Abstract
Colorectal Cancer (CRC) is the second leading cause of cancer-related mortality and is the fourth most common malignant neoplasm in USA. Escaping apoptosis and cell mutation are the prime hallmarks of cancer. It is apparent that balancing the network between DNA damage and DNA repair is critical in preventing carcinogenesis. One-third of cancers might be prevented by nutritious healthy diet, maintaining healthy weight and physical activity. In this review, an attempt is made to abridge the role of carcinogen in colorectal cancer establishment and prognosis, where special attention has been paid to food-borne mutagens and functional role of beneficial human gut microbiome in evading cancer. Further the significance of tailor-made prebiotics, probiotics and synbiotics in cancer management by bio-antimutagenic and desmutagenic activity has been elaborated. Probiotic bacteria are live microorganisms that, when administered in adequate amounts, confer a healthy benefit on the host. Prebiotics are a selectively fermentable non-digestible oligosaccharide or ingredient that brings specific changes, both in the composition and/or activity of the gastrointestinal microflora, conferring health benefits. Synbiotics are a combination of probiotic bacteria and the growth promoting prebiotic ingredients that purport “synergism.”
Keywords: probiotics, prebiotics, synbiotics, colorectal cancer, polyaromatic hydrocarbons, hetercyclic amines, mutagens, antimutagens, cancer
Introduction
Cancer, the leading cause of mortality across the globe, was responsible for 7.6 million deaths in 2008 (13% of total mortality).1,2 American Cancer Society in collaboration with National Cancer Institute, Centre for Disease Control and Prevention, North American Association of Central Cancer Registries and National Centre for Health Statistics, estimated a total of 1,638,910 new cancer cases and 577,190 deaths due to cancer in US during 2012. Colorectal cancer (CRC), prostrate, lung, stomach, liver and breast cancers are the major types of cancer that are associated with significant mortality every year. CRC is the second leading cause of cancer-related mortality and is the fourth most common malignant neoplasm in USA.3
Risk Factors Accountable for Colorectal Cancer
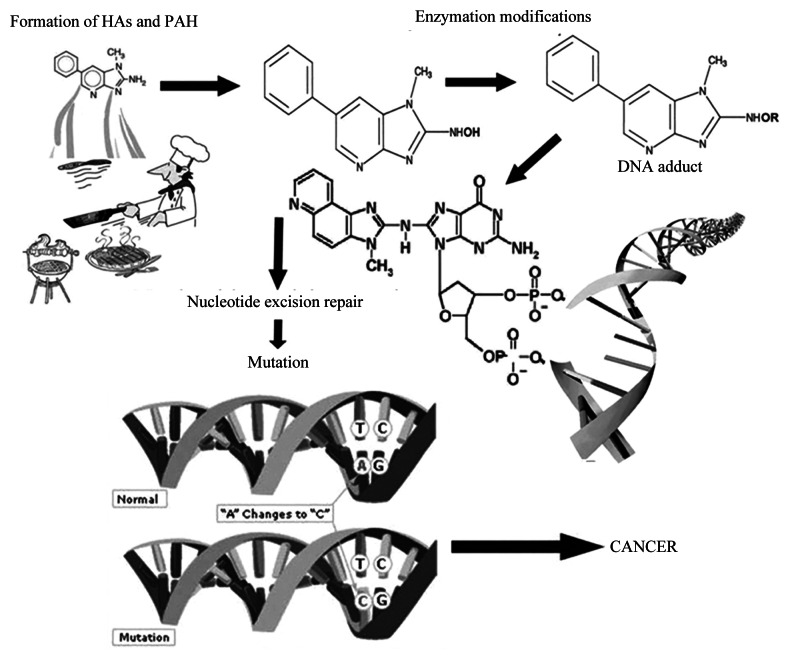
Rapid increase in the global burden of CRC is multifactorial, mainly attributed to certain genetic syndromes and environmental factors, such as dietary habits and life style changes, including, high meat and saturated fat consumption, chronic alcoholism, tobacco consumption and obesity.4 CRC arise by a series of well-defined histological changes (the adenoma-carcinoma sequence), paralleled with mutational activation of oncogenes and loss of heterozygosity of tumor suppressor genes by carcinogenic chemicals and mutagens.5,6 It occurs as a consequence of alteration in the equilibrium between DNA damage and DNA repair leading to cell progeny bearing mutagenic and/ or unrepaired DNA with mismatches that escaped during the DNA repair mechanism. (Fig. 1).7,8 It originates in the inner most intestinal lining and spreads inward to the inner lining, muscle tissue and other organs, leading to metastasis. Although, procarinogens, per se, are not carcinogenic but are converted to later through a series of metabolic reactions by enzymes of cytochrome P450 family and transformed to highly reactive electrophilic compounds which could react with DNA. Moreover, the risk of CRC development depends on the potency of carcinogens, exposure rate and the genetic constitution of the individual.
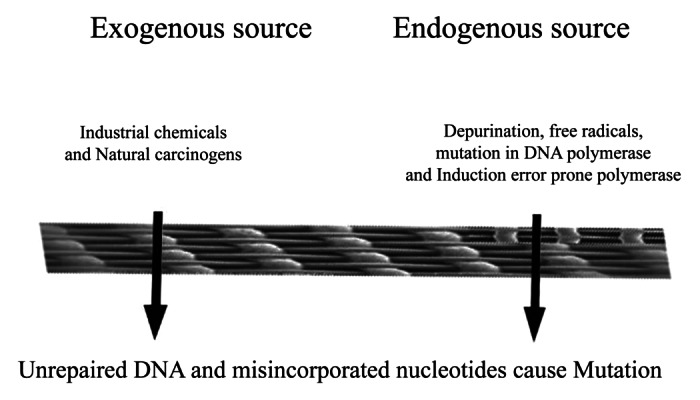
Figure 1. Causes of alterations in the equilibrium between DNA damage and DNA repair and unrepaired DNA escape this equilibrium.
Environmental carcinogens could be exogenous or endogenous in origin and linked to diet and dietary habits, life-style factors (nutrition, tobacco consumption, physical activity, etc.) and occupational hazards.9 Polycyclic aromatic hydrocarbons (PAH), heterocyclic amines (HCA), N-nitroso compounds (NOC), mycotoxins (aflatoxins) and acrylamide are well-known food carcinogens responsible for CRC, breast and prostate cancer, as reported in preclinical and clinical studies (Table 1 and Fig. 2).10-17 These are produced from precursors during food processing methods, for instance, curing, drying, smoking, roasting, refining and fermentation and air pollution.10,11,14 A strong correlation between regular consumption of food cooked at elevated temperatures and increased incidence of CRC was reported previously.10 Epidemiological studies showed that HCA are responsible for adenomatous polyps and the onset symptoms of CRC.18-21 Moreover, dose-dependent relationship was found between cancer risk and the dietary exposure to compounds namely, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,4,8-trimethyl-3H-imidazo[4,5-f]quinoxaline (DiMeIQx), 2-amino-3,8-dimethylimidazo [4,5-f]quinoxaline (MeIQx) and benzo[α]pyrene (BαP).22 Under such inevitable situations where one is continuously exposed to food and environmental carcinogens, the antimutagenic agents could play a vital role in complete elimination of mutagens from the host system. Dietary components from natural resources such as fruits, vegetables and cereals, could be an excellent antimutagenic agents.23 Probiotics and prebiotics gained a lot of attention as antimutagens for their noted carcinogen scavenging and elimination activity. In such scenario, a food supplement/ nutraceutical, principally rich in probiotics, prebiotics and synbiotics would serve as best biological therapy in the removal of food-borne mutagens and carcinogens; thus, preventing CRC.
Table 1. Major mutagens that have been identified in fried food, such as fried beef or fish are as follows.
| Short name | Chemical names | Molecular weight |
|---|---|---|
| Phe-P-1 |
2-amino-5-phenylpyridine |
170 |
| TMIP |
2-amino-n,n,n-trimethyl-imidazo{4,5-f]-pyridine |
176 |
| AαC |
2-amino-9H-pyrido-[2,3-b]-indole |
183 |
| GLU-P-2 |
2-aminodipyrido-[1,2-a:3′,2'-d]-imidazole |
184 |
| Trp-P-2 |
3-amino-1-methyl-5H-pyrido[4,3-b]-indole |
197 |
| MeAαC |
2-amino-3-methyl-9H-pyrido[2,3-b]-indole |
197 |
| IQ |
2-amino-3-methyl-imidazo[4,5-f]-quinoline |
198 |
| IQx |
2-amino-3-methyl-imidazo[4,5-f]-quinoxaline |
199 |
| Trp-P-1 |
3-amino-1,4-dimethyl-5H-pyrido[4,3-b]-indole |
211 |
| 4-MeIQ |
2-amino-3,4-dimethyl-imidazo[4,5-f]-quinoline |
212 |
| 8-MeIQx |
2-amino-3,8-dimethyl-imidazo[4,5-f]-quinoxaline |
213 |
| 4-MeIQx |
2-amino-3,4-dimethyl-imidazo[4,5-f]-quinoxaline |
213 |
| PhIP |
2-amino-1-methyl-6-phenyl-imidazo[4,5-b]-pyridine |
224 |
| 4,8-DiMelQx | 2-amino-3,4,8-trimethyl-imidazo[4,5-f]-quinoxaline | 227 |
Figure 2. Role of food mutagens in causing cancer.
Gut Microbiome in CRC
In spite of CRC treatment by surgery, chemotherapy and radiotherapy, the success rate for cancer treatment is still variable, with high mortality and other adverse side-effects. There is an urgent need to find an alternative solution to this quest. More recently, studies are focused on elucidating the functional accountability of gut microbiota in colon carcinogenesis.24-26
Human gastrointestinal tract (GIT), from small intestine to colon, harbours a variety of bacterial species approximately containing 107 to 1012 cells per gram of the intestinal content.24,27 Babies are born with a sterile intestinal tract that gets swarmed with favorable and unfavorable microbes along with the first feed; and following the childhood, the intestinal microflora remain fairly constant until the alterations are brought by the environmental factors, life style and modified genetic set-up. Human gut microbes are broadly categorized as symbionts, commensals and pathobionts28
Gut microbiota performs vital functions of the host including, immune and nutritional status, thus, assist in health maintenance.29 Equilibrium among various gut bacterial strains and host immunity decide the occurrence of physiological (regulates the presence of resident gut microbiota) and pathological inflammation (depends on the number and virulence of the invading pathogens). Besides these, chronic inflammation profoundly triggers local immune response leading to the release of reactive oxygen species (ROS) and nitric oxide that induce DNA damage and consequently altering tissue homeostasis.30 Cytokines produced during this process play a major role in tissue homeostasis. TNF-α, IL-6, IL-1 and chemokines induce tumor growth by promoting angiogenesis and suppressing immune-mediated tumor elimination; and, IL-10 and TGF-α acts as inhibitor in cancer establishment.30 Thus, altered gut microbiota promote pathogenesis through chronic inflammation, immune evasion and suppression. Colonoscopic studies showed varied distribution of bacterial genera in CRC patients, based on the disease status.31 Significant elevation in Bacteroides/Prevotella population were reported in cancer patients and were correlated with the elevated levels of IL-17 producing cells in the mucosa. A conspicuous difference in the microbial colonization patterns between the tumorous tissue and adjacent non-malignant mucosa suggests that CRC-associated physiological and metabolic changes recruit tumor-foraging commensal-like bacteria (Clostridium spp).32 In the recent years, a great deal of research has been dedicated in understanding the role of specific microbes/microbial community/microbial molecules that confer health benefits under patho-physiological conditions. These microbes may have an apparent competitive advantage in the tumor microenvironment, in replacing pathogenic bacteria in CRC etiology. The dynamic interplay between intestinal microbial ecology (balance between favorable and unfavorable bacteria) and sporadic CRC was investigated by Marchesi et al., which might be an important lead toward the novel microbiome-related diagnostic tools and therapeutic interventions.32
With the fact that in colonic environment, microflora and diet are closely involved in the etiology of CRC, an intense interest has been shown toward the use of probiotics, prebiotics and synbiotics in modulating gut microbiota, host metabolism and thereby aiding in cancer prevention.33,34 Hence the concept of probiotics, prebiotics and synbiotics, having a myriad of health-promoting effects is becoming a revolution. They have shown to alleviate lactose intolerance, lower serum cholesterol level, exert anticancer effect, improve constipation, enhance immunity, regulate obesity and relieve of vaginitis.35,36 Studies focusing on the anti-cancerous activity of probiotics, prebiotics and synbiotics against colorectal, breast and bladder cancer in pre-clinical and clinical trials, have been reported previously.37-39 This review is an attempt to summarize the role of probiotics, prebiotics and synbiotics in the prevention of CRC (Fig. 3).
Figure 3. Mechanism exhibited by probiotics, prebiotics and synbiotics against prevention of colorectal cancer.
Probiotics as Anti-carcinogens and Anti-mutagens: Mechanism of Action
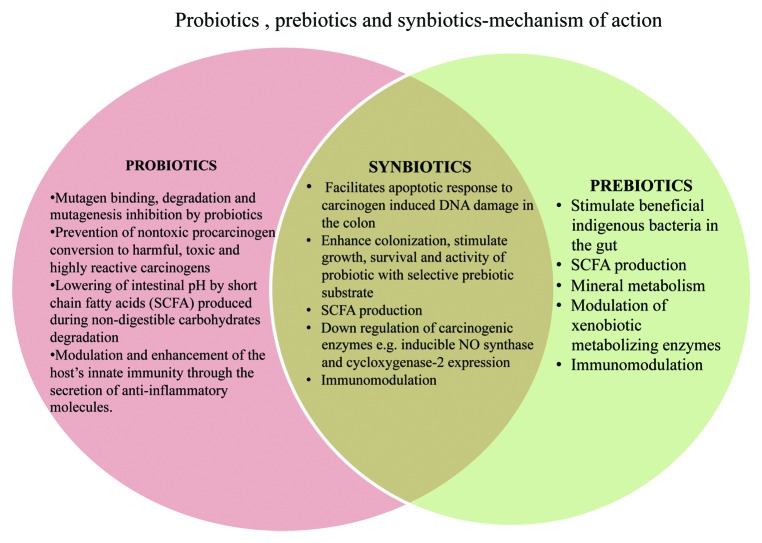
Anticancerous (ACA) and antimutagenic activity (AMA) of probiotics is due to the following:
(1) Mutagen binding, degradation and mutagenesis inhibition by probiotics
(2) Prevention of nontoxic procarcinogen conversion to harmful, toxic and highly reactive carcinogens
(3) Lowering of intestinal pH by short chain fatty acids (SCFA) produced during non-digestible carbohydrate degradation
(4) Modulation and enhancement of the host’s innate immunity through the secretion of anti-inflammatory molecules.
Mutagen binding, degradation and mutagenesis inhibition by probiotics
Potential probiotic strains bind the mutagens through the cell surface and peptidoglycans (sugar and protein moieties) and exert AMA and ACA.40-44 Cellular fraction and cell wall of Streptococcus cremoris Z-25 binds 3-amino-1, 4-dimethyl-5H-pyrido-[4,3-b]indole (Trp-P-1) and 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2).43 The cell wall skeleton of S. cremoris Z-25, L. acidophilus IFO13951 and B. bifidum IF014252 binds Trp-P-1, 2-amino-6-methyldipyrido[1,2-a:3′,2'-d]imidazole (Glu-P-1), 2-amino-5-phenylpyridine (Phe-P-1), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ) and MeIQx. Similarly, the AMA of the proteolytic variant of L. helveticus was due to the peptides released from the fermented milk, whereas, the non-proteolytic variant did not show similar effects.44 However, the exact mechanism of mutagen binding by peptides and its elimination was not elucidated and warrants further studies. In another study, four strains of L. gasseri and B. longum showed high binding and AMA against Trp-P-1, Trp-P-2, Glu-P-1, IQ and MeIQ.40 Binding was dependent on the chemical nature of the mutagen, pH, bacterial strain and the complex array of polysaccharide on the cell wall receptor sites. Sreekumar and Hosono emphasized on importance of using multiple probiotics strains in removing the broad spectrum of mutagens and carcinogens.40
AMA of “Natto” (a Bacillus subtilis-fermented soybean product) against HCAs was due to the binding of HCAs to the bacterial cell-wall structures45 that was dependent on the strain, mutagen chemical nature, pH, incubation time, metal ions, concentration of sodium chloride and alcohol, enzymes and acetylation of Trp-P-1 and IQ.45 Similarly, aflotoxin B1 (AFB1) binding studies to viable, heat- and acid-treated cells of L. rhamnosus GG implicated the involvement of cell wall in capturing AFB1 and is mainly attributed to cell wall carbohydrate components and hydrophobic and electrostatic interactions.46 Further binding of AFB1 to lactobacilli and bifidobacteria was found to be reversible.47
Culture free supernatants of L. plantarum KLAB21 (Kimchi, a Korean fermented food) showed high AMA against N-methyl-N'-nitro-N-nitrosoguanidine (MNNG), NQO, 4-nitro-O-phenylenediamine (NPD) and AFB1.48 AMA against MNNG was due to three secretory glycoproteins (16, 11 and 14 kD).42 Detoxification of mutagenic compounds (AαC, PhIP, IQ, MeIQx and DiMeIQx) by different LAB was reported49 and highest detoxification effect was observed for L. helveticus and S. thermophiles, which was seven to eight times more effective than L. kefir and L. plantarum. L. bulgaricus and S. thermophilus exhibited high AMA against 4-NQO and 2-aminofluorene than fermented milk extracts by S. thermophilus alone.50 Similarly, soymilk fermented with S. thermophilus, L. acidophilus, B. infantis, B. longum showed higher AMA against 3,2-dimethyl-4-amino-biphenyl (DMABP) due to the production of antimutagenic molecules during bacterial fermentation of milk.51 AMA of three B. longum strains in fermented skim milk against Trp-P-1 and Trp-P-2 increased with time.52 Dose-dependent inhibition of Trp-P-1 by B. longum PS+ strain was due to the involvement of crude polysaccharides in binding and AMA.52 In another study, irreversible mutagen binding and AMA by lactobacilli and bifidobacteria were attributed to the butyrate production, a SCFA, that acts at molecular level, as discussed later in prebiotics section.53 This emphasizes the importance of viable probiotic bacteria consumption. Exopolysaccharides (EPS) produced by L. plantarum 301102 inactivated the mutagen, Trp-P-1.54
AMA of probiotic bacteria is growth phase dependent. LAB and bifidobacteria produced extracellular bioactive compounds with differential AMA against B (α) P and sodium azide (SA) at different times of growth.55 Lactobacilli had higher AMA against B (α) P and SA in the stationary phase, whereas B. adolescentis ATCC 15703 exhibited higher AMA against B (α) P in the exponential phase but showed no activity against SA suggesting a strong correlation between bacterial AMA, growth phase and mutagen type.55
Concisely, mutagen binding by probiotic strains (lactobacilli and bifidobacteria) depends on peptidoglycans, polysaccharides, secretary glycoproteins, the growth phase and mutagen type. Further studies are required for precise understanding of the role of cell surface components of LAB and bifidobacteria in antimutagenesis. With the current advancement in molecular techniques, it is possible to accomplish mechanistic based studies to understand mutagen binding by different probiotics.
Prevention of nontoxic procarcinogens conversion to harmful, toxic and highly reactive carcinogens
Probiotic bacteria along with dietary ingredients helps in detoxification and biotransformation of procarcinogens and carcinogens into less toxic metabolites, thus preventing tumor formation.56 Biotransformation of mutagens/ carcinogens occur in the gut with the help of phase I and phase II enzymes and regulate the toxic, mutagenic and neoplastic effects of environmental carcinogens. These enzymes, in turn, are modulated by dietary agents.61 Phase I enzymes causes bio-activation and phase II enzymes causes the inactivation of mutagen/carcinogen.57 Lactobacillus strains from different commercial dairy products exhibited > 80% antigenotoxicity against 4-NQO.58 Antigenotoxicity of Lactobacillus strains against NQO and MNNG was attributed to strain dependency. L. casei, L. acidophilus, L. rhamnosus, L. delbrueckii subsp bulgaricus and L. plantarum had higher antigenotoxicity against NQO, while L. acidophilus had higher activity against MNNG.59 Antigenotoxic activities were correlated with the spectral modifications observed for procarcinogens/genotoxins after probiotic treatment. However, degraded products were not detected.59 Strains retained their viability during and after the genotoxin exposure probably elucidating the role and necessity of viable bacteria in antigenotoxicity. Antigenotoxicity of L. rhamnosus IMC 501 against 4-NQO was also explored, and biotransformation and detoxification of the mutagen was evaluated.60
In vitro experiments showed that L. casei DN 114001 (Actimel strain) adsorb and metabolized IQ, PhIP and MeIQx.61 The degree of detoxification was correlated with incubation time, cell growth and composition of the growth medium. Cells in the active growth phase showed higher activity.
Potential probiotic human strain L. rhamnosus 231 (Lr 231), was shown to possess antimicrobial activity against several human pathogens.62 Further, this strain exhibited in vitro binding of MNNG and MeIQx and biotransformation; and subsequent detoxification and AMA.63 Administration of viable Lr 231 protected rats from MNNG-induced colon inflammation.64 Levels of azoreductase, nitroreductase activities in the feces were reduced. Glutathione reductase activity was increased while glutathione S-transferase activity was reduced in the Lr 231-fed group.64 This was also evident in the histopathological sections of Lr 231-fed group.64 Therefore, Lr 231 supplementation protected the animals from MNNG-induced inflammation. Safety of this strain is proved in mouse model.65 Researchers are further investigating in detail the mechanism of biotransformation and degradation of different mutagens by Lr 231. Understanding the mechanism involved in biotransformation of mutagens/carcinogens by the probiotic bacteria may offer new ways for the management of mutagen or carcinogen-induced CRC.
Inhibition of procarcinogen conversion to carcinogens
Bifidobacteria and lactobacilli decreased the expression of xenobiotic-metabolizing enzymes compared with bacteroides, clostridia and enterobacteriaceae that mediate carcinogenesis through various enzymes, such as, β-glucuronidase, azoreductase and nitroreducatses.66 Certain strains of L. acidophilus and Bifidobacterium spp lowered the activity of these enzymes and reduced the risk of tumor development.66-69 SCFAs produced from colonic non-digestible carbohydrate fermentation enhance the growth of lactobacilli and bifidobacteria and inhibit the generation of carcinogenic products from procarcinogens by lowering enzyme levels.66,70 Modulation of conversion of procarcinogens to carcinogens by beneficial bacteria, is yet another exciting area that needs further detailed investigation at cellular and molecular level.
Lowering of intestinal pH
Probiotic bacteria produce lactic acid and other SCFAs as metabolic products from non-digestible carbohydrate fermentation in the gut. These SCFAs decreases the load of pathobionts, helps in maintaining homeostasis and lower the intestinal pH.66 It also assists in lowering solubility of bile acids and ammonia absorption and increases mineral absorption.70-73
Activation of the host immune system
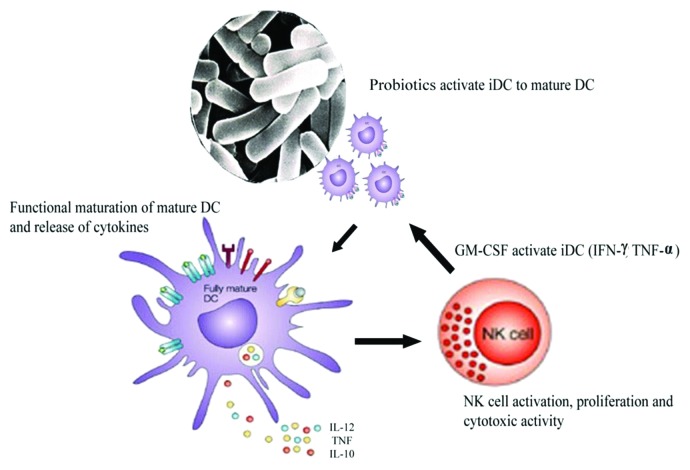
The immune system is a complex cascade, acting and reacting locally at systemic level. Recently, research is focused on understanding the regulation of immune system and the interactions within and between the components of inflammatory cascades.74 Different markers are used to explore these cascades and they are integrated to cope up with the microbial challenges from the environment and to manage common or severe infections.75,76 Dendritic cells (DCs) and natural killer (NK) cells play a critical role in early defense against cancer.77,78 Desmutagenic activities of different LAB strains were reported to regulate myeloid DCs maturation, polarizing the subsequent T-cell activity toward Th1, Th2 or even T-reg responses.79,80 Probiotic-induced immune suppression of carcinogenesis is critically reviewed elsewhere; NK cells were effective against tumor cells and low NK cell activity was associated to lower cancer risk.81 Oral administration of L. casei strain Shirota (LcS) to methylcholanthracene-induced tumor in mice, displaying enhanced host innate immunity by stimulating the spleenic NK cell activity; thus, leading to delayed onset of tumor development.82 Intrapleural injection of an inactivated strain of LcS in mice improved the immune system against tumor.83 This anti-tumor effect was reversed by using anti-TNF monoclonal antibodies, implicating the release of tumor necrosis factor- α (TNF-α), as the anti-tumor agent, signifying a cellular immune mediated effect. LAB modulated DCs, which in turn, are potent activators of NK cells.84 DCs encountering probiotics undergo maturation, stimulating NK cells. This desmutagenic potential of probiotics has been recently addressed, and now it’s evident that human monocyte-derived DCs, blood DCs, mouse spleenic and lymph node DCs were matured by IL-12 produced upon LAB induction. Later this molecule IL-12, activates NK cells to produce IFN-γ, which is in concordance with the belief that IL-12 is essential for IFN-γ production in NK cells (Fig. 4).85 In addition to producing SCFA and lowering the intestinal pH, probiotics might also exert beneficial effect by macrophage activation, cytochrome p450 blocking, reduction of carcinogen generation, downregulation of Ras-p21 expression, increase of cell differentiation, inhibition of cyclooxygenase-2 upregulation and inhibition of nitric oxide synthetase.34 Hence, both bioantimutagenic and desmutagenic activity of probiotics could be highly significant in preventing cancer; and needs further elaboration.
Figure 4. Desmutagenic potential of probiotics in preventing cancer. Interaction of probiotics with immature dendritic cells (DC) leading to activation of cascade and NK cell activation, proliferation and cytotoxic activity.
Prebiotics and Cancer
“Prebiotic” is defined as “a selectively fermentable non-digestible oligosaccharide or ingredient that brings specific changes, both in the composition and/ or activity of the gastrointestinal microflora, conferring health benefits.”86-90
Among different prebiotics, fructooligosaccharides (FOS) and galactooligosaccharides (GOS) are the two frequently reported and are “Generally Regarded as Safe” (GRAS). Others include, xylooligosaccharides (XOS), isomaltooligosaccharides (IMOS), glucooligosaccharides, pectin oligosaccharides (POS), mannanooligosacharides (MOS), gentiooligosaccharides (GTO), chitooligosaccharides (CHOS), soy bean oligosaccharides (SOS) and polydextrose that are not commercially available in high purity and the safety of these oligosaccharides are yet to be evaluated.85,887,90
Prebiotics: Anticarcinogens and Mechanism of Action
ACA of prebiotics is mainly due to the following properties:
(1) Stimulation of beneficial indigenous gut bacteria
(2) Production of SCFAs and lactic acid, as fermentation products
(3) Modification of gene expression in cecum, colon and feces
(4) Enhanced micronutrient absorption in the colon
(5) Modulation of xenobiotic metabolizing enzymes
(6) Modulation of immune response
(1) Stimulation of beneficial indigenous gut microbes
Human gut microbiome exhibit high degree of compositional stability and could be manipulated by prebiotics, probiotics and antibiotics.91,92 Prebiotics positively alter the gut microbiome and its dynamics by stimulating lactobacilli and bifidobacteria that bind and eliminate carcinogens from the gut system.
Animal model studies using prebiotic feed supplements have shown a profound effect in prevention and treatment of CRC. Feeding of long-chain inulin-type fructans increased bifidogenic effect, lowered pH and modulated immunity; and reduced the azoxymethane (AOM)-induced colonic pre-neoplastic aberrant crypt foci (ACF), small intestinal and colon tumors in rodent model.93 Similar inhibitory effects of short FOS and inulin were also reported on ACF-induced rat models.94,95 XOS and FOS were observed to inhibit colonic ACF in dimethylhydrazine (DMH)-treated rats by lowering cecal pH and serum triglyceride concentration. It also caused gain in total cecal weight, increased bifidobacterial population and noticeable reduction in the number of ACF in the colon.96 XOS were shown to exhibit higher bifidogenic effect and was more effective than FOS. GOS also significantly inhibited the development of DMH- or AOM-induced colorectal tumors.97,98 In human volunteer studies, administration of 10 g trans-GOS increased the bifidobacterial count and modified the fermentative activity of colonic flora.99
Consumption of inulin, FOS and GOS caused a laxative effect upon reaching the large intestine where they underwent fermentation, stimulated microbial growth resulting in increased bacterial mass, fecal bulking and peristalsis.100,101 Fecal bulking helps in cancer prevention by reducing transit time and mutagen contact time to intestinal lining.
(2) Production of SCFAs and lactic acid, as fermentation products
Colonic bacterial fermentation of prebiotics produces SCFA, which are the key products in maintaining gut health, intestinal morphology and function.70,102 Commonly formed SCFA are acetic, propionic and butyric acid approximately occurring in molar ratio of 60:20:20. Butyrate serves as an energy source for colonocytes and lowers luminal pH. At molecular level, butyrate acts as histone deacetylase inhibitor, promoting epigenetic hyperacetylation of histones and non-histone proteins (regulate the expression of critical cell cycle regulators CDKN1A),103 altering DNA-methylation, which results in enhanced accessibility of transcription factors to nucleosomal DNA, as mentioned previously.102-107 It was also associated with induction of cell differentiation, suppressed proliferation and enhanced apoptosis, to eliminate DNA-damaged cells that might otherwise progress to malignancy both in vitro and in vivo conditions.108-111 Lactate also improves gut health and gut-associated immune defense and increases adsorption surface area.112 Propionate and acetate induces apoptosis in human colorectal carcinoma cell lines through the loss of mitochondrial trans-membrane potential, generation of ROS, caspase-3-processing and nuclear chromatin condensation.102,113 Recently, SCFA has caught greater attention for its ACA and is investigated extensively.69,113-116 Equally, ACA of circulating propionate and acetate is imprecise and needs further investigation.
(3) Enhanced micronutrient absorption in the colon
The small intestine is the principal site of mineral absorption; however, minerals are absorbed throughout the gut, exerting beneficial effects. Consumption of short- and long-chain fructans increases mineral (calcium, selenium), vitamin (vitamin D) and antioxidant absorption that aid in decreasing cancer risk and maintaining normal bowel structure.117 Inulin, oligofructose, FOS, GOS, SOS, resistant starches (RS), sugar alcohols and di-fructose anhydride have positive effect on mineral absorption and metabolism.118 The underlying interplay between prebiotic-mineral absorption and ACA are manifold and includes, increased solubility of minerals due to local production of SCFA, occurrence of SCFA-salt conjugates, augmented absorption surface, increased expression of calcium-binding proteins, degradation of phytic acid-mineral complex that liberate associated minerals, release of bone-modulating factors, such as, phytoestrogens from foods, stabilization of intestinal microflora and intestinal mucus.117-120
(4) Modulation of xenobiotic metabolizing enzymes
Xenobiotic metabolizing enzymes are the indices of carcinogenicity. They are categorised into phase I and phase II that participates in carcinogen activation and metabolism. Phase I enzymes include, cytochrome-b5, cytochrome-b5 reductase, cytochrome P450, cytochrome P450 reductase, cytochrome P450 2E1 while, phase II include glutathione S-transferase (GST), uridine diphospho-glucuronyl transferase and DT-diaphorase that reduces the activation of procarcinogens to reactive carcinogenic intermediates and its elimination from the body.121 Modulation of phase I and phase II enzymes by dietary agents that have chemopreventive potential are explained earlier in the probiotics section.57 Resistant starch (RS) was observed to induce glutathione transferase π in rat colon.66 SCFA also induced glutathione transferase π as determined using Caco-2 cells.121 LAB makes pronounced stimulation of NADPH–dependent ferrihemoprotein reductase activity (cytochrome P450 reductase) in the colonocytes.122 Feeding prebiotics alone or in combination with horse chestnut/flaxseed reduced the β-glucuronidase activity and increased the β-galactosidase and β-glucosidase activity emphasizing the ACA of prebiotics.123 Similar observations of reduced bacterial β-glucuronidase activity and lower amounts of toxic ammonia in faeces were reported in corn hemicelluloses-fed healthy human volunteers.124 Arabinoxylooligosaccharides (AXOS) stimulated carbohydrate-fermenting bacteria to increase the uptake and assimilation of nitrogen and excretion of ammonia through faeces in healthy human volunteers.125 Reduced serum ammonia levels were observed in patients with liver cirrhosis upon XOS intake.126 This interaction of prebiotics, with gut flora and their proposed role of modulation in expression of xenobiotic metabolizing enzymes, together with its ACA, has barely been investigated and needs thorough research in order to understand and reveal the role of phase I and phase II enzymes.57 The protective effect of fructans, prebiotics and probiotics (lactobacilli and bifidobacteria) on AOM-induced carcinogenesis could be contemplated to the downregulation of gene-expression of inducible NO-synthetase and cyclooxygenase-2.127 Regulation of gene expression in the colon by the administration of prebiotics is yet another interesting topic that needs further research.
(5) Modulation of immune response
Prebiotics may indirectly exhibit immunogenic effects by influencing the intestinal microflora and modulates immune parameters like NK cell activity, secretion of IL-10 and interferon and lymphocyte proliferation.128 Consumption of prebiotics may modulate immune parameters in gut-associated lymphoid tissue (GALT), secondary lymphoid tissue and peripheral circulation.129 There is a paucity of reports examining the influence of prebiotics on the GALT for the improvement of human immune system and ACA. GOS was shown to reduce colitis in Smad3-deficient mice by modulating the function and trafficking of NK cells.130 Although, prebiotics are shown to have immunomodulatory effect, more studies are necessary to establish the mechanistic role of prebiotics-induced immunomodulation against cancer.
Dietary Fibers and its Anticarcinogenic Effect
Dietary fibers, similar to prebiotics, possess ACA that could be attributed to mutagen binding, diluting procarcinogens and carcinogens through fecal bulking and SCFA production.131-134 Monomeric composition and chain conformation of dietary fiber influence the rate and extent of fermentation and eventually, its ACA. Consumption of cereals, pulses, fiber-rich fruits and vegetables reduce the incidence of colorectal adenomas.134 Protective effect of dietary fibers against DMH, HCA and PAH were reported using animal models and in vitro assays.133,134 It was speculated that mutagen or carcinogen binding to dietary fibers or prebiotics may involve interaction between free functional groups of mutagens and dietary fibers or prebiotics and might not merely be an adsorption phenomenon.135 Thus, dietary ingredients could help in cancer prevention by modulating biotransformation of mutagen to less toxic compounds, thereby, making carcinogens less active . However, the role of prebiotics and dietary fibers in CRC prevention needs to be confirmed in human subjects. Another leading issue is to constitute the relation between prebiotics and colonic bifidobacterial count. Most of the available data merely imply the relationship between changes in bacterial composition, reduction in ACF and carcinogenesis. However, no concrete evidence about the involvement of the growth stimulation of lactobacilli, bifidobacteria or other healthy microbial inhabitants are available so far and needs a detailed study using advance techniques to establish this relationship.
Synbiotics and Cancer
“Synbiotics” (“syn”-together and “bios”-life) is “a combination of probiotic bacteria and the growth promoting prebiotic ingredient” that purport “synergism”. Kolida and Gibson, proposed two synbiotic concepts:136
(1) Complementary concept: A single or combination of probiotic bacteria, selected based on the specific-desired host benefits, and prebiotics that are independently chosen to stimulate the beneficial gut microbial population. Prebiotics promote growth and activity of the ingested probiotic, but only indirectly as part of its target range.
(2) Synergistic concept: Specific host beneficial probiotics are selected and the prebiotic component is chosen to specifically enhance the survival, growth and activity of the selected probiotic strain (s). However, the prebiotic may also increase the levels of resident gastrointestinal beneficial microbiota of the host.
An ideal synergistic synbiotic supplement should contain an appropriate single or multi strain probiotic and suitable mixture of prebiotics, where the later selectively favors the former and produce additive or synergistic effect.136,137 It should favor the multiplication of the endogenous beneficial bacteria and reduce the number of cancer-promoting bacteria.
Synbiotics: Anticarcinogens and Mechanisms of Action
Anticarcinogenic effect of synbiotics is ambiguous and is still under debate. The possible mechanisms could be:
(1) Facilitating apoptotic response to carcinogen-induced DNA damage in the colon
(2) Enhancing colonization, stimulate growth, survival and activity of probiotics in the presence of selective prebiotic substrate
(3) Increase SCFA production, anti proliferative activity of synbiotics and downregulation of inducible NO-synthase and cycloxygenase-2 enzymes, involved in colon carcinogenesis
(4) Immunomodulation
(5) Modification of colonic bacterial ecosystem, leading to an overall improvement in metabolic activity of the colon and cecum.
Diets rich in olive oil and extracts from freeze-dried fruits and vegetables considerably reduce the intestinal adenomas in mice indicating that calorie restriction and diet modulation would affect the intestinal microbiota and prevent carcinogenesis.138 B. longum with a derivative of inulin (“Raftiline HP”) also brought similar beneficial changes in cecal physiology and bacterial metabolic activity, reduced tumor risk and the incidence of an AOM-induced putative pre-neoplastic colonic lesions in the rodent model.139 Combination of B. lactis and resistant starch (RS) facilitated the acute apoptotic response to a genotoxic carcinogen (AARGC) and colonic fermentative events in rat model.140 RS serves as a metabolic substrate for B. lactis to exert its pro-apoptotic action. Same research group later in another study reported the protective effect of B. lactis and RS individually and their synbiotic combination (B.lactis and RS) in AOM-induced CRC in rodent models.141 Fermentation events were altered by the inclusion of RS into the diet. This supports the complementary synbiotic concept and proves the superior preventive strategy of synbiotics over prebiotic or probiotic alone.136,141
Fructans and synbiotic combination containing fructans with B. lactis (Bb12) and L. rhamnosus GG minifies the AOM-induced colorectal adenomas and carcinogenesis by increasing SCFA production, lowering proliferative activity and the expression of GST placental enzyme pi-type, inducible NO-synthase, cyclooxygenase-2 enzymes, involved in the pathogenesis of CRC.127Anti-tumorigenic effect of synbiotic combination, oligofructose-enriched inulin, L. rhamnosus and B. lactis was due to immune-modulation, and it was demonstrated that peripheral blood mononuclear cells (PBMC) and Peyer’s patches (PP) were the primary tissues that were specifically affected by prebiotics.142 Moreover, prebiotic supplementation alone induced significant immune-modulation in the intestine, whereas probiotic supplementation was primarily effective when provided as a component of synbiotic. These studies stressed the significance of synbiotic containing prebiotics and probiotics in CRC treatment. Same group reported that AOM treatment significantly reduced NK-cell like cytotoxicity in control, probiotic and prebiotic supplemented groups. In synbiotic supplemented group, NK cell like cytotoxicity in PP was prevented compared with control rats.143Additionally, synbiotic and prebiotic supplemented groups stimulated IL-10 production and reduced interferon-γ production in PP. Largely, synbiotic supplementation in carcinogen-treated rats modulated immune function in the PP and coincided with a reduced number of colon tumors by GALT modulation.143
ACA of FOS together with Bifidobacterium strain and lactitol in conjunction with Lactobacillus, were also reported in vitro and in vivo models.144
Beneficial effect of synbiotics against CRC is postulated based on the studies in animal model. Till date, only one human study showing protective effect of synbiotic against colon cancer is published.145A synbiotic preparation containing oligofructose enriched inulin, L. rhamnosus GG (LGG) and B. lactis Bb12 (BB12) were able to reduce the risk of colon cancer in 12-week randomized, double-blinded, placebo-controlled human study. Synbiotic intervention reduced genotoxin exposure and resulted in increased bifidobacteria and lactobacilli and reduced Clostridium perfringens. This intervention also reduced the colorectal proliferation and the capacity of fecal water to induce necrosis in colonic cells and improved epithelial barrier function in polypectomized patients. Synbiotic consumption reduced IL-2 secretion by peripheral blood mononuclear cells (PBMC) and increased the production of interferon-γ.145
With the studies done so far, synbiotics seems to be far superior to probiotics or prebiotic alone in preventing or treating CRC. Combination of Lactobacillus and bifidobacteria strains together with oligosaccharides (GOS, FOS and inulin) gave greater results compared with pro/prebiotics given individually. However, knowledge on the compatibility of strains in a multi-strain synbiotic combination, minimum effective dose to impart the desired health benefits without any side effects and the appropriate biomarkers in the in vivo trials, are lacking. Studies on the synergistic effects of probiotics and prebiotics with the above discussed observation need to be studied in detail for the effective development of synbiotics.
Conclusion
Knowledge available so far based on in vitro and animal-based studies indicate that probiotics, prebiotics and synbiotics are an ideal choice for the prevention of carcinogenesis. With the advancement in molecular techniques and elucidation of gut microbiome, it is possible to understand the definite mechanism of probiotics, prebiotics and synbiotics, individually or collectively, as anticarcinogenic agent that might answer many unsolved queries and will open new avenues and strategies for cancer prevention, based on the dietary intervention. In conclusion, prevention and treatment of CRC using probiotics, prebiotics and synbiotics need a thorough investigation and more detailed study with human clinical trials and evidences.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23919
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr, accessed on day/month/year.
- 2.Bray F, Ren JS, Masuyer E, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Margalit O, Dubois RN. Metronomic topotecan for colorectal cancer: a promising new option. Gut. 2012;62:190–1. doi: 10.1136/gutjnl-2012-302410. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Diet, Nutrition and Prevention of Human Cancer: A Global Perspective. World Cancer Research Fund & American Institute of Cancer Research. Washington, DC: WCRF/AICR. 1997. [Google Scholar]
- 5.Ferguson PJ, Kurowska E, Freeman DJ, Chambers AF, Koropatnick DJ. A flavonoid fraction from cranberry extract inhibits proliferation of human tumor cell lines. J Nutr. 2004;134:1529–35. doi: 10.1093/jn/134.6.1529. [DOI] [PubMed] [Google Scholar]
- 6.Sankpal UT, Pius H, Khan M, Shukoor MI, Maliakal P, Lee CM, et al. Environmental factors in causing human cancers: emphasis on tumorigenesis. Tumour Biol. 2012;33:1265–74. doi: 10.1007/s13277-012-0413-4. [DOI] [PubMed] [Google Scholar]
- 7.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci U S A. 1995;92:5258–65. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–9. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 9.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeh LA. Environmental and chemical carcinogenesis seminars. Cancer Biol. 2004;14:473–86. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Jägerstad M, Skog K. Genotoxicity of heat-processed foods. Mutat Res. 2005;574:156–72. doi: 10.1016/j.mrfmmm.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 11.Felton JS, Knize MG, Wu RW, Colvin ME, Hatch FT, Malfatti MA. Mutagenic potency of food-derived heterocyclic amines. Mutat Res. 2007;616:90–4. doi: 10.1016/j.mrfmmm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Sankpal UT, Pius H, Khan M, Shukoor MI, Maliakal P, Lee CM, et al. Environmental factors in causing human cancers: emphasis on tumorigenesis. Tumour Biol. 2012;33:1265–74. doi: 10.1007/s13277-012-0413-4. [DOI] [PubMed] [Google Scholar]
- 13.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl. 2012;14:365–74. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagao M, Sugimura T. Carcinogenic factors in food with relevance to colon cancer development. Mutat Res. 1993;290:43–51. doi: 10.1016/0027-5107(93)90031-A. [DOI] [PubMed] [Google Scholar]
- 15.Skog K, Enerith Å, Svanberg M. Effects of different cooking methods on the formation of food mutagens in meat. Int J Food Sci Technol. 2003;8:313–23. doi: 10.1046/j.1365-2621.2003.00677.x. [DOI] [Google Scholar]
- 16.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Rashid A, Cleary K, Abbruzzese JL, Friess H, Takahashi S, et al. Detection of 2-amino-1-methyl-6-phenylimidazo [4,5-b]-pyridine (PhIP)-DNA adducts in human pancreatic tissues. Biomarkers. 2006;11:319–28. doi: 10.1080/13547500600667911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishibe N, Sinha R, Hein DW, Kulldorff M, Strickland P, Fretland AJ, et al. Genetic polymorphisms in heterocyclic amine metabolism and risk of colorectal adenomas. Pharmacogenetics. 2002;12:145–50. doi: 10.1097/00008571-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Gunter MJ, Probst-Hensch NM, Cortessis VK, Kulldorff M, Haile RW, Sinha R. Meat intake, cooking-related mutagens and risk of colorectal adenoma in a sigmoidoscopy-based case-control study. Carcinogenesis. 2005;26:637–42. doi: 10.1093/carcin/bgh350. [PubMed: 15579480] [DOI] [PubMed] [Google Scholar]
- 20.Sinha R, Peters U, Cross AJ, Kulldorff M, Weissfeld JL, Pinsky PF, et al. Meat, meat cooking methods and preservation, and risk for colorectal adenoma. Cancer Res. 2005;65:8034–41. doi: 10.1158/0008-5472.CAN-04-3429. [DOI] [PubMed] [Google Scholar]
- 21.Wu K, Giovannucci E, Byrne C, Platz EA, Fuchs C, Willett WC, et al. Meat mutagens and risk of distal colon adenoma in a cohort of U.S. men. Cancer Epidemiol Biomarkers Prev. 2006;15:1120–5. doi: 10.1158/1055-9965.EPI-05-0782. [DOI] [PubMed] [Google Scholar]
- 22.Anderson KE, Sinha R, Kulldorff M, Gross M, Lang NP, Barber C, et al. Meat intake and cooking techniques: associations with pancreatic cancer. Mutat Res. 2002;506-507:225–31. doi: 10.1016/S0027-5107(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson LR, Philpott M, Karunasinghe N. Dietary cancer and prevention using antimutagens. 2004. Toxicol. 2004;198:147–59. doi: 10.1016/j.tox.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 24.Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512–9. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- 25.Quigley EM. Gut microbiota and the role of probiotics in therapy. Curr Opin Pharmacol. 2011;11:593–603. doi: 10.1016/j.coph.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010;31:246–51. doi: 10.1093/carcin/bgp197. [DOI] [PubMed] [Google Scholar]
- 27.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735–44. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 30.Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14, e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 31.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesi JR, Dutilh BE, Hall N, Peters WH, Roelofs R, Boleij A, et al. Towards the human colorectal cancer microbiome. PLoS One. 2011;6:e20447. doi: 10.1371/journal.pone.0020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol Ther. 2006;5:1265–9. doi: 10.4161/cbt.5.10.3296. [DOI] [PubMed] [Google Scholar]
- 34.Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9:854–63. doi: 10.3390/ijms9050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66. doi: 10.1007/10_2008_097. [DOI] [PubMed] [Google Scholar]
- 36.Delzenne NM, Cani PD. Interaction between obesity and the gut microbiota: relevance in nutrition. Annu Rev Nutr. 2011;31:15–31. doi: 10.1146/annurev-nutr-072610-145146. [DOI] [PubMed] [Google Scholar]
- 37.Feyisetan O, Tracey C, Hellawell GO. Probiotics, dendritic cells and bladder cancer. BJU Int. 2012;109:1594–7. doi: 10.1111/j.1464-410X.2011.10749.x. [DOI] [PubMed] [Google Scholar]
- 38.de Moreno de Leblanc A, Perdigón G. The application of probiotic fermented milks in cancer and intestinal inflammation. Proc Nutr Soc. 2010;69:421–8. doi: 10.1017/S002966511000159X. [DOI] [PubMed] [Google Scholar]
- 39.Kado K, Forsyth A, Patel PR, Schwartz JA. Dietary supplements and natural products in breast cancer trials. Front Biosci (Elite Ed) 2012;4:546–67. doi: 10.2741/399. [DOI] [PubMed] [Google Scholar]
- 40.Sreekumar O, Hosono A. Antimutagenicity and the influence of physical factors in binding Lactobacillus gasseri and Bifidobacterium longum cells to amino acid pyrolysates. J Dairy Sci. 1998;81:1508–16. doi: 10.3168/jds.S0022-0302(98)75716-9. [DOI] [PubMed] [Google Scholar]
- 41.Sreekumar O, Hosono A. The heterocyclic amine binding receptors of Lactobacillus gasseri cells. Mutat Res. 1998;421:65–72. doi: 10.1016/S0027-5107(98)00155-9. [DOI] [PubMed] [Google Scholar]
- 42.Rhee CH, Park HD. Three glycoproteins with antimutagenic activity identified in Lactobacillus plantarum KLAB21. Appl Environ Microbiol. 2001;67:3445–9. doi: 10.1128/AEM.67.8.3445-3449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang XB, Ohta Y. Binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria on mutagens. J Dairy Sci. 1991;74:1477–81. doi: 10.3168/jds.S0022-0302(91)78306-9. [DOI] [PubMed] [Google Scholar]
- 44.Matar C, Nadathur SS, Bakalinsky AT, Goulet J. Antimutagenic effects of milk fermented by Lactobacillus helveticus L89 and a protease-deficient derivative. J Dairy Sci. 1997;80:1965–70. doi: 10.3168/jds.S0022-0302(97)76139-3. [DOI] [PubMed] [Google Scholar]
- 45.Rajendran R, Ohta Y. Binding activity of natto (a fermented food) and Bacillus natto isolates to mutagenic-carcinogenic heterocyclic amines. Can J Microbiol. 2001;47:935–42. doi: 10.1139/w01-094. [DOI] [PubMed] [Google Scholar]
- 46.Haskard C, Binnion C, Ahokas J. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem Biol Interact. 2000;128:39–49. doi: 10.1016/S0009-2797(00)00186-1. [DOI] [PubMed] [Google Scholar]
- 47.Peltonen K, el-Nezami H, Haskard C, Ahokas J, Salminen S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J Dairy Sci. 2001;84:2152–6. doi: 10.3168/jds.S0022-0302(01)74660-7. [DOI] [PubMed] [Google Scholar]
- 48.Rhee C, Park H. Antimutagenic activity of Lactobacillus plantarum KLAB21 isolated from kimchi Korean fermented vegetables. Biotechnol Lett. 2001;23:1583–9. doi: 10.1023/A:1011921427581. [DOI] [Google Scholar]
- 49.Stidl R, Sontag G, Koller V, Knasmüller S. Binding of heterocyclic aromatic amines by lactic acid bacteria: results of a comprehensive screening trial. Mol Nutr Food Res. 2008;52:322–9. doi: 10.1002/mnfr.200700034. [DOI] [PubMed] [Google Scholar]
- 50.Bodana AR, Rao DR. Antimutagenic activity of milk fermented by Streptococcus thermophilus and Lactobacillus bulgaricus. J Dairy Sci. 1990;73:3379–84. doi: 10.3168/jds.S0022-0302(90)79033-9. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh ML, Chou CC. Mutagenicity and antimutagenic effect of soymilk fermented with lactic acid bacteria and bifidobacteria. Int J Food Microbiol. 2006;111:43–7. doi: 10.1016/j.ijfoodmicro.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 52.Sreekumar O, Hosono A. The antimutagenic properties of a polysaccharide produced by Bifidobacterium longum and its cultured milk against some heterocyclic amines. Can J Microbiol. 1998;44:1029–36. [PubMed] [Google Scholar]
- 53.Lankaputhra WEV, Shah NP. Antimutagenic properties of probiotic bacteria and of organic acids. Mutat Res. 1998;397:169–82. doi: 10.1016/S0027-5107(97)00208-X. [DOI] [PubMed] [Google Scholar]
- 54.Tsuda H, Hara K, Miyamoto T. Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J Dairy Sci. 2008;91:2960–6. doi: 10.3168/jds.2007-0538. [DOI] [PubMed] [Google Scholar]
- 55.Chalova VI, Lingbeck JM, Kwon YM, Ricke SC. Extracellular antimutagenic activities of selected probiotic Bifidobacterium and Lactobacillus spp. as a function of growth phase. J Environ Sci Health B. 2008;43:193–8. doi: 10.1080/03601230701795262. [DOI] [PubMed] [Google Scholar]
- 56.Pool-Zobel B, Veeriah S, Böhmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 57.Hammons GJ, Lyn-Cook BD. Modulation of biotransformation enzymes in cancer chemoprevention. Int J Cancer Prev. 2004;1:3–14. [Google Scholar]
- 58.Cenci G, Rossi J, Trotta F, Caldini G. Lactic acid bacteria isolated from dairy products inhibit genotoxic effect of 4-nitroquinoline-1-oxide in SOS-chromotest. Syst Appl Microbiol. 2002;25:483–90. doi: 10.1078/07232020260517607. [DOI] [PubMed] [Google Scholar]
- 59.Caldini G, Trotta F, Villarini M, Moretti M, Pasquini R, Scassellati-Sforzolini G, et al. Screening of potential lactobacilli antigenotoxicity by microbial and mammalian cell-based tests. Int J Food Microbiol. 2005;102:37–47. doi: 10.1016/j.ijfoodmicro.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Verdenelli M, Ricciutelli M, Gigli F, Cenci G, Trotta F, Caldini G, et al. Investigation of antigenotoxic properties of probiotic Lactobacillus rhamnosus IMC 501 by gas chromatography-mass spectrometry. Ital J Food Sci. 2010;22:473–8. [Google Scholar]
- 61.Nowak A, Libudzisz Z. Ability of probiotic Lactobacillus casei DN 114001 to bind or/and metabolise heterocyclic aromatic amines in vitro. Eur J Nutr. 2009;48:419–27. doi: 10.1007/s00394-009-0030-1. [DOI] [PubMed] [Google Scholar]
- 62.Ambalam PS, Prajapati JB, Dave JM, Nair BM, Ljungh Å, Vyas BRM. Isolation and characterization of antimicrobial proteins produced by a potential probiotic strain of human Lactobacillus rhamnosus 231 and its effect on selected human pathogens and food spoilage organisms. Microb Ecol Health Dis. 2009;21:211–20. doi: 10.3109/08910600903429052. [DOI] [Google Scholar]
- 63.Ambalam P, Dave JM, Nair BM, Vyas BR. In vitro mutagen binding and antimutagenic activity of human Lactobacillus rhamnosus 231. Anaerobe. 2011;17:217–22. doi: 10.1016/j.anaerobe.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Gosai V, Ambalam P, Raman M, Kothari CR, Kothari RK, Vyas BR, et al. Protective effect of Lactobacillus rhamnosus 231 against N-methyl-N’-nitro-N-nitrosoguanidine in animal model. Gut Microbes. 2011;2:319–25. doi: 10.4161/gmic.18755. [DOI] [PubMed] [Google Scholar]
- 65.Ambalam P, Ramoliya JM, Dave JM, Vyas BRM. Safety assessment of potential probiotic Lactobacillus rhamnosus 231 and Lactobacillus rhamnosus V92 in animal model. International journal of Bioassays. 2013;2:333–7. [Google Scholar]
- 66.Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73(Suppl):451S–5S. doi: 10.1093/ajcn/73.2.451s. [DOI] [PubMed] [Google Scholar]
- 67.Goldin BR, Gorbach SL. Alterations of the intestinal microflora by diet, oral antibiotics, and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes, and glucuronides. J Natl Cancer Inst. 1984;73:689–95. [PubMed] [Google Scholar]
- 68.Yoon H, Benamouzig R, Little J, François-Collange M, Tomé D. Systematic review of epidemiological studies on meat, dairy products and egg consumption and risk of colorectal adenomas. Eur J Cancer Prev. 2000;9:151–64. doi: 10.1097/00008469-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Ouwehand AC, Lagström H, Suomalainen T, Salminen S. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab. 2002;46:159–62. doi: 10.1159/000063075. [DOI] [PubMed] [Google Scholar]
- 70.Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:351–66. doi: 10.1177/0115426506021004351. [DOI] [PubMed] [Google Scholar]
- 71.Cook SI, Sellin JH. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12:499–507. doi: 10.1046/j.1365-2036.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 72.Vince A, Killingley M, Wrong OM. Effect of lactulose on ammonia production in a fecal incubation system. Gastroenterology. 1978;74:544–9. [PubMed] [Google Scholar]
- 73.Jenkins DJ, Woever TM, Collier GR, Ocana A, Rao AV, Buckley G, et al. Metabolic effect of a low glycemic-index diet. Am J Clin Nutr. 1987;46:986–95. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- 74.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 75.Antoine JM. Probiotics: beneficial factors of the defence system. Proc Nutr Soc. 2010;69:429–33. doi: 10.1017/S0029665110001692. [DOI] [PubMed] [Google Scholar]
- 76.Albers R, Antoine JM, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81. doi: 10.1079/BJN20051469. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 78.Takeuchi H, Maehara Y, Tokunaga E, Koga T, Kakeji Y, Sugimachi K. Prognostic significance of natural killer cell activity in patients with gastric carcinoma: a multivariate analysis. Am J Gastroenterol. 2001;96:574–8. doi: 10.1111/j.1572-0241.2001.03535.x. [DOI] [PubMed] [Google Scholar]
- 79.Stagg AJ, Hart AL, Knight SC, Kamm MA. Microbial-gut interactions in health and disease. Interactions between dendritic cells and bacteria in the regulation of intestinal immunity. Best Pract Res Clin Gastroenterol. 2004;18:255–70. doi: 10.1016/j.bpg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 80.Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171–8. doi: 10.4049/jimmunol.168.1.171. [DOI] [PubMed] [Google Scholar]
- 81.Commane D, Hughes R, Shortt C, Rowland I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat Res. 2005;591:276–89. doi: 10.1016/j.mrfmmm.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 82.Zeuthen LH, Christensen HR, Frøkiaer H. Lactic acid bacteria inducing a weak interleukin-12 and tumor necrosis factor alpha response in human dendritic cells inhibit strongly stimulating lactic acid bacteria but act synergistically with gram-negative bacteria. Clin Vaccine Immunol. 2006;13:365–75. doi: 10.1128/CVI.13.3.365-375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takagi A, Matsuzaki T, Sato M, Nomoto K, Morotomi M, Yokokura T. Enhancement of natural killer cytotoxicity delayed murine carcinogenesis by a probiotic microorganism. Carcinogenesis. 2001;22:599–605. doi: 10.1093/carcin/22.4.599. [DOI] [PubMed] [Google Scholar]
- 84.Yasutake N, Matsuzaki T, Kimura K, Hashimoto S, Yokokura T, Yoshikai Y. The role of tumor necrosis factor (TNF)-α in the antitumor effect of intrapleural injection of Lactobacillus casei strain Shirota in mice. Med Microbiol Immunol. 1999;188:9–14. doi: 10.1007/s004300050099. [DOI] [PubMed] [Google Scholar]
- 85.Rizzello V, Bonaccorsi I, Dongarrà ML, Fink LN, Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J Biomed Biotechnol. 2011;2011:473097. doi: 10.1155/2011/473097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 87.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–75. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 88.Roberfroid MB. Prebiotics: the concept revisited. J Nutr. 2007;137(Suppl 2):830S–7S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 89.Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, et al. Dietary prebiotics: Current status and new definition. IFIS Functional Foods Bulletin. 2011;7:1–19. doi: 10.1616/1476-2137.15880. [DOI] [Google Scholar]
- 90.Aachary A, Prapulla SG. Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Comp Rev Food Sci Food Safety. 2011;10:2–16. doi: 10.1111/j.1541-4337.2010.00135.x. [DOI] [Google Scholar]
- 91.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 92.Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–9. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- 93.Verghese M, Rao DR, Chawan CB, Williams LL, Shackelford L. Dietary inulin suppresses azoxymethane-induced aberrant crypt foci and colon tumors at the promotion stage in young Fisher 344 rats. J Nutr. 2002;132:2809–13. doi: 10.1093/jn/132.9.2809. [DOI] [PubMed] [Google Scholar]
- 94.Reddy BS, Hamid R, Rao CV. Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis. 1997;18:1371–4. doi: 10.1093/carcin/18.7.1371. [DOI] [PubMed] [Google Scholar]
- 95.Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998;19:281–5. doi: 10.1093/carcin/19.2.281. [DOI] [PubMed] [Google Scholar]
- 96.Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr. 2004;134:1523–8. doi: 10.1093/jn/134.6.1523. [DOI] [PubMed] [Google Scholar]
- 97.Wijnands MV, Schoterman HC, Bruijntjes JB, Hollanders VM, Woutersen RA. Effect of dietary galacto-oligosaccharides on azoxymethane-induced aberrant crypt foci and colorectal cancer in Fischer 344 rats. Carcinogenesis. 2001;22:127–32. doi: 10.1093/carcin/22.1.127. [DOI] [PubMed] [Google Scholar]
- 98.Wijnands MV, Appel MJ, Hollanders VM, Woutersen RA. A comparison of the effects of dietary cellulose and fermentable galacto-oligosaccharide, in a rat model of colorectal carcinogenesis: fermentable fibre confers greater protection than non-fermentable fibre in both high and low fat backgrounds. Carcinogenesis. 1999;20:651–6. doi: 10.1093/carcin/20.4.651. [DOI] [PubMed] [Google Scholar]
- 99.Bouhnik Y, Flourié B, D’Agay-Abensour L, Pochart P, Gramet G, Durand M, et al. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr. 1997;127:444–8. doi: 10.1093/jn/127.3.444. [DOI] [PubMed] [Google Scholar]
- 100.Den Hond E, Geypens B, Ghoos Y. Effect of high performance chicory inulin on constipation. Nutr Res. 2000;20:731–6. doi: 10.1016/S0271-5317(00)00162-7. [DOI] [Google Scholar]
- 101.Niittynen L, Kajander K, Korpela R. Galacto-oligosaccharides and bowel function. Scand J Food Nutr. 2007;51:62–6. doi: 10.1080/17482970701414596. [DOI] [Google Scholar]
- 102.Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35(Suppl):S35–8. doi: 10.1136/gut.35.1_Suppl.S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jan G, Belzacq AS, Haouzi D, Rouault A, Métivier D, Kroemer G, et al. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002;9:179–88. doi: 10.1038/sj.cdd.4400935. [DOI] [PubMed] [Google Scholar]
- 104.Li H, Gao Z, Zhang J, Ye X, Xu A, Ye J, et al. Sodium butyrate stimulates expression of fibroblast growth factor 21 in liver by inhibition of histone deacetylase 3. Diabetes. 2012;61:797–806. doi: 10.2337/db11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Smith JG, Yokoyama WH, German JB. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci Nutr. 1998;38:259–97. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- 106.Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr. 2004;134:2450S–4S. doi: 10.1093/jn/134.9.2450S. [DOI] [PubMed] [Google Scholar]
- 107.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–19. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 108.Li CJ, Elsasser TH. Butyrate-induced apoptosis and cell cycle arrest in bovine kidney epithelial cells: involvement of caspase and proteasome pathways. J Anim Sci. 2005;83:89–97. doi: 10.2527/2005.83189x. [DOI] [PubMed] [Google Scholar]
- 109.Pool-Zobel BL, Selvaraju V, Sauer J, Kautenburger T, Kiefer J, Richter KK, et al. Butyrate may enhance toxicological defence in primary, adenoma and tumor human colon cells by favourably modulating expression of glutathione S-transferases genes, an approach in nutrigenomics. Carcinogenesis. 2005;26:1064–76. doi: 10.1093/carcin/bgi059. [DOI] [PubMed] [Google Scholar]
- 110.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res. 1997;57:3697–707. [PubMed] [Google Scholar]
- 111.Le Leu RK, Brown IL, Hu Y, Young GP. Effect of resistant starch on genotoxin-induced apoptosis, colonic epithelium, and lumenal contents in rats. Carcinogenesis. 2003;24:1347–52. doi: 10.1093/carcin/bgg098. [DOI] [PubMed] [Google Scholar]
- 112.Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2012;••• doi: 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 113.Hosseini E, Grootaert C, Verstraete W, Van de Wiele T. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev. 2011;69:245–58. doi: 10.1111/j.1753-4887.2011.00388.x. [DOI] [PubMed] [Google Scholar]
- 114.Whitehead RH, Young GP, Bhathal PS. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM1215) Gut. 1986;27:1457–63. doi: 10.1136/gut.27.12.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–43. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 116.Fukuda S, Toh H, Taylor TD, Ohno H, Hattori M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes. 2012;3:449–54. doi: 10.4161/gmic.21214. [DOI] [PubMed] [Google Scholar]
- 117.Scholz-Ahrens KE, Schaafsma G, van den Heuvel EGHM, Schrezenmeir J. Effects of prebiotics on mineral metabolism. Am J Clin Nutr. 2001;73(Suppl):459S–64S. doi: 10.1093/ajcn/73.2.459s. [DOI] [PubMed] [Google Scholar]
- 118.Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Açil Y, et al. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr. 2007;137(Suppl 2):838S–46S. doi: 10.1093/jn/137.3.838S. [DOI] [PubMed] [Google Scholar]
- 119.Coudray C, Demigné C, Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J Nutr. 2003;133:1–4. doi: 10.1093/jn/133.1.1. [DOI] [PubMed] [Google Scholar]
- 120.Van Loo JA. Prebiotics promote good health: the basis, the potential, and the emerging evidence. J Clin Gastroenterol. 2004;38(Suppl):S70–5. doi: 10.1097/01.mcg.0000128928.99037.e6. [DOI] [PubMed] [Google Scholar]
- 121.Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol Toxicol. 2012;52:37–56. doi: 10.1146/annurev-pharmtox-010611-134748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stein J, Schröder O, Bonk M, Oremek G, Lorenz M, Caspary WF. Induction of glutathione-S-transferase-pi by short-chain fatty acids in the intestinal cell line Caco-2. Eur J Clin Invest. 1996;26:84–7. doi: 10.1046/j.1365-2362.1996.113252.x. [DOI] [PubMed] [Google Scholar]
- 123.Hijová E, Bomba A, Bertková I, Strojný L, Szabadosová V, Šoltésová A. Prebiotics and bioactive natural substances induce changes of composition and metabolic activities of the colonic microflora in cancerous rats. Acta Biochim Pol. 2012;59:271–4. [PubMed] [Google Scholar]
- 124.Sugawara M, Suzuki K, Endo K, Kumemura M, Takeuchi M, Mitsuoka T. Effect of the dietary supplementation of corn hemicellulose on faecal flora and bacterial enzyme-activities in human adults. Agric Biol Chem. 1990;54:1683–8. doi: 10.1271/bbb1961.54.1683. [DOI] [Google Scholar]
- 125.Cloetens L, De Preter V, Swennen K, Broekaert WF, Courtin CM, Delcour JA, et al. Dose-response effect of arabinoxylooligosaccharides on gastrointestinal motility and on colonic bacterial metabolism in healthy volunteers. J Am Coll Nutr. 2008;27:512–8. doi: 10.1080/07315724.2008.10719733. [DOI] [PubMed] [Google Scholar]
- 126.Kajihara M, Kato S, Konish M, Yamagishi Y. Horie YIshii H. Xylooligosaccharide decrease blood ammonia levels in patients with liver cirrhosis. Am. J. Gastroen. 2000;95:2514. doi: 10.1111/j.1572-0241.2000.02712.x. [DOI] [Google Scholar]
- 127.Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, et al. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–60. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- 128.Janardhana V, Broadway MM, Bruce MP, Lowenthal JW, Geier MS, Hughes RJ, et al. Prebiotics modulate immune responses in the gut-associated lymphoid tissue of chickens. J Nutr. 2009;139:1404–9. doi: 10.3945/jn.109.105007. [DOI] [PubMed] [Google Scholar]
- 129.Brisbin JT, Gong J, Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim Health Res Rev. 2008;9:101–10. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 130.Gopalakrishnan A, Clinthorne JF, Rondini EA, McCaskey SJ, Gurzell EA, Langohr IM, et al. Supplementation with galacto-oligosaccharides increases the percentage of NK cells and reduces colitis severity in Smad3-deficient mice. J Nutr. 2012;142:1336–42. doi: 10.3945/jn.111.154732. [DOI] [PubMed] [Google Scholar]
- 131.Perrin P, Pierre F, Patry Y, Champ M, Berreur M, Pradal G, et al. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut. 2001;48:53–61. doi: 10.1136/gut.48.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klurfeld DM. Dietary fiber-mediated mechanisms in carcinogenesis. Cancer Res. 1992;52(Suppl):2055s–9s. [PubMed] [Google Scholar]
- 133.Ferguson LR, Harris PJ. Studies on the role of specific dietary fibres in protection against colorectal cancer. Mutat Res. 1996;350:173–84. doi: 10.1016/0027-5107(95)00105-0. [DOI] [PubMed] [Google Scholar]
- 134.Smith-Barbaro P, Hanson D, Reddy BS. Carcinogen binding to various types of dietary fiber. J Natl Cancer Inst. 1981;67:495–7. [PubMed] [Google Scholar]
- 135.Raman M, Nilsson U, Skog K, Lawther M, Nair B, Nyman M. Physicochemical characterisation of dietary fibre components and their ability to bind some process-induced mutagenic heterocyclic amines, Trp-P-1, Trp-P-2, AαC and MeAαC. Food Chem. 2012 doi: 10.1016/j.foodchem.2012.11.111. Accepted. [DOI] [PubMed] [Google Scholar]
- 136.Kolida S, Gibson GR. Synbiotics in health and disease. Annu Rev Food Sci Technol. 2011;2:373–93. doi: 10.1146/annurev-food-022510-133739. [DOI] [PubMed] [Google Scholar]
- 137.Kondepudi KK, Ambalam P, Nilsson I, Wadström T, Ljungh A. Prebiotic-non-digestible oligosaccharides preference of probiotic bifidobacteria and antimicrobial activity against Clostridium difficile. Anaerobe. 2012;18:489–97. doi: 10.1016/j.anaerobe.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 138.Mai V, Colbert LH, Perkins SN, Schatzkin A, Hursting SD. Intestinal microbiota: a potential diet-responsive prevention target in ApcMin mice. Mol Carcinog. 2007;46:42–8. doi: 10.1002/mc.20233. [DOI] [PubMed] [Google Scholar]
- 139.Rowland IR, Rumney CJ, Coutts JT, Lievense LC. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis. 1998;19:281–5. doi: 10.1093/carcin/19.2.281. [DOI] [PubMed] [Google Scholar]
- 140.Le Leu RK, Brown IL, Hu Y, Bird AR, Jackson M, Esterman A, et al. A synbiotic combination of resistant starch and Bifidobacterium lactis facilitates apoptotic deletion of carcinogen-damaged cells in rat colon. J Nutr. 2005;135:996–1001. doi: 10.1093/jn/135.5.996. [DOI] [PubMed] [Google Scholar]
- 141.Le Leu RK, Hu Y, Brown IL, Woodman RJ, Young GP. Synbiotic intervention of Bifidobacterium lactis and resistant starch protects against colorectal cancer development in rats. Carcinogenesis. 2010;31:246–51. doi: 10.1093/carcin/bgp197. [DOI] [PubMed] [Google Scholar]
- 142.Roller M, Pietro Femia A, Caderni G, Rechkemmer G, Watzl B. Intestinal immunity of rats with colon cancer is modulated by oligofructose-enriched inulin combined with Lactobacillus rhamnosus and Bifidobacterium lactis. Br J Nutr. 2004;92:931–8. doi: 10.1079/BJN20041289. [DOI] [PubMed] [Google Scholar]
- 143.Roller M, Rechkemmer G, Watzl B. Prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillius rhamnosus and Bifidobacterium lactis modulates intestinal immune funcation in rats. J Nutr. 2004;134:1153–6. doi: 10.1093/jn/134.1.153. [DOI] [PubMed] [Google Scholar]
- 144.Iannitti T, Palmieri B. Therapeutical use of probiotic formulations in clinical practice. Clin Nutr. 2010;29:701–25. doi: 10.1016/j.clnu.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr. 2007;85:488–96. doi: 10.1093/ajcn/85.2.488. [DOI] [PubMed] [Google Scholar]