Abstract
Background:
Short sleep duration has been linked to impaired glucose metabolism in many experimental studies. Moreover, studies have reported indications of an increased metabolic stress following sleep restriction.
Objective:
We aimed to investigate the effects of partial sleep deprivation on markers of glucose metabolism. Additionally, we aimed to investigate if short sleep duration induces a state of endocrine stress.
Design:
A randomized crossover design, with 2 experimental conditions: 3 consecutive nights of short sleep (SS, 4 h/night) and long sleep (LS, 9 h/night) duration.
Subjects and Measurements:
In 21 healthy, normal-weight male adolescents (mean ± SD age: 16.8 ± 1.3 y) we measured pre- and post-prandial glucose, insulin, C-peptide, and glucagon concentrations. Furthermore, we measured fasting cortisol, 24-h catecholamines, and sympathovagal balance.
Results:
Fasting insulin was 59% higher (P = 0.001) in the SS than the LS condition as was both fasting (24%, P < 0.001) and post-prandial (11%, P = 0.018) C-peptide. Pre- and post-prandial glucose and glucagon were unchanged between conditions. The homeostasis model assessment of insulin resistance (HOMA-IR) index was 65% higher (P = 0.002) and the Matsuda index was 28% lower (P = 0.007) in the SS condition compared to the LS condition. The awakening cortisol response and 24-h norepinephrine were not affected by sleep duration, whereas 24-h epinephrine was 24% lower (P = 0.013) in the SS condition. Neither daytime nor 24-h sympathovagal balance differed between sleep conditions. Short wave sleep was preserved in the SS condition.
Conclusion:
Short-term sleep restriction is associated with decreased insulin sensitivity in healthy normal-weight adolescent boys. There were no indications of endocrine stress beyond this.
Citation:
Klingenberg L; Chaput JP; Holmbäck U; Visby T; Jennum P; Nikolic M; Astrup A; Sjödin A. Acute Sleep Restriction Reduces Insulin Sensitivity in Adolescent Boys. SLEEP 2013;36(7):1085-1090.
Keywords: Sleep deprivation, sleep quality, glucose metabolism, teenagers
INTRODUCTION
Observational studies have shown an association between short sleep duration and impaired glucose metabolism and diabetes on both adults1 and adolescents.2–4 These findings have been supported by experimental studies in adults showing that endocrine glucose allostasis is acutely impaired after acute sleep restriction5–9 and poor sleep quality.10 The mechanisms by which short sleep duration leads to impaired glucose allostasis are still unclear. A decrease in insulin sensitivity11 and increased cortisol and catecholamine levels,5 both of which are proxies for endocrine stress, have been proposed by some investigators as possible mechanisms. Thus, increased endocrine stress could be an underlying mechanism for the impaired glucose metabolism observed in response to partial sleep deprivation in humans.
The present study aimed to investigate, for the first time in a group of adolescents, the effects of three consecutive nights of partial sleep deprivation on markers of glucose allostasis. Since knowledge on this relation in teenagers has only been described in cross-sectional studies,2–4 we need to ascertain both the temporal sequence of the relation and the possible causal relation in this particular age group. Adolescents are interesting not only because of the lack of experimental studies addressing this age group, but also because adolescents have had decreased sleep duration over the past decade.12 Furthermore, the widespread use of technology, especially among teenagers,13 has delayed the onset of sleep, possibly introducing daytime napping as well. Consequently, this group is subjected to disruption of the normal circadian rhythmicity of not only sleep but also the hormonal systems involved in the regulation of blood glucose. From a long-term health perspective, this group is also interesting because of the possible risk for developing type 2 diabetes in later life. Identifying early risk factors for reduced insulin sensitivity is crucial in determining the root causes of adult type 2 diabetes. Experimental evidence determining the causal relation between behavioral issues (such as sleep) and impaired glucose metabolism will address this issue and provide knowledge about possible specific countermeasures to the risk of onset of type 2 diabetes at an early stage in life. Moreover, women short sleepers have previously been found to have lower risk of incident diabetes than men14; thus, it is probable that they are more resistant to allostatic overload following short sleep than men. Thus, investigating male subjects is of further interest in relation to the risk of type 2 diabetes. We hypothesize that acute partial sleep deprivation reduces insulin sensitivity in adolescent boys.
METHODS
Twenty-one healthy, normal-weight male adolescents with body mass index (BMI) < 25 kg/m2 (age adjusted),15 (mean ± SD BMI, 21.0 ± 1.8 kg/m2), aged 15 to 19 y (mean ± SD age, 16.8 ± 1.3 y) were recruited through postings and by word of mouth. Volunteers were excluded from participation for any of the following reasons: smoking, unstable body weight (± 3 kg) during the 6 months preceding testing, regular physical exercise (> 3 h/week), excessive intake of alcohol (> 7 drinks/week), substance abuse, excessive intake of caffeine (> 300 mg/day), metabolic diseases, medication use that could interfere with the outcome variables, eating disorders, highly restrained eating behavior (score ≥ 10 for cognitive dietary restraint on the Three-Factor Eating Questionnaire), irregular eating pattern (e.g., skipping breakfast), transmeridian travelling in the past month, and inability to comply with the protocol. In addition, each participant completed the Pittsburgh Sleep Quality Index (PSQI) questionnaire,16 a self-rated questionnaire that assesses sleep quality and disturbances over the preceding month. A total score > 5 is associated with poor sleep and thus served as an exclusion criterion. All participants (and parents when under 18 y of age) gave written informed consent to participate. The study was approved by the ethical committee of the Capital Region of Denmark. The study is described in details elsewhere.17
Using a within-subject experimental design, each participant was engaged, during 3 consecutive nights, in each of the 2 following conditions in a random order: (1) short sleep (SS, 4 h/ night from 03:00 to 07:00) and (2) long sleep (LS, 9 h/night from 22:00 to 07:00). Nine hours of sleep per night was chosen because it is the recommended duration of sleep for this age group (US National Sleep Foundation). These 2 experimental conditions were separated by 3-4 weeks. Between the experimental conditions the participants were asked to maintain normal activities and particularly not to change sleeping, eating, or physical activity patterns. Vigorous physical activity was not allowed 24 h before testing and during the test days. This was done to standardize the subjects and to limit pre-trial interference. Furthermore, a normal sleep schedule had to be respected for 3 d before the first testing day in each condition in order to minimize the influence of potential pre-trial sleep restriction. Upon arrival the subjects were asked questions about their sleeping habits as well as mental and physical health in the days preceding the trial.
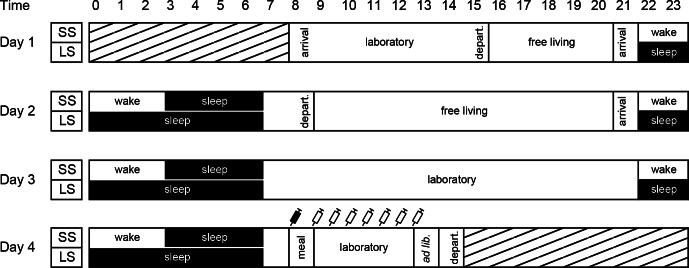
The 3 nights were spent at the department's laboratory under strictly controlled conditions. From midday on day 1 and during daytime on day 2, the participants were free to leave the laboratory. On day 3 and 4, the subjects stayed in the laboratory. Figure 1 shows a schematic overview of the study protocol. Trained personnel monitored the subjects visually on a regular basis to ensure their safety and compliance to the protocol. The personnel only interacted with the subjects to ensure that there were no protocol violations (e.g., making contact at any sign of sleeping during waking hours). Individually adjusted energy balanced diets were provided during the intervention period at the same time point in both conditions. Energy requirements were calculated using the WHO formula for this age group.18 The diets were identical and served at the same time points in both conditions.
Figure 1.
Study outline. Hatched areas represent time periods outside the study protocol. The participants spent both sleeping and wake time in the laboratory on day 1 and 2 to be accustomed to the facilities. Syringe symbols denote blood samplings (filled syringe denote fasting sample). Meals were given at the same time points in both conditions: breakfast at 09:00, lunch at 13:00, snack at 15:30, dinner at 18:00, and snack at 21:00. Meals outside the laboratory were provided by our research kitchen staff. In the free living periods, the participants were encouraged to engage in everyday activities, but abstain from scheduled physical activity. ad lib., ad libitum meal test; arrival/depart., arrival or departure from the laboratory; laboratory, participants stayed at the laboratory, LS, long sleep condition (9 h from 22:00-07:00); meal, breakfast meal, SS, short sleep condition (4 h from 03:00-07:00).
A 24-h urine collection was done during day 3 for analyses of catecholamines (determined by solid phase extraction by ion exchange followed by high performance liquid chromatography with electrochemical detection [HPLC-EC]). Saliva samples were collected using a Salivette (Sarstedt) tube immediately after awakening the last morning (sample 1) and 30 min after awakening for analysis of cortisol (determined by solid phase extraction followed by ultra-high performance liquid chromatography tandem mass spectrometry [LC-MS/MS]).
Blood samples were drawn in the fasting state and every 30 min for 3 h following a standardized breakfast meal, consisting of 25% of the individual daily energy requirement and with an energy distribution of 11 E% protein, 27 E% fat, and 62 E% carbohydrate. Both the amount and distribution of macronutrients correspond to an average Danish breakfast meal. Blood samples were analyzed for plasma glucose (measured enzymatically, ABX Pentra 400, Montpellier, France): serum insulin and C-peptide (determined by chemiluminescent immunometric assay, Immulite, Los Angeles, CA), and plasma glucagon (determined by competitive RadioImmunoAssay, Euro Diagnosticas, Malmö, Sweden). For values under the limit of detection (LOD) we used two-thirds of the LOD. The statistical analyses were carried out with and without these samples. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as follows: (fasting glucose × fasting insulin)/22.5, while the Matsuda index was calculated as follows: 10,000/√((fasting glucose × fasting insulin) × (mean glucose0-210 × mean insulin0-210)).
Sleep was monitored by polysomnography (TrackitTM, Lifelines Neurodiagnostic Systems Inc., Troy, IL, USA) during the whole intervention period (day 1 to 4) in both conditions, and recordings were scored offline according to standard criteria (American Academy of Sleep Medicine, 2007). The hypnograms were carefully examined for daytime napping. Minor instances of sleep (5-10 min) during the day were allowed, whereas longer periods of sleep were regarded as protocol violation and reason for exclusion (evaluated individually for each participant). Only one person was excluded on the basis of daytime napping. Because of technical difficulties with the electrodes, only 13 subjects had complete data in both conditions to evaluate sleep stages. However, we had complete data on sleep length.
Sympathovagal balance (low frequency to high frequency ratio, LF/HF-ratio) was assessed using an ActiHeart device (Camntech, Cambridge, UK) as described elsewhere.19
Pairwise comparisons were done using a multiple linear regression model with robust estimation of variance (Huber-White estimation model), with order of treatment as fixed explanatory variable. Differences in repeated measurements of substrates were tested using a random coefficient model. For statistical evaluation, Stata version 11 (StataCorp, College Station, Texas, USA) was used. Data are presented as means ± SEM unless otherwise specified. A P-value < 0.05 was considered significant.
RESULTS
The participants slept on average 506 ± 42 and 243 ± 15 min during the LS and SS conditions, respectively. The duration of slow wave sleep (SWS) in the subpopulation was preserved in the SS condition (SS, 132.2 ± 22.2 min and LS, 140.5 ± 37.7 min, P = 0.20), whereas REM sleep (REMS) was reduced in the SS condition (SS, 46.6 ± 19.7 min and LS, 124.7 ± 24.0 min, P < 0.001). Number of state transitions per hour did not differ between conditions (SS, 8.7 ± 3.1 and LS; 7.1 ± 2.8, P = 0.18), while the number of awakenings per hour tended to be higher in the SS condition but did not reach statistical significance (SS, 2.0 ± 1.4 and 1.4 ± 0.7, P = 0.080).
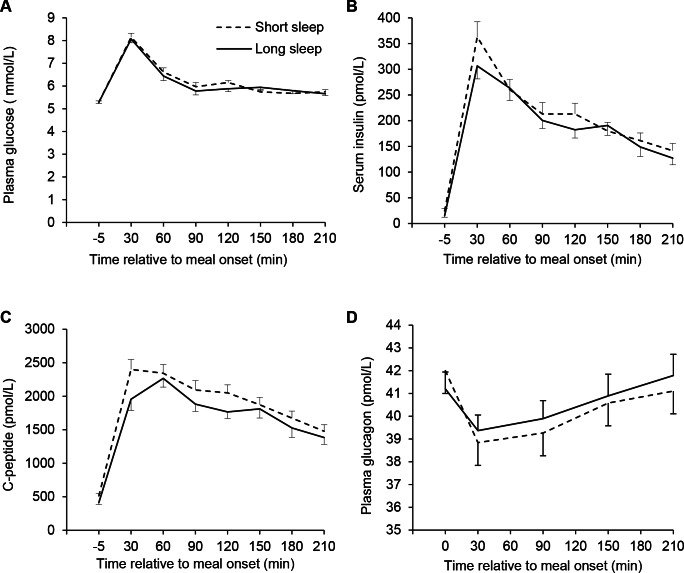
Baseline values and area under/over the curve (AUC/AOC, trapezoid method) calculations for plasma glucose, insulin, C-peptide, and glucagon are presented in Table 1, and profiles are depicted in Figure 2. Overall, HOMA-IR was higher in the SS condition than the LS condition, and the Matsuda index was lower in the SS condition. Insulin was higher in the fasted state, while C-peptide was higher both in the fasting state and using the AUC in the SS compared to the LS condition.
Table 1.
Fasting values, AUC/AOC calculations, and glucose metabolism indexes for the short and long sleep conditions
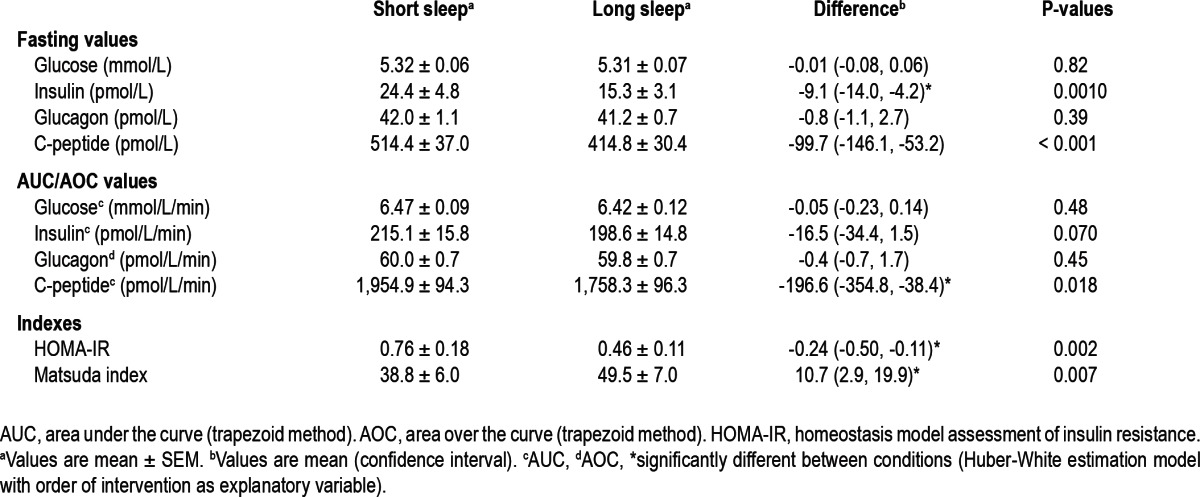
Figure 2.
Profiles of glucose (A), insulin (B), C-peptide (C), and glucagon (D) before and after the standardized meal challenge in the morning after 3 consecutive nights of either short sleep (4 h/night) or long sleep (9h/night). Significant higher fasting levels in the SS condition for insulin and C-peptide as well as area under the curve (trapezoid rule) for C-peptide (Huber-White estimation model with order of intervention as explanatory variable). No time × condition effect in a random coefficient model (P = 0.71, 0.68, 0.60, and 0.47 for glucose, insulin, C-peptide, and glucagon, respectively). Panel E: *Significant difference between conditions in sample 1 (Huber-White estimation model with order of intervention as explanatory variable). Values are mean ± SEM.
Cortisol at awakening was 26% lower in the SS condition (SS, 6.8 ± 5.0 nmol/L and LS, 8.6 ± 4.5 nmol/L, P = 0.030), but there was no difference at 30 min (SS, 13.5 ± 5.3 nmol/L and LS, 13.9 ± 4.8 nmol/L, P = 0.74). Likewise, the response in saliva cortisol between awakening and 30 min later was not different (SS, 6.7 ± 4.2 nmol/L and LS, 5.4 ± 5.8 nmol/L, P = 0.41). For 24-h urine catecholamine values, only epinephrine differed between conditions, with a lower value in the SS condition (SS, 0.055 ± 0.006 μmol/L and LS, 0.068 ± 0.007 μmol/L, P = 0.013), while norepinephrine was unaltered by sleep condition (SS, 0.23 ± 0.017 μmol/L and LS, 0.22 ± 0.018 μmol/L, P = 0.42).
Sympathovagal balance was higher in the SS condition than the LS condition during the night time period (22:00-03:00), where the subjects were either awake (SS condition) or sleeping (LS condition). The LF/HF ratio was 1.79 ± 0.67 and 1.31 ± 0.53 (P = 0.006) for the SS and LS conditions, respectively. The opposite was seen during sleep (03:00-07:00), where the sympathovagal balance was 1.27 ± 0.66 and 1.58 ± 0.81 (P = 0.049) for the SS and LS conditions, respectively. However, neither daytime (P = 0.78) nor 24-h (P = 0.64) sympathovagal balance differed between conditions.
DISCUSSION
The present study suggests that, despite maintained SWS, three nights of moderate sleep restriction acutely decreases insulin sensitivity in healthy, lean adolescent boys. The indication of an increased post-prandial inhibition of glucagon further substantiates this finding. These results are in line with previous observational studies on adolescents,2–4 and thus underline the temporal sequence of the relation found in the cross-sectional studies. Furthermore, our results are in agreement with adult experimental studies using clamp technique,11 intravenous glucose tolerance test,8 and ad libitum meal setting.20 The combination of elevated insulin levels (preprandially), elevated C-peptide levels (pre- and postprandially), and unchanged glucose levels (pre- and postprandial) indicates a reduced sensitivity to insulin. These changes may be just transient effects of acute sleep restriction. If sustained, however, this may ultimately increase the risk of developing type 2 diabetes.
In our study, SWS was maintained despite considerable restricted sleep in the SS condition but with a substantial reduction in REMS. Previous studies have established that the amount of SWS can indeed be preserved in a sleep restricted situation,9,21 and that curtailment of SWS only is associated with insulin resistance.10 However, in spite of no significant change in SWS in our teenagers, a marked reduction in insulin sensitivity was observed, suggesting that total sleep time and not only SWS affects the mechanisms involved in glucose metabolism.
In contrast to our expectations, we did not observe an effect of sleep restriction on the 30 min response in saliva cortisol in the morning. In fact a lower cortisol concentration at awakening in the SS condition was observed. Similar results on morning cortisol levels as a result of sleep restriction have been reported earlier.22,23 Whereas morning levels of cortisol can be affected by the means of awakening (e.g., spontaneous or by the staff), it has also been suggested that a lower cortisol level upon awakening reflects a blunting of the normal circadian pulse of cortisol.23 The lower fasting cortisol level could, however, simply be an effect of an altered mid-sleep time point (05:00 in SS vs. 02:30 in LS), which could shift the circadian cortisol curve somewhat forward.24 Other studies have reported a higher than normal increase of cortisol in the evening resulting from sleep deprivation.5,22 It can be speculated that these observations reflect an impairment of the negative-feedback control of the hypothalamic-pituitary-adrenal axis,25 which can be an underlying mechanism to the decreased insulin sensitivity.26 We did not measure cortisol levels in the evening; thus, additional disturbances in the circadian rhythmicity of cortisol in our teenagers are still a possible mechanism involved in the decreased insulin sensitivity observed.27
It is not clear to us why the subjects experience a lower negative sympathovagal balance during sleep in the SS condition than the LS condition. However, this is at least partly supported by the finding of a lower 24-h epinephrine level in the SS condition. If SWS is crucial in maintaining allostasis, and SWS is maintained, short sleep per se might not impose an allostatic overload beyond the decreased insulin sensitivity in these teenagers. However, a better understanding of the rhythmicity of both sympathovagal balance and hormonal stress markers in teenagers are needed to further elucidate this.
There are some limitations in this study that deserve attention. We did not assess sleep duration in the days before each trial. It is possible that sleep varied between subjects and between conditions. Since blood samples were performed on the morning of day 4, the subjects were standardized in the previous three days, limiting the possible confounding effect of any pre-trial variations in sleep. Furthermore, 9 h of sleep per night in the LS condition might not maximize the amount of sleep for all participants in this age group. Thus, some participants are potentially kept in a very mild sleep deprived state in this condition. Subjective data on sleepiness (data published elsewhere17), however, revealed that the participants on average felt rested in the LS condition, suggesting a negligible impact on the outcome. Blood glucose levels and the hormones responsible for the allostatic regulation thereof are subjected to circadian control regardless of the sleep/wake cycle.28 A continuous measurement over 24 h would elucidate any effect on the circadian rhythmicity besides what is caused by sleep restriction. This is particularly of relevance in studies where mid-sleep time point is not synchronized as in the present one. In studies where mid-sleep time point has been synchronized to maintain normal circadian rhythm, evidence of reduced insulin sensitivity is, however, also present.5,8,11 Moreover, misaligning the circadian rhythmicity by either delaying or advancing the sleep/wake cycle has also been shown to cause disturbance of the glucose-insulin metabolism.29 Thus, allostatic overload on the glucose regulatory system is probably caused by both disturbances in the circadian rhythmicity and sleep duration. In that sense, the results from the present study can potentially be a result of circadian disturbances as well as sleep restriction. In order to mimic the sleep restriction behavior in teenagers, however, we chose to synchronize wakeup time. Additionally, we measured the hormonal response to a test meal. More robust methodologies (e.g., clamp techniques) would provide a way to quantifying β-cell sensitivity to a glucose load (hyperglycemic clamp) and of the tissue sensitivity to insulin (euglycemic insulin clamp).30 However, these techniques are rather invasive, and we found them not feasible in our population of young teenagers.
In summary, short sleep induces signs of decreased insulin sensitivity, despite maintained SWS and in the absence of signs of a global metabolic stress. However, glucose allostasis was maintained, due to compensatory increased insulin secretion. It could be speculated that our subjects because of their young age and normal weight status have a more efficient regulatory ability. Future work is needed to confirm our observations in this young population and to determine whether the reported effects associated with partial sleep deprivation are maintained on a chronic basis also with a more moderate restriction of sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study is part of the OPUS project. OPUS is an acronym of the project “Optimal well-being, development and health for Danish children through a healthy New Nordic Diet” and is supported by a grant from the Nordea Foundation. The OPUS Centre, Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen. The funding organization played no role in the design, implementation, analysis, or interpretation of the data. The trial was registered at clinicaltrials.gov as NCT01198431. The authors thank the participants and their parents for their excellent collaboration; the staff at the Department of Nutrition, Exercise and Sports for their skilled technical assistance; and also Mrs. Helle Lüthje Leonthin at the Center for Sleep Medicine for her excellent work in analyzing the sleep data. Experiments were performed at the Department of Nutrition, Exercise and Sports, Faculty of Science, University of Copenhagen.
ABBREVIATIONS
- BMI
body mass index
- SS
short sleep
- LS
long sleep
- LOD
limit of detection
- LF/HF ratio
low frequency to high frequency ratio
- SWS
slow wave sleep
- REMS
rapid eye movement sleep
- AUC
area under the curve
- AOC
area over the curve
- HOMA-IR
homeostasis model assessment of insulin resistance
REFERENCES
- 1.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koren D, Levitt Katz LE, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–7. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews KA, Dahl RE, Owens JF, Lee L, Hall M. Sleep duration and insulin resistance in healthy black and white adolescents. Sleep. 2012;35:1353–8. doi: 10.5665/sleep.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javaheri S, Storfer-Isser A, Rosen CL, Redline S. Association of short and long sleep durations with insulin sensitivity in adolescents. J Pediatr. 2011;158:617–23. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiegel K, Leproult R, Van CE. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 6.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donga E, van Dijk M, van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95:2963–8. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 8.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmid SM, Jauch-Chara K, Hallschmid M, Schultes B. Mild sleep restriction acutely reduces plasma glucagon levels in healthy men. J Clin Endocrinol Metab. 2009;94:5169–73. doi: 10.1210/jc.2009-0969. [DOI] [PubMed] [Google Scholar]
- 10.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105:1044–9. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The National Sleep Foundation. Teens and sleep. Washington: 2006. [Google Scholar]
- 13.The National Foundation. Technology use and sleep. Washington: 2011. [Google Scholar]
- 14.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 17.Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr. 2012;96:240–8. doi: 10.3945/ajcn.112.038638. [DOI] [PubMed] [Google Scholar]
- 18.WHO. WHO Technical Report Series. Geneva: WHO; 1985. Energy and protein requirements. Reports of a joint FAO/WHO/ UNU expert consultation. [Google Scholar]
- 19.Chaput JP, Visby T, Nyby S, et al. Video game playing increases food intake in adolescents: a randomized crossover study. Am J Clin Nutr. 2011;93:1196–203. doi: 10.3945/ajcn.110.008680. [DOI] [PubMed] [Google Scholar]
- 20.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Disturbed glucoregulatory response to food intake after moderate sleep restriction. Sleep. 2011;34:371–7. doi: 10.1093/sleep/34.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leproult R, Copinschi G, Buxton O, Van CE. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20:865–70. [PubMed] [Google Scholar]
- 23.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–6. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Griefahn B, Robens S. The cortisol awakening response: a pilot study on the effects of shift work, morningness and sleep duration. Psychoneuroendocrinology. 2008;33:981–8. doi: 10.1016/j.psyneuen.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–9. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–26. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 27.Dallman MF, Strack AM, Akana SF, et al. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–47. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 28.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–42. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 30.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]