Abstract
Sleep apnea is a common chronic disease that is associated with coronary heart disease, stroke, heart failure and mortality, although the ability of sleep apnea treatment to reduce cardiovascular morbidity and mortality has not been demonstrated. In contrast to patients seeking treatment in sleep disorders centers, as many as half of individuals with moderate to severe sleep apnea in the general population do not report excessive sleepiness; however, if treatment of sleep apnea were shown to reduce cardiovascular disease risk, this would provide a strong rationale for treatment of sleep apnea even in the absence of daytime sleepiness. This article summarizes the status of clinical trials evaluating the potential cardiovascular benefits of sleep apnea treatment and discusses the challenges of conducting such trials, and introduces the International Collaboration of Sleep Apnea Cardiovascular Trialists (INCOSACT), a clinical research collaboration formed to foster cardiovascular sleep research.
Citation:
Gottlieb DJ; Craig SE; Lorenzi-Filho G; Heeley E; Redline S; McEvoy RD; Durán-Cantolla J. Sleep apnea cardiovascular clinical trials— current status and steps forward: the International Collaboration of Sleep Apnea Cardiovascular Trialists. SLEEP 2013;36(7):975-980.
Keywords: Cardiovascular disease, clinical trials, sleep apnea
Sleep apnea is among the most common chronic diseases of adults. Despite differences in methodology and disease definition, the prevalence of sleep apnea is consistently high across different cultures and populations. In a representative U.S. adult population sample from the state of Wisconsin, the prevalence of sleep apnea was estimated to be 9% of women and 24% of men aged 30-60 years,1 with even higher prevalence reported in the elderly. A high prevalence of sleep apnea has been reported in studies from around the world.2–5 By the 1980s, an association of severe sleep apnea with mortality and cardiovascular disease was recognized. Over the subsequent two decades, a large body of observational research from community-based epidemiologic studies has established an association of sleep apnea with increased incidence of coronary heart disease, stroke, heart failure, and mortality after adjusting for established cardiovascular disease risk factors.6–11 Both human and animal physiologic studies have identified mechanisms whereby sleep apnea might cause cardiovascular morbidity and mortality, including increased sympathetic nervous system activity, hypoxic and oxidative stress, systemic inflammation, impaired glucose handling, and mechanical factors increasing cardiac filling and ventricular transmural pressure.12,13 These findings provide biological plausibility to a causal association of sleep apnea with cardiovascular disease. It is important to note that, in contrast to patients seeking treatment in sleep disorders centers, as many as half of individuals with moderate to severe sleep apnea in the general population do not report excessive sleepiness,14 and this is also true of samples of consecutive patients with established cardiovascular disease or heart failure. Should a causal association exist between sleep apnea and cardiovascular disease, this would provide a strong rationale for treatment of sleep apnea even in the absence of daytime sleepiness.
To date, however, the ability of sleep apnea treatment to reduce cardiovascular morbidity and mortality has not been demonstrated. Several observational studies comparing obstructive sleep apnea (OSA) patients treated with continuous positive airway pressure (CPAP) versus those not treated have found elevated cardiovascular risk only in those with untreated OSA15–18; however, lack of treatment in these studies was self-selected based on refusal of or non-adherence to therapy, raising the possibility that increased cardiovascular risk was related to other factors associated with poor adherence to prescribed medical therapy. Multiple small, single-center randomized clinical trials evaluating intermediate physiologic endpoints have pointed to beneficial effects of treatment with CPAP on blood pressure, glucose tolerance or insulin resistance, subclinical measures of atherosclerosis, and left ventricular ejection fraction. A number of negative trials have also been reported and the possibility of positive publication bias further dampens enthusiasm for studies of this type to inform clinical practice. Moreover, even if the effect of CPAP is correctly estimated by these studies, caution must be exercised in extrapolating from these intermediate endpoint studies to clinical outcomes of interest. In this regard, the Canadian Positive Airway Pressure (CANPAP) Trial is particularly instructive. After small trials indicated that CPAP treatment of Cheyne-Stokes respiration in chronic heart failure resulted in dramatic improvements in left ventricular ejection fraction, the CANPAP study was conducted as a multicenter, randomized clinical trial to establish the benefits of this therapy on transplant-free survival. Although an improvement in ejection fraction was observed, there was no improvement in transplant-free survival and a trend toward increased early mortality in the CPAP-treated arm.19
Such considerations underscore the importance of well-designed, multicenter, randomized clinical trials (RCTs) to evaluate the impact of sleep apnea treatment on cardiovascular morbidity and mortality. To date, few such studies have been undertaken. Almost two decades after the Wisconsin Sleep Cohort Study opened the eyes of the medical community to the high prevalence of sleep apnea, it is difficult to attribute the paucity of sleep apnea randomized clinical trials to the relative youth of the field of sleep medicine. The difficulty in conducting such clinical trials should not be minimized, however. Cardiovascular clinical trials require large numbers of patients treated for a period of years in order to accrue a sufficient number of disease endpoints for meaningful analysis, necessitating coordination of data collection across multiple sites and requiring substantial expertise and resources. In addition to the issues of regulatory compliance, harmonization of study procedures, subject recruitment, and secular changes in therapy that exist for any clinical trial, treatment trials for sleep apnea pose several unique challenges.
First, there is a lack of standardization in the approach to characterizing baseline presence and severity of sleep apnea, with regard to both the parameters measured and the definition of sleep apnea. While diagnosis of sleep apnea has been traditionally based on polysomnography, this technique is expensive and requires the availability of specialized facilities, both of which would hinder the conduct of large, multicenter international randomized clinical trials. Moreover, limited channel, home-based sleep apnea testing, typically measuring airflow, respiratory effort, and pulse oximetry, is now widely used in the clinical diagnosis of sleep apnea in the U.S. and throughout the world. While there is not a consensus regarding the parameters needed for adequate and consistent characterization of sleep apnea for inclusion of subjects in clinical trials, the use of simple portable monitoring devices may offer an affordable approach to large-scale screening such as would be required for international randomized clinical trials. Indeed, while it may be important to discriminate central from obstructive sleep apnea in the setting of patients with chronic heart failure20 or stroke, simple oximetry might be a sufficient screening tool in settings where discrimination of central from obstructive sleep apnea is not needed.
Another challenge in sleep apnea clinical trials relates to the use of a placebo treatment. While there is uncertainty regarding the role of sleep apnea treatment in modifying long-term cardiovascular risk, there is consensus that sleep apnea treatment improves symptoms of sleep apnea, in particular excessive sleepiness. A threshold of symptoms below which it is ethical to withhold effective symptomatic therapy needs to be established, although potential biases introduced by excluding highly symptomatic patients must be considered. Beyond the ethics of placebo use, there is the question of which non-therapeutic comparator is appropriate. Short-term randomized trials investigating neurocognitive function or intermediate cardiometabolic endpoints have utilized various comparators, including sham CPAP, subtherapeutic CPAP, placebo pills, nasal dilator strips, or a non-treatment control group without placebo. While sham devices have been validated as a placebo for CPAP, both patients and providers may be less willing to participate in a long duration clinical trial when the comparator is perceived as burdensome. Sham CPAP resulted in somewhat higher dropout rates over a 6-month trial,21 a problem that would likely increase in longer-term studies. Moreover, it is unclear whether they might themselves have an adverse impact on sleep quality and thus potentially bias results. At present, there is not consensus on the ideal comparator in sleep apnea clinical trials.
Adherence to therapy over a period of years, as is needed in studies of cardiovascular morbidity and mortality, is a challenge in clinical trials generally. Adherence may be particularly difficult to achieve in sleep apnea cardiovascular trials, as patients with few or no sleep apnea symptoms are generally less tolerant of CPAP than are symptomatic sleep apnea patients. The relative improvement in study efficiency by utilizing run-in periods to identify early non-adherers is unclear. Short-term trials have often utilized intensive participant contact to enhance CPAP adherence; however, trials that more closely approximate the actual practice of sleep medicine are more practical and better suited to inform clinical decision-making. While an arbitrary threshold of at least four hours per night is often used to identify adequate CPAP adherence, the relation of duration of CPAP use to improved quality of life or cardiovascular benefit is uncertain. As contemporary CPAP devices provide accurate adherence data, there is an opportunity to embed studies of adherence predictors within the framework of large randomized clinical trials and to carefully assess the relation of adherence to clinical outcomes.
Despite these challenges, the past several years have seen an increasing number of randomized clinical trials of sleep apnea treatment. We have conducted a search of major worldwide clinical trials registry databases, including all registries contributing data to the World Health Organization Clinical Trials Registry Platform, and included all trials submitted to the registry as of November 2012. The search was designed to return all studies including the key words “sleep apnea” plus any of the following terms: cardiovascular, cerebrovascular, stroke, or vascular. These studies were then individually reviewed to identify those that were randomized trials with a planned enrollment of ≥ 50 subjects, an intervention targeting sleep apnea, and a cardiovascular disease outcome. (Although early studies, including the CANPAP trial, may not have been registered, the International Committee of Medical Journal Editors guidelines require that all clinical trials beginning after 2005 be prospectively registered in order to be considered for publication, and it is therefore unlikely that any currently active, large clinical trials would be unregistered.) This search identified 39 randomized clinical trials of sleep apnea treatment initiated after 2000 with a target enrollment of at least 50 subjects and endpoints of blood pressure, cardiac function, functional status following stroke, or composite cardiovascular disease endpoints. These include 13 completed trials comprising 2,859 subjects (Table 1) and 27 on-going trials with a planned enrollment of 11,236 subjects (Tables 2 and 3). This search identified multiple additional ongoing randomized trials assessing the impact of OSA therapy on biomarkers of cardiovascular risk, such as measures of oxidative stress, endothelial function, or metabolic syndrome. The majority of the trials with disease outcomes (17 of the 27 active studies) have blood pressure as the main outcome (Table 2). Because blood pressure is a continuous measure and changes can be detected over a relatively short period of time, such studies can generally be completed in less time, with fewer subjects and at lower cost than would be required for studies of discrete disease endpoints such as myocardial infarction or stroke. In contrast to the average sample size of approximately 200 subjects in completed cardiovascular sleep apnea studies, early studies demonstrating that statin therapy compared to placebo reduced the risk of incident or recurrent coronary events enrolled over 4,000 subjects31; to demonstrate a reduction in mortality with statins, over 9,000 subjects were enrolled.32 Similar sample sizes may be needed to clearly demonstrate cardiovascular risk reduction following sleep apnea treatment.
Table 1.
Registered randomized clinical trials of sleep apnea treatment and cardiovascular endpoints (completed studies)

Table 2.
Registered randomized clinical trials of sleep apnea treatment and blood pressure endpoints (ongoing studies)
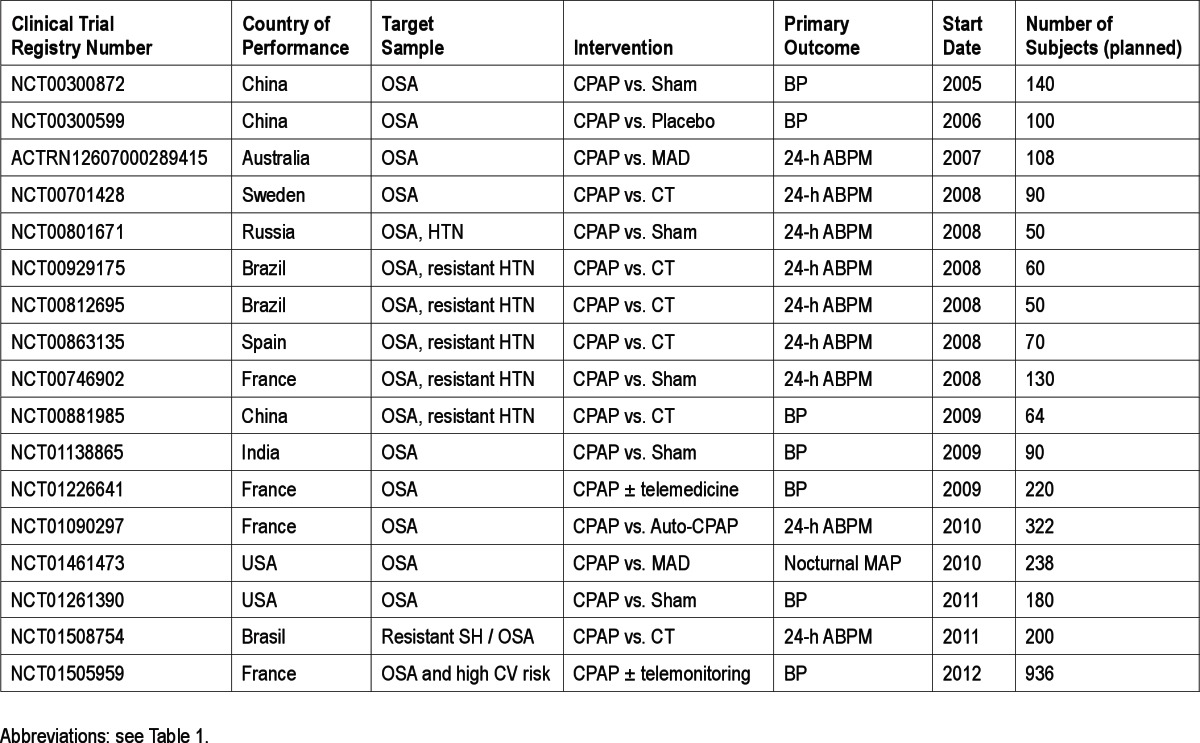
Table 3.
Registered randomized clinical trials of sleep apnea treatment and cardiac endpoints (ongoing studies)

While samples of this magnitude have not yet been enrolled in sleep apnea cardiovascular trials, several recently completed studies have begun to raise the bar. The Spanish Sleep and Breathing Network, started in the mid-1990s, has been very active in conducting clinical trials based on the approach of a central committee that identifies relevant clinical questions, establishes funding streams, and selects participating centers for each project, providing technical support and guidance to researchers and pre-submission review of manuscripts. This network has recently published a randomized clinical trial assessing a composite incident cardiovascular disease and hyper-tension endpoint in 723 non-sleepy patients with sleep apnea, randomized to treatment with CPAP versus conservative therapy for a median of 4.0 years.23 Reflecting both the relatively low power of even such an ambitious study and the potential importance of treatment adherence, the 17% reduction in risk observed in the CPAP-treated group was not statistically significant, although a subgroup analysis suggested a significant benefit in CPAP-adherent (≥ 4 h/night) subjects. The Oxford group recently reported results of the Multicentre OSA Interventional Cardiovascular Trial (MOSAIC), which investigated the effects of CPAP on cardiovascular risk score, sleepiness and quality of life in 391 minimally symptomatic patients.29 After 6 months of follow-up, the CPAP-treated group had a significant reduction in daytime sleepiness and self-assessed health status, but no improvement in cardiovascular risk score. Two large multicenter studies of adaptive pressure-support servoventilation treatment of sleep apnea in patients with heart failure with reduced ejection fraction are currently in progress. Both the North America-based Adaptive Servoventilation for Treatment of OSA and CSA in Heart Failure (ADVENT-HF) study, enrolling heart failure patients with central sleep apnea or non-sleepy OSA, and the Europe-based Treatment of Predominant Central Sleep Apnoea by Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) study, enrolling heart failure patients with predominant central sleep apnea, will evaluate the impact of treatment on the clinically important outcomes of mortality and cardiovascular hospitalization. The largest ongoing RCT, the Sleep Apnea Cardiovascular Endpoints (SAVE) study, has recruited over 2,000 of a planned 2,500 patients with minimally symptomatic OSA and ischemic heart disease or cerebrovascular disease to study the impact of CPAP therapy on combined vascular events and mortality. This study has partnered with existing sleep clinical trials networks and with non-sleep clinical trialists who have experience in running large clinical trials in other fields of medicine. Reflecting a cautious interest in the area of sleep apnea cardiovascular trials, the US National Institutes of Health have recently funded three planning grants, the Heart Biomarker Evaluation in Apnea Treatment (HeartBEAT, NCT01086800), Best Apnea Interventions in Research (BestAIR, NCT01261390), and the Sleep Apnea in TIA/Stroke (SleepTight, NCT01446913) studies to evaluate design approaches for a large scale clinical trial of CPAP for cardiovascular risk reduction, including effectiveness of various recruitment strategies, methods for optimizing adherence, use of control treatments, intermediate endpoints most responsive to intervention, and the use of oxygen as an alternative to CPAP.
Even as these important trials are ongoing, some argue that clinical trials of sleep apnea treatment to prevent cardiovascular disease are unethical and that all patients with OSA should be treated to prevent cardiovascular complications. While there should be little debate that treatment is appropriate to reduce the symptoms of daytime sleepiness and nocturnal sleep disruption that often accompany sleep apnea, we believe that there is uncertainty with respect to the cardiovascular benefits of treatment of sleep apnea. Conversely, large-scale sleep apnea cardiovascular trials might be considered premature, as epidemiologic studies show inconsistent associations of sleep apnea with cardiovascular risk across age and gender groups, while biomarkers identifying subsets of sleep apnea patients with increased cardiovascular risk are lacking. Although there remain important questions to be addressed with observational studies, whose answers may inform the design of future clinical trials, the data supporting an association of sleep apnea with cardiovascular risk are sufficiently compelling, and the magnitude of the potential public health risk sufficiently great, that it is time to complete the inferential loop by demonstrating that effective treatment of sleep apnea will prevent cardiovascular morbidity and mortality.
To further this aim, an international collaboration of investigators, loosely modeled on the Spanish Sleep and Breathing Network, has been established. This International Collaboration of Sleep Apnea Cardiovascular Trialists (INCOSACT) has the express goal of fostering international multicenter randomized clinical trials of sleep apnea treatment, with a particular focus on studies to reduce sleep apnea-related cardiovascular risk. The consortium aims to promote new research ideas and help researchers obtain funding by fostering academic-industry partnerships and providing a credible framework to support the conduct of clinical trials. It will facilitate the sharing of expertise and, where appropriate, the standardization of methodology for diagnosis, treatment and monitoring of participants, and will help investigators negotiate the complex regulatory landscape that confronts international clinical trials. INCOSACT membership includes senior and junior investigators actively involved in the conduct of Phase 3 sleep apnea cardiovascular clinical trials (or Phase 2 trials expressly designed to test feasibility issues relevant to Phase 3 studies) as well as cardiovascular clinical trialists, and has representation from multiple continents and regions. An Executive Committee, whose members will rotate every 2-3 years, holds quarterly meetings by phone or video conference and annual meetings at an international sleep or respiratory conference. The INCOSACT secretariat is presently hosted by the American Sleep Medicine Foundation. An INCOSACT website is being developed (www.incosact.org) and will ultimately contain both public information regarding international sleep apnea clinical trials and a secure location for communication and document sharing among INCOSACT members. While the focus will be on large randomized clinical trials, the consortium will encourage and support the conduct of meta-analyses of data collected in smaller clinical trials and would welcome ideas for collaboration on mechanistic research trials that could be embedded as sub-studies within larger research frameworks, facilitating basic research including genetic and biomarker assessments that might otherwise be prohibitively expensive. By promoting large scale clinical research, INCOSACT hopes to assist investigators to answer some of the key remaining questions in cardiovascular sleep research.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Gottlieb has served as a consultant to ResMed Corporation. Dr. Heeley and Dr. McEvoy have received research support for the SAVE Trial from Philips Respironics, ResMed Inc., and Fisher and Paykel Healthcare. Dr. Redline has received research support from Philips Respironics, ResMed Foundation, and ResMed Inc.
ABBREVIATIONS
- ABPM
ambulatory blood pressure monitoring
- ACS
acute coronary syndrome
- ADHF
acute decompensated heart failure
- AF
atrial fibrillation
- ASV
adaptive servoventilation
- CHD
coronary heart disease
- CPAP
continuous positive airway pressure
- CSA
central sleep apnea
- CT
conservative therapy
- CV
cardiovascular
- HF
heart failure
- HTN
hypertension
- INCOSACT
International Collaboration of Sleep Apnea Cardiovascular Trialists
- LVEF
left ventricular ejection fraction
- MAD
mandibular advancement device
- MI
myocardial infarction
- OSA
obstructive sleep apnea
- RCT
randomized clinical trial
- TIA
transient ischemic attack
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occur-rence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151:1459–65. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 3.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119:62–9. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 4.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163:685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 5.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11:441–6. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb DJ, Yenokyan G, Newman AB, et al. A prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea and incident stroke: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall NS, Wong KK, Liu PY, et al. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement. Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 13.Drager LF, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: an emerging risk factor for atherosclerosis. Chest. 2011;140:534–42. doi: 10.1378/chest.10-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in moderate to severe sleep disordered breathing. Sleep. 2005;28:472–7. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 15.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–65. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 16.Milleron O, Pilliere R, Foucher A, et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25:728–34. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 18.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–84. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 19.Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 20.Naughton MT, Lorenzi-Filho G. Sleep in heart failure. Prog Cardiovasc Dis. 2009;51:339–49. doi: 10.1016/j.pcad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egea CJ, Aizpuru F, Pinto JA, et al. Cardiac function after CPAP therapy in patients with chronic heart failure and sleep apnea: a multicenter study. Sleep Med. 2008;9:660–6. doi: 10.1016/j.sleep.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 24.Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37:1128–36. doi: 10.1183/09031936.00034410. [DOI] [PubMed] [Google Scholar]
- 25.Lozano L, Tovar JL, Sampol G, et al. Continuous positive airway pressure treatment in sleep apnea patients with resistant hypertension: a randomized, controlled trial. J Hypertens. 2010;28:2161–8. doi: 10.1097/HJH.0b013e32833b9c63. [DOI] [PubMed] [Google Scholar]
- 26.Durán-Cantolla J, Aizpuru F, Montserrat JM, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. 2010;341:c5991. doi: 10.1136/bmj.c5991. [DOI] [PubMed] [Google Scholar]
- 27.Pépin JL, Tamisier R, Barone-Rochette G, Launois SH, Lévy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182:954–60. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 28.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136:991–7. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67:1090–6. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 30.Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. 2011;42:1062–7. doi: 10.1161/STROKEAHA.110.597468. [DOI] [PubMed] [Google Scholar]
- 31.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 32.The long-term intervention with pravastatin in ischaemic disease (lipid) study group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]


