Abstract
Tuo et al. (2012) demonstrated tumor necrosis factor-inducible gene 6 recombinant protein (TSG-6) arrest of focal retinal lesions on a Ccl2 and Cx3cr1 double deficient mouse (DKO) on rd8 background (hereon referred to as DKO rd8). DKO rd8, a model of focal retinal degeneration with earlier onset and higher penetrance than Ccl2 and Cx3cr1 single knockout strains, demonstrates characteristic features of AMD such as focal photoreceptor atrophy, retinal pigmented epithelium (RPE) degeneration, elevated ocular A2E levels and complement deposition in addition to retinal dystrophy. The discovery of the accidently introduced Crb1 mutation (rd8) in the C57BL/6N strain has led to the recent opinion that DKO rd8 is not a model of AMD but solely a model of Crb1‑associated retinal degeneration. Differences between DKO rd8 and Crb1rd8 photoreceptor and RPE pathology, as well as increased A2E and immune dysfunction, show that DKO rd8 recapitulates some AMD‑like features in addition to rd8 retinal dystrophy. The appearance of rd8 lesions and Ccl2/Cx3cr1 lesions and the amelioration of most Ccl2/Cx3cr1 lesions in intervention studies show DKO rd8 to be a useful and appropriate model for therapeutic compound screening, such as the case with anti-inflammatory TSG‑6.
Keywords: age-related macular degeneration, TSG-6, Ccl2, Cx3cr1, rd8, animal model
Introduction
Recently, Tuo et al. (2012) used a Ccl2−/−/Cx3cr1−/− double knockout (DKO) on C57BL/6N genetic background to show tumor necrosis factor-inducible gene 6 recombinant protein (TSG-6) arrest of focal retinal lesions.1 The DKO strain was generated as a model for focal retinal degeneration after report of senescent Ccl2 single knockout mice developing key characteristics of human age-related macular degeneration (AMD) and the discovery of an association between CX3CR1 single nucleotide polymorphisms and AMD.2-5 The Ccl2 and Cx3cr1 deficient DKO strain exhibits AMD‑like features such as retinal pigmented epithelium (RPE) alteration, photoreceptor atrophy, immune activation and A2E elevation and thus has been suggested as an appropriate model for human AMD.6,7 As compared with independently-generated Ccl2 and Cx3cr1 single knockout strains, DKO shows earlier onset, higher penetrance and more lesions (Fig. 1). Therefore, this strain has served as a valuable tool for screening therapies for human AMD.
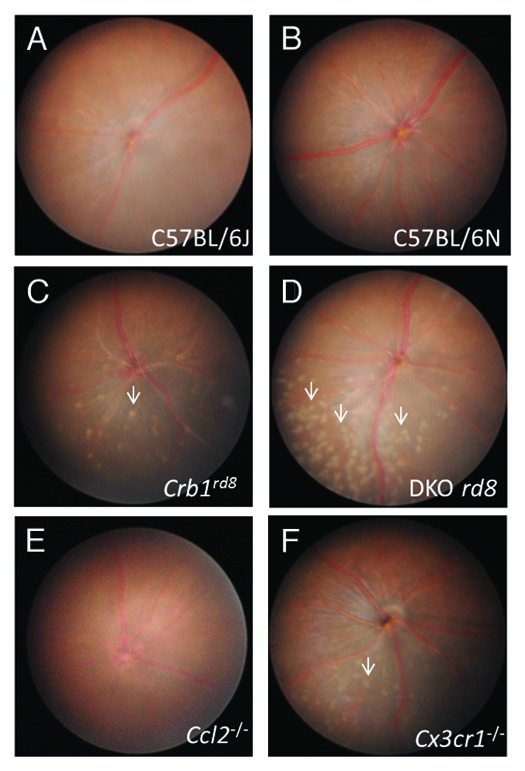
Figure 1. Fundus photos of C57BL/6J, C57BL/6N, Crb1rd8, DKO rd8, Ccl2−/−, Cx3cr1−/− at two months. (A) C57BL/6J shows no phenotype, (B) C57BL/6N shows very little phenotype, (C) Crb1rd8 displays a few, small retinal lesions in the inferior nasal quadrant. (D) DKO rd8 shows focal retinal lesions at a greater number than Crb1rd8 and the rd8-containing C57BL/6N. (E) Ccl2−/− exhibits no lesions. (F) Cx3cr1−/− has retinal lesions that are greater in number than Crb1rd8, but markedly less than DKO rd8.
The recent finding of the accidently introduced crumbs-like 1 (Crb1) mutation and Crb1 rd8-associated retinal dystrophy in the C57BL/6N background however, has challenged DKO as an AMD mouse model.8,9 CRB1 is thought to be involved in maintaining the integrity of the external limiting membrane and mutations in CRB1 result in genetic retinal disorders such as retinitis pigmentosa and Leber congenital amaurosis in humans.10 The Crb1rd8 mutation results in irregular retinal lesions in the inferior nasal quadrant of the fundus and thus is suggested to have potential implications for all ocular vision research models on C57BL/6N background, including DKO (hereon referred to as DKO rd8). Although it is critical to create retinal degeneration on rd8 background, the DKO rd8 mice develop not only retinal dystrophy (a few, mild Crb1rd8 lesions in C57B/6N mouse) but also additional focal retinal degenerative lesions. While the retinal lesions in these mouse strains may appear clinically similar (Fig. 1), differences in photoreceptor and RPE pathologies, A2E levels and immune response with Crb1rd8 mice show DKO rd8 to be more consistent with human AMD than with solely Crb1rd8-associated retinal diseases.
Differences in DKO rd8 and Crb1rd8 Pathology
Analysis of C57BL/6 mouse lines and embryonic stem cells revealed the presence of the Crb1 mutation in homozygous form in the C57BL/6N strain, but not the C57BL/6J strain.8 This finding indicates a “founder effect” mutation in C57BL/6N, suggesting that transgenic and knockout models created on the C57BL/6N background carry the Crb1 mutation and thus may present rd8-associated pathology.8 Crb1rd8-associated pathology is characterized by fundus lesions in the inferior nasal quadrant corresponding to abnormal Müller cells, retinal folds and pseudorosettes between the inner and outer nuclear layers. Within these retinal dystrophic lesions there is shortening and disarray of focal photoreceptor inner and outer segments (Fig. 2C).11 It is thought that the rd8 mutation results in truncation of the transmembrane and cytoplasmic domain of Crb1, disrupting the adhesion of adherens junction proteins to the external limiting membrane (ELM, comprised of zonula adherens that link photoreceptors and Müller cells) and affecting the development of photoreceptor inner and outer segments.11 In the Crb1rd8 mouse, loss of adherens junctions and the Müller cell apical processes underlying the junctions of the ELM is observed. Crb1 is largely absent or diffuse in Müller cell apical processes, which are aberrantly prominent in the inner nuclear layer. Preservation of normal inner and outer segment length is seen in areas adjacent to the region of degeneration.11 These findings suggest the primary pathology of Crb1rd8 mice to be developmental defects in Müller cells and photoreceptor inner and outer segment shortening, resulting in the secondary pathology of retinal dysplasia and degeneration of focal photoreceptors. Thus, Crb1rd8 is a model of retinal dystrophy resulting from malformed Müller cell apical villi.12
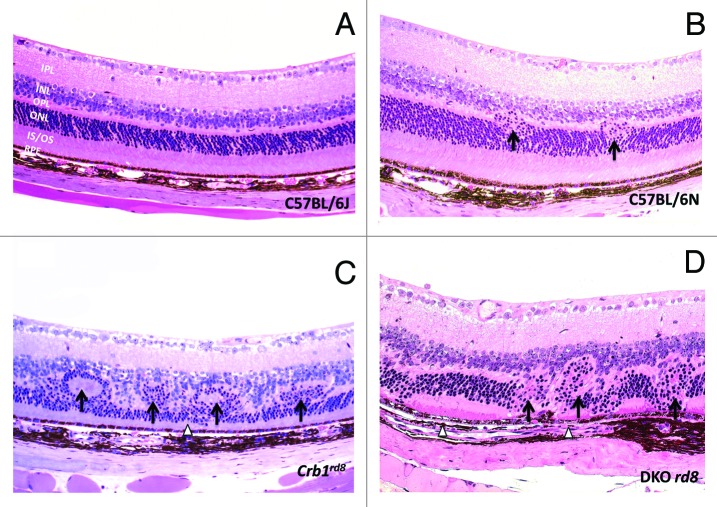
Figure 2. Photomicrographs of C57BL/6J, C57BL/6N, Crb1rd8 and DKO rd8 retina. (A) C57BL/6J strain that does not carry the rd8 mutation shows normal ocular architecture. (B) The C57BL/6N strain displays small rd8-associated dystrophic lesions showing migration of the ONL into the OPL (arrows), preserved photoreceptor IS/OS, and intact RPE. (C) Crb1rd8 displays pseudorosettes and retinal folds in addition to retinal dystrophy involving the inner and ONL (arrows) and thinning of IS/OS (arrowhead). (D) In addition to the rd8-associated lesions observed in C57BL/6N, DKO rd8 shows severe ONL disorganization (arrows), focal loss of IS/OS, and hypopigmentation and vacuolation of RPE (arrowheads). IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS/OS, inner segments and outer segments; RPE, retinal pigmented epithelium. (hematoxylin and eosin, original magnification, 200×)
DKO rd8 mice also show photoreceptor pathology (Fig. 2D).6 However, in addition to photoreceptor dystrophic lesions, RPE degeneration appears to be a featured pathological event in DKO rd8. Whereas retinal folds and pseudorosettes are frequently seen in Crb1rd8 mice (Fig. 2C), these retinal lesions are seldom found in DKO rd8 (Fig. 2D). While differences in mouse and human physiology limit the formation of drusen proper,13 infrequent appearance of drusen-like lesions in DKO rd8, which has not been reported in Crb1rd8 mice, demonstrate distinct retinal pathology between the two strains.14 In general, C57BL/6N mice seem to have much less severe retinal phenotypes with exception of the foci of mild retinal dystrophy (Fig. 2B) compared with the Crb1rd8 mice (Bo Chang, personal communication).
In addition to photoreceptor degeneration, RPE alteration is a cardinal feature of human AMD.15,16 One of the prominent histopathologies in DKO rd8 is abnormal RPE cells, which display hypopigmentation, depigmentation, vacuolation and atrophy.6,14 Contrastingly, RPE histology is relatively preserved in Crb1rd8 mice and in patients with Leber congenital amaurosis (LCA), a disease associated with Crb1 mutation, even in the advanced stages.10 These observations suggest that Crb1rd8-associated and DKO rd8-associated pathologies may not operate through identical mechanisms.
Elevated Lipofuscin Biochemical Marker A2E in DKO rd8
Accumulation of the lipofuscin fluorophore A2E, a well-known biochemical marker of human AMD, is seen in DKO rd8. A2E accumulation is associated with retinal aging and is known to activate the complement system as well as induce RPE apoptosis and neurodegeneration.17-19 Compared with age-matched C57BL/6N mice, a 3- to 4‑fold increase of ocular A2E is seen in DKO rd8.6,14 Contrastingly, Crb1rd8 and C57BL/6J mice do not show elevated A2E levels (Fig. 3). The generation of A2E is considered to be the consequence of burst oxidative stress that is not related in mechanism to the function of Crb1, a component of the molecular scaffold.20
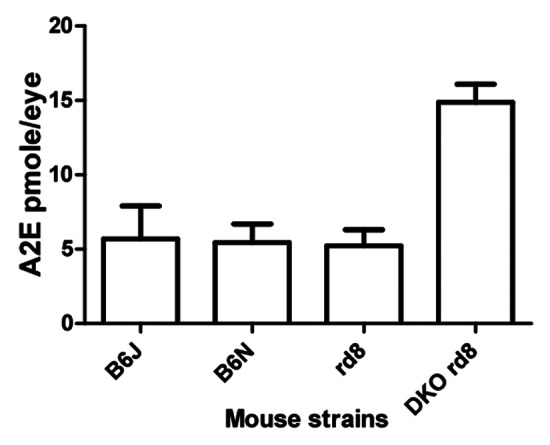
Figure 3. Quantification of A2E in C57BL/6J, C57BL/6N, Crb1rd8 and DKO rd8 strains at 2 mo. DKO rd8 shows an A2E increase of more than 3-fold compared with the other three strains. *p < 0.05 in DKO rd8 compared with age-matched C57BL/6J, C57BL/6N and Crb1rd8.
Immunopathology of DKO rd8
The DKO rd8 model expresses a spectrum of immunological features of AMD. Increased deposition of complement factors, altered secretion of chemokines and cytokines, and the accumulation of activated microglia and macrophages in retinal lesions have been linked to human AMD and can correspondingly be demonstrated in the DKO rd8. Expression of CD46, a regulatory protein of complement proteins C3 and C4, suggests the presence of activated complement.6 Elevated expression of C3d is seen in RPE and photoreceptors of DKO rd8 and the recruitment of microglia and macrophages in subretinal and retinal lesions is also observed.21 Consistent with AMD patients, DKO rd8 mice demonstrate increased expression of anti-retinal autoantibodies.21
Moreover, the DKO rd8 strain shows greater macrophage infiltration and complement factor deposition than Crb1rd8 or C57BL/6N (Fig. 4 and B), suggesting that immune dysfunction or activation of innate immunity, which is documented in human AMD, seen in DKO rd8 cannot reasonably be accounted for by the rd8 mutation alone. These results suggest that the immunopathology seen in DKO rd8 should not be thought of as solely secondary immune response to rd8-associated retinal dystrophy, and indicate that DKO rd8 recapitulation of AMD-like immunopathology is not the same as rd8-mediated immunopathology.
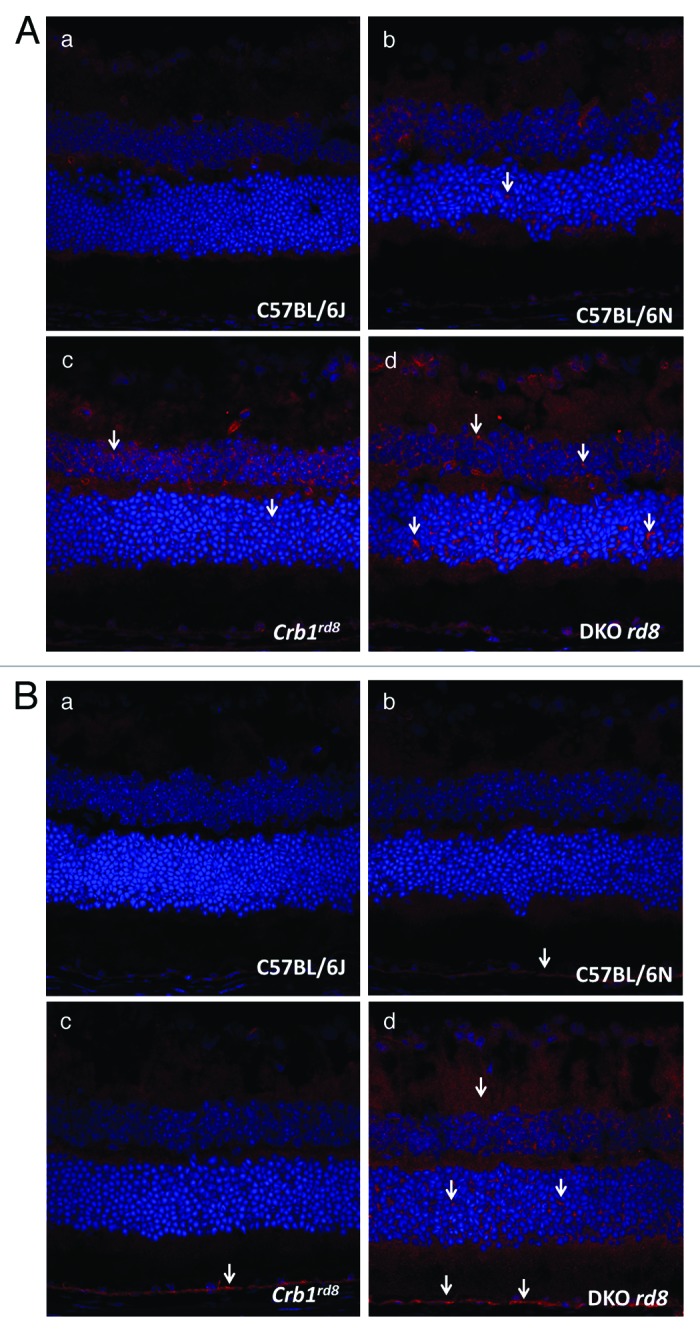
Figure 4. (A) Photomicrographs of macrophages (arrows) in C57BL/6J, C57BL/6N, Crb1rd8 and DKO rd8 retina detected by immunohistochemistry and confocal microscopy. (A) There are few macrophages in C57BL/6J wild-type mouse. (B) C57BL/6N shows increased macrophages compared with wild-type. (C) Crb1rd8 displays more macrophage infiltration relative to C57BL/6N. (D) DKO rd8 exhibits greater macrophage infiltration than Crb1rd8 and C57BL/6N. (original magnification, 400×). (B) Photomicrographs of complement C3d (arrows) in C57BL/6J, C57BL/6N, Crb1rd8 and DKO rd8 retina detected by immunohistochemistry and confocal microscopy. (A) No complement C3d in C57BL/6J wild-type mouse. (B) C57BL/6N shows minimal C3d deposition in RPE compared with wild-type. (C) Crb1rd8 displays elevated C3d deposition in RPE relative to C57BL/6N. (D) DKO rd8 exhibits greater C3d deposition than Crb1rd8 and C57BL/6N, significantly in the RPE and neuroretina. (original magnification, 400×)
Ccl2−/−/Cx3cr1−/− and Crb1rd8 Interaction
As evidenced by differences in photoreceptor atrophy and RPE degeneration, DKO and Crb1rd8 pathology are distinct. Ccl2−/−/Cx3cr1−/− deficiency and the rd8 mutation thus produce two different types of lesions, which, despite arising from distinct mutations, nonetheless interact and modify each other at some level. Independently established Ccl2−/−/Cx3cr1−/− double deficient mice on B6J background could not replicate the retinal phenotypes observed in our DKO rd8; interestingly, these mice can develop retinal AMD-like lesions under oxidative stress (Heping Xu, EVER abstract, 2012). However, it has been reported that Ccl2 and Cx3cr1 single deficiency generate a retinal AMD phenotype in aging mice and additionally, that only 81% of rd8 mice show a retinal phenotype compared with 100% of DKO rd8 mice.6,8,14 This suggests that Ccl2 and Cx3cr1 double deficiencies may add other lesions to the early onset retinal dystrophy caused by rd8 alone. A recent study of Ccl2 and Cx3cr1 single knockouts reports that despite similar accumulation of subretinal macrophages in both strains, Ccl2 and Cx3cr1 differentially modulate AMD-like lesions. Particularly, it is suggested that Cx3cr1 results in more pronounced phenotype of rd8-associated degeneration, a finding that was maintained in the Ccl2 and Cx3cr1 double knockout (Heping Xu, EVER abstract, 2012, Ulrich F. Luhmann, EVER abstract 2012).
Crb1rd8 mice primarily show retinal lesions at the ELM whereas DKO rd8 show lesions in retinal inner and outer segment layer, suggesting that Ccl2 and Cx3cr1 double deficiencies could play a role in the high penetrance of rapid photoreceptor and RPE degeneration seen in DKO rd8. Intervention studies conducted on DKO rd8 show reduction or stabilization of these deeper retinal lesions but may have little or no effect on rd8 lesions in the ELM.13,22,23 Study of an independently-generated Ccl2−/−/Cx3cr1−/−/Crb1rd8/rd8 strain by Luhmann et al. discredited DKO rd8 as a model for AMD, citing the primary role of rd8 in the observed early onset retinal degeneration.9 While the rd8 mutation may contribute critically to photoreceptor degeneration, the RPE pathology, immune dysfunction, and A2E accumulation as manifested in DKO rd8 still require further explanation. Thus, interaction between Ccl2, Cx3cr1 and rd8 originated from C57BL/6N is possible and further investigation is needed to elucidate the exact mechanisms and how they fit in to a model for AMD.
DKO rd8 as a Model for AMD
When discussing the implications of the rd8 mutation in the DKO rd8 mouse model, consideration must be given to the influence of the genetic background of any engineered mouse model for disease. The generation of knockout mice using 129 and C57BL/6N strains exposed the model to the possibility of “passenger” genes, residual regions of 129-derived genetic material that were transported along with the “knocked out” gene onto C57BL/6N background, having created potential sites of genetic variability.24 Genetic confounders arising from traditional knockout technology using 129 and C57BL/6 may have substantial impact on phenotype, as the two mouse strains have significant genetic and physiological differences and 129 is known to have a multitude of its own mutations.24
Currently, there is no clear model for recapitulating the full clinical features of AMD.7 It must be taken into consideration that mice lack macula and thus, no murine model can ever fully recapitulate human AMD. Oxidative damage models dependent on phototoxicity or high fat diet alone or in combination do not show all characteristic RPE changes, increased levels of A2E or complement deposition.25,26 Induced choroidal neovascularization using matrigel, laser photocoagulation or elevated VEGF expression fail to model mechanisms behind the presentation of disease and thus do not result in increases of A2E or complement deposition.27-30 Immunization with carboxyethylpyrrole (CEP)-adducted protein in mice shows RPE alteration and complement deposition, yet retinal lesions independent of macrophage accumulation are observed and choroidal neovascularization does not develop in this model.31,32 Nonetheless, a growing body of evidence implicating AMD as an immunological disease necessitates that any mouse model feature immunopathological mechanisms.33,34 While the genetic roles and interactions of Ccl2, Cx3cr1 and rd8 are not fully elucidated, the recapitulation of certain features of human AMD can be demonstrated in DKO rd8. Within known AMD mouse models, DKO rd8 is a model to date that demonstrates focal RPE alteration, photoreceptor atrophy, enhanced A2E accumulation, and dysregulation of the immune system.6,14,34
While the identification of rd8 and rd8-associated retinal phenotype does impact the power of DKO rd8 as a model for AMD, the use of the model as self-control by treating one eye and using the contralateral eye as the control allows for appropriate experimental interventional studies. Thus, DKO rd8 may still serve as a suitable and relevant model for compound screening on focal retinal degenerative diseases including AMD. In intervention studies on DKO rd8, treatment is shown to alleviate photoreceptor and RPE lesions that can be attributed to Ccl2 and Cx3cr1 double deficiencies while leaving rd8 lesions unaffected. Such is the case with the report by Tuo (2012) that TSG-6 arrested lesions in the retina.
Disclosure of Potential Conflicts of Interest
The authors state no conflicts of interest.
Acknowledgments
This work was supported by the NEI intramural fund.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/22949
References
- 1.Tuo J, Cao X, Shen D, Wang Y, Zhang J, Oh JY, et al. Anti-inflammatory recombinant TSG-6 stabilizes the progression of focal retinal degeneration in a murine model. J Neuroinflammation. 2012;9:59. doi: 10.1186/1742-2094-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–7. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 3.Combadière C, Feumi C, Raoul W, Keller N, Rodéro M, Pézard A, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen M, Forrester JV, Xu H. Dysregulation in retinal para-inflammation and age-related retinal degeneration in CCL2 or CCR2 deficient mice. PLoS One. 2011;6:e22818. doi: 10.1371/journal.pone.0022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raoul W, Feumi C, Keller N, Lavalette S, Houssier M, Behar-Cohen F, et al. Lipid-bloated subretinal microglial cells are at the origin of drusen appearance in CX3CR1-deficient mice. Ophthalmic Res. 2008;40:115–9. doi: 10.1159/000119860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, et al. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–36. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramkumar HL, Zhang J, Chan CC. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD) Prog Retin Eye Res. 2010;29:169–90. doi: 10.1016/j.preteyeres.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, et al. The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci. 2012;53:2921–7. doi: 10.1167/iovs.12-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luhmann UF, Lange CA, Robbie S, Munro PM, Cowing JA, Armer HE, et al. Differential modulation of retinal degeneration by Ccl2 and Cx3cr1 chemokine signalling. PLoS One. 2012;7:e35551. doi: 10.1371/journal.pone.0035551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Hollander AI, Heckenlively JR, van den Born LI, de Kok YJ, van der Velde-Visser SD, Kellner U, et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203. doi: 10.1086/321263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehalow AK, Kameya S, Smith RS, Hawes NL, Denegre JM, Young JA, et al. CRB1 is essential for external limiting membrane integrity and photoreceptor morphogenesis in the mammalian retina. Hum Mol Genet. 2003;12:2179–89. doi: 10.1093/hmg/ddg232. [DOI] [PubMed] [Google Scholar]
- 12.van de Pavert SA, Sanz AS, Aartsen WM, Vos RM, Versteeg I, Beck SC, et al. Crb1 is a determinant of retinal apical Müller glia cell features. Glia. 2007;55:1486–97. doi: 10.1002/glia.20561. [DOI] [PubMed] [Google Scholar]
- 13.Shen D, Cao X, Zhao L, Tuo J, Wong WT, Chan CC. Naloxone ameliorates retinal lesions in Ccl2/Cx3cr1 double-deficient mice via modulation of microglia. Invest Ophthalmol Vis Sci. 2011;52:2897–904. doi: 10.1167/iovs.10-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CC, Ross RJ, Shen D, Ding X, Majumdar Z, Bojanowski CM, et al. Ccl2/Cx3cr1-deficient mice: an animal model for age-related macular degeneration. Ophthalmic Res. 2008;40:124–8. doi: 10.1159/000119862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2:552–77. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 16.Mettu PS, Wielgus AR, Ong SS, Cousins SW. Retinal pigment epithelium response to oxidant injury in the pathogenesis of early age-related macular degeneration. Mol Aspects Med. 2012;33:376–98. doi: 10.1016/j.mam.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–9. [PubMed] [Google Scholar]
- 18.Zhou J, Jang YP, Kim SR, Sparrow JR. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci U S A. 2006;103:16182–7. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Kim SR, Westlund BS, Sparrow JR. Complement activation by bisretinoid constituents of RPE lipofuscin. Invest Ophthalmol Vis Sci. 2009;50:1392–9. doi: 10.1167/iovs.08-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006;84:4–15. doi: 10.1111/j.1600-0420.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 21.Ross RJ, Zhou M, Shen D, Fariss RN, Ding X, Bojanowski CM, et al. Immunological protein expression profile in Ccl2/Cx3cr1 deficient mice with lesions similar to age-related macular degeneration. Exp Eye Res. 2008;86:675–83. doi: 10.1016/j.exer.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuo J, Ross RJ, Herzlich AA, Shen D, Ding X, Zhou M, et al. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuo J, Pang JJ, Cao X, Shen D, Zhang J, Scaria A, et al. AAV5-mediated sFLT01 gene therapy arrests retinal lesions in Ccl2(-/-)/Cx3cr1(-/-) mice. Neurobiol Aging. 2012;33:433–, e1-10. doi: 10.1016/j.neurobiolaging.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisener-Dorman AF, Lawrence DA, Bolivar VJ. Cautionary insights on knockout mouse studies: the gene or not the gene? Brain Behav Immun. 2009;23:318–24. doi: 10.1016/j.bbi.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cousins SW, Espinosa-Heidmann DG, Alexandridou A, Sall J, Dubovy S, Csaky K. The role of aging, high fat diet and blue light exposure in an experimental mouse model for basal laminar deposit formation. Exp Eye Res. 2002;75:543–53. doi: 10.1006/exer.2002.2047. [DOI] [PubMed] [Google Scholar]
- 26.Dithmar S, Sharara NA, Curcio CA, Le NA, Zhang Y, Brown S, et al. Murine high-fat diet and laser photochemical model of basal deposits in Bruch membrane. Arch Ophthalmol. 2001;119:1643–9. doi: 10.1001/archopht.119.11.1643. [DOI] [PubMed] [Google Scholar]
- 27.Shen D, Wen R, Tuo J, Bojanowski CM, Chan CC. Exacerbation of retinal degeneration and choroidal neovascularization induced by subretinal injection of Matrigel in CCL2/MCP-1-deficient mice. Ophthalmic Res. 2006;38:71–3. doi: 10.1159/000090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Zhao L, Li Y, Liu Y, Xiao W, Song Y, et al. A subretinal matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest Ophthalmol Vis Sci. 2010;51:6009–17. doi: 10.1167/iovs.09-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, et al. Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol. 1998;153:1641–6. doi: 10.1016/S0002-9440(10)65753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–44. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–8. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollyfield JG, Perez VL, Salomon RG. A hapten generated from an oxidation fragment of docosahexaenoic acid is sufficient to initiate age-related macular degeneration. Mol Neurobiol. 2010;41:290–8. doi: 10.1007/s12035-010-8110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–68. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008;30:97–110. doi: 10.1007/s00281-008-0112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]