Abstract
Malaria is an infectious disease that threatens half of the world’s population. This debilitating disease is caused by infection from parasites of the genus Plasmodium. Insecticides, bed nets and drug therapies have lowered the prevalence and death rate associated with malaria but this disease continues to plague many populations around the world. In recent years, many organizations have suggested developing methods for a complete eradication of malaria. The most straightforward and effective method for this potential eradication will be through the development of a low-cost vaccine. To achieve eradication, it will be necessary to develop new vaccine candidates and novel systems for both the production and delivery of these vaccines. Recently, the green algae Chlamydomonas reinhardtii has been used for the recombinant expression of malaria vaccine candidates including the transmission blocking vaccine candidate Pfs48/45. Here, we discuss the potential of this research on the future development of a low-cost malaria vaccine candidate.
Keywords: malaria, transmission blocking vaccine, Pfs48/45, Plasmodium falciparum, Chlamydomonas reinhardtii, chloroplast engineering, protein expression, algae
Malaria is a widespread infectious disease that threatens half of the world’s population and leads to nearly a million deaths annually. This debilitating illness causes high fevers, shaking and flu-like symptoms that are particularly a threat to children and pregnant women.1 In addition, the prevalence of malaria is highest in Africa where 89% of worldwide malaria deaths occur. Unfortunately, the high level of incidence of malaria in Africa is coupled in many countries with a reduced opportunity to provide the relatively expensive therapies and medical supplies required to treat those most in need. In order to combat malaria and save lives, particularly in the African regions, it will be necessary to develop malaria treatment and prevention therapies that are both inexpensive and easy to distribute and use.
The use of insecticides, bed nets and drug therapies undoubtedly have and will continue to play a crucial role in lowering the prevalence and death rate associated with malaria. However, even with these methods many populations are still at risk. Through advancements in medical research and technologies over the past several decades, we have reached a point where scientific knowledge may allow us to develop therapies that can truly provide global protection from this deadly disease well into the future. In recent years, programs such as the Gates Foundation have proposed the complete eradication of malaria. While a campaign of this magnitude is proving to be difficult, it has the best chance of success by pairing current therapies with the development of new therapies, particularly a vaccine. Vaccines have proven highly successful in the past in eradicating other diseases such as small pox and polio.2
Malaria Vaccines
The first step in developing a vaccine for malaria is understanding the biology of the causative agent, parasites of the genus Plasmodium. One member of this genus, P. falciparum, is thought to be responsible for most instances of malaria and related mortality; therefore vaccine research has focused largely on this species. An improved comprehension of this organism’s biology is critical to identifying antigenic protein candidates that occur during different stages of its complex lifecycle, including its interactions with the mosquito biological vector and the human host.2 Development of a vaccine has been complicated by the genetic diversity identified within many candidate proteins already being targeted as vaccines. While this protein complexity and diversity within P. falciparum represents a significant challenge in identifying vaccine candidates, it also offers opportunity due to a wide variety of potential targets spanning the various stages of this organism’s lifecycle. Ideally, a candidate vaccine or a suite of vaccines will be developed that when administered overcome this complexity and diversity to offer a stronger level of immunological protection to those exposed to the P. falciparum parasite and ultimately provide better protection against malaria.
Over the past several decades, scientists have focused primarily on three types of vaccine candidates—preerythrocytic, erythrocytic and transmission blocking vaccines. Each of these potential candidates targets a different stage of the Plasmodium lifecycle. The preerythrocytic vaccines would inhibit infection, thus preventing the parasite from infecting the blood, while the erythrocytic vaccine could reduce or eliminate the symptomatic illnesses associated with the disease. Transmission blocking vaccines would prevent the reproduction of the Plasmodium parasite in the mosquito, thereby protecting entire communities by stopping the transmission of the parasite to the human host from the mosquito.2 While each of these potential vaccines would likely help to lower the incidence of malaria, it will likely take a combination of all three strategies to truly offer a widespread and effective end to the disease.
While understanding the biology of P. falciparum and identifying potential vaccine candidates are the first steps toward the development of a vaccine, the second challenge will be identifying a reliable, efficient and cost-effective method to produce that vaccine and make it available to the people in need. There are several potential recombinant protein expression systems that have been used for many years in the creation of vaccines; however, in the case of some of P. falciparium proteins identified as malaria vaccine candidates these prokaryotic and eukaryotic expression systems have proven to be less than ideal systems due largely to low recombinant protein expression levels, lack of protein solubility and the addition of unwanted post-translational modifications. Thus, one of the key aspects to the future development of potential malaria vaccine candidates will be the identification of a versatile expression host capable of expressing a large suite of complex P. falciparum proteins in as close to their native state as possible.
Algae as a Malaria Vaccine Platform
In our recent paper, “Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii,” we demonstrate that the chloroplast of C. reinhardtii has the correct biochemical environment to allow for the expression, folding, and disulfide bond formation of the antigenic domain of one of the more complex P. falciparum vaccine candidates, Pfs48/45.3 This surface protein plays a role in the male/female gamete interaction within the mosquito midgut. It is a member of a family of surface proteins that contain a 6-cysteine conserved domain structure and is believed to work in conjunction with a second member of this family of surface proteins, Pfs230, to allow for reproduction. Pfs48/45 contains a glycosylphosphatidylinositol (GPI) anchor that attaches the protein to the surface of the gamete creating a platform for the soluble Pfs230 protein to attach and allow for zygote formation.4,5
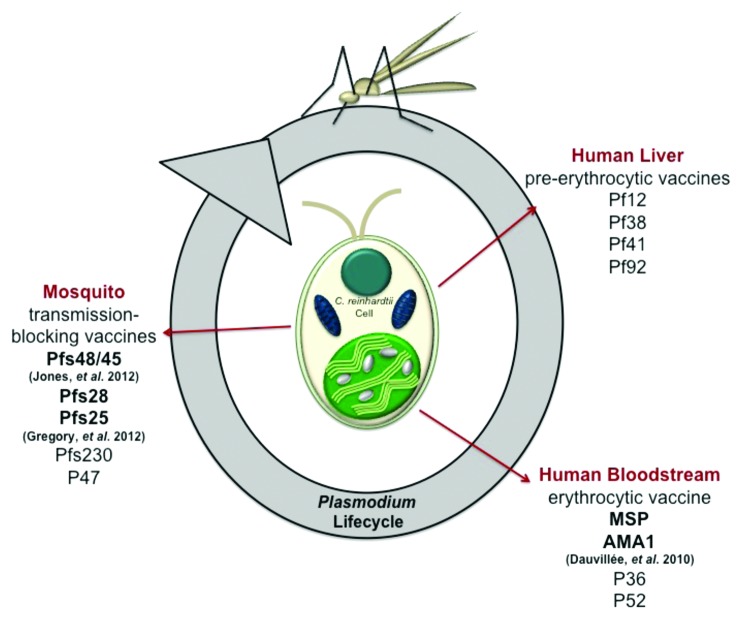
The 6-cysteine domain structure present in both Pfs48/45 and Pfs230 is also found within surface proteins associated with the other life stages of the P. falciparum lifecycle including the preerythrocytic sporozoites and the erythrocytic merozoites.4 Since exposure to Pfs48/45 induces natural immunity and antibodies elicited by both Pfs48/45 and Pfs230 have been shown to induce transmission-blocking immunity, it is possible that the other surface proteins within this family may also elicit an effective immune response.5 Thus, these other surface proteins represent additional potential candidates for malaria vaccine development. Our finding that C. reinhardtii can be used for the recombinant expression of Pfs48/45 may also indicate that this host is a suitable expression platform for these other proteins (Fig. 1).
Figure 1. Potential of Chlamydomonas reinhardtii as a recombinant protein expression platform for the generation of malaria vaccine candidates. Pfs48/45 is a member of a family of proteins expressed throughout the Plasmodium lifecycle. Due to their structural similarities, other members of this family have the potential to be expressed in C. reinhardtii (non-bold print). C. reinhardtii has already been shown to express other candidate Plasmodium proteins including Pfs28, Pfs25, MSP and AMA1 (bold print).
However, the success of Pfs48/45 as a candidate malaria vaccine protein and the use of C. reinhardtii as an expression platform for other antigenic proteins containing the 6-cysteine conserved domain structure requires further study of recombinant expression factors involved in the production of these antigenic proteins. Three factors that should be carefully considered are solubility, stability and cost-effective scale up that would allow for inexpensive and straightforward distribution of the vaccine.
While the expression of Pfs48/45 in C. reinhardtii was readily verified upon concentration and purification, improving the solubility of this protein within the chloroplast could greatly enhance C. reinhardtii as an expression platform for this entire family of proteins. Based on western blot analysis, the c.r.Pfs48/45 protein appeared to have a higher concentration within the total protein fraction compared with the soluble fraction and was not as readily apparent prior to concentration in the soluble protein extract. We hypothesize that one of the reasons for this is that the natural function of Pfs48/45 in Plasmodium is to act as a membrane anchor. The C-terminal domain expressed in the chloroplast contained the codon-optimized sequence for the GPI domain found in the Pfs48/45 surface protein. Since this GPI domain is designed to embed within the surface of the gamete, it is possible that it could also interact with a membrane component of the C. reinhardtii chloroplast. This would account for the higher content of c.r.Pfs48/45 within the total protein of the western blot, compared with the soluble fraction. It is possible that without this membrane interaction the protein is less stable and more susceptible to degradation by proteases.
Clearly, elucidating the role of the GPI during expression of Pfs48/45 within the chloroplast of C. reinhardtii will be important for increasing the abundance of stable soluble recombinant protein in attempts to meet the demand for a potential vaccine. While this will help in designing ideal growth and purification methods, we could also potentially utilize the GPI domain in a different way to help produce a more prolific vaccine. One example could be to specifically design and use a native plastid anchor domain within the recombinant protein to enhance its interaction with the plastid membrane even further. While this may at first increase the percentage of recombinant protein in the insoluble fraction, through further genetic engineering it may be possible to develop methods to release this stable protein from the membrane and increase its overall yield. Even without the potential for removing it from the plastid membrane, the anchored c.r.Pfs48/45 protein could also be considered in conjunction with a second protein (Pfs230) that could be co-expressed within the chloroplast. Since Pfs230 is known to have transmission blocking activity, is naturally more soluble than Pfs48/45 and naturally interacts with Pfs48/45 within the P. falciparum host, it seems reasonable that providing a C. reinhardtii chloroplast host that already contains a membrane embedded Pfs48/45, could provide a better expression platform for the expression of the much larger and more complex protein Pfs230. Expressing these two proteins together could perhaps lead to a multivalent vaccine that produces higher induction of transmission-blocking antibodies during the immune response.
One of the other major considerations for the expression of Pfs48/45 or any other recombinant protein being considered as a malaria vaccine candidate is the cost-effective scale up of the protein, and the ability to easily distribute these candidate vaccines without added requirements such as refrigeration to avoid degradation. While scale was not directly addressed in our research article, it is important to emphasize that one of the benefits of using an algal based recombinant protein expression system is the inherent ability of algal systems to produce large-scale cultures without the need for high levels of nutrients or energy supplementation. These same qualities are the reason why algae are being studied for their potential in biofuels. Although our research focused largely on the expression of the c.r.Pfs48/45 recombinant protein in a non-photosynthetic mutant strain, we were also able to show that the same protein could be expressed within a photosynthetic strain. The development of an algae expression platform for the production of malaria vaccine candidates would be greatly enhanced by the use of photosynthetic conditions due to lower costs from minimal nutrient requirements in the media, avoidance of expensive antibiotics in culture and an increased versatility in where these algae can be grown to produce the candidate vaccines. For instance, in a photosynthetic strain the vaccine candidate could be produced in a simple and affordable photobioreactor with minimal nutrients, and grown under natural sunlight in Africa, rather than being produced in distant facilities that require long-distance shipping that would increase overall costs and reduce availability to people who are most in need of vaccine access.
Another factor in creating a cost-effective vaccine will be improving the overall yield of recombinant proteins from C. reinhardtii. Yield improvements are likely to occur through consideration of three aspects of protein expression—transcription, translation and protein degradation. Transcription is controlled through the use of promoters, where a strong promoter is likely to increase the overall yield of the protein. In the c.r.Pfs48/45 research, the main promoter used was designed from the psbA gene, an abundant protein of the photosynthetic membranes. While this promoter has been reliable as a strong promoter in the chloroplast, there may be an opportunity to develop and utilize other promoters (including those that are inducible) to further increase yield. This same idea may also apply to the 5′ and 3′ untranslated regions of the recombinant mRNA, as these have been shown to be critical factors in directing high levels of translation of recombinant proteins. An inducible system may also help to minimize protein degradation from proteases and other enzymes located in the cell as the induction of the recombinant protein could be better controlled and occur over a shorter period of time. It could also be useful to identify proteases in the chloroplast and create additional mutations in these to help prevent the degradation of recombinant vaccine proteins.6
Future of Algae and Malaria Vaccines
The use of the chloroplast in C. reinhardtii as an expression platform is similar to other plant based systems that have proven to be capable of expressing and accumulating large quantities of recombinant proteins.6,7 Algae and plant expression systems offer a unique opportunity to produce therapeutic proteins, particularly vaccines, as oral therapeutics. The production of an oral vaccine for malaria in C. reinhardtii could significantly reduce the production cost, as purification is a major component of recombinant vaccine costs, and because unpurified proteins should have better protein stability and significantly easier distribution and administration.6 Ideally, an algae protein expression platform would not only be capable of expressing an edible vaccine for malaria but would be safe enough to be grown by people living in regions with high rates of Plasmodium infection.
While the expression of the C-terminal domain of Pfs48/45 in algae may bring us one step closer to producing a transmission-blocking malaria vaccine, the complexity and diversity of the P. falciparum lifecycle may require the development of multiple vaccine candidate proteins that when taken together give the human host a more versatile and complete protective immune response against the invading pathogen. Another recent paper from our laboratory, and a previous paper published in 2010 demonstrate that C. reinhardtii can also express two other transmission-blocking vaccine candidates, Pfs25 and Pfs28 as well as two erythrocytic stage antigen candidates from the rodent infecting species P. berghei, the apical major antigen (AMA1) and the major surface protein (MSP1).8,9 The expression of five different Plasmodium protein vaccine candidates, including those that are either erythrocytic or transmission blocking, demonstrate the versatility of C. reinhardtii in producing malaria vaccine candidate proteins. As a basis for the future, these research articles set a foundation for further attempts at expressing a wide variety of Plasmodium antigenic proteins and the development of a versatile, cost-effective, and bioavailable vaccine that could truly bring the world one step closer to the elusive eradication of malaria.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/22577
References
- 1.Center for Disease Control. Malaria. 2012 August 9; Available from: www.cdc.gov/MALARIA/
- 2.Thera MA, Plowe CV. Vaccines for malaria: how close are we? Annu Rev Med. 2012;63:345–57. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones CS, Luong T, Hannon M, Tran M, Gregory JA, Shen Z, et al. Heterologous expression of the C-terminal antigenic domain of the malaria vaccine candidate Pfs48/45 in the green algae Chlamydomonas reinhardtii. Appl Microbiol Biotechnol. 2012 doi: 10.1007/s00253-012-4071-7. In press. [DOI] [PubMed] [Google Scholar]
- 4.Arredondo SA, Cai M, Takayama Y, MacDonald NJ, Anderson DE, Aravind L, et al. Structure of the Plasmodium 6-cysteine s48/45 domain. Proc Natl Acad Sci U S A. 2012;109:6692–7. doi: 10.1073/pnas.1204363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Schaijk BCL, van Gemert GJ, van de Vegte-Bolmer M, Eksi S, Williamson KC, Sauerwein RW. Expression and GPI anchoring of P230 in ∆Pf48/45 Plasmodium falciparum sexual stage parasites. In: The Plasmodium 6-cysteine protein family in sexual and sporozoite stages: targets for malaria vaccine development. Dissertation. 2012; Radboud University. Netherlands. [Google Scholar]
- 6.New JS, Westerveld D, Daniell H. Chloroplast-derived therapeutic and prophylactic vaccines. In: Wang A, Ma S, eds. Molecular Farming in Plants: Recent Advances and Future Prospects. New York:Springer, 2012:69-87. [Google Scholar]
- 7.Clemente M, Corigliano MG. Overview of plant-made vaccine antigens against malaria. J Biomed Biotechnol. 2012;2012:1–8. doi: 10.1155/2012/206918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregory JA, Li F, Tomosada LM, Cox CJ, Topol AB, Vinetz JM, et al. Algae-produced Pfs25 elicits antibodies that inhibit malaria transmission. PLoS One. 2012;7:e37179. doi: 10.1371/journal.pone.0037179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dauvillée D, Delhaye S, Gruyer S, Slomianny C, Moretz SE, d’Hulst C, et al. Engineering the chloroplast targeted malarial vaccine antigens in Chlamydomonas starch granules. PLoS One. 2010;5:e15424. doi: 10.1371/journal.pone.0015424. [DOI] [PMC free article] [PubMed] [Google Scholar]