Abstract
Certain randomized, placebo-controlled trials of oral supplementation with B. infantis 35624 have demonstrated the amelioration of symptoms of irritable bowel syndrome. Potential GI colonization by B. infantis 35624 or effects of supplementation on resident GI microbiota may pertain to these clinical observations. In this study, fecal excretion of B. infantis 35624 before, during and after 8 weeks of daily treatment was compared in subjects with IBS who received either the encapsulated oral supplement (n = 39) or placebo (n = 37) and in healthy subjects who received the supplement (n = 41). Secondarily, changes in assessed fecal microbiota and IBS symptoms were determined. Supplementation significantly increased fecal B. infantis 35624 excretion vs. placebo in IBS subjects; excretion in healthy subjects receiving supplement was quantitatively similar. Fecal levels of the probiotic declined and approached baseline once dosing ceased, documenting that colonization is transient. Although supplementation increased numbers of B infantis 35624 within the GI tract, limited changes in 10 other fecal taxa were observed either in healthy subjects or those with IBS. No impact on IBS symptoms was observed. Detection of bacterial DNA in fecal samples suggests that the probiotic is able to survive transit through the GI tract, although strain selective culture techniques were not performed to confirm viability of B. infantis 35624 in the feces. Continuous probiotic administration was necessary to maintain steady-state transit. Given the complex spectrum of GI microbiota, however, monitoring perturbations in selected taxa may not be not a useful indicator of probiotic function.
Keywords: bifidobacterium infantis 35624, probiotics, irritable bowel syndrome, symptoms, fecal microbiota, polymerase chain reaction
Introduction
The human GI (gastrointestinal) tract harbors a complex array of microbes that play a multifactorial role in maintaining human health. GI microbiota metabolize nondigestible foods, produce essential nutrients, support the immune system and prevent pathogen colonization. Bifidobacterium species and lactobacilli are thought to contribute to a beneficial balance of GI microbiota1 and exert positive effects on host cell metabolism, immune system function, vitamin production, pathogen inhibition and blood cholesterol reduction.2
Irritable bowel syndrome (IBS) is a gastrointestinal disorder characterized by abdominal pain/discomfort, bloating and abnormal bowel habits. IBS prevalence has been estimated at 7–15% in Western countries3,4 and twice as many women as men seek treatment. Although the etiology of IBS is not well understood, evidence from culture-based and molecular techniques indicates that the condition is associated with a disturbed intestinal microbiota.5-8 The bacterial species Bifidobacterium spp, Lactobacillus spp and Veillonella spp and the groups Bifidobacterium catenulatum and Clostridium coccoides appear to be affected,8 and the relative abundance of several 16s rRNA gene phylotypes is also altered.5,7 Consequently, the use of probiotics to modulate the balance of intestinal microbiota and, potentially, to restore homeostasis is an active area of research.
A variety of probiotic microbes, administered either as individual strains9-15 or as mixtures,16-23 has been evaluated in randomized placebo-controlled trials. Various clinical outcomes have been examined, including effects on IBS symptoms and/or quality of life, as well as markers of putative microbial modes of action, such as changes in the composition of the intestinal microbiota,20,24 enzymatic activity,16,19 fermentation, production of volatile fatty acids19 and modulation of the immune response.20 Studies differed in the choice and number of strains employed, mode of administration, dose, duration of treatment and population characteristics and results have been mixed; however, a subset of randomized, placebo-controlled trials have demonstrated the amelioration of IBS symptoms25-29 and a few provide evidence for a concomitant microbial mode of action.20,30,31
Our laboratory is interested in the effects of oral supplementation with Bifidobacterium longum subsp infantis 35624 (B. infantis 35624) on the composition of the intestinal microbiota. This study was a randomized, double-blind, placebo-controlled trial of the effects of eight weeks of daily administration of encapsulated B. infantis 35624 in subjects with IBS and healthy controls. The primary outcome measure was levels of fecal excretion of the probiotic microbe assessed by quantitative PCR (qPCR). Secondarily, the impact of supplementation on the relative abundance of assessed fecal microbiota and effects on IBS symptoms were evaluated.
Results
Subject Disposition and Demographic Characteristics
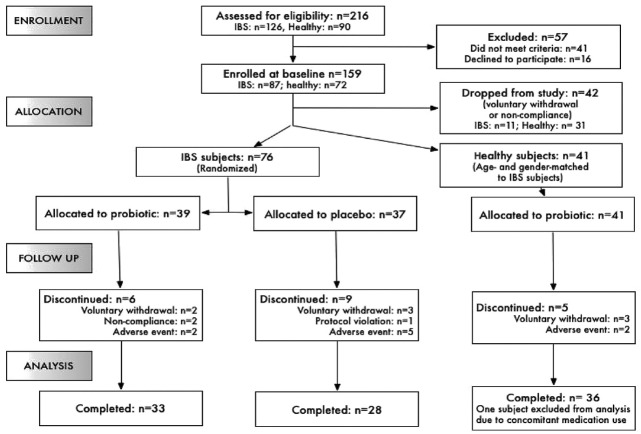
Of 216 people assessed for eligibility, 159 (87 with and 72 without IBS) were enrolled. Following the baseline period, 117 were allocated to treatment groups: 76 subjects with IBS were randomly allocated to either the probiotic supplement (n = 39) or the placebo (n = 37) and 41 healthy subjects (age- and gender-matched to IBS subjects) were allocated to the probiotic supplement (Fig. 1). Thirty-three subjects with IBS in the treatment group, 28 subjects with IBS in the placebo group and 35 subjects without IBS completed the study and were included in the per-protocol efficacy analyses. Reasons for discontinuation included voluntary withdrawal, non-compliance with the protocol or adverse events experienced during the study (Fig. 1).
Figure 1. Subject Flow through the study.
Distributions by age, sex and ethnicity in subjects allocated to treatment were similar in all groups (Table 1). The study population was predominantly female (95 of 117 or 81% of subjects) and Caucasian (92 of 117 or 79% of subjects). Overall mean ages were 45.5 y (range 21–65 y) for subjects with IBS and 44.1 y (range 20–65 y) for subjects without IBS. Mean weights in all groups were close to 80 kg. No difference was found in the percentage of subjects with IBS who smoked allocated to either treatment (20.5% in the probiotic supplement group and 18.9% in the placebo group). Approximately 27% of subjects without IBS were smokers.
Table 1. Demographics of study subjects allocated to treatment.
| Parameter | Subjects with IBS | Healthy subjects | |||
|---|---|---|---|---|---|
| |
B. infantis 35624 |
Placebo |
P-value |
B. infantis 35624 |
|
| Age (years) |
Mean ± SE |
47.0 ± 1.96 |
43.2 ± 2.01 |
0.19 |
44.1 ± 1.78 |
| |
Range |
21–65 |
21–64 |
|
20–65 |
| Height (cm) |
Mean ± SE |
166 ± 1.05 |
164 ± 1.08 |
0.40 |
167 ± 1.29 |
| |
Range |
152–178 |
150–178 |
|
150–180 |
| Weight (kg) |
Mean ± SE |
80.9 ± 3.10 |
83.6 ± 3.18 |
0.55 |
82.8 ± 3.18 |
| |
Range |
46–144 |
57–126 |
|
52–133 |
| Gender – N (%) |
Female |
31 (79.5) |
31 (83.8) |
0.63 |
33 (80.5) |
| |
Male |
8 (20.5) |
6 (16.2) |
|
8 (19.5) |
| Race – N (%) |
Caucasian |
28 (71.8) |
30 (81.1) |
0.46 |
34 (82.9) |
| |
African-American |
10 (25.6) |
7 (18.9) |
|
6 (14.6) |
| |
Latino |
1 (2.6) |
0 (0.0) |
|
0 (0.0) |
| |
Asian-Oriental |
0 (0.0) |
0 (0.0) |
|
1 (2.4) |
| Smoker – N (%) |
Yes |
8 (20.5) |
7 (18.9) |
0.86 |
11 (26.8) |
| No | 31 (79.5) | 30 (81.1) | 30 (73.2) | ||
Fecal excretion of B. infantis 35624
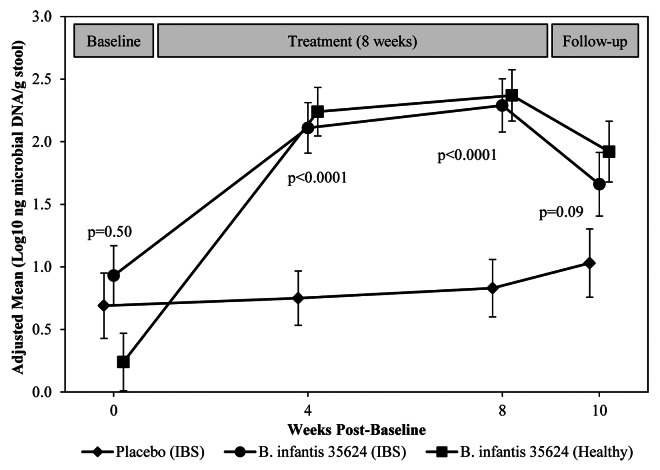
Prior to treatment, B. infantis 35624 was detected by qPCR (EPS-1 probe) in feces of all three study groups (mean ≤ 1 log10 ng DNA/g stool) (Fig. 2). After four and eight weeks of treatment, fecal levels of B. infantis 35624 from IBS subjects who received the probiotic rose significantly over those from subjects that received placebo (Fig. 2). In subjects with IBS and healthy controls who received the active supplement, fecal levels of the probiotic strain approached a plateau in the range of 2 to 2.5 log10 ng DNA/g stool during the treatment phase. Excretion of the probiotic strain declined in both these groups in the two-week follow-up period after treatment termination, although not to baseline levels. Comparable results were obtained when two other B. infantis 35624-specific probes (BI01615 and BI02420t) were employed in post-hoc qPCR analyses of microbial DNA in frozen fecal samples, which served to validate the results (data not shown).
Figure 2. Mean log10 ng microbial DNA per g fecal sample by qPCR analysis of B. infantis 35624 DNA using the EPS-1 primer probe just prior to treatment (baseline), after weeks 4 and 8 of treatment and at two weeks post-treatment. P values compare subjects with IBS who received either the probiotic supplement or placebo.
Effects of supplementation on assessed fecal microbiota
Quantitative PCR analysis of 10 assessed taxa was performed to determine whether supplementation with B. infantis 35624 would potentially alter the composition of the fecal microbiota (Tables 2–5). In subjects with IBS, probiotic supplementation resulted in a statistically significant increase in fecal levels of Clostridium coccoides-Eubacterium rectale group after eight weeks of treatment relative to placebo (5.69 log10 ng DNA/g stool in the treatment group relative to 5.53 log10 ng DNA/g stool in the placebo group) (Table 2). No statistically significant (p < 0.05) differences were observed among the other taxa examined. In the group of healthy subjects, changes from baseline were examined, though the statistical significance of such changes was not determined due to the lack of a healthy group control. The largest numerical differences that occurred were in the Bifidobacterium catenlatum group where mean levels increased from baseline as much as 0.40 log10 ng DNA/g stool (Table 4).
Table 2. Assessed fecal microbiota in subjects with IBS at baseline and after four and eight weeks of daily treatment with B. infantis 35624 or placebo and after 2 weeks of post-treatment follow-up.
| Microbiota | Study Visit | Treatment groups | |||
|---|---|---|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
Placebo (log 10 ng DNA g−1) |
|
|
| |
Mean (SE) |
Mean (SE) |
Difference (SE) |
p-value |
|
| Bifidobacterium spp |
Baseline |
4.30 (0.211) |
4.33 (0.229) |
|
|
| |
Week 4 |
4.40 (0.096) |
4.42 (0.105) |
-0.01 (0.142) |
0.923 |
| |
Week 8 |
4.53 (0.117) |
4.43 (0.128) |
0.09 (0.173) |
0.599 |
| |
Follow-up |
4.42 (0.116) |
4.40 (0.126) |
0.02 (0.171) |
0.916 |
| Lactobacillus group |
Baseline |
3.57 (0.096) |
3.75 (0.104) |
|
|
| |
Week 4 |
3.59 (0.058) |
3.51 (0.064) |
0.08 (0.087) |
0.358 |
| |
Week 8 |
3.58 (0.079) |
3.46 (0.086) |
0.12 (0.117) |
0.323 |
| |
Follow-up |
3.39 (0.082) |
3.41 (0.089) |
-0.01 (0.121) |
0.925 |
| Veillonella spp |
Baseline |
2.30 (0.174) |
2.55 (0.189) |
|
|
| |
Week 4 |
2.28 (0.112) |
2.20 (0.123) |
0.08 (0.166) |
0.612 |
| |
Week 8 |
2.43 (0.102) |
2.17 (0.112) |
0.26 (0.152) |
0.093 |
| |
Follow-up |
2.27 (0.120) |
2.21 (0.131) |
0.07 (0.178) |
0.710 |
| Desulfovibrio gp. |
Baseline |
3.02 (0.279) |
2.74 (0.302) |
|
|
| |
Week 4 |
2.76 (0.099) |
2.65 (0.108) |
0.11 (0.147) |
0.455 |
| |
Week 8 |
2.93 (0.126) |
2.71 (0.136) |
0.22 (0.186) |
0.243 |
| |
Follow-up |
2.79 (0.146) |
2.52 (0.158) |
0.27 (0.215) |
0.219 |
| Ruminococcus productus- Clostridium coccoides |
Baseline |
2.49 (0.124) |
2.67 (0.134) |
|
|
| |
Week 4 |
2.58 (0.059) |
2.64 (0.064) |
-0.06 (0.087) |
0.488 |
| |
Week 8 |
2.65 (0.051) |
2.64 (0.056) |
0.01 (0.076) |
0.897 |
| |
Follow-up |
2.55 (0.066) |
2.59 (0.072) |
-0.04 (0.098) |
0.655 |
| Clostridium coccoides- Eubacterium rectale gp. |
Baseline |
5.63 (0.062) |
5.77 (0.067) |
|
|
| |
Week 4 |
5.58 (0.043) |
5.60 (0.048) |
-0.02 (0.065) |
0.712 |
| |
Week 8 |
5.69 (0.045) |
5.53 (0.050) |
0.15 (0.068) |
0.025 |
| Follow-up | 5.62 (0.051) | 5.59 (0.056) | 0.03 (0.076) | 0.696 | |
Post-baseline means are least-squares means from the statistical mixed model.
Table 5. Assessed fecal microbiota in healthy subjects at baseline and after four and eight weeks of daily treatment with B. infantis 35624 and after 2 weeks of post-supplementation follow-up.
| Microbiota | Study Visit | Treatment |
|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
| |
|
Mean (SE) |
| Clostridium perfringens gp. |
Baseline |
1.21 (0.135) |
| |
Week 4 |
1.50 (0.102) |
| |
Week 8 |
1.42 (0.102) |
| |
Follow-up |
1.32 (0.103) |
| Enterococcus spp |
Baseline |
2.24 (0.110) |
| |
Week 4 |
2.31 (0.079) |
| |
Week 8 |
1.98 (0.093) |
| |
Follow-up |
2.18 (0.098) |
| Bacteroides-Prevotella-Porphymonas |
Baseline |
5.73 (0.039) |
| |
Week 4 |
5.81 (0.030) |
| |
Week 8 |
5.68 (0.029) |
| Follow-up | 5.74 (0.034) |
Healthy subjects received B. infantis 35624 supplementation for eight weeks. No supplement was administered at baseline or during follow-up (weeks 9 and 10).
Table 4. Assessed fecal microbiota in healthy subjects at baseline and after four and eight weeks of daily treatment with B. infantis 35624 and after 2 weeks of post-supplementation follow-up.
| Microbiota | Study Visit | Treatment |
|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
| |
|
Mean (SE) |
| Bifidobacterium spp |
Baseline |
4.12 (0.202) |
| |
Week 4 |
4.43 (0.092) |
| |
Week 8 |
4.37 (0.113) |
| |
Follow-up |
4.34 (0.111) |
| Lactobacillus group |
Baseline |
3.53 (0.092) |
| |
Week 4 |
3.50 (0.056) |
| |
Week 8 |
3.37 (0.076) |
| |
Follow-up |
3.25 (0.078) |
| Veillonella spp |
Baseline |
2.21 (0.167) |
| |
Week 4 |
2.42 (0.109) |
| |
Week 8 |
2.39 (0.099) |
| |
Follow-up |
2.36 (0.117) |
| Desulfovibrio gp |
Baseline |
2.89 (0.267) |
| |
Week 4 |
2.83 (0.096) |
| |
Week 8 |
2.83 (0.122) |
| |
Follow-up |
2.83 (0.141) |
| Ruminococcus productus-Clostridium coccoides |
Baseline |
2.53 (0.118) |
| |
Week 4 |
2.63 (0.056) |
| |
Week 8 |
2.55 (0.049) |
| |
Follow-up |
2.55 (0.063) |
| Clostridium coccoides-Eubacterium rectale gp |
Baseline |
5.58 (0.059) |
| |
Week 4 |
5.70 (0.042) |
| |
Week 8 |
5.64 (0.044) |
| |
Follow-up |
5.57 (0.049) |
| Bifidobacterium catenlatum gp |
Baseline |
1.29 (0.361) |
| |
Week 4 |
1.62 (0.084) |
| |
Week 8 |
1.69 (0.127) |
| Follow-up | 1.45 (0.158) |
Healthy subjects received B. infantis 35624 supplementation for eight weeks. No supplement was administered at baseline or during follow-up (weeks 9 and 10).
Table 3. Assessed fecal microbiota in subjects with IBS at baseline and after four and eight weeks of daily treatment with B. infantis 35624 or placebo and after 2 weeks of post-supplementation follow-up.
| Microbiota | Study Visit | Treatment groups | |||
|---|---|---|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
Placebo (log 10 ng DNA g−1) |
|
|
| |
|
Mean (SE) |
Mean (SE) |
Difference (SE) |
p-value |
| Bifidobacterium catenlatum gp. |
Baseline |
1.96 (0.377) |
1.55 (0.409) |
|
|
| |
Week 4 |
1.44 (0.088) |
1.36 (0.095) |
0.07 (0.129) |
0.566 |
| |
Week 8 |
1.63 (0.132) |
1.62 (0.143) |
0.01 (0.195) |
0.945 |
| |
Follow-up |
1.53 (0.166) |
1.51 (0.178) |
0.02 (0.243) |
0.948 |
| Clostridium perfringens gp. |
Baseline |
1.38 (0.141) |
1.23 (0.153) |
|
|
| |
Week 4 |
1.01 (0.107) |
1.13 (0.116) |
-0.13 (0.158) |
0.428 |
| |
Week 8 |
1.06 (0.106) |
1.24 (0.115) |
-0.18 (0.157) |
0.265 |
| |
Follow-up |
1.15 (0.108) |
1.35 (0.117) |
-0.19 (0.159) |
0.227 |
| Enterococcus spp |
Baseline |
2.31 (0.115) |
2.34 (0.125) |
|
|
| |
Week 4 |
2.35 (0.082) |
2.38 (0.090) |
-0.03 (0.122) |
0.809 |
| |
Week 8 |
2.25 (0.097) |
2.21 (0.105) |
0.04 (0.143) |
0.770 |
| |
Follow-up |
1.99 (0.102) |
1.92 (0.111) |
0.07 (0.151) |
0.665 |
| Bacteroides-Prevotella- Porphymonas |
Baseline |
5.80 (0.040) |
5.86 (0.044) |
|
|
| |
Week 4 |
5.81 (0.031) |
5.78 (0.034) |
0.02 (0.045) |
0.639 |
| |
Week 8 |
5.78 (0.030) |
5.76 (0.033) |
0.03 (0.044) |
0.541 |
| Follow-up | 5.74 (0.034) | 5.70 (0.038) | 0.03 (0.051) | 0.494 | |
Post-baseline means are least squares means from the statistical mixed model.
Clinical Symptoms of IBS
No significant difference between groups of subjects with IBS allocated to the probiotic supplement or placebo was found in symptoms of abdominal pain, bloating, urgency, incomplete evacuation, straining, gas or for overall symptom severity after four and eight weeks of treatment (Table 6). Mean scores for the severity of abdominal pain, bloating, urgency and gas all were generally close to 2, where 0 = no symptoms, 1 = very mild symptoms and 2 = mild symptoms. In both the probiotic supplementation and the placebo groups, IBS symptoms were scored as less severe during treatment relative to the baseline phase, and the degree of perceived improvement was similar in both groups (Table 6).
Table 6. Mean subjective ratings1 of irritable bowel syndrome (IBS) symptom severity at baseline and during weeks 4 and 8 of treatment with B. infantis 35624 or placebo recorded in subjects with IBS. Post-baseline means are least squares meaans from the statistical mixed model.
| Symptom | Study Visit | Treatment Groups | |||
|---|---|---|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
Placebo (log 10 ng DNA g−1) |
|
|
| |
|
Mean (SE) |
Mean (SE) |
Difference (SE) |
p-value |
| Abdominal pain |
Baseline |
2.39 (0.141) |
2.50 (0.145) |
|
|
| |
Week 4 |
2.15 (0.164) |
1.97 (0.171) |
0.18 (0.237) |
0.438 |
| |
Week 8 |
1.99 (0.161) |
1.73 (0.170) |
0.26 (0.234) |
0.261 |
| Bloating |
Baseline |
2.47 (0.156) |
2.42 (0.160) |
|
|
| |
Week 4 |
2.15 (0.160) |
2.01 (0.166) |
0.14 (0.230) |
0.541 |
| |
Week 8 |
2.01 (0.157) |
1.89 (0.166) |
0.13 (0.229) |
0.576 |
| Urgency |
Baseline |
2.38 (0.136) |
2.20 (0.139) |
|
|
| |
Week 4 |
2.04 (0.157) |
1.96 (0.163) |
0.08 (0.227) |
0.728 |
| |
Week 8 |
1.95 (0.163) |
1.70 (0.173) |
0.25 (0.238) |
0.287 |
| Incomplete evacuation |
Baseline |
2.24 (0.170) |
2.18 (0.174) |
|
|
| |
Week 4 |
1.94 (0.158) |
1.94 (0.165) |
0.00 (0.228) |
0.992 |
| |
Week 8 |
1.77 (0.165) |
1.65 (0.175) |
0.12 (0.240) |
0.611 |
| Straining |
Baseline |
1.98 (0.171) |
1.90 (0.176) |
|
|
| |
Week 4 |
1.77 (0.159) |
1.65 (0.165) |
0.12 (0.229) |
0.592 |
| |
Week 8 |
1.57 (0.166) |
1.56 (0.176) |
0.01 (0.242) |
0.965 |
| Gas |
Baseline |
2.45 (0.158) |
2.29 (0.162) |
|
|
| |
Week 4 |
2.15 (0.154) |
2.09 (0.160) |
0.06 (0.223) |
0.797 |
| |
Week 8 |
2.08 (0.166) |
1.87 (0.175) |
0.21 (0.242) |
0.384 |
| Overall symptom severity |
Baseline |
2.54 (0.149) |
2.66 (0.153) |
|
|
| |
Week 4 |
2.44 (0.171) |
2.07 (0.178) |
0.37 (0.248) |
0.134 |
| Week 8 | 2.20 (0.165) | 1.82 (0.174) | 0.38 (0.241) | 0.114 | |
1 Symptom severity scoring scale: 0, none; 1, very mild; 2, mild; 3, moderate; 4, severe; 5, very severe
Subjects experienced 1–2 bowel movements per day on average and mean scores for the level of satisfaction with their bowel habits during the treatment phase ranged between 2 and 3, where a score of 2 = somewhat satisfied and a score of 3 = somewhat dissatisfied (Table 7). The IBS composite score, derived from the sum of scores from two symptoms (abdominal discomfort, bloating/distension) and the bowel habit satisfaction score, showed a decreasing trend in both groups (suggesting perceived improvements over time) but with no statistically difference between them (Table 7).
Table 7. Mean number of bowel movements per day, subjective ratings of bowel habit satisfaction, composite scores of IBS symptom relief and global assessments of IBS symptom relief with B. infantis 35624 or placebo in subjects with IBS. Post-baseline means are least squares means from the statistical mixed model.
| Parameter | Study Visit | Treatment Groups | |||
|---|---|---|---|---|---|
| |
|
B. infantis 35624 (log 10 ng DNA g−1) |
Placebo (log 10 ng DNA g−1) |
|
|
| |
|
Mean (SE) |
Mean (SE) |
Difference (SE) |
p-value |
| Number of daily bowel movements 1 |
Baseline |
1.66 (0.155) |
1.74 (0.159) |
|
|
| |
Week 4 |
1.69 (0.085) |
1.46 (0.088) |
0.23 (0.123) |
0.059 |
| |
Week 8 |
1.91 (0.222) |
1.58 (0.234) |
0.34 (0.323) |
0.297 |
| Overall appearance of bowel movements 2 |
Baseline |
3.58 (0.189) |
3.62 (0.194) |
|
|
| |
Week 4 |
3.66 (0.156) |
3.51 (0.161) |
0.14 (0.225) |
0.524 |
| |
Week 8 |
3.93 (0.187) |
3.45 (0.201) |
0.48 (0.274) |
0.080 |
| Bowel habit satisfaction score 3 |
Baseline |
2.72 (0.162) |
3.00 (0.167) |
|
|
| |
Week 4 |
2.66 (0.197) |
2.41 (0.209) |
0.25 (0.287) |
0.381 |
| |
Week 8 |
2.36 (0.188) |
2.21 (0.199) |
0.15 (0.275) |
0.575 |
| IBS composite score 4 |
Baseline |
7.57 (0.431) |
7.92 (0.443) |
|
|
| |
Week 4 |
6.98 (0.501) |
6.38 (0.533) |
0.60 (0.731) |
0.410 |
| Week 8 | 6.39 (0.468) | 5.80 (0.495) | 0.59 (0.682) | 0.385 | |
(1) The number of bowel movements was recorded daily during weeks 4 and 8 of treatment. (2) Appearance of bowel movements was scored on a seven-point Bristol Stool Form Scale, as follows: 1, separate hard lumps, like nuts (difficult to pass); 2, sausage-shaped but lumpy; 3, sausage- or snake-like, cracked surface; 4, sausage- or snake-like, smooth surface; 5, soft blobs with clear cut edges (passed easily); 6, fluffy pieces with ragged edges, a mushy stool; 7, watery stool, entirely liquid. (3) Bowel habit satisfaction was scored at the end of weeks 4 and 8. Scoring scale: 0, very satisfied; 1, satisfied; 2, somewhat satisfied; 3, somewhat dissatisfied; 4, dissatisfied; 5, very dissatisfied. (4) IBS composite score derived from the sum of scores for abdominal pain, bloating and bowel habit satisfaction.
Adverse events
Administered products were generally well tolerated. Three non-treatment emergent adverse events (AE) of ear pain, headache and cold symptoms (two subjects) within the IBS group and one non-treatment emergent AE of head congestion within the healthy group resulted in discontinuation from the study. The percentage of subjects reporting AEs was consistent across treatment groups and across healthy and IBS subjects, with the highest proportion of subjects reporting AEs in the IBS placebo group (38%) compared with 33% in the IBS probiotic treatment group and 32% in the healthy probiotic treatment group. The IBS placebo group reported the most AEs (42) as compared with the IBS probiotic treatment group (20) and the healthy probiotic treatment group (21). The most common treatment-emergent AEs reported involved the respiratory system in healthy subjects and the digestive system in subjects with IBS.
Discussion
This 12-week, prospective, placebo-controlled, randomized trial evaluated the effects of 8 weeks of daily supplementation of B. infantis 35624 in capsule form in subjects with and without IBS. The primary outcome measures were the level of fecal excretion of B. infantis 35624 before, during and after treatment in people with IBS and in healthy controls. Secondarily, changes in fecal levels of 10 assessed microbial taxa were determined in people with and without IBS and the severity of self-reported gastrointestinal symptoms was compared between subjects with IBS that received either the probiotic supplement or placebo.
Daily administration of the encapsulated probiotic supplement (109 cfu per capsule) for eight weeks in subjects with IBS resulted in a significant increase (approximately 1.5 log ng DNA/g stool) relative to placebo in the levels of fecal excretion of this microbe as measured by quantitative PCR (p < 0.0001); similar changes vs. baseline were observed for healthy subjects. Fecal excretion of the probiotic in the IBS placebo group was relatively consistent from Week 4 to Week 8, which suggests that the rates of supplementation and excretion from the lower GI tract had reached steady-state by Week 4. Similar excretion patterns were observed in post-hoc analyses with two other selective probes (BI01615 and BI02420t) (data not shown).
PCR amplifies DNA that is present and its detection does not definitively confirm microbial viability; however, it has been established that B. infantis 35624 survives transit through the GI tract in studies of patients with ulcerative colitis.32 Specifically, using B. infantis 35624 transformed with a rifampicin resistance gene as a marker for enumeration, the investigators demonstrated that the strain had established itself in the colon of these patients, reaching levels of 105–108 cfu, depending on the individual; the strain could be recovered from fecal samples and colon biopsies. Consequently, the recovery of B. infantis 35624 DNA from fecal samples in our study suggests that the encapsulated probiotic survived degradation by gastric acid and bile salts in the upper GI tract.
In the present study, fecal excretion of B. infantis 35624 declined (although not quite to baseline levels) in the two weeks following treatment cessation in both healthy subjects and subjects with IBS, whereas excretion levels were relatively unchanged in the group of subjects with IBS who received placebo. These trends were validated by analysis of stored, frozen stool samples by using two other distinct probes specific for this organism in independent q-PCR analyses (data not shown). We have observed similar trends in fecal excretion of B. infantis 35624 in a seven-week oral supplementation study (two week baseline, three weeks of administration and two weeks of follow-up), using lipid-based and powder-based capsule formulations of this probiotic microbe (unpublished data). Other investigators, using mixtures of probiotic strains, also demonstrated that fecal excretion of the component probiotic microbes rose during the period of administration and declined thereafter.16 Hence, consistent administration of the probiotic supplement is necessary to maintain a steady-state level of transit through the GI-tract.
Both culture-based and molecular analyses suggest that the intestinal microbiota of IBS patients is perturbed. For example, culture-based studies showed lower levels of lactobacilli and bifidobacteria and heightened levels of anaerobic Clostridium spp in IBS patients.33 Real-time PCR analyses demonstrated significant inter-individual heterogeneity in the fecal microbiota of IBS patients, but suggested that the bacterial species Bifidobacterium spp, Lactobacillus spp and Veillonella spp and the groups Bifidobacterium catenulatum and Clostridium coccoides are affected in these patients.8 Subdividing by bowel habit revealed lower levels of lactobacilli in diarrhea-predominant patients and higher levels of Veillonella spp in constipation-predominant patients.8
One possible mechanism of action of probiotics is modulation of the composition of intestinal microbiota, which could be evidenced by shifts in the relative proportions fecal microbes following supplementation. Our q-PCR analysis of 10 assessed taxa of potential clinical importance revealed limited evidence for a probiotic-induced change in GI tract microbiota with B. infantis 35624. In healthy subjects, fecal levels of Lactobacillus group decreased at eight weeks relative to baseline, a decrease that persisted through the post-supplementation period. A modest increase in Bifidobacterium spp relative to baseline levels was observed at week 4 only. One caveat is that evidence for a change in fecal microbiota in the healthy group is confounded by the lack of a placebo control. In subjects with IBS, no major changes in the 10 assessed taxa were apparent in the supplement group relative to the placebo control group, except for an increase in Clostridium coccoides-Eubacterium rectale after eight weeks of probiotic supplementation.
One of the limitations of RT qPCR analysis is that the full spectrum of microbiota is not covered, as the quantified microbes are predetermined. Hence, an effect of probiotic supplementation on the composition of the GI tract microbiota could be missed. Indeed, using RT qPCR, other investigators failed to find a significant impact on as many as 300 taxa in fecal samples from 55 IBS patients following 24 week of daily supplementation with a multispecies probiotic (Lactobacillus rhamnosus GG, Lactobacillus rhamnosus Lc705, Propionbacterium freudenreichii subsp shermanii JS and Bifidobacterium breve Bb99), except for an increase in Bifidobacterium spp in the placebo group.19 However, in a subsequent a placebo-controlled, 24-week intervention of the probiotic mixture in 42 IBS subjects, RT q-PCR analysis of just 8 bacterial targets selected for their putative IBS association revealed specific alterations in Clostridium thermosuccinogenes (85%), Ruminococcus torques (93%) and Ruminococcus torques (94%).34 Using a custom-made microarray of approximately 5500 oligonucleotide probes representing over 1000 currently known intestinal microbial species, this group of investigators found that daily supplementation with a multispecies probiotic (Lactobacillus rhamnosus GG, Lactobacillus rhamnosus Lc705, Propionbacterium freudenreichii subsp shermanii JS and Bifidobacterium animalis subsp lactis Bb12) for 20 weeks reduced IBS symptoms and stabilized the fecal microbiota relative to baseline during the latter 10 weeks of treatment, based on a logarithmic similarity index between time-points.20
Consequently, our study does not allow firm conclusions about the impact of B. infantis 35624 supplementation on the GI tract microbiota. However, exploratory studies from our laboratory, which characterized fecal microbiota using terminal restriction fragment polymorphism (t-RFLP) analysis of microbial DNA, showed that controlled daily administration of 1010 cfu B. infantis 35624 in milk for three weeks helped normalize the intestinal microbiota. The analysis of fecal samples from 24 subjects (13 with IBS and 11 controls) revealed 3 DNA fragments associated exclusively with IBS, 29 fragments that differed in proportion between IBS patients and healthy controls prior to treatment and a probiotic-associated shift to equivalent proportions of 16 of these 29 fragments in the microbiota of both groups (unpublished data). Hence, more robust and comprehensive surveys (and, possibly, longer interventions) may be needed to detect potential changes in GI tract microbiota associated with B. infantis 35624 supplementation
Although this investigation was not designed specifically to assess the impact of B. infantis 35624 on IBS symptoms, subjective symptom diaries were collected. We found no evidence of IBS symptom relief associated with daily supplements of 109 cfu of encapsulated B. infantis 35624 over the course of eight weeks. Symptom severity declined comparably in both the probiotic and placebo groups, consistent with a placebo effect. The IBS symptom results from this study are not in agreement with two previously published studies in which significant improvement in IBS symptoms was observed for B. infantis 35624.14,30 The reason for this difference between studies is not known. It is widely recognized that placebo response rates across randomized controlled trials of therapies in IBS are high.36
In summary, our investigation demonstrated that during 8 weeks of daily supplementation with 109 cfu of the encapsulated B. infantis 35624, fecal excretion of this microbe rose to comparable levels both in healthy subjects and subjects with IBS, whereas excretion remained relatively unchanged in subjects with IBS that received placebo. This suggests that the probiotic is able to survive transit through the GI tract, although strain selective culture techniques were not performed to confirm viability of B. infantis 35624 in the feces. Excretion declined once supplementation ceased, which indicates that continued administration is necessary to maintain transit and documents that colonization is transient. Despite exposure to exogenous B. infantis 35624, few significant treatment-related shifts occurred among 10 assessed taxa represented in the fecal microbiota of healthy subjects and subjects with IBS, nor was any significant impact on IBS symptoms noted in this particular study. As mentioned above previous studies demonstrated a significant improvement in IBS symptoms with supplementation of B. infantis 35624.14,30 We conclude that further fecal microbiota studies with more comprehensive microbial surveys and additional doses of B. infantis 35624 administered for longer durations are needed to further explore the role of the intestinal microbiota composition and metabolic processes on relief of IBS symptoms.
Patients and Methods
Study design and population
This was single-center, double-blind, randomized trial of daily supplementation of the probiotic, B. infantis 35624 or placebo, for eight weeks in people with and without IBS. The primary objective was to determine the effects of administration of the encapsulated probiotic microbes on fecal excretion of B. infantis 35624 in IBS and healthy subjects. Secondarily, effects of probiotic supplementation on assessed taxa in fecal samples from people with and without IBS were examined, as well as effects on gastrointestinal symptoms in people with IBS.
The study population was recruited from the population at large and comprised three groups: subjects with IBS, randomized by gender and IBS subtype (diarrhea-predominant symptoms, constipation-predominant symptoms or mixed symptoms) to either (1) the probiotic supplement or (2) the placebo and (3) healthy controls, matched to subjects with IBS by age (± 5 y) and gender, who received the probiotic treatment. The study protocol was approved by an Investigational Review Board and performed in compliance with the US Code of Federal regulations on Good Clinical Practices (21 CFR 10.90, 50, 56 and 812) and the World Medical Association Declaration of Helsinki (1996 amendment). All participants signed informed consent prior to study enrollment.
Enrollment proceeded until at least 36 subjects (target of 6 males, 30 females) were allocated to the three study groups. Eligible subjects were between 18 and 65 y of age and had not participated in a trial of an investigational new drug in the previous 30 d. They were in generally good health (eligibility determined by a physician-investigator at screening on the basis of medical and medication history) except that those with IBS met the Rome II diagnostic criteria,35 specifically: for at least 12 weeks (not necessarily consecutive) of the preceding 12 mo or for at least three weeks (not necessarily consecutive) of the preceding three months, they experienced abdominal discomfort with at least two of three features: (1) relieved by defecation and/or (2) onset associated with a change in frequency of stool and/or (3) onset associated with a change in the appearance or form of stool; and had symptoms consistent with IBS [altered stool frequency (fewer than three bowel movements a week or more than three bowel movements a day); altered stool form; abnormal stool passage (straining, urgency, incomplete passage); passage of mucus; feelings of bloating or abdominal distension]. Eligible subjects were willing to refrain from taking foods or dietary supplements containing live bacteria for two weeks prior to enrollment and during the course of the study and to maintain their current dietary habits, including (if pertinent) their established regimen of dietary fiber supplements or regimen of prescribed medications (excluding MAO inhibitors). Eligible female subjects were not pregnant or nursing and were willing to use contraception and to undergo pregnancy testing at baseline and during the treatment phase. Exclusion criteria were age > 55 y with no sigmoidoscopy or colonoscopy in the prior 5 y; dependence on stimulant laxatives or antidiarrheals; use of systemic steroidal agents, opiates or narcotic analgesics in the previous month; use of antiseizure medication in the previous three months; and either the use of systemic medication or evidence of significant acute or coexisting chronic illness, which in the judgment of the study investigator, would preclude study participation.
Opiates and narcotic analgesics, mono-amine oxidase (MAO inhibitors), serotonin/5-hydroxytrypyamine (5-HT)3/5-HT4 agonists or antagonists, antipsychotics and anti-seizure medications were not allowed to be used during the study. Acetaminophen may have been used for non-IBS pain management (e.g., headache, etc). Laxatives and anti-diarrheals were allowed during the study period as long as they were not needed more than once per week.
The 12-week study had three phases: (1) a two-week baseline phase when no treatment was administered; (2) an 8-week treatment phase in which one daily capsule of either B. infantis 35624 (109 cfu per capsule, range 108–1010 cfu/capsule) or placebo were self-administered, according to group assignment and (3) a two-week, follow-up phase of no further treatment. Fecal samples [two acceptable (i.e., volume sufficient for DNA extraction) samples per subject] were collected at the end of the baseline phase, following four and eight weeks of treatment and at the end of the follow-up phase (a total of eight samples per subject).
Probiotic supplement and placebo preparation
The probiotic supplement was prepared by mixing a freeze-dried preparation of B. infantis 35624 with the excipient formulation under controlled humidity. The ratio of freeze-dried B. infantis 35624 to excipient was sufficient to produce a capsule product with a mean count of 1 × 109 cfu per capsule (range 1 × 108–1 × 1010 cfu/capsule, accounting for variability in the plate count method). The placebo capsules were prepared under the same conditions but with the exclusion of the freeze-dried of B. infantis 35624. Both capsule formulations were evaluated in the previously described capsule disintegration test14 to ensure that the contents within the capsule would be released appropriately within the GI tract. Additionally, concurrent stability testing was performed to assure that the B. infantis 35624 was viable at the target concentration throughout the in-life study period.
Extraction and purification of DNA from fecal samples
Fecal samples less than 24 h old were obtained from all subjects and stored at 4°C prior to processing. Samples were diluted 1/10 with sterile saline and homogenized using a stomacher device (IUL Masticator). A portion of the homogenized sample was used to obtain DNA using the QIAamp DNA Stool Mini Kit (QIAGEN Inc.) according to the manufacturer’s instructions. Purified DNA was quantified using the Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen Corp.) as specified by the manufacturer.
qPCR analyses of fecal bacterial phylotypes
Fecal microbiota were assessed by qPCR within 24 h of sample collection using primers for B. infantis 35624, Bifidobacterium spp, Lactobacillus gp, Veillonella spp, Desulfovibrio gp., Ruminococcus productus-Clostridium coccoides, Clostridium coccoides-Eubacterium rectale gp., Bifidobacterium catenulatum gp., Clostridium perfringens gp., Enterococcus spp and Bacteroides-Prevotella-Porphyromonas. The iCycler iQ Real-Time Detection System (Bio-Rad), a component of iCycler Optical System Interface software (version 2.3, Bio-Rad) was used for the qPCR analysis as described in the literature.8
The primary outcome measure was the level of fecal B. infantis 35624 (log10 ng DNA g−1 sample) before, during and after daily supplementation with either B. infantis 35624 (109 cfu/capsule) or placebo. The principal primer/probe used for this analysis was selective for B. infantis 35624 based on sequence information from one of the household genes coding for the extracellular polysaccharide matrix (EPS-1). This primer was determined using Primer 3 software and was subsequently shown not to produce a PCR product with either B. longum (ATCC 15707) or B. infantis (ATCC 15697). After the completion of the initial analyses with the EPS-1 probe, further validation was performed by qPCR analyses of stored, frozen fecal samples using 2 other probe/primer sets, designated BIO1615 and BIO2420t. Standards with known concentrations of 16S RNA gene copies were used for comparative purposes. Data presented are the mean values of duplicate analyses for the same DNA extracts in independent runs.
Clinical outcome measures
A secondary outcome measure was the severity of gastrointestinal symptoms reported by subjects with IBS. Gastrointestinal symptoms experienced by subjects with IBS were recorded in diaries daily (throughout the baseline phase and during the fourth and eight week of treatment). Symptoms were graded on a six-point scale, where 0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe and 5 = very severe. The following symptoms were assessed daily: abdominal pain/discomfort; bloating/distension; urgency; straining; incomplete bowel evacuation; flatulence; and an overall rating of symptom severity. Subjects recorded the number of bowel movements per day and stool consistency (Bristol Stool Form Scale) and rated their level of satisfaction with bowel habits (where 0 = very satisfied, 1 = satisfied, 2 = somewhat satisfied, 3 = somewhat dissatisfied, 4 = dissatisfied and 5 = very dissatisfied). A composite score of IBS symptoms was derived from the sum of scores for abdominal pain/discomfort, bloating/distension and bowel habit satisfaction during baseline, week 4 and week 8 of dosing.
Safety assessments
Adverse events (AEs) and serious adverse events (elicited, volunteered or observed) were monitored and recorded throughout the study. Each subject was able to request health-related information or report any AE experienced via a 24-h toll-free telephone number. Any reported events were recorded and followed-up. In addition, personnel at the study site interviewed each subject at each visit to assess AEs.
Statistical analyses
Study size calculations were based on the primary outcome measure: total B. infantis 356245 measured by q-PCR. It was estimated that 32 subjects per IBS treatment group completing the study would provide approximately 85% power to detect a 1-logarithm difference in mean values between supplement and placebo groups, based on an error variance of 1.92 and the 5% significance level (2-sided). Statistical analyses of the fecal microbiota (q-PCR) were performed on the per-protocol population. Baseline characteristics were summarized with descriptive statistics and assessed for treatment imbalance among IBS subjects with chi-square or Fisher’s exact test for categorical variables (e.g., gender); and by t-test for continuous variables (e.g., age). The post-baseline fecal microbiota and symptom severity data in IBS subjects were analyzed separately using a linear mixed-model with Treatment (supplementation and placebo) and Subject included as fixed and random effects, respectively and baseline as a continuous covariate. Microbiota count data were log transformed prior to statistical analysis. For symptom severity score data, baseline was computed as the average of repeated measurements during the seven days prior to randomization. All treatment comparisons were made with 2-sided tests at α = 0.05.
Acknowledgments
The authors acknowledge and would like to thank Deborah Hutchins, PhD ELS (Hutchins and Associates LLC) for technical writing assistance and Christi A. Messer, Pharm D (The Procter and Gamble Company) for editorial assistance.
Glossary
Abbreviations:
- AE
adverse event
- CFR
Code of Federal Regulations
- EPS
extracellular polysaccharide
- IBS
irritable bowel syndrome
- MAO
monoamine oxidase
- qPCR
quantitative polymerase chain reaction
- rDNA
ribosomal DNA
- t-RFLP
terminal restriction fragment polymorphism
- GI
gastrointestinal
Disclosure of Potential Conflicts of Interest
The study was funded by The Procter and Gamble Company, which sells B. infantis 35624 under the trade name, Align®. D.C., PhD and R.D.G., PhD are employees of The Procter and Gamble Company. E.M.M.Q., MD has served as a speaker, a consultant and an advisory board member to Procter and Gamble and has received funding from The Procter and Gamble Company. Writing support provided by Deborah Hutchins, PhD ELS was funded by The Procter and Gamble Company.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/24196
References
- 1.Saulnier DM, Kolida S, Gibson GR. Microbiology of the human intestinal tract and approaches for its dietary modulation. Curr Pharm Des. 2009;15:1403–14. doi: 10.2174/138161209788168128. [DOI] [PubMed] [Google Scholar]
- 2.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 3.Andrews EB, Eaton SC, Hollis KA, Hopkins JS, Ameen V, Hamm LR, et al. Prevalence and demographics of irritable bowel syndrome: results from a large web-based survey. Aliment Pharmacol Ther. 2005;22:935–42. doi: 10.1111/j.1365-2036.2005.02671.x. [DOI] [PubMed] [Google Scholar]
- 4.Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–75. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- 5.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, et al. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9:95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyra A, Rinttilä T, Nikkilä J, Krogius-Kurikka L, Kajander K, Malinen E, et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol. 2009;15:5936–45. doi: 10.3748/wjg.15.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malinen E, Rinttilä T, Kajander K, Mättö J, Kassinen A, Krogius L, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100:373–82. doi: 10.1111/j.1572-0241.2005.40312.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan MA, O’Morain CA. Bacterial supplementation in the irritable bowel syndrome. A randomised double-blind placebo-controlled crossover study. Dig Liver Dis. 2000;32:294–301. doi: 10.1016/S1590-8658(00)80021-3. [DOI] [PubMed] [Google Scholar]
- 10.Niedzielin K, Kordecki H, Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2001;13:1143–7. doi: 10.1097/00042737-200110000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Sen S, Mullan MM, Parker TJ, Woolner JT, Tarry SA, Hunter JO. Effect of Lactobacillus plantarum 299v on colonic fermentation and symptoms of irritable bowel syndrome. Dig Dis Sci. 2002;47:2615–20. doi: 10.1023/A:1020597001460. [DOI] [PubMed] [Google Scholar]
- 12.Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr. 2005;24:925–31. doi: 10.1016/j.clnu.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 13.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–9. doi: 10.1016/S0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 14.Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O’Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 15.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–32. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 16.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;152:735–41. doi: 10.1016/S0923-2508(01)01254-2. [DOI] [PubMed] [Google Scholar]
- 17.Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2008;32:147–52. doi: 10.1016/j.gcb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22:387–94. doi: 10.1111/j.1365-2036.2005.02579.x. [DOI] [PubMed] [Google Scholar]
- 19.Kajander K, Krogius-Kurikka L, Rinttilä T, Karjalainen H, Palva A, Korpela R. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:463–73. doi: 10.1111/j.1365-2036.2007.03391.x. [DOI] [PubMed] [Google Scholar]
- 20.Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27:48–57. doi: 10.1111/j.1365-2036.2007.03542.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17:895–904. doi: 10.1046/j.1365-2036.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–96. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 23.Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29:97–103. doi: 10.1111/j.1365-2036.2008.03848.x. [DOI] [PubMed] [Google Scholar]
- 24.Michail S, Kenche H. Gut microbiota is not modified by randomized, double-blind, placebo-controlled trial of VSL#3 in diarrhea-predominant irritable bowel syndrome. Probiotics Antimicrob Proteins. 2011;3:1–7. doi: 10.1007/s12602-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner DM, Moeller MJ, Chey WD, Schoenfeld PS. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am J Gastroenterol. 2009;104:1033–49, quiz 1050. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 26.Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. doi: 10.1186/1471-230X-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650–61. doi: 10.3748/wjg.14.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325–32. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 29.Nikfar S, Rahimi R, Rahimi F, Derakhshani S, Abdollahi M. Efficacy of probiotics in irritable bowel syndrome: a meta-analysis of randomized, controlled trials. Dis Colon Rectum. 2008;51:1775–80. doi: 10.1007/s10350-008-9335-z. [DOI] [PubMed] [Google Scholar]
- 30.O’Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541–51. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 31.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–8. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 32.von Wright A, Vilpponen-Salmella T, Pages Llopis M, Collins K, Kiely B, Shanahan F, et al. The survival and colonic adhesion of Bifidobaterium infantis in patients with ulcerative colitis. Int Dairy J. 2002;12:197–200. doi: 10.1016/S0958-6946(01)00162-5. [DOI] [Google Scholar]
- 33.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–94. [PubMed] [Google Scholar]
- 34.Lyra A, Krogius-Kurikka L, Nikkilä J, Malinen E, Kajander K, Kurikka K, et al. Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol. 2010;10:110. doi: 10.1186/1471-230X-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furman DL, Cash BD. The role of diagnostic testing in irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:105–19. doi: 10.1016/j.gtc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Ford AC, Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:144–58. doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]