Abstract
Introduction:
Self-reported sleep disturbances are associated with an increased risk of depression in younger and older adults, but associations between objective assessments of sleep/wake disturbances via wrist actigraphy and risk of depression are unknown.
Methods:
Depressive symptoms (Geriatric Depression Scale [GDS]), self-reported (questionnaires), and objective (actigraphy) sleep parameters were measured at baseline in 2,510 nondepressed men 67 y or older. Depressive symptoms were reassessed an average of 3.4 ± 0.5 y later.
Results:
Of the 2,510 men without evidence of depression at baseline, 116 (4.6%) were depressed (GDS ≥ 6) at the follow-up examination. After adjusting for multiple potential confounders, including baseline depressive symptoms (GDS 0-5), there was evidence of an association between poor self-reported sleep quality and higher odds of being depressed at follow-up (multivariable odds ratio [MOR] = 1.53, 95% confidence interval (CI) 1.00-2.33). In age- and site-adjusted models, objectively measured reduced sleep efficiency (odds ratio [OR] = 1.88, 95% CI 1.13-3.13), prolonged sleep latency (OR = 1.77, 95% CI 1.04-3.00), greater nighttime wakefulness (OR = 1.48, 95% CI 1.01-2.18) and multiple long-wake episodes (OR = 1.69, 95% CI 1.15-2.47) were associated with increased odds of depression at follow-up, but these associations were attenuated and no longer significant after further adjustment for number of depressive symptoms at baseline. Self-reported excessive daytime sleepiness and objectively measured total sleep time were not associated with depression status at follow-up. Excluding baseline antidepressant users from the analyses did not alter the results.
Conclusions:
Among nondepressed older men, poor self-reported sleep quality was associated with increased odds of depression several years later. Associations between objectively measured sleep disturbances (e.g., reduced sleep efficiency, prolonged sleep latency, greater nighttime wakefulness, and greater long-wake episodes) and depression several years later were largely explained by a greater burden of depressive symptoms at baseline.
Citation:
Paudel M; Taylor BC; Ancoli-Israel S; Blackwell T; Maglione JE; Stone K; Redline S; Ensrud KE; for the Osteoporotic Fractures in Men Study Group. Sleep disturbances and risk of depression in older men. SLEEP 2013;36(7):1033-1040.
Keywords: Actigraphy, depression, elderly, sleep quality
INTRODUCTION
Prior studies have suggested that up to 50% of older adults endorse symptoms of poor or insufficient sleep, such as difficulty falling asleep, fragmented sleep, early morning awakening, and daytime sleepiness.1–4 Depression is also common among older adults, with an estimated prevalence of 8-16%.1,5–8 In older populations, both sleep disturbances and depression are associated with greater risk of adverse health-related outcomes, such as poor health status,2,6 disability,6 poorer physical functioning,9,10 falls, and fractures.11 However, the association between poor sleep and depression is complex, bidirectional in nature, and not thoroughly understood.2
The results of a recently published meta-analysis suggests that insomnia is associated with an approximately 2-fold increased risk of incident depression,5,12–14 whereas results from studies including older adults reported that the risk may be 3-fold.5,12,15–17 However, all of these previous studies relied on self-reported assessments of sleep disturbances and/or insomnia. Studies examining associations between electroencephalographic (EEG) sleep parameters and subsequent risk of depression have suggested that dysregulations in rapid eye movement (REM) sleep patterns are associated with recurrent depressive episodes18,19 and that disturbances in polysomnographic parameters (such as reduced REM latency and slow wave sleep) are associated with a greater lifetime risk of depression.20 The results of these studies suggest that disturbances in EEG sleep parameters may precede depressive episodes. It is important to note that these studies enrolled participants from clinical and not community-based settings, and thus findings are subject to selection biases. To our knowledge, the longitudinal associations between objective (actigraphic) assessments of sleep disturbances and incident depression in a large community-based cohort have not been assessed.
In a prior cross-sectional study, we examined the association between depressive symptoms (as assessed by the Geriatric Depression Scale [GDS]), subjective sleep disturbances (as assessed by the Pittsburgh Sleep Quality Index [PSQI] and Epworth Sleepiness Scale [ESS]), and objective assessments of sleep/wake disturbances using wrist actigraphy using data from the Outcomes of Sleep Disorders in Older Men (MrOS Sleep) study.21,22 Results from those analyses suggested a strong association between greater level of depressive symptoms and self-reported poor sleep quality and excessive daytime sleepiness, and a moderate association between greater level of depressive symptoms and objectively measured prolonged sleep latency. Our results were confirmed in a similar analysis using data from the Study of Osteoporotic Fractures in community-dwelling older women.22
In this study, we tested the hypothesis that objective sleep/ wake disturbances, as well as self-reported sleep disturbances, are associated with incident depression in older men. We assessed several measures of self-reported and objective sleep disturbances and depressive symptoms at baseline in 2,510 community-dwelling, nondepressed men age 65 y and older enrolled in the MrOS Sleep study and then reassessed depressive symptoms an average of 3.4 y later.
METHODS
Participants
From March 2000 through April 2002, 5,994 men were recruited for participation in the baseline examination of the prospective Osteoporotic Fractures in Men (MrOS) Study.23 Men were recruited from population-based listings in six areas of the United States: Birmingham, Alabama; the Monongahela Valley near Pittsburgh, Pennsylvania; Minneapolis, Minnesota; Palo Alto, California; San Diego, California; and Portland, Oregon.24 Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded.
From December 2003 through March 2005, active participants were invited to enroll in an ancillary study: MrOS Sleep. Of the 5,994 men enrolled in the overall MrOS Study, 3,135 (68%) were enrolled in the MrOS Sleep Study (> 100% of recruitment goal). Of the 2,859 men who did not participate in this ancillary study, 344 died before the sleep visit; 36 had already stopped participating in the study; 332 were not invited because recruitment goals had already been met; 150 were not eligible due to current continuous positive airway pressure or mouthpiece use, open tracheotomy, or use of oxygen therapy; and 1,997 refused participation. Of those who participated in MrOS Sleep, 2,989 men had at least 72 h (3 nights) of wrist actigraphy data, and measures of depressive symptoms at baseline. The 2,510 men who were categorized as nondepressed at baseline (GDS score < 6) and had repeat measures of depressive symptoms at a follow-up examination an average of 3.4 y later comprised the analytical cohort for this study. The institutional review board at each center approved the study protocol, and written informed consent was obtained from all patients.
Measures of Sleep Parameters
Actigraphy
Sleep parameters were measured at the baseline examination using an octagonal sleep watch actigraph (Ambulatory Monitoring, Inc., Ardsley, NY), a small device used to detect movement and provide information on sleep-wake patterns. Actigraphs are similar in appearance to a wristwatch and were placed on the wrist of the nondominant hand. Participants were instructed to wear the actigraph for a minimum of 5 nights, securely fastened around their nondominant wrist, to be removed only when bathing or during water sports. Accelerometers within the actigraph measured movement several times per sec and stored the information digitally in 1-min epochs. Actigraphy has been shown to provide a reliable estimate of sleep-wake activity, and is highly correlated with polysomnography (PSG), which remains the gold standard.25 In a prior analysis using a subset of MrOS Sleep data, actigraphy data was moderately correlated with PSG data, with intraclass correlation coefficients (ICC) ranging from 0.32 to 0.57.26 Data were collected in three modes but are reported here using the digital integration mode (also known as proportional integration mode),15 which was used to analyze the actigraphy data.27 Details of the actigraphy scoring algorithms used in the study have been published elsewhere.28,29
Participants completed sleep diaries for the time they wore the actigraph. These diaries included time into and time out of bed and times the actigraph was removed. This information was used in editing the actigraphy data files to set intervals for when the participant was in bed trying to sleep (after “lights off”) and to delete times when the actigraph was removed. Interscorer reliability for editing the actigraphy data files has been found to be high in our group (ICC = 0.95) and actigraphy has been shown to have good concordance with total sleep time (TST) from PSG.29,30
Sleep/wake parameters used in analyses included sleep efficiency: the percentage of time (0-100%) the participant was sleeping from sleep onset until the last min scored as sleep (the following morning), sleep latency: the time from when the participant reported “lights off” until the time scored as sleep onset, time awake after sleep onset: the number of min scored as wake from sleep onset until the end of the last sleep episode while in bed, TST: the h per night spent sleeping while in bed after “lights off”, and long-wake episodes: the number of awakenings (≥ 5 min) while in bed. All actigraphy measurements were averaged over the total number of nights the actigraph was worn. Because most men did not complete the requested 5 nights of actigraphy data, we restricted our primary analyses to men who had at least 72 h of actigraphy recordings.
For primary analyses, sleep parameters were expressed as dichotomous variables based on consideration of clinical relevance of values for older adults, validated cut-points, and/ or comparability to prior MrOS Sleep publications21,31–33: poor sleep efficiency (< 70% versus ≥ 70%), prolonged sleep latency (≥ 1 versus < 1 h), greater nighttime wakefulness (awakening after sleep onset ≥ 1.5 versus < 1.5 h), and multiple long-wake episodes (≥ 8 versus < 8). TST was expressed as a three-level predictor (≤ 5 h (short sleep duration), 5-8 h (normal sleep duration), > 8 h (long sleep duration), based on clinical relevance and the presumption that there is a U-shaped association between TST and depression.34 In secondary analyses we expressed sleep/wake parameters as continuous variables.
We computed an objective sleep/wake disturbances summary score by counting the number of times a participant was classified as having a sleep/wake disturbance, as defined previously, on each on the actigraphy parameters. Scores ranged from 0 to 5.
Self-Reported Measures of Sleep and Daytime Sleepiness
Participants were asked to complete the PSQI and the ESS. The PSQI is a validated measure of self-reported sleep quality and sleep disturbances over the past month. The questionnaire is divided into sections that assess self-reported sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. Global PSQI scores range from 0 to 21, and a standard cutoff greater than 5 is indicative of poor sleep quality. This cutoff has a sensitivity of 89.6% and a specificity of 86.5% in distinguishing good sleepers from poor sleepers,35–37 and has been shown to have good reliability, internal consistency, and construct validity in the MrOS cohort.38 In primary analyses we expressed PSQI as a dichotomous predictor: > 5 versus ≤ 5, and in secondary analyses we expressed PSQI as a continuous variable.
The ESS is a self-administered questionnaire that classifies self-reported daytime sleepiness in people with sleep disorders. Patients are asked to rate how likely (from 1 to 3, with 1 being unlikely and 3 being highly likely) they are to doze off in eight typical daily situations. ESS scores range from 0 to 24, with a standard cutoff of > 10 indicative of excessive daytime sleepiness.39,40 The ESS has been validated in the MrOS cohort.38 In primary analyses we expressed ESS as a dichotomous cut-point: > 10 versus ≤ 10, and in secondary analyses we expressed ESS as a continuous variable.
Measurement of Depression
Depressive symptoms were evaluated at baseline and at the follow-up visit using the 15-item GDS. The GDS is designed specifically for elderly patients and consists of 15 yes or no questions assessing depressive symptoms. A standard cutoff of six or more symptoms is used to define clinically significant depression; this cutoff has a sensitivity of 91% and a specificity of 65% compared with diagnoses by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.41,42 For this analysis we assessed whether sleep disturbances at baseline were associated with incident depression at a follow-up examination, and therefore, men with depression at baseline (GDS ≥ 6) were excluded.
Other Measures
A trained technician interviewed participants at each examination. Information was obtained on health status,43 alcohol intake, caffeine consumption,44 smoking status, walking for exercise, and impairments in instrumental activities of daily living (IADL).45,46 Information from a previous visit was used to assess age, race/ethnicity, living situation, and education. Participants were asked to report all medications (prescription and nonprescription) used within the past 30 days. All prescription and non-prescription medications were entered into an electronic database and each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).47 Participants were asked about a history of physician-diagnosed stroke, parkinsonism, diabetes mellitus, chronic obstructive lung disease, congestive heart failure, chronic kidney disease, and myocardial infarction. Cognitive function was assessed using the Modified Mini-Mental State Examination.48 Body weight was measured using a balance beam or digital scale. Height was measured using a wall-mounted stadiometer. Height and weight were used to calculate body mass index (kg/m2).
Statistical Analysis
Differences in baseline characteristics according to depression status at the follow-up examination were compared using analysis of variance for normally distributed continuous data, Kruskal-Wallis for skewed continuous data, and chi-square tests for categorical data.
For primary analyses, the associations between sleep disturbances at baseline and odds of being depressed at follow-up were examined using logistic regression in the entire cohort (n = 2,510), and in a subset of participants who reported not using antidepressants at the baseline examination (n = 2,352). Secondary analyses expressed sleep disturbance parameters as continuous variables and calculated odds ratios per standard deviation increase/decrease in sleep parameters.
Models included an age- and clinic site-adjusted base model, the base model further adjusted for number of baseline GDS symptoms, and the base model that further adjusted for multiple potential confounders. Covariates thought to be associated with depression or with sleep disturbances were considered for selection in multivariable models if they showed a significant association at P ≤ 0.10 in univariate analyses. Covariates included in multivariable-adjusted models were: age, clinic site, number of baseline GDS symptoms, health status, education, alcohol use, use of benzodiazepines at baseline, use of antidepressants at baseline (in the cohort that included baseline antidepressant users), cognitive function, walking for exercise, impairments in IADL, and selected medical conditions.
Several sensitivity analyses were conducted and included analyses examining change in GDS symptoms, and analyses excluding men who reported using antidepressants or benzodiazepines at the baseline examination. Because results of sensitivity analyses were consistent with the primary analysis, only results of the primary analysis are shown. Statistical analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of the Study Population
Of the 3,135 men who attended the sleep visit, the analytic cohort consisted of 2,510 men who had complete measures of self-reported and objective sleep and depression and who were classified as nondepressed (GDS < 6) at baseline. Of the 625 men who participated in the sleep examination but who were not included in the analytical cohort, 306 (49%) remained actively enrolled in the study beyond the sleep examination but did not have follow-up data, 272 (44%) died prior to the follow-up visit, 26 had been terminated from the study after the sleep visit, and 21 refused participation in the follow-up visit. On average, men who died or did not have follow-up data had a greater burden of depressive symptoms at baseline, compared with men who were included in the analytic cohort (1.89 versus 1.28 symptoms). Furthermore, compared with men included in the analytical cohort, the 306 surviving enrolled men not included in the analytical cohort were on average older (77.0 versus 75.9 y), and more likely to report poorer health status at baseline (25.6% versus 8.7%).
At the follow-up examination, 1,899 men were not depressed (zero to two depressive symptoms), 495 (19.7%) had some depressive symptoms (three to five), and 116 (4.6%) were depressed with six or more depressive symptoms. The mean and median (inter-quartile range) change in GDS score (computed as follow-up score − baseline score) was 0.44 and 0 (0-1) symptoms respectively (data not shown). Actigraphy data were collected for an average of 4.7 ± 0.7 days.
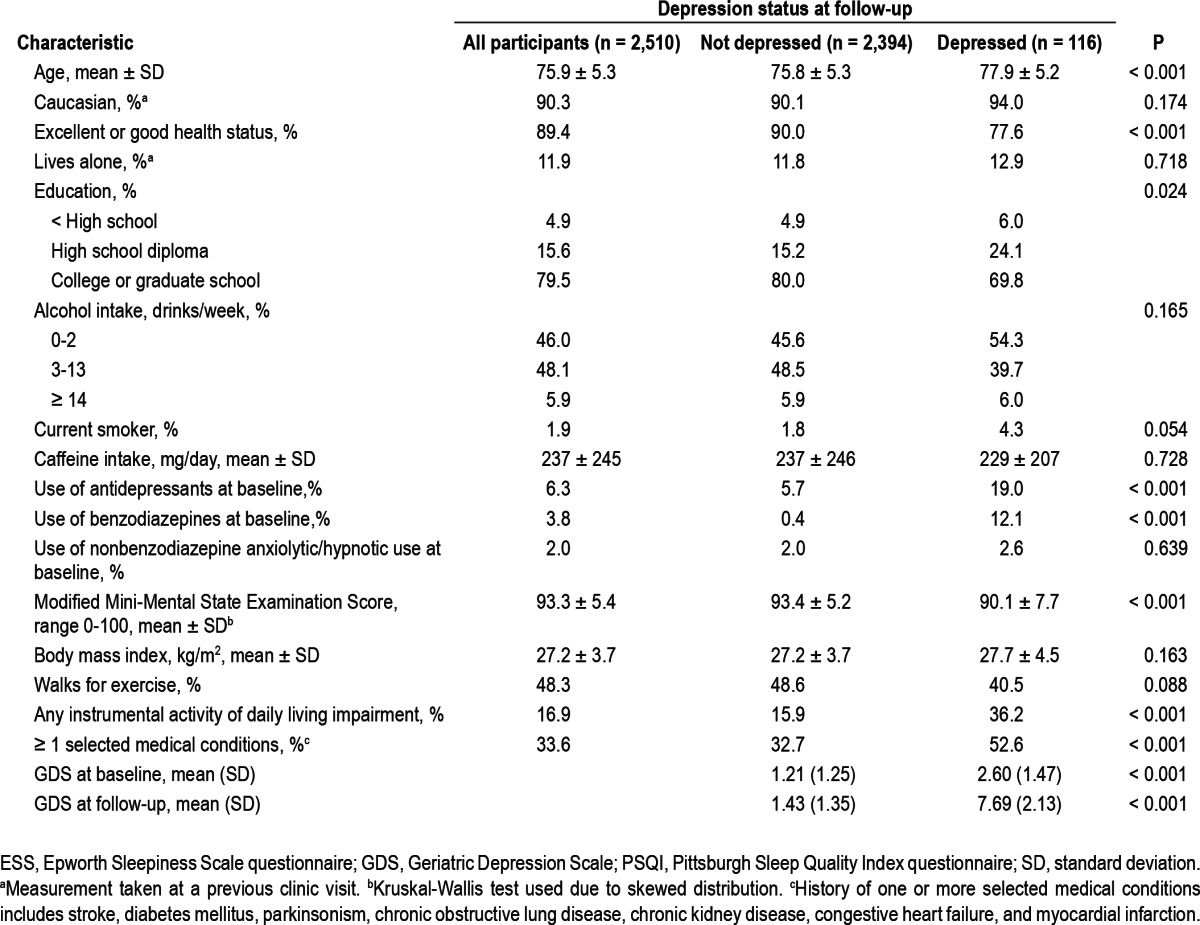
Characteristics of participants according to depression status at follow-up are presented in Table 1. In general, older age, poorer health status, lower educational achievement, current smoking, higher rate of antidepressant use and benzodiazepine use, poorer cognitive function, lower rate of regular walking for exercise, greater impairments in activities of daily living, lower physical activity, and one or more selected medical conditions were associated with depressed status at the follow-up examination.
Table 1.
Baseline characteristics of 2,510 participants according to depression status at follow-up
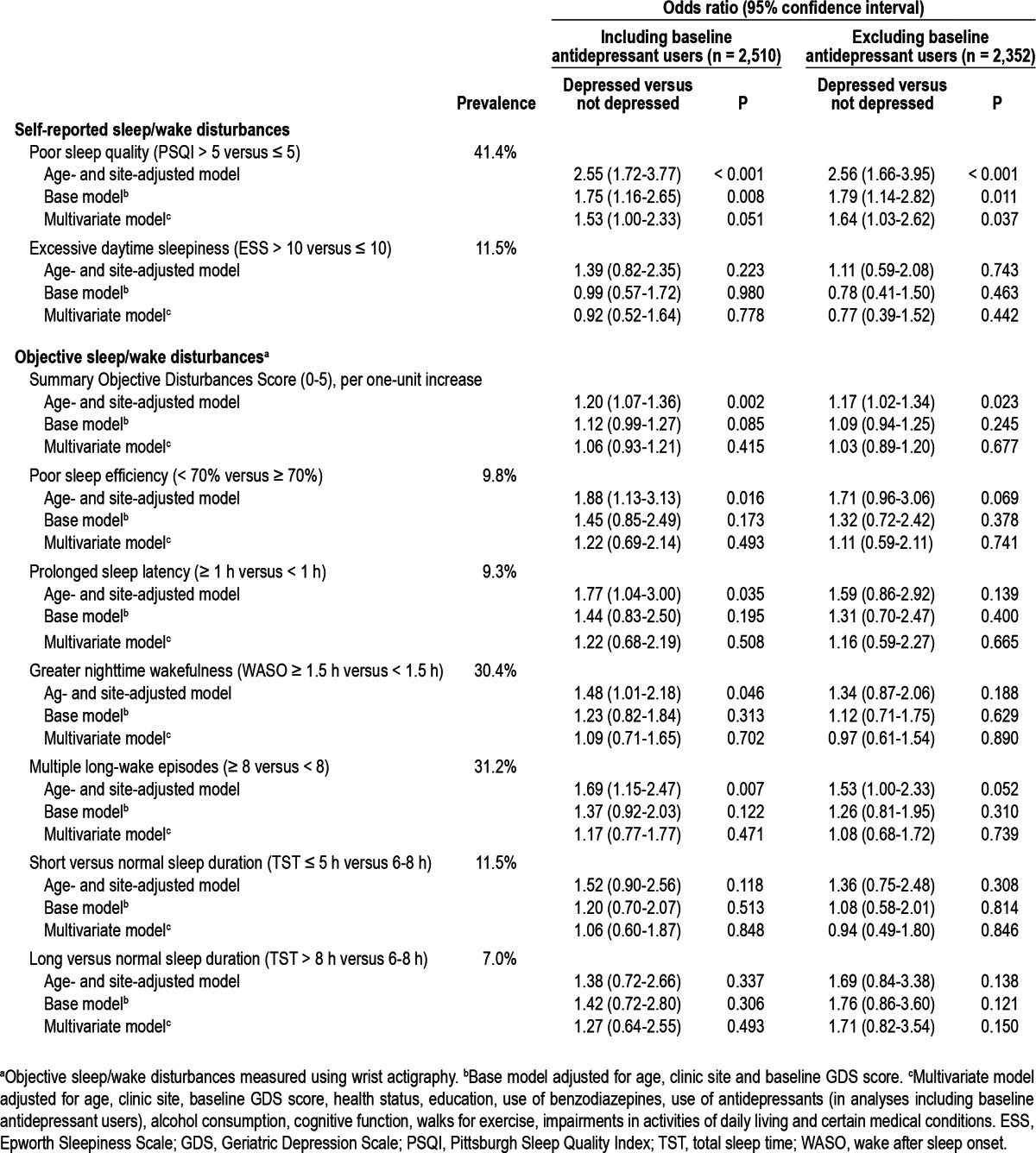
Sleep/wake disturbances were also prevalent in this population. Objective assessments of sleep/wake disturbances via wrist actigraphy suggested that 246 men (9.8%) had sleep efficiency < 70%, 234 (9.3%) had sleep latency ≥ 1 h, 289 (11.5%) slept an average of < 5 h per night, and 176 (7.0%) slept an average of > 8 h per night. Also, 763 men (30.4%) were awake for more than 90 min after sleep onset during the night, and 783 (31.2%) had more than eight long-wake episodes during the night (Table 2). The objective sleep/wake disturbances summary score ranged from 0 to 5, with 1,362 men (54%) having no objective sleep/wake disturbances, 427 (17%) having one objective sleep/wake disturbance, and 721 (29%) having two or more objective sleep/wake disturbances. Similarly, there was also a high prevalence of self-reported sleep/wake disturbances in this cohort, with 1,040 men (41.4%) reporting poor sleep quality (PSQI > 5), and 288 (11.5%) reporting excessive daytime sleepiness (ESS > 10) (data not shown).
Table 2.
Association between sleep/wake disturbances and odds of depression at follow-up
Longitudinal Association of Self-Reported Sleep Disturbances and Depression
In general, poor sleep quality (PSQI > 5) at baseline was associated with a 2.6-fold increased odds of depression at follow-up, in age- and site-adjusted models (OR = 2.55, 95% CI 1.72-3.77) (Table 2). Results were attenuated by approximately 39.6% after adjustment for number of baseline GDS symptoms, but remained significant. After further adjustment for health status, education, use of antidepressants or benzodiazepines at baseline, alcohol consumption, cognitive function, walking for exercise, IADL, and comorbidities, results were further attenuated, but persisted (OR = 1.53, 95% CI 1.00-2.33, P = 0.05). Results in analyses excluding baseline antidepressant users were similar (multivariable OR = 1.64, 95% CI 1.03-2.62, P = 0.037).
There was no association between excessive daytime sleepiness (ESS > 10) and odds of depression in age- and site-adjusted models, or in multivariable adjusted models (Table 2). Results were similar in analyses excluding baseline antidepressant users.
Longitudinal Associations of Objective Sleep/Wake Disturbances and Depression
In age- and site-adjusted models, we observed statistically significant associations between objective sleep/wake summary score (per one-unit increase), sleep efficiency < 70% versus ≥ 70%, sleep latency ≥ 1 h versus < 1 h, awakening after sleep onset ≥ 1.5 h versus < 1.5 h, and eight or more versus fewer than 8 long wake episodes and greater odds of being depressed (Table 2). Results however were attenuated and no longer statistically significant after further adjustment for the number of baseline depressive symptoms, as well as after additional adjustment for multiple potential confounders (Table 2). Tests for an interaction between subjective poor sleep quality and objective summary score were not statistically significant (P for interaction = 0.389). Furthermore, results were similar and remained nonsignificant in analyses that excluded baseline antidepressant users. We did not observe statistically significant associations between sleep duration categories and depression status at follow-up in any of the models.
Additional Analyses
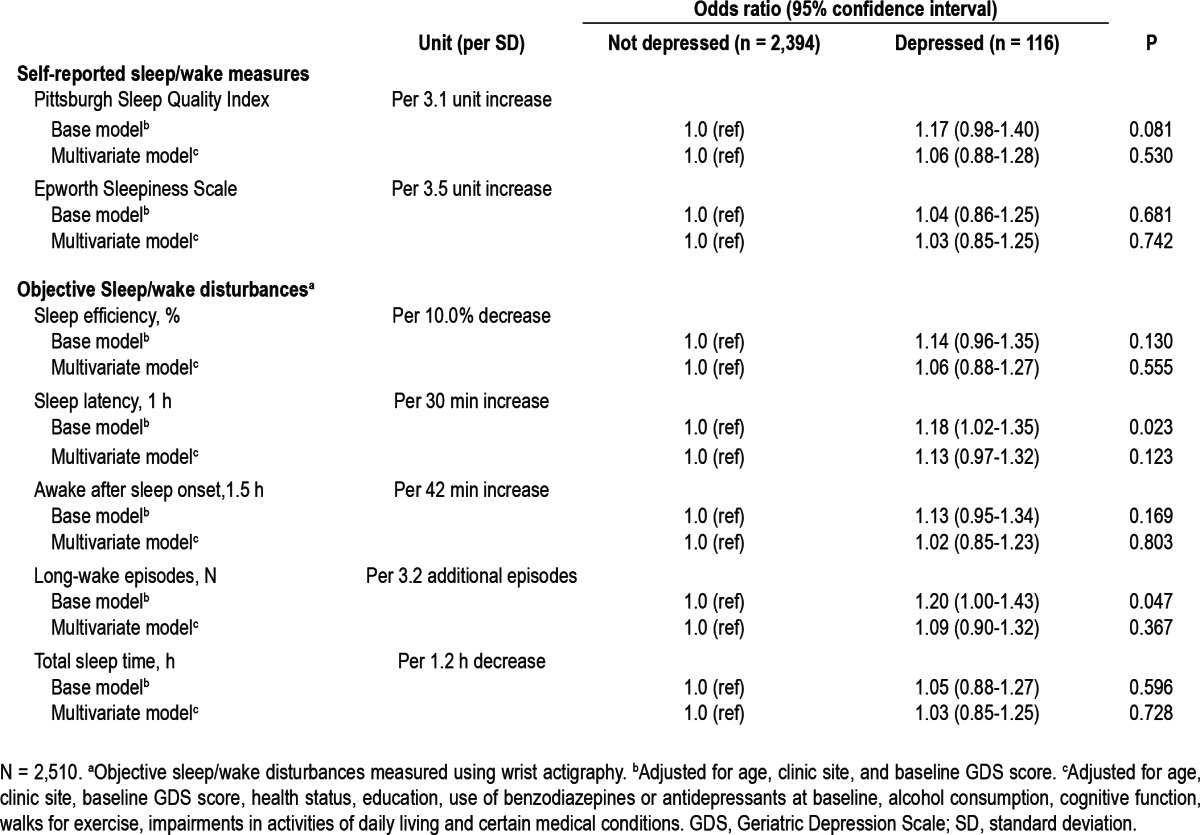
When the sleep parameters were expressed as continuous variables, there was evidence of linear associations of increasing sleep latency, increasing numbers of long-wake episodes, and worsening PSQI with higher odds of depression at follow-up, in models adjusted for age, site, and baseline GDS score (Table 3). After further adjustment for multiple potential confounders, these associations were attenuated and were no longer significant. There was no evidence of linear associations of sleep efficiency, awakening after sleep onset, TST, and ESS with odds of depression at follow-up.
Table 3.
Association between continuous sleep/wake parameters and odds of depression at follow-up
DISCUSSION
Poor self-reported sleep quality at baseline was independently associated with greater odds of incident depression at follow-up in older, community-dwelling men. Men with poor sleep efficiency, prolonged sleep latency, greater nighttime wakefulness, multiple long-wake episodes, and greater burden of objectively measured sleep/wake disturbances also had greater odds of being depressed at the follow-up examination. These associations were mostly explained by having a greater number of depressive symptoms at baseline among men with these sleep disturbances. Furthermore, we did not observe an association of objectively assessed TST or reports of daytime sleepiness with depression status at follow-up.
Although we were unable to identify other studies that have examined the longitudinal associations between actigraphy-assessed sleep/wake disturbances and risk of developing depression, our findings regarding self-reported sleep disturbances are generally consistent with previously published studies. A meta-analysis including longitudinal studies across a variety of age groups found that in comparison with participants without insomnia, nondepressed participants with insomnia or insomnia symptoms have a 2-fold increased risk of developing depression.5 Although we did not specifically assess insomnia in our study, our results also suggested that the odds of being depressed at follow-up ranged from 1.3 to 2.5-fold higher among men with poor sleep quality at baseline. Furthermore, we observed that greater burden of depressive symptoms at baseline and health-related factors partially explained the association between poor sleep quality and risk of depression at follow-up. Because we did not ascertain a self-reported history of depression in the study, we are unable to discern whether burden of depressive symptoms at baseline indicate participants who were not ‘depressed’ according to a GDS score < 6, but who had a history of depressive illness. It is also possible that depressive symptoms at baseline were attributable to sleep disturbances at baseline because the association between sleep and depression may be bidirectional in nature. Furthermore, there was no evidence of linearity in multivariable adjusted models examining associations between self-reported sleep quality (using the PSQI) and odds of depression at follow-up. This suggests that a threshold rather than linear association exists between sleep quality and depression. We also did not find any evidence of an interaction between poor subjective sleep quality (PSQI > 5 versus ≤ 5) and objective sleep/wake disturbances summary score, which suggests that in this cohort, associations between objective sleep/wake measures did not differ by presence/absence of subjective poor sleep quality.
Prior studies using objective measures (PSG) of sleep have observed that dysregulations in REM sleep patterns are associated with recurrent depressive episodes18,19 and that disturbances in PSG parameters (such as reduced REM latency and slow wave sleep) are associated with a greater lifetime risk of depression.20 Our current analysis did not use PSG parameters.
Our findings also suggest that the associations between objectively assessed poor sleep efficiency, prolonged sleep latency, greater nighttime wakefulness and multiple long-wake episodes, and increased odds of depression at follow-up are largely explained by a higher burden of depressive symptoms at baseline among older men with these sleep disturbances. The results of our previously published cross-sectional analyses21 showed independent associations between greater level of depressive symptoms (expressed as GDS 0-2; 3-5; ≥ 6) and higher odds of poor sleep quality, excessive daytime sleepiness, and objectively assessed prolonged sleep latency at baseline. The clinical implications of our findings, both cross-sectional and longitudinal, suggest that older men presenting with reports of disturbed sleep should be evaluated for prevalent depressive symptoms and depression. The underlying mechanisms explaining why poor self-reported sleep quality, but not objective assessments of sleep/wake disturbances, is an independent predictor of future depression are not understood. Future studies should continue to focus efforts on furthering our understanding of the mechanisms underlying sleep and depression pathways and the direction of the associations.
The strengths of this study include a large sample size; excellent follow-up (> 90%), participants from six different areas of the United States; and participant selection that was not contingent on presence of a sleep disorder, depression, or other medical conditions; a comprehensive set of measurements, including validated measures of both self-reported and objective sleep/wake parameters; and robustness of results to sensitivity analyses.
There are also several limitations to note. Because the cohort included only older, community-dwelling, primarily Caucasian men, these results may not apply to other populations. Also, these analyses rely on a self-reported questionnaire to assess depressive symptoms and depression. Although this scale has performed well in validation studies, it is not a clinical measure of depression; therefore, studies using clinical measures of depression would improve our understanding of the sleep/wake-depression association. Information on history of depression prior to baseline was not assessed, and we are unable to discern whether the men in our study have had prior depressive episodes. Furthermore, approximately 75% of the cohort experienced no change or only a one-unit change in GDS symptoms during follow-up. Although these men were generally healthy at baseline, there remains the question about how sensitive the GDS is to change in symptoms in older men. Future studies should further evaluate the sensitivity of the GDS to change, and confirm results using other measures of depression. Future studies should also examine longitudinal associations using alternative objective parameters (such as those obtained with PSG), as well as explore the interrelationships between objective and subjective measures of sleep. The follow-up time was relatively short, and some of the associations may reflect reverse causality.
There are also a few limitations to note regarding actigraphy. Actigraphy algorithms may be less reliable in people who have insomnia, and will result in overestimations of periods of sleep. It is also important to note that there was a modest correlation between actigraphy and PSG in our cohort (ICC = 0.32-0.57).26 We did not assess insomnia in our cohort, but are confident that if this bias exists, it likely has an attenuating effect on our results.
CONCLUSION
In summary, in a cohort of nondepressed, community-dwelling older men at baseline, poor subjective sleep quality was associated with depression at follow-up. Associations of objectively measured reduced sleep efficiency, prolonged sleep latency, greater nighttime wakefulness, and greater long-wake episodes with depression at follow-up were mostly explained by having a greater number of depressive symptoms at baseline in men with these sleep disturbances.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Ancoli-Israel has been a consultant or served on the advisory board of Ferring Pharmaceuticals, GlaxoSmithKline, Merck, Neurocrine Biosciences, Pfizer, Respironics/Philips, Sanofi-Aventis, Somaxon, and had a grant from Litebook, Inc. Dr. Corey-Bloom has received research funds from Elan, SIENA, PRANA, and Teva. Dr. Redline has received research equipment and/or research support from Philips Respironics and ResMed Inc. and ResMed Foundation. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health (NIH) funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. This work was also supported with resources and the use of facilities at the Minneapolis VA Medical Center. The views expressed herein do not necessarily represent the views of the Department of Veterans Affairs or the United States Government.
REFERENCES
- 1.Ancoli-Israel S. Sleep and aging: prevalence of disturbed sleep and treatment considerations in older adults. J Clin Psychiatry. 2005;66:24–30. [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53:S264–71. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 3.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135:10–9. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: a systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 7.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 8.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Spira AP, Covinsky K, Rebok GW, et al. Poor sleep quality and functional decline in older women. J Am Geriatr Soc. 2012;60:1092–8. doi: 10.1111/j.1532-5415.2012.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan CM, Kroenke K, Counsell SR, et al. Treatment of depression improves physical functioning in older adults. J Am Geriatr Soc. 2005;53:367–73. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- 11.Whooley MA, Kip KE, Cauley JA, Ensrud KE, Nevitt MC, Browner WS. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:484–90. doi: 10.1001/archinte.159.5.484. [DOI] [PubMed] [Google Scholar]
- 12.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 13.Ohayon MM, Roth T. What are the contributing factors for insomnia in the general population? J Psychosom Res. 2001;51:745–55. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 14.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokoyama E, Kaneita Y, Saito Y, et al. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010;33:1693–702. doi: 10.1093/sleep/33.12.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London. Br J Gen Pract. 1993;43:445–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Kupfer DJ, Ehlers CL, Frank E, Grochocinski VJ, McEachran AB. EEG sleep profiles and recurrent depression. Biol Psychiatry. 1991;30:641–55. doi: 10.1016/0006-3223(91)90010-j. [DOI] [PubMed] [Google Scholar]
- 19.Kupfer DJ. Sleep research in depressive illness: clinical implications--a tasting menu. Biol Psychiatry. 1995;38:391–403. doi: 10.1016/0006-3223(94)00295-E. [DOI] [PubMed] [Google Scholar]
- 20.Giles DE, Kupfer DJ, Rush AJ, Roffwarg HP. Controlled comparison of electrophysiological sleep in families of probands with unipolar depression. Am J Psychiatry. 1998;155:192–9. doi: 10.1176/ajp.155.2.192. [DOI] [PubMed] [Google Scholar]
- 21.Paudel ML, Taylor BC, Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–35. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. J Am Geriatr Soc. 2012;60:635–43. doi: 10.1111/j.1532-5415.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell T, Ancoli-Israel S, Redline S, Stone KL. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–67. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambulatory Monitoring. Action-W User's Guide. 2012 [Google Scholar]
- 28.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell T, Ancoli-Israel S, Redline S, Stone KL. Factors that may influence the classification of sleep-wake by wrist actigraphy: the MrOS Sleep Study. J Clin Sleep Med. 2011;7:357–67. doi: 10.5664/JCSM.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ensrud KE, Blackwell TL, Redline S, et al. Sleep disturbances and frailty status in older community-dwelling men. J Am Geriatr Soc. 2009;57:2085–93. doi: 10.1111/j.1532-5415.2009.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ensrud KE, Blackwell TL, Ancoli-Israel S, et al. Sleep disturbances and risk of frailty and mortality in older men. Sleep Med. 2012;13:1217–25. doi: 10.1016/j.sleep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fava M. Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 2004;65:27–32. [PubMed] [Google Scholar]
- 35.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 36.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF, III, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 38.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67A:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 40.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 41.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 2012;5:165–73. [Google Scholar]
- 42.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Ware J, Kosinski M, Keller S. SF-12: How to score the SF-12 Physical and Mental Health Summary Scores. 1998 [Google Scholar]
- 44.Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–29. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 45.Pincus T, Summey JA, Soraci SA, Jr, Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 46.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987:1–115. [PubMed] [Google Scholar]
- 47.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 48.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]


