Abstract
beclin 1, the mammalian homologue of the yeast Atg6, is a key autophagy-promoting gene that plays a critical role in the regulation of cell death and survival of various types of cells. However, recent studies have observed that the expression of beclin 1 is altered in certain diseases including cancers. The causes underlying the aberrant expression of beclin 1 remain largely unknown. We report here that microRNAs (miRNAs), a class of endogenous, 22–24 nucleotide noncoding RNA molecules able to affect stability and translation of mRNA, may represent a previously unrecognized mechanism for regulating beclin 1 expression and autophagy. We demonstrated that beclin 1 is a potential target for miRNA miR-30a, and this miRNA could negatively regulate beclin 1 expression resulting in decreased autophagic activity. Treatment of tumor cells with the miR-30a mimic decreased, and with the antagomir increased, the expression of beclin 1 mRNA and protein. Dual luciferase reporter assay confirmed that the miR-30a binding sequences in the 3′-UTR of beclin 1 contribute to the modulation of beclin 1 expression by miR-30a. Furthermore, inhibition of beclin 1 expression by the miR-30a mimic blunted activation of autophagy induced by rapamycin. Our study of the role of miR-30a in regulating beclin 1 expression and autophagy reveals a novel function for miRNA in a critical cellular event with significant impacts in cancer development, progression and treatment, and in other diseases.
Keywords: beclin 1, autophagy, microRNA, miR-30a, gene expression
Introduction
Autophagy, a conserved, programmed response to metabolic and environmental stress found in yeast, plants, worms, flies, mice and man,1 has been known to play a critical role in the regulation of survival and death of various types of cells.2 Recent studies have implicated autophagy in a number of physiologic and pathophysiologic processes such as aging, cancers and neurodegenerative diseases.3 The process of autophagy involves formation of double-membrane vesicles (autophagosomes) that engulf organelles and cytoplasm, then fuse with the lysosome to form the autolysosme, where the contents are degraded and recycled for protein and ATP synthesis.4,5 The formation of the autophagosome is mediated by a series of autophagy-promoting gene products that function at different stages of autophagy.6 beclin 1, the mammalian homologue of the yeast Atg6, is a key autophagy-promoting gene whose product is part of a lipid kinase (class III Phosphoinositide 3-kinase) complex that participates in the early stage of autophagosome formation.7,8 Beclin 1 is a ~60-kDa coiled-coli protein also able to interact with bcl-2, an anti-apoptotic protein. Although ubiquitously expressed, it has been known that the expression of beclin 1 is altered in certain diseases. For example, in early Alzheimer disease beclin 1 expression is decreased;9 in contrast, neurodegeneration causes upregulation of beclin 1.10 In several types of human cancers, the expressions of beclin 1, both protein and mRNA, were also found to be aberrant.11-13 Yet, the causes underlying the altered expression of this key autophagy-promoting gene remain largely unknown. In the present study we sought to explore the role of microRNAs (miRNAs) in the regulation of expression of beclin 1. MiRNAs are a class of endogenous, 22–24 nucleotide RNA molecules with the ability to induce mRNA degradation, translational repression, or both, via pairing with partially complementary sites in the 3′ UTR of the targeted genes.14-17 It is estimated that more than 1,000 miRNAs exist in mammalian cells, and that 30% of all genes are regulated by miRNAs.18-20 Because of their capacity to target numerous mRNAs, miRNAs can regulate the expression of genes in a number of pathways that are associated with tumor initiation, development and progression.21,23 We report here that the autophagy-promoting gene beclin 1 is a potential target for miR-30a, and this miRNA may play a regulatory role in autophagic response through modulating the expression of beclin 1.
Results
beclin 1 is a putative target for miR-30a. To explore the possible role of miRNAs in regulating autophagy, we first analyzed the miRNA expression profiling in tumor cells treated with HBSS (nutrient deprivation) or rapamycin. Nutrient deprivation and rapamycin treatment are known to activate autophagy in various types of cells.26,27 Using a miRNA microarray (OSUCCC-microRNA version 4.0) analysis, we observed a differential expression of 13 miRNAs in T98G and MDA-MB-468 cells treated with HBSS or rapamycin, as compared with the untreated cells or the cells treated with vehicle. The heat map of these miRNA expression profiles of those samples is shown as Supplemental data (Fig. S1). We then conducted an in silico search for miRNA binding sites using the PicTar algorithm (http://pictar.bio.nyu.edu). Among those 13 miRNAs that were differentially expressed in the HBSS or rapamycin-treated cells, we found in the 3′-UTR of beclin 1 the consensus sequences for miR-30a, implying that beclin 1 is a potential target for miR-30a. To verify the change of miR-30a expression following HBSS or rapamycin treatment, we performed qRT-PCR analysis of the endogenous miR-30a expression. Figure 1 shows a 10% and 35% reduction of miR-30a expression in cells subjected to nutrient depletion or rapamycin treatment, respectively. These results suggest a possible role for this miRNA in targeting beclin 1 in response to stresses.
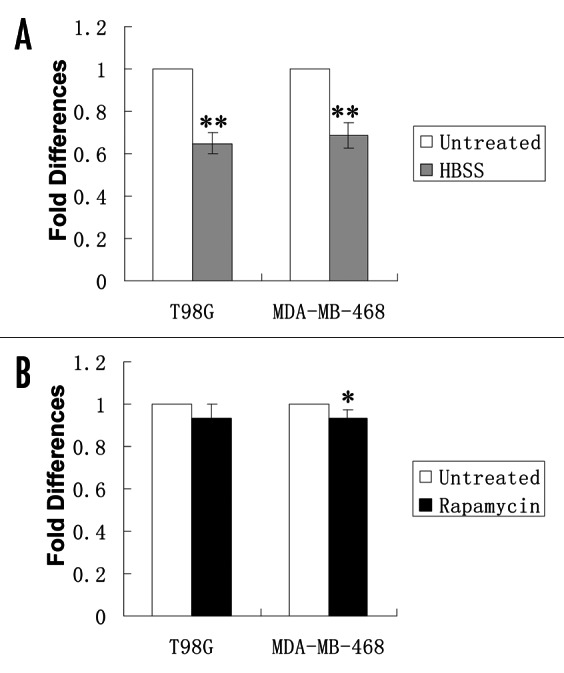
Figure 1. Effects of HBSS and rapamycin on endogenous miR-30a expression. T98G and MDA-MB-468 cells were treated with HBSS for 4 h or rapamycin (200 nM) for 12 h. At the end of treatment, endogenous miR-30a expression was analyzed by Real-time RT-PCR, as described in “Material and Methods.” Small nuclear RNA (RNU66) was used as internal control. The expression level of miR-30a was calculated using the MxPro software (Version 4.00, Stratagene). Results shown are the mean ± SD of triplicate determinations from one of three identical experiments. *p < 0.05 vs. control; **p < 0.01 vs. control.
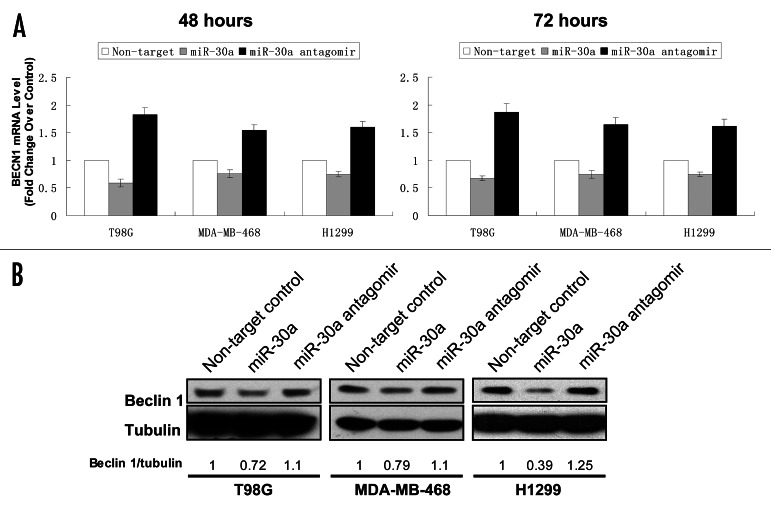
MiR-30a negatively regulates beclin 1 expression. To obtain experimental evidence supporting beclin 1 gene as a target for miR-30a, we next examined the effect of miR-30a on beclin 1 expression using a mimic and an antagomir of this miRNA. In these experiments, T98G, MDA-MB-468 and H1299 cells were transfected with a miR-30a mimic or an antagomir, and then the expressions of beclin 1 mRNA and protein were analyzed by qTR-PCR and western blot, respectively. As shown in Figure 2, transfection with the miR-30a mimic caused a 25–40% decrease in beclin 1 mRNA (Fig. 2A), and a 25–60% decrease in beclin 1 protein (Fig. 2B); by contrast, treatment with the miR-30a antagomir resulted in a 55–85% increase in beclin 1 mRNA (Fig. 2A) and a 10–25% increase in beclin 1 protein (Fig. 2B). Using the mimic and antagomir of miR-30a, these experiments demonstrated a suppressive role for this miRNA in beclin 1 expression.
Figure 2. Effect of miR-30a on the expression of beclin 1. (A) T98G, MDA-MB-468 and H1299 cells were transfected with a mimic or antagomir of miR-30a (100 nM) or a control RNA (100 nM). Forty-eight and 72 hours later, total RNAs were extracted from the treated cells and quantitative real-time RT-PCR analyses of beclin 1 mRNA were performed as described in “Material and Methods.” beclin 1 mRNA levels of the cells treated with a control RNA were arbitrarily set at 1, and beclin 1 mRNA levels of the cells treated with the miR-30a mimic were normalized to the control. Results shown are the mean ± SD of triplicate determinations from one of three identical experiments. (B) T98G, MDA-MB-468 and H1299 cells were treated as described in (A). Forty-eight hours later, cell lysates were prepared from the transfected cells. Equal amounts (25 μg proteins) of cell lysates were separated by 8% SDS-PAGE, and then transferred onto nitrocellulose membranes. The membranes were immunoblotted with a monoclonal anti-beclin 1 antibody. Detection of beclin 1 was performed using enzyme-linked chemiluminescence. 〈-tubulin was used as a loading control. Protein expression was quantified using the ImageJ software. Beclin 1/tubulin ratios of the samples treated with a control RNA was arbitrarily set at 1, and the Beclin 1/tubulin ratios of the miR-30a mimic or antagomir-treated samples were normalized to the control. Results shown are the mean ± SD of triplicate determinations from one of three identical experiments.
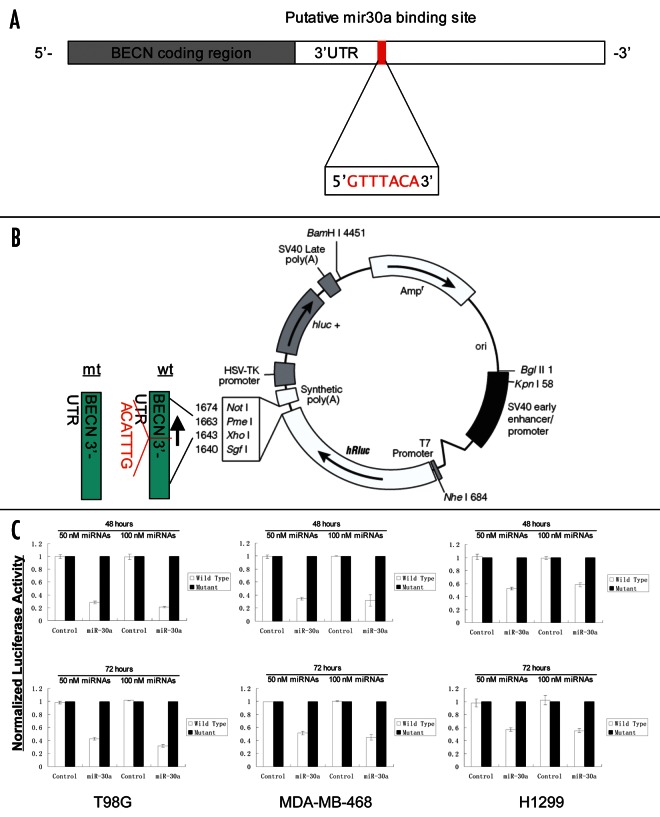
To validate the predicted consensus sequences for miR-30a in the beclin 1-3′ UTR, and determine whether these miR-30a binding sequences directly contributed to the negative regulation of beclin 1 expression, we tested the effects of miR-30a on activity of a reporter gene using the vectors that either contained wild-type miR-30a targeting sequences (psiCHECKTM2-WT-BECN-3′-UTR) or deletion mutant (psiCHECKTM2-MT-BECN-3′-UTR) (Fig. 3B). As shown in Figure 3C, cotransfection of T98G, MDA-MB-468 and H1299 cells with 50 nM or 100 nM of the miR-30a mimic resulted in a 40–80% reduction in the activity of the reporter gene vector containing the wild-type miR-30a targeting sequences (psiCHECKTM2-WT-BECN-3′-UTR), in comparison to that of the vector with the deletion mutant (psiCHECKTM2-MT-BECN-3′-UTR). In contrast, the nontargeting control RNA did not have any effect on the reporter activity of either of the vectors (Fig. 3C). These results demonstrated that the miR-30a binding sequences in the beclin 1-3′ UTR is the region required for the miR-30a-mediated inhibition of beclin 1 expression.
Figure 3.beclin 1 is a target for miR-30a. (A) The miR-30a consensus sequences in the beclin 1 3′-UTR. (B) Construction of the psiCHECKTM2-WT-BECN-3′-UTR and psiCHECKTM2-MT-BECN-3′-UTR. The 1,574 bp fragment of the beclin 1 3′-UTR containing the miR-30a consensus sequences was inserted into the psiCHECKTM2 dual luciferase reporter plasmid at the 3′ end of the coding sequence of R. reniformis luciferase. The deletion mutant (psiCHECKTM2-MT-BECN-3′-UTR) was generated using mutagenesis PCR method. (C) Luciferase reporter assays. Cells were cotransfected with either psiCHECKTM2-WT-BECN-3′-UTR or psiCHECKTM2-MT-BECN-3′-UTR vector and a miR-30a mimic or a nontargeting control RNA. Firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega), and Renilla luciferase activity was normalized to firefly luciferase activity. Results shown are the mean ± SD of triplicate determinations from one of three identical experiments.
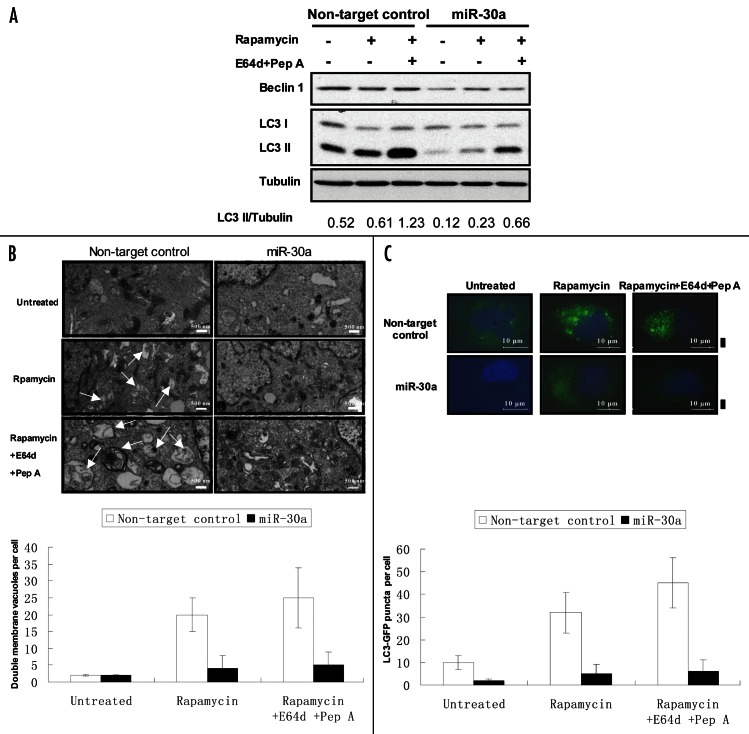
Effect of miR-30a on autophagic activity. Beclin 1 plays an essential role in activating autophagy. To determine the functional consequence of modulation of beclin 1 expression by miR-30a, we tested the effect of the miR-30a mimic on autophagic response to rapamycin. Figure 4 shows that treatment of T98G cells with rapamycin activated autophagy, as evidenced by the increases in LC3-II amount (Fig. 4A), in double membrane vacuoles in the cytoplasm (Fig. 4B), and in formation of GFP-LC3 aggregation (Fig. 4C). Notably, tumor cells transfected with the mimic of miR-30a showed a remarkable reduction in beclin 1 expression, and a blunted autophagic response to rapamycin, as evidenced by the lower LC3-II levels (Fig. 4A), fewer double membrane vacuoles (Fig. 4B), and less GFP-LC3 aggregations (Fig. 4C), indicating that inhibition of beclin 1 expression by miR-30a leads to -suppression of autophagic activity. To confirm that the effect of miR-30a on autophagy was mediated through beclin 1, we tested the effect of miR-30a on the resveratrol-induced autophagy in MCF-7 cells, which was reported to be beclin 1-independent.28Figure 5 shows that miR-30a mimic had no effect on autophagy induced by resveratrol in MCF-7 cells.
Figure 4. Effect of miR-30a on autophagic response to rapamycin. (A) T98G cells transfected with a mimic of miR-30a or a nontargeting control RNA were treated with rapamycin (200 nM) in the presence or absence of lysosomal protease inhibitors E64D (10 μg/ml) and pepstatin A (10 μg/ml). At the end of treatment, formation of LC3-II was detected by immunoblotting with an anti-MAP-LC3 antibody as described in “Materials and Methods.” (B) T98G cells were treated as described in (A), and then were harvested by trypsinization, fixed and embedded in spur resin. Ninety nm-thin sections were cut and examined at 80 Kv with a JEOL 1200EX transmission electron microscope. Arrows indicate autophagic vacuoles. Double membrane vacuoles per cell were determined by counting 20 cells for each sample and average numbers of double membrane vacuoles were shown. The bars are the mean ± S.D. (C) GFP-LC3-expressing T98G cells were treated as described in (A). At the end of treatment, cells were fixed with 4% formaldehyde for 15 min and inspected at 60x magnification for numbers of GFP-LC3 puncta. LC3-GFP puncta per cell were determined by counting 20 cells for each sample and average numbers of puncta per cell were shown. The bars are the mean ± S.D. Results shown are the representative of three identical experiments.
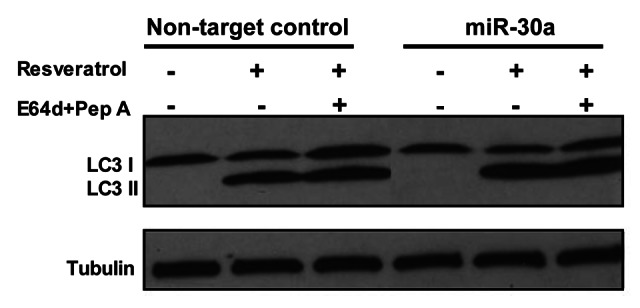
Figure 5. miR-30a does not affect the resveratrol-induced beclin 1-independent autophagy. MCF-7 cells transfected with a mimic of miR-30a or a nontargeting control RNA were treated with resveratrol (50 μM) in the presence or absence of lysosomal protease inhibitors E64D (10 μg/ml) and pepstatin A (10 μg/ml). At the end of treatment, formation of LC3-II was detected by immunoblotting with an anti-MAP-LC3 antibody as described in “Materials and Methods.”
Discussion
It is known that altered autophagic activity is associated with a number of diseases such as cancer,18,29,30 and that the beclin 1-mediated autophagy plays an important role in the regulation of cell survival and death.8,31 However, beclin 1 expression has been found to be aberrant in certain diseases including cancers.9-13 How autophagy and beclin 1 expression are regulated is not fully understood. The current study reports our finding that miRNAs can control the expression of beclin 1 expression, thereby modulating autophagic activity. Through the use of mimic and antagomir, we demonstrate that the miRNA miR-30a is able to modulate autophagy through inhibiting beclin 1 expression, and this effect is mediated via the miR-30a consensus sequences contained in the 3′-UTR of beclin 1. To our knowledge, this is the first report on the role of miRNAs in regulating autophagy, an important cellular process that is involved in many physiological and pathophysiologic events including cancer.
The role of miR-30a in the regulation of beclin 1 expression is evidenced by our experiments showing that transfection of tumor cells with the miR-30a mimic resulted in decreases in both mRNA and protein of beclin 1 (Fig. 2), and in reductions of the activity of a reporter gene plasmid containing the consensus sequences for miR-30a (Fig. 3). By contrast, in the absence of the miR-30a consensus sequences the inhibitory effect of miR-30a on the reporter activity was abolished (Fig. 3C). Furthermore, we showed that transfection of cells with an antagomir of miR-30a caused an increase in beclin 1 mRNA and protein (Fig. 2), providing additional evidence for a possible role of miR-30a in controlling beclin 1 expression. Inhibition of beclin 1 expression by miR-30a leads to suppression of autophagic activity, as transfection with the miR-30a mimic also blunted autophagic response of tumor cells to rapamycin (Fig. 4), an activator of autophagy.32 Additionally, the role of miR-30a in modulating autophagy and beclin 1 expression is supported by our analysis of the endogenous miRNA expression, which showed a various degrees of reduction of miR-30a expression in the cells subjected to different treatments (Fig. 1), although Beclin 1 protein levels appeared unchanged following those treatments (Fig. 4A). This is probably because although downregulation of miR-30a can slow down the degradation of beclin 1 mRNA or/and de-repress translation of Beclin 1 protein, the change of the endogenous miR-30a caused by rapamycin is not sufficient to upregulate Beclin 1 protein level. In fact, an increase in Beclin 1 has not so far been found to be a requisite for activation of autophagy. The results of the current study mainly depend on use of the mimic and antagomir to miR-30a; nevertheless, whether and how endogenous miR-30a actually regulates beclin 1 expression, and whether the expression of Beclin 1 protein is indeed regulated by miR-30a, would need further investigation. Additionally, the specific effect of miR-30a on Beclin 1-mediated autophagy was supported by our results showing that the resveratrol-induced autophagy in MCF-7 cells, which was reported to be Beclin 1-independent,28 was not affected by treatment with miR-30a mimic (Fig. 5).
In this study we have found that beclin 1 expression is negatively regulated by miR-30a. As a series of gene products such as Atg5, Atg7 and Atg10 participate in activating autophagy,6 it is likely that other autophagy-promoting genes are also regulated by miRNAs, given the pervasive regulatory functions of these small RNA molecules in biology. The results reported here were obtained with cancer cell lines in this study. Nevertheless, aberrant expression of miR-30a and some other miRNAs have been indeed found in human cancers.33 It would be important and interesting to further analyze if a direct correlation between the aberrant expression of endogenous miR-30a and altered expression of beclin 1 exists in cancer specimens and cell lines, although such a study would require a statistical analysis of an adequate size of samples to make a sound conclusion. However, it remains to be determined whether or not miR-30a also participates in regulating beclin 1 expression and autophagy in nonmalignant cells. Involvement of miRNA in regulating autophagy is -undoubtedly worth further investigation, and these studies should yield new insights into how autophagy is regulated, and the association of this cellular response with various diseases.
The evidence for the importance of both autophagy and miRNAs in cancers has been emerging in recent years. It is becoming increasingly recognized that altered autophagy is associated with tumor formation and progression, and with the altered response to several forms of cancer treatments.34 For example, we and others observed that autophagy played an essential role in survival of malignant cells under environmental, metabolic or therapeutic stress conditions.35,36 We found that induction of autophagy protects cancer cell viability under cellular stress such as nutrient deprivation.35 Katayama et al. reported that autophagy-associated ATP surge protected cancer cell viability and contributed to resistance to cytotoxic drugs such as etoposide and temozolomide.36 Also, aberrant expressions of miRNAs are known to impact on many patho-physiologic processes, including proliferation, apoptosis and stress response,37,38 and are implicated in tumor initiation, development and progression. For instance, expression of miR-21 has been reported to promote tumor cell proliferation and growth, and these effects are mediated through upregulation of bcl-2 expression.39 Our recent comparison of miRNA expression profilings between multidrug resistant and drug sensitive cancer cells revealed a differential expression pattern, and we further found that miR-27a and miR-451 are involved in the modulation of expression of MDR1 gene, whose product is the multidrug transporter, P-glycoprotein.25 The results reported here suggest that miRNAs have the potential of modulating autophagy through regulation of the expression of the key autophagy genes such as beclin 1, providing evidence for a new role of miRNAs in a cellular process with the importance that has been recognized increasingly in cancer biology.
Materials and Methods
Cell culture and reagents
Human breast cancer cell lines MDA-MB-468 and MCF-7, lung cancer cell line H1299, and glioma cell line T98G were purchased from American Type Culture Collection. MDA-MB-468 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies, Cat. No. 31053036); MCF-7 and H1299 cells were maintained in RPMI 1640 medium (Invitrogen Life Technologies, Cat. No. 22400089); and T98G cells were maintained in Ham’s F-10 (Invitrogen Life Technologies, Cat. No. 11550043)/DMEM (10:1) medium. All the media contained 10% (v/v) fetal bovine serum (Sigma, Cat. No. F4135), 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen Life Technologies, Cat. No. 15140163). Cell lines were cultured in a 5% CO2-humidified incubator at 37°C.
The mimic (Cat. No. C-30050503) and antagomir (Cat. No. IH-300505-05) of miR-30a and a control miRNA (Cat. No. CN-001000-01) were purchased from Dharmacon Inc., Hank’s buffered salt solution (HBSS) was purchased from Invitrogen Life Technologies. (Cat. No. 14025092). Other reagents were purchased from Sigma-Aldrich.
MiRNA microarray profiling
Total RNAs from cells were extracted using TriZol Reagent (Invitrogen Life Technologies, Cat. No. 10296010). RNA labeling and hybridization on miRNA microarray were performed as described previously.24 Briefly, 5 μg of total RNA from each sample was biotin-labeled by reverse transcription using 5′ biotin end-labeled random octomer oligo primer. Hybridization of biotin-labeled cDNA was carried out on miRNA microarray chip (OSU version 4.0, Ohio State University, Columbus, OH), which contains 1,600 miRNA oligo probes derived from 474 human and 373 mouse miRNA genes and printed in duplicates. Hybridization signals were detected by biotin binding of a Streptavidin-Alexa 647 conjugate using an Axon Scanner 4000B. The images were quantified using GenePix 6.0 software (Axon Instrument Inc.,).
Real-time RT-PCR analysis of miRNA
TaqMan® miRNA assays were performed to determine the endogenous mature miRNA expression. Briefly, miRNAs were converted to cDNA using TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems, Cat. No. 4366596), followed by TaqMan® based quantative PCR on the Stratagene 3005P Real-TimePCR system using the miRNA-specific primers from Applied Biosystems. Small nuclear RNA (RNU66) was used as internal control. The expression level of miRNA was calculated using MxPro software (Version 4.00, Stratagene).
Dual luciferase reporter assay
The 1,574 bp fragment of the beclin 1 3′-UTR containing the miR-30a targeting sequence (GTTTACA) was cloned into the psiCHECKTM-2 dual Luciferase reporter plasmid (Promega, Cat. No. C8021) at the 3′ end of the coding sequence of R. reniformis luciferase to produce psiCHECKTM2-WT-BECN-3′-UTR using PCR (primer Forward: 5′GCA AGC CAG ACA GGA AAA AG3′; Reverse: 5′AAC ATC AAG GGG GAA AAT CC3′). To produce the deletion mutant (psiCHECKTM2-MT-BECN-3′-UTR) of the miR30a targeting site, mutagenesis PCR was performed with the following condition: 15 cycles of 30 s at 95°C, 15 cycles of 30 s at 55°C, and 2 min/kb template plasmid DNA at 68°C (BECN1 miR-30a mutagenesis primer Forward: 5′-CCT TAA CGA AAA TTT CCT ATG TCT GTC TAT TGG TAT GC-3′; BECN1 miR-30a mutagenesis primer Reverse: 5′-GCA TAC CAA TAG ACA GAC ATA GGA AAT TTT CGT TAA GG-3′). The accuracy of the plasmid inserts was determined by complete sequencing analysis.
For the reporter assays, cells were cultured to approximately 80% confluence in a 6-well plate, and then cotransfected with either psiCHECKTM2-WT-BECN-3′-UTR or psiCHECKTM2-MT-BECN-3′-UTR vector and the miR-30a mimic for 48 or 72 hours. Firefly and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay system (Promega, Cat. No. E1910), and Renilla luciferase activity was normalized to firefly luciferase activity.
MiRNAs transfection. Cells in exponential phase of growth were plated in 60 mm plates at 1 x 106 cells/plate and cultured overnight, then transfected with a mimic or antagomir of miR-30a, or a nontargeting control RNA (100 nM) using Lipofectamine 2000 and OPTI-MEM I reduced serum medium (Invitrogen Life Technologies, Cat. No. 51985034), according to the manufacturer’s protocol. The effects of miR-30a and the nontarget control RNA were examined by western blot and real-time RT-PCR.
Western blot analysis
Cells were washed with PBS containing Protease Inhibitor Cocktail (Pierce Biotechnology Inc., Cat. No. 78429), and then lysed in CelLyticTM MT Cell Lysis Reagent (Sigma-Aldrich, Cat. No. C3228). The lysates were transferred to 1.5 ml Eppendorf tubes and clarified by centrifugation at 16,000 xg for 25 min at 4°C. Equal amounts (25 μg) of cell lysates were resolved by SDS-PAGE, and then transferred to nitrocellulose membranes. The membranes were incubated in 5% nonfat milk in TBST-0.1% Tween-20 at room temperature for 1 h, then immunoblotted with the respective antibodies. Detection by enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol (ECL; Pierce Biotechnology Inc., Cat. No. 32209). Quantifications of protein bands were performed using the ImageJ software (http://rsb.info.nih.gov/ij).
Real-time RT-PCR
Total RNAs were extracted from cells with TriZol Reagent (Invitrogen Life Technologies, Cat. No. 10296010) following the manufacturer’s protocol. First strand cDNA synthesis and amplification were performed using Omniscript RT Kit (QIAGEN, Cat. No. 205113). The following human beclin 1 primers were used: forward: 5′-CAA GAT CCT GGA CCG TGT CA-3′; reverse: 5′-TGG CAC TTT CTG TGG ACA TCA-3′.11 The β-actin primers were as follows: forward: 5′-GCC AAC ACA GTG CTG TCT GG-3′; reverse 5′-GCT CAG GAG GAG CAA TGA TCT TG-3′.25 SYBR Green quantitative PCR amplifications were performed on the Stratagene 3005P Real-TimePCR system. Reactions were carried out in a 25-μl volume containing 12.5 μl of 2x SYBR Green PCR Master Mix. The thermal profile for the real-time PCR was 95°C for 3 min followed by 40 cycles of 95°C for 20 s, 59°C for 30 s and 70°C for 30 s. The fold-changes for beclin 1 expression were calculated using the MxPro software (Version 4.00, Stratagene).
Fluorescence microscopy
Cells were cotransfected with GFP-LC3 vector (1 μg/ml) and a miR-30a mimic (100 nm) using Lipofectamine 2000 (Invitrogen Life Technologies, Cat. No. 11668019). Forty-eight hours later, cells were fixed in 4% formaldehyde for 10 minutes. Cells were then washed thrice with PBS, and observed under a fluorescence microscope (Zeiss Axiovert 200M) with 400x lens.
Electron microscopy
Cells harvested by trypsinization were fixed in 2.5% gluteraldehyde/4% paraformaldehyde in 0.1 M cacodylate buffer, and then post-fixed in 1% osmium tetroxide buffer. After dehydration in a graded series of acetone, the cells were embedded in spur resin. Thin sections (90 nm) were cut on a Reichert Ultracut E microtome. Sectioned grids were stained with saturated solution of uranyl acetate and lead citrate. Sections were examined at 80 kV with a JEOL 1200EX transmission electron microscope.
Supplementary Material
Acknowledgments
Supported by grants from the U.S. Public Health Service CA66077 and Department of Defense BC050789.
Glossary
Abbreviations:
- miRNA
microRNA
- UTR
untranslated region
- HBSS
hank’s buffered salt solution
- LC3
microtubule-associated protein 1 light chain 3
- RT-PCR
reverse transcriptase-polymerase chain reaction
- PBS
phosphate-buffered saline
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TBST
tris-buffered saline
Note
Supplementary material can be found at: www.landesbioscience.com/journals/autophagy/article/9064
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/9064
References
- 1.Kim J, Klionsky DJ. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu Rev Biochem. 2000;69:303–42. doi: 10.1146/annurev.biochem.69.1.303. [DOI] [PubMed] [Google Scholar]
- 2.Yu L, Strandberg L, Lenardo MJ. The selectivity of autophagy and its role in cell death and survival. Autophagy. 2008;4:567–73. doi: 10.4161/auto.5902. [DOI] [PubMed] [Google Scholar]
- 3.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–5. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5:886–97. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 6.Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, et al. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 2007;17:839–49. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 9.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–9. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlich S, Shohami E, Pinkas-Kramarski R. Neurodegeneration induces upregulation of Beclin 1. Autophagy. 2006;2:49–51. doi: 10.4161/auto.2156. [DOI] [PubMed] [Google Scholar]
- 11.Miracco C, Cosci E, Oliveri G, Luzi P, Pacenti L, Monciatti I, et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int J Oncol. 2007;30:429–36. [PubMed] [Google Scholar]
- 12.Shen Y, Li DD, Wang LL, Deng R, Zhu XF. Decreased expression of autophagy-related proteins in malignant epithelial ovarian cancer. Autophagy. 2008;4:1067–8. doi: 10.4161/auto.6827. [DOI] [PubMed] [Google Scholar]
- 13.Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai Z, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–75. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32:189–97. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat Struct Mol Biol. 2009;16:144–50. doi: 10.1038/nsmb.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, et al. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell. 2005;120:623–34. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Lytle JR, Yario TA, Steitz JA. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5′ UTR as in the 3′ UTR. Proc Natl Acad Sci U S A. 2007;104:9667–72. doi: 10.1073/pnas.0703820104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 19.Robins H, Li Y, Padgett RW. Incorporating structure to predict microRNA targets. Proc Natl Acad Sci U S A. 2005;102:4006–9. doi: 10.1073/pnas.0500775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sood P, Krek A, Zavolan M, Macino G, Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci U S A. 2006;103:2746–51. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–84. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 22.Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–8. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- 23.Blower PE, Verducci JS, Lin S, Zhou J, Chung JH, Dai Z, et al. MicroRNA expression profiles for the NCI-60 cancer cell panel. Mol Cancer Ther. 2007;6:1483–91. doi: 10.1158/1535-7163.MCT-07-0009. [DOI] [PubMed] [Google Scholar]
- 24.Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A. 2004;101:9740–4. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, et al. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–8. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin S, White E. Role of autophagy in cancer: management of metabolic stress. Autophagy. 2007;3:28–31. doi: 10.4161/auto.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–62. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–29. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- 29.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson KM, Weiss GJ. MicroRNAs and cancer: past, present, and potential future. Mol Cancer Ther. 2008;7:3655–60. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 31.Wang J. Beclin 1 bridges autophagy, apoptosis and differentiation. Autophagy. 2008;4:947–8. doi: 10.4161/auto.6787. [DOI] [PubMed] [Google Scholar]
- 32.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat Rev Drug Discov. 2007;6:304–12. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 33.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–70. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 34.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–34. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–23. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- 36.Katayama M, Kawaguchi T, Berger MS, Pieper RO. DNA damaging agent-induced autophagy produces a cytoprotective adenosine triphosphate surge in malignant glioma cells. Cell Death Differ. 2007;14:548–58. doi: 10.1038/sj.cdd.4402030. [DOI] [PubMed] [Google Scholar]
- 37.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 38.Harfe BD. MicroRNAs in vertebrate development. Curr Opin Genet Dev. 2005;15:410–5. doi: 10.1016/j.gde.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–803. doi: 10.1038/sj.onc.1210083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.