Abstract
The microbial communities found in the mammalian large intestine and rumen efficiently degrade many recalcitrant substrates that are resistant to the host’s digestive enzymes. These communities are known from molecular profiling to be highly diverse at the species and strain level, but it may be that only certain specialized organisms (“keystone species”) have the ability to initiate degradation of such substrates, thus releasing energy on which the rest of the community depends. We have recently reported that Ruminococcus bromii has a superior ability to degrade certain forms of particulate resistant starch (RS) when compared with other highly abundant species of amylolytic bacteria found in the human colon and have presented evidence that this bacterium provides an example of a keystone species within the microbial community with respect to RS fermentation. The concept of keystone species can be equally relevant to other activities, e.g., those involved in stabilizing the community.
Keywords: keystone species, microbiota, resistant starch, anaerobic bacteria, microbial consortia, human colon, cross-feeding
Introduction
“Some are more equal than others”—this famous quotation from George Orwell’s Animal Farm referred to the extra benefits claimed by the leaders of his supposedly egalitarian (farmyard) societies. It could also be taken, however, to refer to a greater contribution of certain community members compared with others in generating resources on which the whole community depends. The current tendency toward wholly sequence-based descriptions of microbial communities provides little definitive information on the functional roles of the multitude of different phylotypes that make up the community. This can result in a somewhat neutral description of the community in which importance is equated, by default, to relative abundance. On the other hand where functional information is available, typically from cultured representatives, it emerges that some key metabolic or enzymatic capabilities may be limited to a small number of organisms, whose impact on the community may therefore be disproportionately large in relation to their numerical abundance. In some situations such organisms may be said to have a “keystone” role, meaning that their absence would, for example, greatly decrease the degradation and utilization of an important substrate, thus affecting the remainder of the microbial community.
Ruminococcus bromii as a Keystone Species in the Fermentation of Dietary Resistant Starches
A good example of such a keystone species within the human colonic microbiota was reported recently by Ze et al. (2012).1 Dietary resistant starch is often the single largest source of energy contributing to bacterial growth in the human colon, depending of course on diet composition. Ze et al. (2012)1 demonstrated an exceptional ability of the human colonic Firmicutes species Ruminococcus bromii to degrade particulate resistant starches (RS). They showed that the amylases of R. bromii strain L2–63 caused extensive degradation of RS even when this strain was inoculated into an RS-containing medium that did not support its growth. In contrast strains of three other amylolytic bacteria from the human colon, Eubacterium rectale, Bacteroides thetaiotaomicron or Bifidobacterium adolescentis, although able to grow on the medium with other added carbon sources, showed a limited ability to utilize boiled RS3 starch. In all three cases, however, co-inoculation with R. bromii L2–63 on this medium led to greatly increased starch utilization. In Ze et al., (2012),1 good growth of R. bromii was only obtained in medium containing 30% rumen fluid, but we have subsequently used a modified medium that allows it to grow in the absence of rumen fluid (Fig. 1). Co-culture of the non-starch degrading species Anaerostipes hadrus2 with R. bromii can be seen to result in the removal of reducing sugar that accumulated in the R. bromii monoculture (Fig. 1). This increased the utilization of total sugar by the co-culture (p < 0.001) and resulted in stimulation of A. hadrus growth, monitored by qPCR.3,4 We have also examined consortia comprising either four (including R. bromii) or three (without R. bromii) amylolytic bacteria, plus Anaerostipes hadrus (Fig. 2). It can be seen that utilization of the boiled RS3 starch was stimulated almost 3 fold when R. bromii was present in the consortium (p < 0.001). qPCR analysis demonstrated that although R. bromii grew within this defined community, its growth was more limited than in the monoculture (Fig. 1). Other species therefore benefitted from the amylolytic action of this primary degrader by competing for the soluble breakdown products, and this competition is assumed to explain the more limited growth of R. bromii and reduced overall starch utilization by comparison with the co-culture shown in Figure 1. Butyrate, which is produced by E. rectale and A. hadrus but not by R. bromii, increased in the presence of R. bromii, as did the net consumption of acetate which is linked to butyrate production by these species.5 The mechanisms that allow R. bromii to degrade particulate RS with such high efficiency are under investigation. The organization of amylases in Gram-positive bacteria6,7 clearly differs from the sus paradigm developed for starch utilization by Gram-negative Bacteroides species8 which appears more suited to the sequestration of soluble molecules.9,10
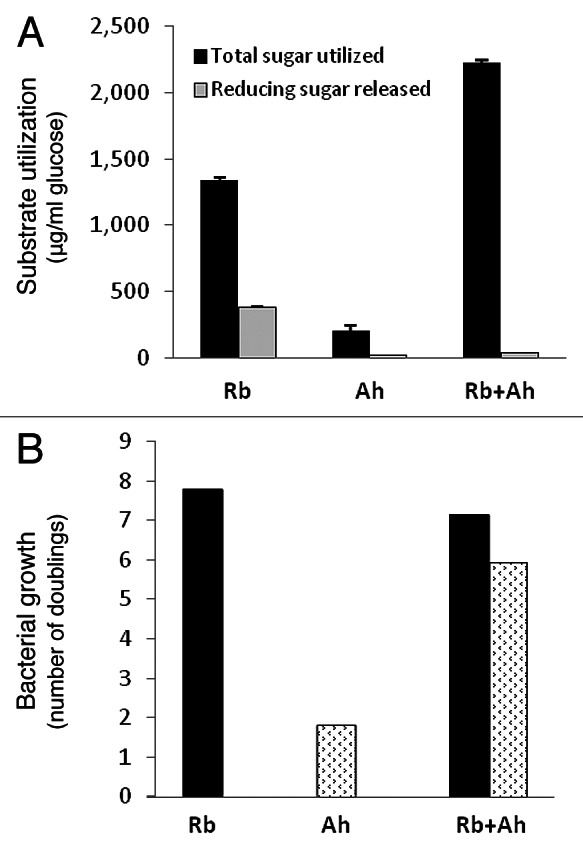
Figure 1. Co-culture of Ruminococcus bromii L2–63 (Rb) with the non-amylolytic bacterium Anaerostipes hadrus SS2/1 (Ah). (A) shows total sugar utilization and reducing sugar accumulation (as glucose equivalents) within cultures after 48 h incubation at 37°C (compared with zero time controls). (B) shows bacterial growth estimated by qPCR and expressed as doublings in 48h (log2 nt48/nt0, where n is the estimated number of rRNA gene copies). Data are means of triplicate cultures. Incubation was in anaerobic medium containing 0.2% boiled RS3. The medium is the same as the modified YCFA medium described in Ze et al., (2012)1 except that it contains 1% (instead of 0.25%) casitone and 0.25% (instead of 0.1%) yeast extract, additional filter-sterilized vitamins11 and a trace element solution.28 The primers used for qPCR detection here and in Figure 2 were described previously.3,4
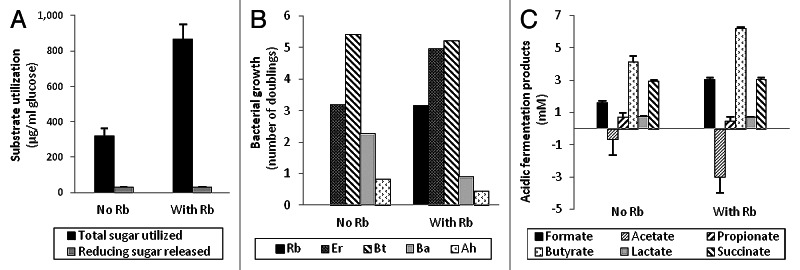
Figure 2. Stimulation of RS3 degradation in a five-membered bacterial consortium by R. bromii L2–63 (Rb). Two consortia comprising: five strains (B. thetaiotaomicron 5482 (Bt), B. adolescentis L2–32 (Ba), E. rectale A1–86 (Er), A. hadrus SS2/1 (Ah) and R. bromii L2–63) (“with Rb”); or four strains (the same mix without R. bromii) (“no Rb”) were inoculated into anaerobic medium containing 0.2% boiled RS3 and incubated for 48h at 37°C. (A) Total sugar utilization and reducing sugar accumulation (as glucose equivalents) within cultures (compared with zero time controls). (B) Bacterial 16S rRNA gene copies, estimated by qPCR using specific primer combinations, expressed as doublings (see Figure 1 legend). (C) Acidic fermentation products (mM). Data are means of triplicate cultures.
Remarkably, the study of Ze et al., (2012)1 appears to be the first report of cultural work on R. bromii since Herbeck and Bryant examined growth requirements in 1974.11 R. bromii is one of the most abundant species in the healthy human colon, and three recent studies12-14 showed that its representation in the faecal microbiota was increased in most volunteers when given diets containing RS2 or RS3 resistant starches. In their carefully controlled dietary study, Walker et al., (2011)13 detected “R-ruminococci” in fecal samples from 12 out of 14 obese male volunteers examined. Remarkably, the two exceptions were also the only two people to have residual unfermented starch in their fecal samples. Using a follow-up sample from one of these volunteers, Ze et al., (2012)1 were able to show that addition of R. bromii restored RS3 degradation in vitro, whereas E. rectale, B. adolescentis and B. thetaiotaomicron had little effect.
Functional Redundancy vs. Niche Specialization: Consequences of Inter-Individual Variation
At first sight, the concept of keystone species appears to contradict a developing view that functional redundancy is the dominant feature of gut microbial communities. High throughput sequence analyses indicate there is a greater degree of variability within the gut microbial community at the phylogenetic level than at the level of gene categories identified from metagenome sequencing.15,16 This leads to the proposition that the phylogenetic diversity can be treated mainly as “noise”, with core functions being performed by a large number of alternative phylotypes. To take one possible example, many Firmicute species within the human gut microbiota utilize the same pathway for butyrate formation. Several ecologically and nutritionally distinct groups of butyrate producers can be identified5 but different species within these functional groups, although known to vary between individuals,3 might be considered essentially interchangeable as agents in the delivery of butyrate to the gut epithelium. On the other hand, we know that these species are not precisely equivalent; variations in substrate utilization and metabolic capabilities that are known to occur between strains and species of Roseburia,17 for example, may indeed have consequences for the community and the host. In other words, common sets of genes will often occur in organisms that are ecologically quite distinct. Returning to starch utilization, discussed above, putative amylases belonging to glycoside hydrolase family 13 are abundant in the metagenome and can be found in the great majority of sequenced genomes from human intestinal bacteria. Many of these enzymes are likely to be concerned with utilizing soluble starches and oligosaccharides, however, and the characteristics that allow an organism to degrade particulate resistant starch apparently occur only in rather few “keystone” species.
The question of functional redundancy is particularly important in relation to inter-individual variation. Given a high degree of functional redundancy, much of the remarkable variation in the gut microbiota that is seen between individuals at the phylotype level would have little functional significance. By definition, however, variation between individuals in the occurrence of keystone species could have major consequences for their health and for responses to dietary components, as illustrated by the limited fermentation of RS3 starch seen in two individuals lacking R. bromii by Walker et al., (2011).13 A healthy, balanced gut microbiota would therefore be defined to a large degree by the possession of a set of important keystone species.
Keystone Species in General
Classic work on the rumen, where the major source of energy for microbial growth typically comes from lignocellulosic plant cell walls, revealed only a small number of microbial species with the ability to degrade cellulose.18 Interestingly these include two species of ruminococci, R. flavefaciens and R. albus, along with the fibrolytic Gram-negative species Fibrobacter and certain anaerobic eukaryotes (fungi and protozoa). Although rumen cellulose breakdown is therefore not attributable to any single species, it has been shown that the bulk of the community depends critically on these primary cellulolytic organisms for the release of soluble growth substrates.19 Interestingly the only human colonic bacterium so far shown to be able to degrade crystalline cellulose is another species of Ruminococcus, R. champanellensis. It has been proposed that subjects whose colonic microbiota are capable of degrading this type of cellulose are characterized by possession of this species.20,21 It is intriguing, but probably not coincidental, that the same family of Gram-positive bacteria (Ruminococcaceae) includes potential primary degraders of two very different substrates, lignocellulose and resistant starch. Ruminococcus spp were found to represent a 4-fold higher proportion of bacterial 16S rRNA sequences associated with particulate material from human fecal samples than in the liquid phase22 suggesting that their niche involves tight adherence to particles. Specialised cell surface structures and enzyme complexes involved in adhesion and degradation are the key to microbial degradation of such particulate substrates, as has been established for the cellulolytic ruminococci found in the rumen.23,24 In the case of another recalcitrant substrate, mucin, however, the candidate keystone species Akkermansia muciniphila, comes from a different phylum (Verrucomicrobia).25 In each of these cases newly available genome sequence data will facilitate discovery of the mechanisms used by these intriguing and specialized bacteria to exploit their particular niches.
The concept of keystone species can be readily applied to the release of energy from recalcitrant substrates; however it is likely to prove relevant also to other types of microbial interaction that occur within complex gut communities. Returning to the rumen, bacteria that utilize lactate for example play a key role in stabilizing the community by preventing the drop in pH that results from lactate accumulation. There is evidence that such microbially-mediated buffering applies also to the human colonic microbiota26 where only certain species have the ability to convert lactate into butyrate, acetate or propionate.27 The keystone role of such species therefore resides in their stabilizing impact on the gut environment. A case can also be made for other specialist groups such as hydrogen-utilizers whose activities have wide-ranging effects on the rest of the community. In reality it may turn out that we should be talking about “keystone groups” rather than “keystone species” as it would be remarkable if such activities were always limited to a single species. The taxonomic detail is however less important than the insights that can be gained into the functioning and stability of complex gut communities.
It is clearly important to identify such keystone species in gut microbial communities and to be able to monitor their populations using metagenomic data and also by more targeted approaches. Variation in the populations and activities of such species both between individuals and within individuals (with diet, age and disease) can help to explain and predict the behavior of the microbial ecosystem and the complex interplay between diet, gut microbiota and health.
Acknowledgments
The authors would like to acknowledge support from the Scottish Government Food, Land and People program and from the Society for Applied Microbiology. Thanks are also due to David Ramsbottom, Andrea Quartieri and Ilaria Pesci for valuable discussion of the composition of the growth medium.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/23998
References
- 1.Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–43. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen-Vercoe E, Daigneault M, White A, Panaccione R, Duncan SH, Flint HJ, et al. Anaerostipes hadrus comb. nov., a dominant species within the human colonic microbiota; reclassification of Eubacterium hadrum Moore et al. 1976. Anaerobe. 2012;18:523–9. doi: 10.1016/j.anaerobe.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–14. doi: 10.1111/j.1462-2920.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 4.Ramirez-Farias C, Slezak K, Fuller Z, Duncan A, Holtrop G, Louis P. Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101:541–50. doi: 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- 5.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay AG, Scott KP, Martin JC, Rincon MT, Flint HJ. Cell-associated α-amylases of butyrate-producing Firmicute bacteria from the human colon. Microbiology. 2006;152:3281–90. doi: 10.1099/mic.0.29233-0. [DOI] [PubMed] [Google Scholar]
- 7.O’Connell Motherway M, Fitzgerald GF, Neirynck S, Ryan S, Steidler L, van Sinderen D. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl Environ Microbiol. 2008;74:6271–9. doi: 10.1128/AEM.01169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol. 2012;10:323–35. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 11.Herbeck JL, Bryant MP. Nutritional features of the intestinal anaerobe Ruminococcus bromii. Appl Microbiol. 1974;28:1018–22. doi: 10.1128/am.28.6.1018-1022.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abell GCJ, Cooke CM, Bennett CN, Conlon MA, McOrist AL. Phylotypes related to Ruminococcus bromii are abundant in the large bowel of humans and increase in response to a diet high in resistant starch. FEMS Microbiol Ecol. 2008;66:505–15. doi: 10.1111/j.1574-6941.2008.00527.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011;5:220–30. doi: 10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martínez I, Kim J, Duffy PR, Schlegel VL, Walter J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS One. 2010;5:e15046. doi: 10.1371/journal.pone.0015046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncan SH, Aminov RI, Scott KP, Louis P, Stanton TB, Flint HJ. Proposal of Roseburia faecis sp. nov., Roseburia hominis sp. nov. and Roseburia inulinivorans sp. nov., based on isolates from human faeces. Int J Syst Evol Microbiol. 2006;56:2437–41. doi: 10.1099/ijs.0.64098-0. [DOI] [PubMed] [Google Scholar]
- 18.Hungate RE. The rumen and its microbes. 1966. Academic Press Inc., New York. [Google Scholar]
- 19.Dehority BA. Effects of microbial synergism on fibre digestion in the rumen. Proc Nutr Soc. 1991;50:149–59. doi: 10.1079/PNS19910026. [DOI] [PubMed] [Google Scholar]
- 20.Robert C, Bernalier-Donadille A. The cellulolytic microflora of the human colon: evidence of microcrystalline cellulose-degrading bacteria in methane-excreting subjects. FEMS Microbiol Ecol. 2003;46:81–9. doi: 10.1016/S0168-6496(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 21.Chassard C, Delmas E, Robert C, Bernalier-Donadille A. The cellulose-degrading microbial community of the human gut varies according to the presence or absence of methanogens. FEMS Microbiol Ecol. 2010;74:205–13. doi: 10.1111/j.1574-6941.2010.00941.x. [DOI] [PubMed] [Google Scholar]
- 22.Walker AW, Duncan SH, Harmsen HJM, Holtrop G, Welling GW, Flint HJ. The species composition of the human intestinal microbiota differs between particle-associated and liquid phase communities. Environ Microbiol. 2008;10:3275–83. doi: 10.1111/j.1462-2920.2008.01717.x. [DOI] [PubMed] [Google Scholar]
- 23.Rincon MT, Cepelnik T, Martin JC, Lamed R, Bayer EA, Flint HJ, et al. Unconventional mode of attachment of the Ruminococcus flavefaciens cellulosome to the bacterial cell wall. J Bacteriol. 2005;187:7569–78. doi: 10.1128/JB.187.22.7569-7578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincon MT, Dassa B, Flint HJ, Travis AJ, Jindou S, Borovok I, et al. Abundance and diversity of dockerin-containing proteins in the fiber-degrading rumen bacterium, Ruminococcus flavefaciens FD-1. PLoS One. 2010;5:e12476. doi: 10.1371/journal.pone.0012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–8. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belenguer A, Duncan SH, Holtrop G, Anderson SE, Lobley GE, Flint HJ. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl Environ Microbiol. 2007;73:6526–33. doi: 10.1128/AEM.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan SH, Louis P, Flint HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. 2004;70:5810–7. doi: 10.1128/AEM.70.10.5810-5817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macfarlane GT, Hay S, Gibson GR. Influence of mucin on glycosidase, protease and arylamidase activities of human gut bacteria grown in a 3-stage continuous culture system. J Appl Bacteriol. 1989;66:407–17. doi: 10.1111/j.1365-2672.1989.tb05110.x. [DOI] [PubMed] [Google Scholar]


