Abstract
In recent years, the importance of the mucus layer in the colon has become increasingly clear. Disturbance of the mucus layer has been implicated in a variety of intestinal diseases. We have recently investigated the importance of the mucus layer in colon ischemia-reperfusion (IR). Using a newly developed human and rat colon IR model, we showed that colon ischemia leads to mucus barrier breakdown. This allowed intraluminal bacteria to interact with the colonic epithelium, which was associated with an inflammatory response. Intriguingly, we found goblet cells to respond immediately by expelling their mucin granules into the gut lumen, which flushed bacteria from the colonic crypts and resulted in rapid restoration of the mucus layer during reperfusion. Our study might explain why ischemic colitis tends to have favorable outcomes and can often be treated conservatively.
Keywords: colonic diseases, mucosal injury, intestinal barrier function, ischemia-reperfusion, mucosal barrier
The Mucus Layer
The distal colon harbours an impressive density of 1012 bacteria per gram of luminal content.1 In the healthy colon, the gut microbiota has a profound influence on human physiology and the gut microbiota complements our biology in a way that is mutually beneficial.2 Disturbances in physiology however potentially lead to intrusion of bacteria into the host internal milieu, resulting in inflammation. To prevent direct exposure to intraluminal microbiota, the colon is equipped with several lines of defense. The mucus layer is the first anatomical site at which the host encounters the gut microbiota. This layer creates a physical and chemical barrier that prevents adherence of microbiota to the epithelium (Fig. 1A).3-5 The properties of mucus are derived from the major gel-forming glycoprotein components called mucins,4,5 which are continuously produced by specialized goblet cells that are found in large numbers in the colonic epithelium. In the endoplasmic reticulum (ER) and Golgi, mucins are oligomerized and glycosylated to provide mucins their viscous and protective properties.4 MUC2 is the most abundantly expressed secretory mucin in the colon and is stored in bulky apical granules of the goblet cells, which form the characteristic goblet cell thecae.4 The importance of the mucus layer has been acknowledged in a variety of studies. Loss of the mucus layer in MUC2 deficient mice has been shown to cause spontaneous colitis,6 and to increase susceptibility to lethal infectious colitis induced by pathogenic and commensal flora.7 In addition, DSS-induced chronic colonic inflammation in mice, a model for human IBD, is preceded by mucus barrier breakdown and adherence of microbiota to the epithelium.8
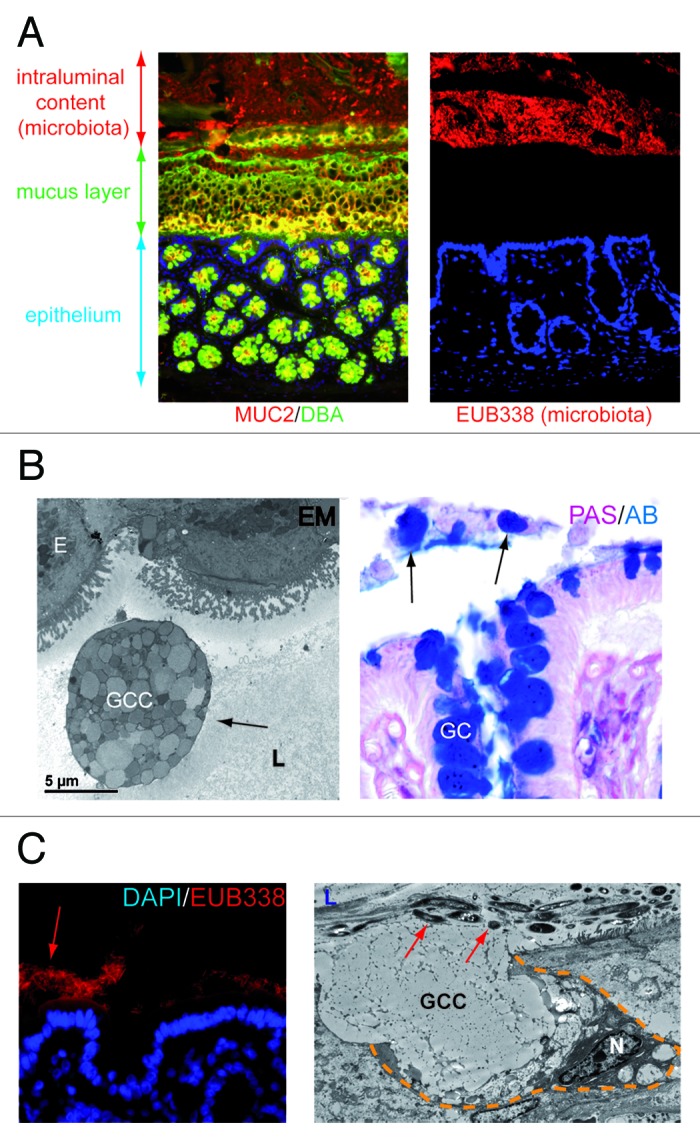
Figure 1.(A) The colonic mucus layer provides a physical barrier between intraluminal microbiota and the colonic epithelium. Left panel: immunofluorescent staining of healthy rat colon. Healthy mucus is composed of highly glycosylated MUC2 (MUC2, red; Dolichus Biflorus Agglutinin (DBA), green; overlay, yellow). Right panel: the mucus layer provides a physical barrier preventing interaction of intraluminal bacteria (stained in red, EUB388 stains rDNA of bacteria) with the colonic epithelial cells (blue, nuclei of the cells in the colonic mucosa). (B) Goblet cells are released into the gut lumen upon colonic ischemia. Electron microscopy picture (left panel) and PAS/AB staining (right panel) of human colon exposed to ischemia. Note the presence of entire goblet cell contents in the colonic lumen (arrows), which might indicate impaired dissemination of goblet cell granules. (E, epithelial cell; GC, Goblet cell; GCC, Goblet cell contents; L, lumen) (C) Loss of the mucus layer allows physical interaction of bacteria with epithelial cells which triggers goblet cell mucus release. Left panel: fluorescent in situ hybridization with EUB388, which stains rDNA of bacteria, shows intraluminal bacteria in close proximity with the colonic epithelium in rat colon exposed to ischemia. Right panel: Electron microscopy reveals that goblet cells release their contents (GCC, goblet cell contents) into the crypt lumen (L) upon IR in an attempt to flush bacteria (red arrows) from the crypts. (N, nucleus; yellow dashed line demarcates a goblet cell).
Colon Ischemia and Reperfusion Results in Mucus Barrier Breakdown
Colon ischemia/reperfusion (IR) is the most common form of intestinal ischemia and is observed in a variety of situations including infections and vasculitis, as well as in patients undergoing aortic surgery or cardiac bypass surgery.9-12 In addition, intestinal hypoxia is a well-known pathophysiological phenomenon in the (chronically) inflamed mucosa, such as mucosal inflammation observed in IBD.13,14 To study the pathophysiology of human colonic IR we recently developed a human experimental colon IR model,15 analogous to a recently developed model for human small intestinal IR.16-18 The first interesting observation was that human colon tissue was far more resistant to ischemia than human jejunum.18 The epithelial lining of the human colon remained intact even after 60 min of ischemia, with or without reperfusion. In addition, apoptosis of colonocytes was limited to only few cells in the crypt tips, whereas in the human small intestine IR was associated with massive apoptosis of epithelial cells in the villus tips and crypts.17,18
Although epithelial cell damage was limited, we showed that colonic ischemia was particularly associated with damage of the mucus layer, the important first line of defense against intraluminal microbiota.15 It is not fully understood why detachment of the mucus layer from the colonocytes occurred in colon exposed to IR. First, it might simply be a consequence of detachment of epithelial cells at the tip of the ischemically damaged colonic epithelium. Second, it might be a consequence of massive secretory activity of goblet cells creating a strong outward flow that not only flushes bacteria from the crypts, but also the mucus layer. In addition, epithelial hypoxia might change the conditions involved in attachment and proper dispersion of mucus, which further compromises the mucus layer. For example, it has been demonstrated that expansion and dispersion of mucus is bicarbonate-dependent, due to the cationic nature of mucins.19,20 It can be envisioned that the bicarbonate depletion due to an acidic milieu in hypoxic epithelial cells leads to impaired degranulation and dispersion of goblet cells. Interestingly, we observed “entire” goblet cell contents in the colonic lumen exposed to IR (Fig. 1B), which supports the latter theory.
Loss of the colonic mucus layer was associated with bacterial adherence to epithelial cells (Fig. 1C, left panel) and translocation of bacteria into the colonic crypts. This study therefore supports previous studies showing the importance of the mucus layer in preventing bacterial adherence and penetration. Intriguingly, goblet cells react rapidly in response to these bacterial threats with expulsion of their mucus containing granules (Fig. 1C, right panel). This so called goblet cell “compound exocytosis” following colonic ischemia is an accelerated and acute release of centrally stored mucin granules from goblet cells.4,21 This has been described as a mechanism to reinforce the barrier and exclude pathogens from the normally sterile crypts. Compound exocytosis results in a 100–1000 fold expansion in mucin volume upon hydration and contributes to the rapid replacement of a degraded mucus barrier.4 Since bacteria were rapidly flushed from the colonic crypts, compound exocytosis seems an efficient mechanism to prevent excessive exposure to microbiota to the epithelium, thereby preventing intestinal inflammation. The protective effect of the mucus layer on ischemia-induced bacterial translocation and consequently inflammation might explain why patients with colonic ischemia tend to have a mild course as compared with small intestinal IR, where the mucus layer is thinner.12,22-24 In addition, mucus organization can explain why ischemia of the right-sided colon is often more severe than left-sided colonic ischemia, since thickness of the inner mucus layer increases from the proximal to distal colon.12
As soon as 240 min after initiation of reperfusion of the colon, the mucus layer covering epithelial cells was restored.15 Bacteria were no longer observed in close proximity with epithelial cells. We did observe, as expected, an inflammatory response during reperfusion, however it is plausible that this response would be far more extensive without the protective response of the goblet cells to bacterial threats. To answer the question whether mucus release prevents excessive bacterial translocation and inflammation, it is important to identify -and interfere with- the environmental factor that triggers mucus release. There is a large list of environmental stimuli that alter the rate of mucin release (for example the release of inflammatory cytokines, prostaglandins, cholinergic stimuli, lipopolysaccharide, bile salts, nucleotides, nitric oxide, vasoactive intestinal peptides and neutrophil elastase) (reviewed by McGuckin et al.).4 A difficult factor in identifying the involvement of one of these factors during IR is that many of these phenomena are hallmarks of intestinal IR. In particular, exposure to bacteria and their pro-inflammatory component lipopolysaccharide is a well known trigger for goblet cells to increase their secretory rate, especially in the airway epithelium.21 In our study, exposure to bacteria seems a plausible trigger for compound exocytosis, since increased goblet cell secretory activity occurred simultaneously with bacterial penetration into the otherwise sterile colonic crypts. To add mechanistic data to prove this hypothesis, a logical step would be to study goblet cell secretory activity in germ-free animals exposed to IR. However, these animals have both disturbed mucin quantity and altered mucin quality (sulphation and sialylation) due to the absence of microbiota,25-27 which hampers evaluation of the selective effects of ischemia and reperfusion. Future studies are needed to mechanistically prove that microbial sensing triggers goblet cell compound exocytosis and thereby rapid restoration of the mucus layer.
Adding up our data, we conclude that the colon has efficient mechanisms to prevent excessive exposure to intraluminal contents following ischemia-induced mucus barrier loss. An interesting question is whether the consequences of repetitive ischemic hits or chronic intestinal ischemia can also be counteracted by goblet cell mucus release. We observed that reloading of goblet cells following IR was relatively slow, since goblet cells were only partly refilled even after 240 min of reperfusion. This is in line with the large size of the MUC2 proteins, which slows down the biosynthesis and formation of new mucin polymers.28,29 In addition, it should be noted that IR of both the human and rat colon was accompanied by considerable endoplasmic reticulum stress, as assessed by splicing of X-box binding protein-1 (unpublished data). IR-induced ER stress can impair mucus barrier recovery in two ways.
First, ER stress induces a translational block, which is mediated by PERK-dependent phosphorylation of eukaryotic translation-initiation factor 2α (eIF2α).30,31 The translational block alleviates ER stress by inhibiting protein processing in the ER. In the human and rat colon, increased expression of several components of this ER-stress pathway were observed, including increased expression of GADD34 (unpublished data). The observed ER stress during colon IR, which is associated with decreased protein synthesis, might have played a role in the slow refilling of goblet cell granules during reperfusion.
Second, the ER is crucial for mucin oligomerization and N-glycosylation, which provides mucins their viscous and protective properties.32,33 ER stress can therefore also be associated with decreased mucus quality. Loss of O-glycosylation of mucins has been shown to induce spontaneous colitis in mice,34 and reduced glycosylation is observed in ulcerative colitis in man as well.32,35 In addition, impaired glycosylation has recently been linked to endoplasmic reticulum stress in mice and man.32 Lastly, mucin oligosaccharide chains are often terminated with sialic acid or sulfate groups, which account for their acidic properties.5 This is particularly relevant, since the acidic nature of mucus makes it more resistant to bacterial degradation.5,36 It is therefore important that future studies will be directed at investigating whether prolonged IR or chronic IR with severe ER stress leads to decreased mucus quality, bacterial translocation and deregulated inflammatory responses.
Conclusion
In conclusion, we have provided first clues on the pathophysiology of human and rat colon ischemia-reperfusion. We showed that the epithelium in the colon is quite resistant to ischemia. However, breakdown of the mucus barrier facilitates bacterial penetration deep into the colonic crypts and in between epithelial cells. We also showed that bacterial penetration is associated with goblet cell compound exocytosis; the rapid release of centrally stored mucins. This is a protective response directed at clearing potential threats from the colonic crypts. Goblet cell compound exocytosis resulted in rapid clearance of bacteria from the colonic crypts and restoration of the mucus layer within 240 min of reperfusion.
Improved understanding of the etiology of ischemic colitis is essential to develop new preventive and/or therapeutic strategies. In addition, such knowledge might improve treatment modalities for inflammatory bowel disease (IBD), since epithelial hypoxia is a well-known pathophysiological phenomenon in the inflamed intestine of patients with IBD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23866
References
- 1.Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol. 2010;10:735–44. doi: 10.1038/nri2850. [DOI] [PubMed] [Google Scholar]
- 2.Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 3.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105:15064–9. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–78. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 5.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73:1131S–41S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 6.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–29. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 2010;6:e1000902. doi: 10.1371/journal.ppat.1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansson ME, Gustafsson JK, Sjöberg KE, Petersson J, Holm L, Sjövall H, et al. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Gastroenterological Association American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology. 2000;118:951–3. doi: 10.1016/S0016-5085(00)70182-X. [DOI] [PubMed] [Google Scholar]
- 10.Longstreth GF, Yao JF. Epidemiology, clinical features, high-risk factors, and outcome of acute large bowel ischemia. Clin Gastroenterol Hepatol. 2009;7:1075–80, e1-2, quiz 1023. doi: 10.1016/j.cgh.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Feuerstadt P, Brandt LJ. Colon ischemia: recent insights and advances. Curr Gastroenterol Rep. 2010;12:383–90. doi: 10.1007/s11894-010-0127-y. [DOI] [PubMed] [Google Scholar]
- 12.Brandt LJ, Feuerstadt P, Blaszka MC. Anatomic patterns, patient characteristics, and clinical outcomes in ischemic colitis: a study of 313 cases supported by histology. Am J Gastroenterol. 2010;105:2245–52, quiz 2253. doi: 10.1038/ajg.2010.217. [DOI] [PubMed] [Google Scholar]
- 13.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–7. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grootjans J, Hundscheid IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK, et al. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut. 2012;62:250–8. doi: 10.1136/gutjnl-2011-301956. [DOI] [PubMed] [Google Scholar]
- 16.Derikx JP, Matthijsen RA, de Bruïne AP, van Dam RM, Buurman WA, Dejong CH. A new model to study intestinal ischemia-reperfusion damage in man. J Surg Res. 2011;166:222–6. doi: 10.1016/j.jss.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruïne AP, van Bijnen AA, et al. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol. 2010;176:2283–91. doi: 10.2353/ajpath.2010.091069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, et al. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529–39, e3. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–22. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdugo P. Goblet cells secretion and mucogenesis. Annu Rev Physiol. 1990;52:157–76. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 21.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 22.Montoro MA, Brandt LJ, Santolaria S, Gomollon F, Sánchez Puértolas B, Vera J, et al. Workgroup for the Study of Ischaemic Colitis of the Spanish Gastroenterological Association (GTECIE-AEG) Clinical patterns and outcomes of ischaemic colitis: results of the Working Group for the Study of Ischaemic Colitis in Spain (CIE study) Scand J Gastroenterol. 2011;46:236–46. doi: 10.3109/00365521.2010.525794. [DOI] [PubMed] [Google Scholar]
- 23.Elder K, Lashner BA, Al Solaiman F. Clinical approach to colonic ischemia. Cleve Clin J Med. 2009;76:401–9. doi: 10.3949/ccjm.76a.08089. [DOI] [PubMed] [Google Scholar]
- 24.Green BT, Tendler DA. Ischemic colitis: a clinical review. South Med J. 2005;98:217–22. doi: 10.1097/01.SMJ.0000145399.35851.10. [DOI] [PubMed] [Google Scholar]
- 25.Enss ML, Schmidt-Wittig U, Müller H, Mai UE, Coenen M, Hedrich HJ. Response of germfree rat colonic mucous cells to peroral endotoxin application. Eur J Cell Biol. 1996;71:99–104. [PubMed] [Google Scholar]
- 26.Kandori H, Hirayama K, Takeda M, Doi K. Histochemical, lectin-histochemical and morphometrical characteristics of intestinal goblet cells of germfree and conventional mice. Exp Anim. 1996;45:155–60. doi: 10.1538/expanim.45.155. [DOI] [PubMed] [Google Scholar]
- 27.Fontaine N, Meslin JC, Lory S, Andrieux C. Intestinal mucin distribution in the germ-free rat and in the heteroxenic rat harbouring a human bacterial flora: effect of inulin in the diet. Br J Nutr. 1996;75:881–92. doi: 10.1079/BJN19960194. [DOI] [PubMed] [Google Scholar]
- 28.Axelsson MA, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–70. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- 29.Johansson ME, Hansson GC. The goblet cell: a key player in ischaemia-reperfusion injury. Gut. 2012;62:188–9. doi: 10.1136/gutjnl-2012-302582. [DOI] [PubMed] [Google Scholar]
- 30.Koritzinsky M, Magagnin MG, van den Beucken T, Seigneuric R, Savelkouls K, Dostie J, et al. Gene expression during acute and prolonged hypoxia is regulated by distinct mechanisms of translational control. EMBO J. 2006;25:1114–25. doi: 10.1038/sj.emboj.7600998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–64. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–63. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- 34.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–66. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Podolsky DK, Fournier DA. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988;95:379–87. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- 36.Robertson AM, Wright DP. Bacterial glycosulphatases and sulphomucin degradation. Can J Gastroenterol. 1997;11:361–6. doi: 10.1155/1997/642360. [DOI] [PubMed] [Google Scholar]


