with PI displaying a significant decrease in regional cerebral blood flow in frontal, parietal, and occipital cortices and basal ganglia during sleep.9 Despite their contrasting findings, the functional imaging studies both provided evidence that brain regions with altered function explain the pathophysiology and the clinical symptoms accompanied by chronic insomnia. However, the relatively few patients involved in these two studies could be a limitation.10 Several magnetic resonance imaging (MRI) studies have been conducted with patients with PI. They too yielded controversial results. Although one study reported smaller hippocampal volumes in patients with PI compared with NCs,11 the other study did not reveal any difference in volume.12
Voxel-based morphometry (VBM) is an automated technique that has grown in popularity since its introduction,13,14 reflecting the fact that it provides a comprehensive assessment of anatomical differences throughout the brain.14 An earlier optimized VBM study showed that patients with PI had gray matter (GM) deficits in the left orbitofrontal cortex and precuneus compared with NCs.15 However, optimized VBM does have a circularity problem15 because the registration requires an initial tissue classification and vice versa.16 SPM8-based VBM has been refined through the development of a registration method termed Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL), which is a more sensitive means of identifying differences of GM and white matter (WM).16 Thus, SPM8-based VBM provides more accurate localization than optimized VBM in terms of supporting precise intersubject alignment and segmentation performance throughout the iterative unified model.16,17
The current study used SPM8-based VBM to evaluate GM and WM changes in patients with PI and to investigate the relationships between structural brain changes and characteristic clinical features as well as cognitive function.
METHODS
Patients
Forty patients who had sleep onset and/or maintenance insomnia were recruited from the sleep disorder clinic of the university hospital (Samsung Seoul Hospital) located in Seoul, South Korea. Inclusion criteria were an age of 40-70 y, conformity to the definition of PI by the International Classification of Sleep Disorders-2 (ICSD-2),18 and a duration of insomnia ≥ 1 y.
NCs who were age-, sex-, and education status-matched to the patients with PI were recruited through an advertisement in a local community. The history of medical and sleep disorders was evaluated with a clinical interview and the history of psychiatric disorders was evaluated with the Structured Clinical Interview for DSM-IV in the patients with PI and the healthy control patients. All patients completed a sleep diary for at least 2 weeks and a number of self-questionnaires, such as the Pittsburgh Sleep Quality Index (PSQI), Beck Depression Inventory-II (BDI-II), Insomnia Severity Index (ISI), and Epworth Sleepiness Scale (ESS). In addition, all patients completed a physical examination, blood tests that included complete blood counts, chemistry battery, and serum electrolytes and toxic screening, overnight polysomnography (PSG), brain MRI, and neuropsychological tests. PSG was performed within 2 weeks after the patient was first enrolled in this study. PSG was started between 10:30pm and 12:00pm according to the usual bedtime of the patient and ended when the patient awoke in the morning. The brain MRI and neuropsychological test were performed within 2 weeks after the PSG.
Exclusion criteria for study patients and controls were: (1) a total sleep time < 7 h on history only for NCs; (2) obstructive sleep apnea syndrome (OSA, apnea-hypopnea index greater than 5/h); (3) moderate to severe periodic limb movement during sleep (PLMS, total PLM index > 25/h); (4) abnormal sleep-wake rhythms; (5) hypertension, diabetes, heart, or respiratory diseases; (6) history of cerebrovascular disease; (7) other neurological (neurodegenerative diseases, epilepsy, head injury) or psychiatric diseases (psychosis, current depression); (8) alcohol or illicit drug abuse or current intake of psychoactive medications; and (9) a structural lesion on brain MRI. To avoid the possible effect of medications on the results, three patients who had a history of taking antidepressants, anxiolytic agents, or sleeping pills were excluded. Five patients were excluded due to definite sleep disorders confirmed by PSG; four with moderate to severe OSA (apnea-hypopnea index, 18.3-40.2/h) and 1 with periodic limb movement disorder (total index 50.1/h, movement arousal index 10.5/h). Seven subjects (five with PI and two NCs) who showed diffuse brain atrophy or lacunar infarctions on brain MRIs were also excluded. Finally, 27 patients with PI and 27 NCs were included in the study. Informed consent was obtained from all participants for all tests in the protocol, and the institutional review board of our hospital authorized the study protocol and design. Nineteen patients with PI and 17 control patients were commonly involved in the previous study.12
Overnight PSG
The day before the sleep studies, patients were asked to refrain from drinking alcohol or caffeinated beverages. Sleep studies were recorded using a Remlogic device (Embla Systems, Denver, CO, USA). A night PSG was performed using a four-channel electroencephalogram (EEG, C3/A2; C4/A1; O1/ A2; O2/A1); a four-channel electrooculogram; an electromyo-gram of the submental, intercostal, and anterior tibialis muscles; and an electrocardiogram with surface electrodes. A thermistor for monitoring nasal airflow, a nasal air pressure monitor, an oximeter for measuring oxygen saturation, piezoelectric bands for determining thoracic and abdominal wall motion, and a body position sensor were attached to each patient. Patients were recorded on videotape using an infrared video camera and were continuously observed by a PSG technician. Sleep architecture was scored in 30-sec epochs, and sleep staging was interpreted according to previously published criteria.19 Apneas and hypopneas were defined by previously established criteria. Obstructive apnea was defined as a reduction in airflow greater than 90% that lasted at least 10 sec, during which there was evidence of a persistent respiratory effort. A central apnea was defined as a reduction in airflow of more than 90% that lasted at least 10 sec, during which there was no evidence of respiratory effort. A hypopnea was defined as a reduction in airflow by a reduction of airflow by 30% for more than 10 sec, which was accompanied by a 4% or greater oxygen desaturation. Arousals were classified as breathing-related arousals (occurring within 3 sec following apnea, hypopnea, or snoring) and other type of arousals (spontaneous arousal, periodic limb movements-associated arousals).20
Magnetic Resonance Imaging
MRI was performed using a GE Signa 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI, USA). All patients underwent spoiled gradient echo (SPGR), T1-weighted, T2-weighted, and fluid attenuated inversion recovery (FLAIR) imaging protocols. Coronal SPGR MRI data were obtained using the following scanning variables: 1.6-mm thickness, no gap, 124 slices, repetition time/echo time (TR/TE) = 30/7 ms, flip angle (FA) = 45°, number of excitations (NEX) = 1, matrix = 256 × 192, and field of view (FOV) = 22 × 22 cm. The voxel dimension of SPRG MR images was 0.86 × 0.86 × 1.6 mm. Oblique coronal FLAIR MRI was performed using a 4.0-mm slice thickness, 1.0-mm gap, 32 slices, TR/TE = 10,002/127.5 msec, 1 NEX, matrix = 256 × 192, and FOV = 20 × 20 cm. Oblique coronal T2-weighted magnetic resonance images were obtained with a 3.0-mm slice thickness, 0.3-mm gap, 56 slices, TR/TE = 5,300/99 msec, FA = 90°, 3 NEX, matrix = 256 × 192, and FOV = 20 × 20 cm.
Voxel-Based Morphometry
All the VBM preprocessing and statistical analyses were performed using the SPM8 software (Welcome Department of Cognitive Neurology, Institute of Neurology, University College London, UK; http://www.fil.ion.ucl.ac.uk/spm) on Matlab 2008a (Mathworks, Sherborn, MA, USA) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html). The original images were reoriented into sagittal alignment. Next, the images were simultaneously corrected for bias-field inhomogeneity, registered by linear (12-parameter affine) and nonlinear transformations, and segmented into GM, WM, and cerebrospinal fluid by an integrated one-pass procedure (the unified segmentation). The method was further refined by accounting for partial volume effects,16 by applying adaptive maximum a posteriori estimations,21 and a hidden Markov random field model22 as previously described.23 Thus, the model could avoid the circularity problem of the optimized VBM procedure.24 To identify both the regional differences in the volume of a particular tissue that requires information about absolute volumes to be preserved and the relative concentration of GM or WM structures in the spatially normalized images with the deformation fields within 1.5 × 1.5 × 1.5 voxel sizes, obtained by the DARTEL suite of tools that allows a highly accurate intersubject registration of brain images, we applied a further optional preprocessing step, which is usually referred to as modulation. It can be used to detect differences GM or WM volumes, whereas the unmodulated GM and WM images allow identify differences of GM and WM concentrations. To achieve the modulated images, GM and WM images were imported into DARTEL, and nonlinear deformations for their optimal alignment were estimated by alternating between building a template and registering the tissue class images with the template. Subsequently, Jacobian-scaled (modulated) warped tissue class images were created. After finishing the preprocessing, we checked the data quality using the modules ‘display one slice for all images’ and ‘check sample homogeneity using covariance.’ Finally, the GM and WM partitions were smoothed with an 8-mm full-width at half-maximum Gaussian kernel and were used for statistical analysis.
Neuropsychological Assessments
Patients underwent a battery of neuropsychological tests and an individual standardized intelligence test. Neuropsychological tests consisted of the Korean California Verbal Test (K-CVLT) and the Rey Complex Figure Test (RCFT) for memory function, the digit span tests from the Wechsler Memory Scale-Revised, the Corsi Block tapping tests (forward and backward), the Trail Making Tests A and B, the digit symbol test for attention and working memory, the Stroop test and the Controlled Oral Word Association Test (COWAT) for executive function, and the Korean Boston naming test (K-BNT) for verbal function. It took approximately 2.5 h to complete all the tests. Detailed information and test procedures were described previously.12
Statistical Analyses
The demographics and PSG data between the patients with PI and NCs were compared using the Mann-Whitney U-test. Two-sample t-tests were used to compare the GM between patients with PI and NCs using analysis of covariance, with age and sex as covariates in SPM8. Each patient's intracranial volume (ICV) measurement was used to correct variations in individual head size and to obtain more focused results. The statistical threshold was corrected for multiple comparisons with extent threshold of 100 voxels and more than 50 voxels, respectively.
The correlation analyses were performed by multiple regressions. Variables were demographics (age, education years, insomnia duration, body mass index, and sleep questionnaire scores), PSG and neuropsychological parameters, and a self-reported psychometric questionnaire (SCL-90R). There was no a priori hypothesis for the correlations between GM and the previously mentioned parameters and no significant results were reported after correction for multiple comparisons in patients with PI.15 To test the hypotheses of regional specific covariates effects, the estimates were compared using two linear contrasts (positive or negative correlation). Covariates were age, sex, and ICV.
All results are presented as Montreal Neurological Institute (MNI) coordinates. The MNI coordinates for relevant anatomical names of the selected voxel or cluster(s) in databases (xBrain, Google Scholar and Pubmed) were obtained by an xjView toolbox25 compatible with SPM8.
RESULTS
Clinical Characteristics
The details of the demographics and PSG parameters are summarized in Table 1. All subjects were right-handed. The mean age of the patients with PI (52.3 y) and NCs (51.7 y) was not statistically significant (P = 0.478). The sex ratio (female 92.5% vs. 85%, same respective order) was also not significantly different (P = 0.646). Subjective sleep quality was determined by the PSQI, which was assessed during the previous 2 weeks of the study; the mean PSQI was significantly higher in patients with PI than in the NCs.
Table 1.
Characteristics of subjects
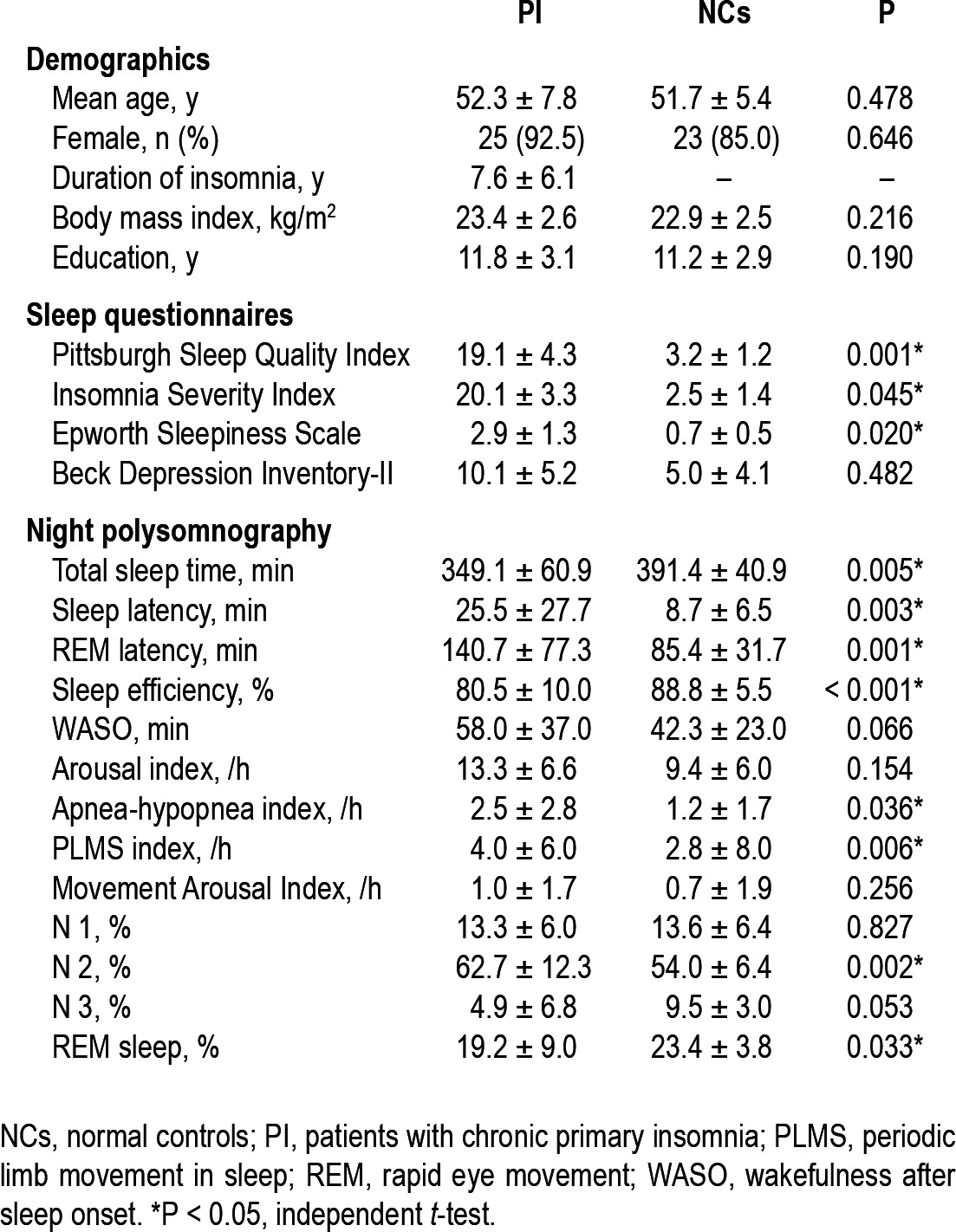
Patients with PI reported severe insomnia symptoms (mean ISI, 20.1). Mean score of BDI-II seemed to be higher, albeit nonsignificantly, in patients with PI (10.1) than in NCs (5.0). Although patients with PI had shorter total sleep time than NCs (349.1 vs. 391.4 min, respectively, P = 0.005), much longer sleep latency (25.5 vs. 8.7 min, respectively, P = 0.003), and lower sleep efficiency (80.5% vs. 88.8%, respectively, P < 0.001), arousal index of the patients with PI was not definitely higher than NCs (13.3 vs. 9.4/h, respectively, P = 0.154), and wake after sleep onset (WASO) was not different (58.0 vs. 42.3 min, respectively, P = 0.066).
Neuropsychological Tests
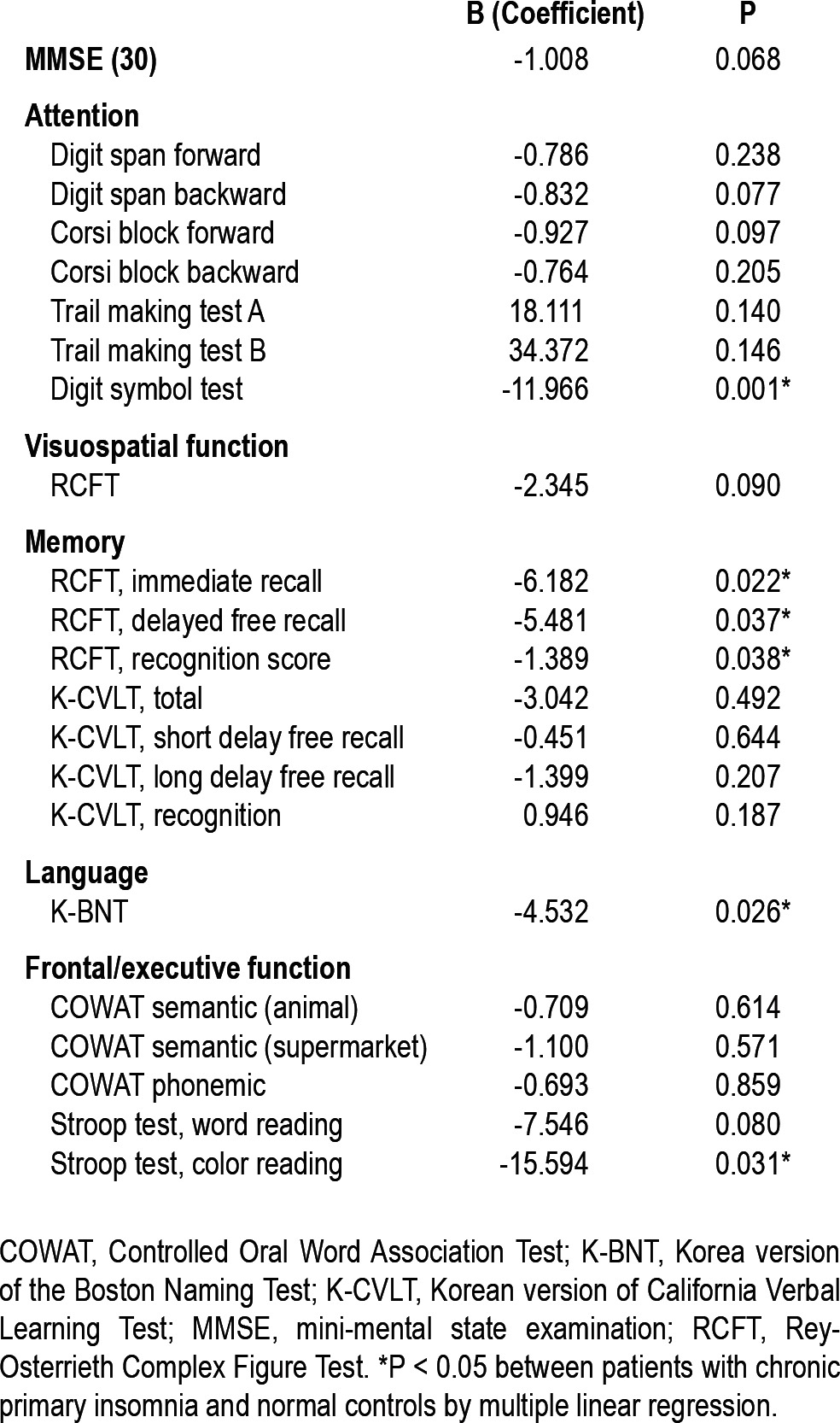
Multiple regression analyses were performed after adjusting for age, sex, and years of education to compare cognition between the patients with PI and NCs. The patients with PI displayed significantly lower scores on tests of attention and frontal/executive function and showed impaired nonverbal memory and language function compared with NCs (Table 2).
Table 2.
Comparison of neuropsychologic results between patients with chronic primary insomnia and normal controls
Group Differences of Regional GM and WM
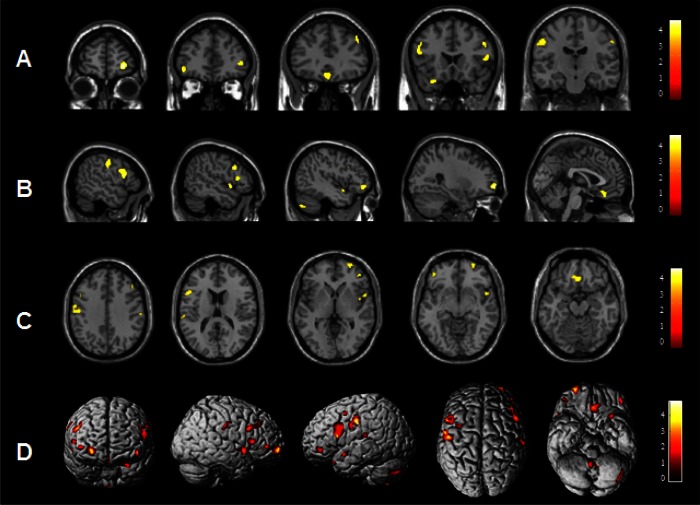
Patients with PI showed significant reduction of GMC in left or right dorsolateral prefrontal cortices (right superior frontal gyrus, left middle frontal gyrus, and bilateral inferior frontal gyri), pericentral cortex (left precentral gyrus and bilateral post-central gyri), and superior temporal gyrus compared to NCs with the cluster threshold of 100 voxels at the level of uncorrected P < 0.001. When the cluster threshold more than 50 voxels was applied, GMC decreased in the larger brain areas with the same anatomical coordinates as the results of the cluster threshold of 100 voxels and decreased additionally in medial frontal gyri and cerebellum. Table 3 and Figure 1 showed the results with the cluster threshold more than 50 voxels.
Table 3.
Brain regions showing significant decrease of gray matter concentration in patients with chronic primary insomnia compared with normal controls
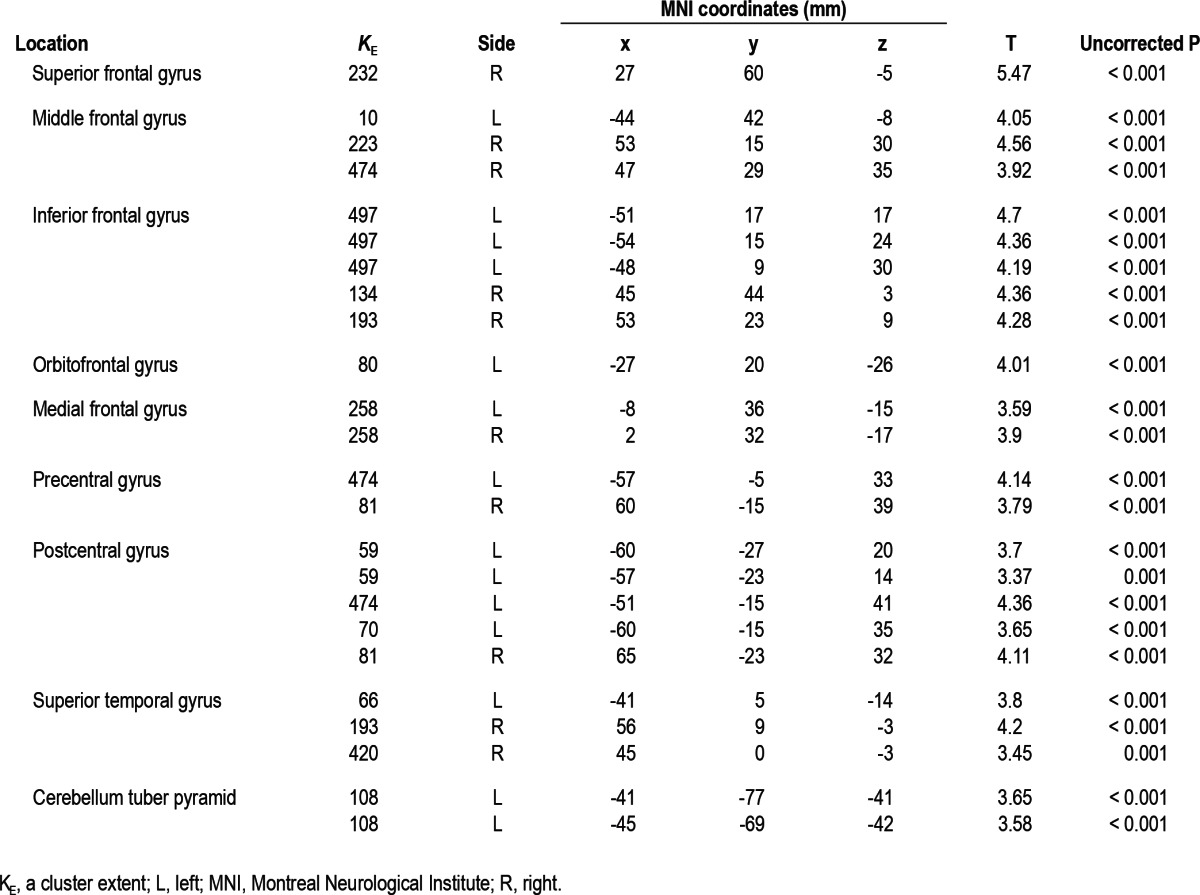
Figure 1.
A statistical brain map showing a decrease in gray matter concentration (GMC) in patients with chronic primary insomnia (PI) compared with normal controls (NCs). GMCs were significantly decreased in PI compared with NCs (uncorrected at P < 0.001, two-sample t-test) in the following brain structures: right superior frontal gyrus, left orbitofrontal gyrus, right inferior frontal gyrus, right medial frontal gyrus, right middle frontal gyrus, right precentral gyrus, left postcentral gyrus (A) coronal; left postcentral gyrus, left inferior frontal gyrus, right middle frontal gyrus, right inferior frontal gyrus, right superior temporal gyrus, left middle frontal gyrus, left cerebellum, right superior frontal gyrus, left medial frontal gyus (B) sagittal; left postcentral gyrus, right middle frontal gyrus, right precentral gyrus, left inferior frontal gyrus, right superior frontal gyrus, right inferior frontal gyrus, right superior temporal gyrus, left middle frontal gyrus, left medial frontal gyrus (C) axial. The overall areas with reduced GMCs were shown in a three-dimensional brain surface rendering view (D). The results were displayed with the cluster threshold more than 50 voxels. Scales in the color bar are t scores. The left side of the images represents the left hemisphere of the brain.
In addition, no brain region revealed significant GMV reduction at the cluster threshold of 100 voxels. However, in the analysis with the cluster threshold more than 50 voxels, GMV was decreased in left medial frontal gyrus where GMC reduced (x: y: z = -2, 29, -15, KE, cluster extent = 195) and in left middle temporal gyrus (x: y: z = -51, -15, 38, KE = 41) at the level of uncorrected P < 0.001.
However, none of the brain regions showing either GMC or GMV decrease in patients with PI continued to be significant after applying correction for multiple comparisons.
There were no brain regions showing increased GMC or GMV and insignificant WM changes in patients with PI compared with NCs in neither cluster thresholds.
Regression Analyses of GM Concentrations with Other Clinical Variables in Patients with PI
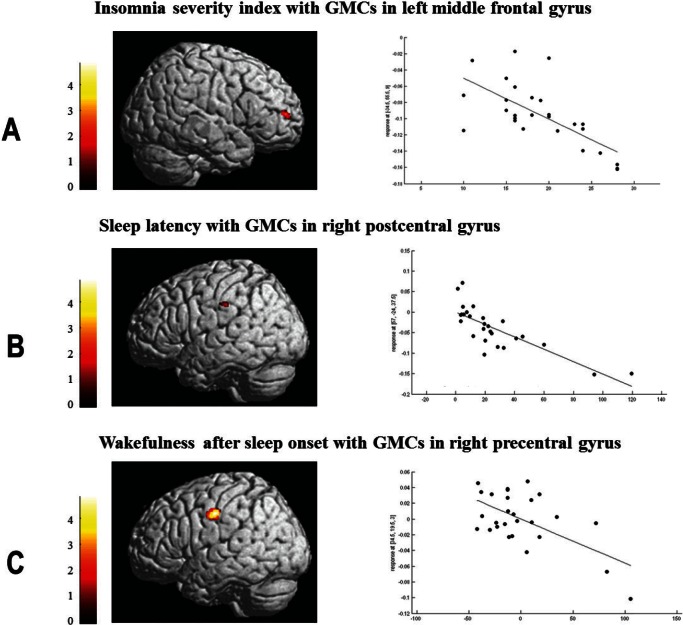
GMCs in the left middle frontal gyrus were negatively correlated with ISI and WASO by multiple regressions. Prolonged sleep latency was related to the reduced GMCs in the right post-central gyrus and longer WASO was associated with the decrease of GMCs in the right precentral gyrus (Table 4 and Figure 2). Demographics, other PSG parameters except sleep latency and WASO, neuropsychological scores, and SCL-90R scores did not show significant correlations with GM of PI patients.
Table 4.
Multiple regression analyses between gray matter deficits and characteristics of patients with chronic primary insomnia
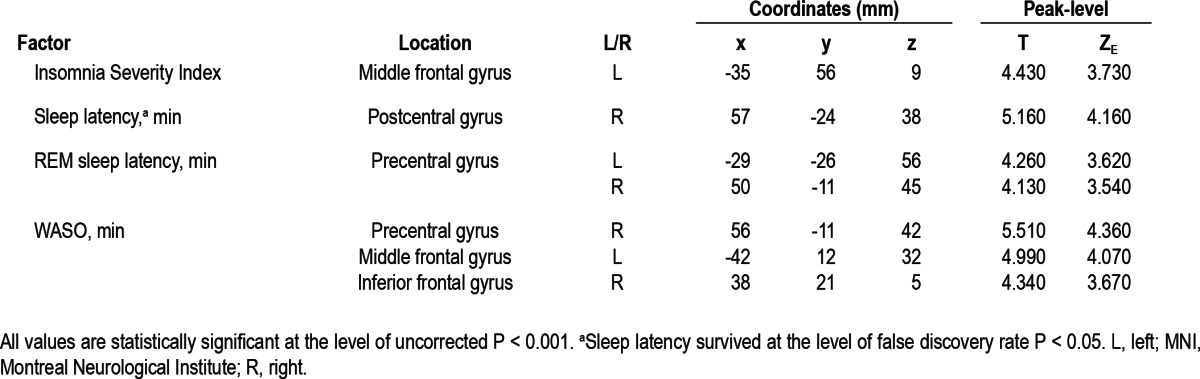
Figure 2.
Significant brain regions showing negative correlation with characteristics of patients with chronic primary insomnia. With the controlled confounders of age, sex, and intracranial volume, a negative correlation was observed between gray matter concentrations (GMCs) in the left middle frontal gyrus and the Insomnia Severity Index (r = -0.613, P = 0.014) (A), between GMCs in the right postcentral gyrus and the sleep latency (r = -0.411, P = 0.019) (B), and between GMCs in the right precentral gyrus and the wakefulness after sleep onset (r = -0.443, P = 0.018) (C).
In NCs, there was no significant correlation between GM and data.
DISCUSSION
This study explored the alterations of GM in the brains of patients with PI using a unified segmentation model and DARTEL method of VBM analysis. Brain regions with GM deficit may suggest the relationship with clinical symptoms and cognitive dysfunctions in patients with PI.
GM Deficits in the Dorsolateral Prefrontal Cortex in Patients with PI
The dorsolateral prefrontal cortex is involved in executive functions.26 Task assessments of shifting attention and working memory have revealed deficits among individuals with chronic insomnia more often than not.2–6 When a task of sustained attention requires a response choice, individuals with chronic insomnia tend to display consistent attention deficits.2–4,6,27,28
We observed a significant reduction of GMCs in the dorso-lateral prefrontal cortex (bilateral superior, middle, and inferior frontal gyri) in the patients with PI. These patients showed significantly lower scores in the digit symbol test, Stroop test, and color reading test, which indicated that the patients with PI had impaired attention and frontal lobe function compared to NCs even after controlling for age, sex, and years of education. Moreover, the ISI and WASO of the patients with PI were negatively correlated with GMCs in the middle and/or inferior frontal gyri. A first published VBM study in patients with PI reported that there was no correlation between insomnia severity and duration and GM deficit in prefrontal cortex.15 There was no prior hypothesis regarding correlation between regional GM deficit and clinical and sleep data in patients with PI. However, these preliminary results showing significantly negative correlation may support the possible relationship of the prefrontal cortex with sleep quality.
Nonverbal memory was significantly impaired in patients with PI compared with NCs. The data from numerous studies support the role of sleep for memory registration and consolidation. Visual declarative memory performance has been significantly associated with total sleep time, sleep efficiency, duration of nonrapid eye movement sleep (NREM) and number of NREM-rapid eye movement sleep cycles.29,30 We observed that Rey-Osterrieth Complex Figure Test scores, which reflect nonverbal memory function, were significantly associated with total sleep time (r = 0.765, P = 0.012) and sleep efficiency (r = 0.691, P = 0.038) in patients with PI. This suggests that a lack of sleep and poor sleep quality may cause non-verbal memory dysfunction.
GM deficits in the dorsolateral prefrontal cortex may provide the anatomical substrates to be related to attention deficit, frontal lobe dysfunction, and nonverbal memory decline of patients with PI, which might be associated with poorer sleep.
GM Deficits in Precentral and Postcentral Gyri in Patients with PI
Although no structural imaging study presented the GM changes in the precentral gyrus in individuals with chronic insomniacs, functional alterations related to precentral areas had been reported. An electroencephalography study using high-density source mapping in individuals with chronic insomnia revealed hypoactivation of the premotor region, ventromedial prefrontal cortex, and anterior cingulate.31 Individuals with chronic insomnia had disturbed intracortical excitability, as evidenced by a larger motor-evoked potential size to stimulation of precentral area and by an attenuated intracranial facilitation in a transcranial magnetic stimulation study.32 This abnormal excitability persisted despite sleep therapy that effectively improved sleep quality,32 which implied that individuals with chronic insomnia might have accompanying functional and structural abnormalities in the pre-central area. In the current study we found that reduced GMC in the right precentral gyrus was significantly related to longer duration of WASO. This finding suggests that disturbed nocturnal sleep might have a harmful effect on the precentral area.
The postcentral gyrus is the main receptive region for external stimuli as the location of the primary somatosensory cortex. Individuals with chronic insomnia have difficulty disengaging from the awakening process at sleep onset.33 Recently, the post-central gyrus was implicated with the default mode network,34 which are functional brain hubs showing coupled signal fluctuations in the absence of external stimuli during restful waking and sleep.35 Our patients had more prolonged sleep latencies on PSG than NCs, and sleep latency was negatively correlated with GMCs in the right postcentral gyrus. These observations suggest that alterations of GM in the postcentral gyrus in patients with PI are related to difficulty in sleep initiation of patients due to a reduced capacity to disengage from external information processing.
GM Deficits in Lateral Temporal Cortices in Patients with PI
The superior temporal cortex contains the primary auditory area, which is responsible for sound processing. Normal activation of the auditory cortex is decreased to help maintain sleep in response to external stimuli.36 However, individuals with chronic insomnia are hypervigilant and ruminative according to neurocognitive models,37,38 and show an excessive hyper-arousal of the central nervous system throughout the night in electrophysiological studies.39 We found the reduction of GMC in the left and right superior temporal gyri in patients with PI, which might suggest the altered processing of external stimuli in the auditory cortex, which results in a hyperarousal state during sleep and related sleep onset, and the ensuing maintenance of insomnia.
Comparison with a Previous VBM Study
Recently Altena et al.15 reported the first VBM results in patients with chronic primary insomnia.15 They reported smaller GM volumes in the left orbitofrontal and parietal cortices in insomnia patients and the negative correlation between orbito-frontal GM and insomnia severity, without any correlation with mood ratings.15 We observed similar GM changes in the orbito-frontal gyrus, not in the precuneus. Orbitofrontal involvement in sleep vulnerability appears a robust finding.40 The ISI scores of our patients with PI were inversely correlated to GM in the dorsolateral prefrontal cortices. Several factors may be responsible for these partial discrepancies. The subject characteristics between the current and prior studies could be different. Volume reduction in the orbitofrontal cortex has been related to major depression41 and advanced age.42 In the study of Altena et al.,15 in only one patient the diagnosis of major depression with a prior history of antidepressant medication use was made. We also excluded patients who had a history of any kind of psychiatric disease and use of antidepressants, hypnotic agents, or anxiolytic agents to avoid chronic mood or medication effects on the brain. Mean age of patients in their study15 was older (60.3 y) than our patients with PI (52.3 y). Aging also may have influenced the results. Another factor may be difference in analysis methods between the two VBM studies. They used optimized VBM analysis,15 which is known to have a circularity problem.17 To avoid the problem of registration,24 we adopted SPM8-based VBM embedded in a newly developed registration method (DARTEL), which is more sensitive to identify differences of GM and WM.16 Different methodologies may lead to different results in neuroimaging studies.
Previous VBM studies by Altena et al.15 demonstrated the areas showing only GMV reduction in patients with chronic insomnia. In the current study, we observed the decreases in both GMC and GMV and brain regions showing volume changes were much smaller than that with concentration reduction. In optimized VBM, GMC in the local unit (i.e., voxel) can be transformed to the GMV through the commonly known “modulation” process while accounting for regional stretching and compression occurring during coregistration.17 The GMC is generally interpreted as GM tissue density relative to WM whereas GMV as absolute volume regardless of WM.43 Quantifying these two measures does not necessarily lead to overlapped results due to their different underlying properties and rather complements aspects of brain structural alterations.17 According to previous neuroimaging studies,43–46 we therefore reported the results of both metrics.
It should be noted that the current study has several limitations. We presented the results of group differences and correlation analyses with a statistical threshold of P < 0.001 uncorrected level. When multiple comparison analyses had been performed using family-wise error or false discovery rate, no brain regions remained significant. Sleep latencies on PSG showing negative correlation with GMC in patients with PI had survived after correction of multiple comparisons. Because there was no a priori hypothesis for the correlations between GM and the demographics, PSG parameters, and neuropsychological factors, we performed regression analyses between them. To the best of our knowledge, insomnia severity was reported to be negatively correlated with left orbitofrontal GM volume without correction.15
Although GM deficits in our patients with PI might not be large enough to survive in the analyses of multiple comparison, it seems to be clear that structural changes of brain may be associated with the chronic insomnia. As the other study15 pointed out, GM deficit might precede the development of chronic insomnia because GM reductions were not correlated with insomnia duration, as in our study. GM reduction as well as a lack of sleep or poorer sleep quality in the patients with PI might be responsible for clinical features and cognitive dysfunction in chronic insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Drs. Joo and Noh contributed equally to the preparation of the manuscript. This study was supported by a Grant of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (No. A110097), and by the Global Frontier R&D Program on Human-centered Interaction for Coexistence funded by the National Research Foundation of Korea grant funded by the Korean Government (MEST) (NRF-M1AXA003-2011-0031688).
Footnotes
A commentary on this article appears in this issue on page 965.
REFERENCES
- 1.Roth T, Roehrs T, Pies R. Insomnia: pathophysiology and implications for treatment. Sleep Med Rev. 2007;11:71–9. doi: 10.1016/j.smrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Edinger JD, Means MK, Carney CE, Krystal AD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altena E, Van Der Werf YD, Strijers RL, Van Someren EJ. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. J Sleep Res. 2008;17:335–43. doi: 10.1111/j.1365-2869.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 4.Varkevisser M, Kerkhof GA. Chronic insomnia and performance in a 24-h constant routine study. J Sleep Res. 2005;14:49–59. doi: 10.1111/j.1365-2869.2004.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Edinger JD, Glenn DM, Bastian LA, Marsh GR. Slow-wave sleep and waking cognitive performance II: Findings among middle-aged adults with and without insomnia complaints. Physiol Behav. 2000;70:127–34. doi: 10.1016/s0031-9384(00)00238-9. [DOI] [PubMed] [Google Scholar]
- 6.Hauri PJ. Cognitive deficits in insomnia patients. Acta Neurol Belg. 1997;97:113–7. [PubMed] [Google Scholar]
- 7.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 9.Smith MT, Perlis ML, Chengazi VU, et al. Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep. 2002;25:325–35. [PubMed] [Google Scholar]
- 10.Dang-Vu TT, Desseilles M, Petit D, Mazza S, Montplaisir J, Maquet P. Neuroimaging in sleep medicine. Sleep Med. 2007;8:349–72. doi: 10.1016/j.sleep.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Riemann D, Voderholzer U, Spiegelhalder K, et al. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–8. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noh HJ, Joo EY, Kim ST, et al. The relationship betwen hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol. 2012;8:130–8. doi: 10.3988/jcn.2012.8.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2:244–52. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- 14.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 15.Altena E, Vrenken H, Van Der Werf YD, van den Heuvel OA, Van Some-ren EJ. Reduced orbitofrontal and parietal gray matter in chronic insomnia: a voxel-based morphometric study. Biol Psychiatry. 2010;67:182–5. doi: 10.1016/j.biopsych.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 18.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 19.The International Classification of Sleep Disorders. Diagnostic and Coding Manual. Rochester, MN: American Sleep Disorders Association; 1997. [Google Scholar]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Bethesda, MD: Neurological Information Network; 1968. [Google Scholar]
- 21.Gholipour A, Estroff JA, Sahin M, Prabhu SP, Warfield SK. Maximum a posteriori estimation of isotropic high-resolution volumetric MRI from orthogonal thick-slice scans. Med Image Comput Comput Assist Interv. 2010;13:109–16. doi: 10.1007/978-3-642-15745-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–86. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 23.Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging. 2005;24:1548–65. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- 24.Gaser C. Partial volume segmentation with adaptive maximum a posteriori (MAP) approach. Neuroimage. 2009;47:S121. [Google Scholar]
- 25.Cui X, Li J. xjView — a viewing program for SPM. 2007. Available at: http://people.hnl.bcm.tmc.edu/cuixu/xjView.
- 26.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 27.Sugerman JL, Stern JA, Walsh JK. Daytime alertness in subjective and objective insomnia: some preliminary findings. Biol Psychiatry. 1985;20:741–50. doi: 10.1016/0006-3223(85)90153-2. [DOI] [PubMed] [Google Scholar]
- 28.Schneider-Helmert D. Twenty-four-hour sleep-wake function and personality patterns in chronic insomniacs and healthy controls. Sleep. 1987;10:452–62. doi: 10.1093/sleep/10.5.452. [DOI] [PubMed] [Google Scholar]
- 29.Maquet P, Smith C, Stickgold R. Sleep and brain plasticity. Oxford: Oxford University Press; 2003. [Google Scholar]
- 30.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 31.Szelenberger W, Niemcewicz S. Event-related current density in primary insomnia. Acta Neurobiol Exp (Wars) 2001;61:299–308. doi: 10.55782/ane-2001-1405. [DOI] [PubMed] [Google Scholar]
- 32.van der Werf YD, Altena E, van Dijk KD, et al. Is disturbed intracortical excitability a stable trait of chronic insomnia? A study using transcranial magnetic stimulation before and after multimodal sleep therapy. Biol Psychiatry. 2010;68:950–5. doi: 10.1016/j.biopsych.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Bastien CH, St-Jean G, Morin CM, Turcotte I, Carrier J. Chronic psycho-physiological insomnia: hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep. 2008;31:887–98. doi: 10.1093/sleep/31.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–13. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukunaga M, Horovitz SG, van Gelderen P, et al. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging. 2006;24:979–92. doi: 10.1016/j.mri.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 36.Czisch M, Wetter TC, Kaufmann C, Pollmacher T, Holsboer F, Auer DP. Altered processing of acoustic stimuli during sleep: reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuroimage. 2002;16:251–8. doi: 10.1006/nimg.2002.1071. [DOI] [PubMed] [Google Scholar]
- 37.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 38.Morin CM. Insomnia: psychological assessment and management. New York: Guilford Press; 1993. [Google Scholar]
- 39.Merica H, Blois R, Gaillard JM. Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci. 1998;10:1826–34. doi: 10.1046/j.1460-9568.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- 40.Stoffers D, Moens S, Benjamins J, van Tol M-J, Penninx BWJH, Veltman DJ, et al. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bremner JD, Vythilingam M, Vermetten E, et al. Reduced volume of orbitofrontal cortex in major depression. Biol Psychiatry. 2002;51:273–9. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- 42.Resnick SM, Lamar M, Driscoll I. Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Ann N Y Acad Sci. 2007;1121:562–75. doi: 10.1196/annals.1401.027. [DOI] [PubMed] [Google Scholar]
- 43.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol. 2012;121:649–58. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasic N, Walter H, Höse A, Wolf RC. Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord. 2008;109:107–16. doi: 10.1016/j.jad.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–62. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 46.Yamada M, Hirao K, Namiki C, et al. Social cognition and frontal lobe pathology in schizophrenia: a voxel-based morphometric study. Neuroimage. 2007;35:292–8. doi: 10.1016/j.neuroimage.2006.10.046. [DOI] [PubMed] [Google Scholar]