Abstract
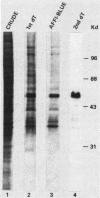
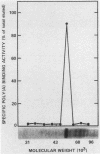
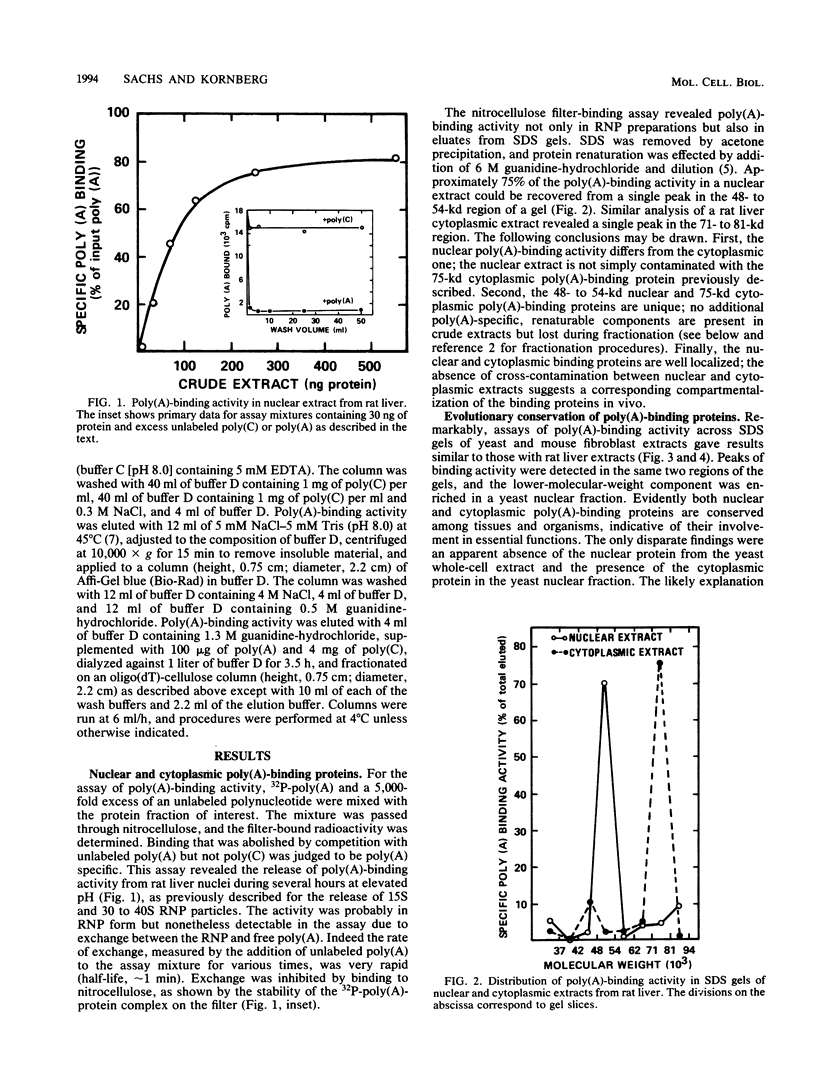
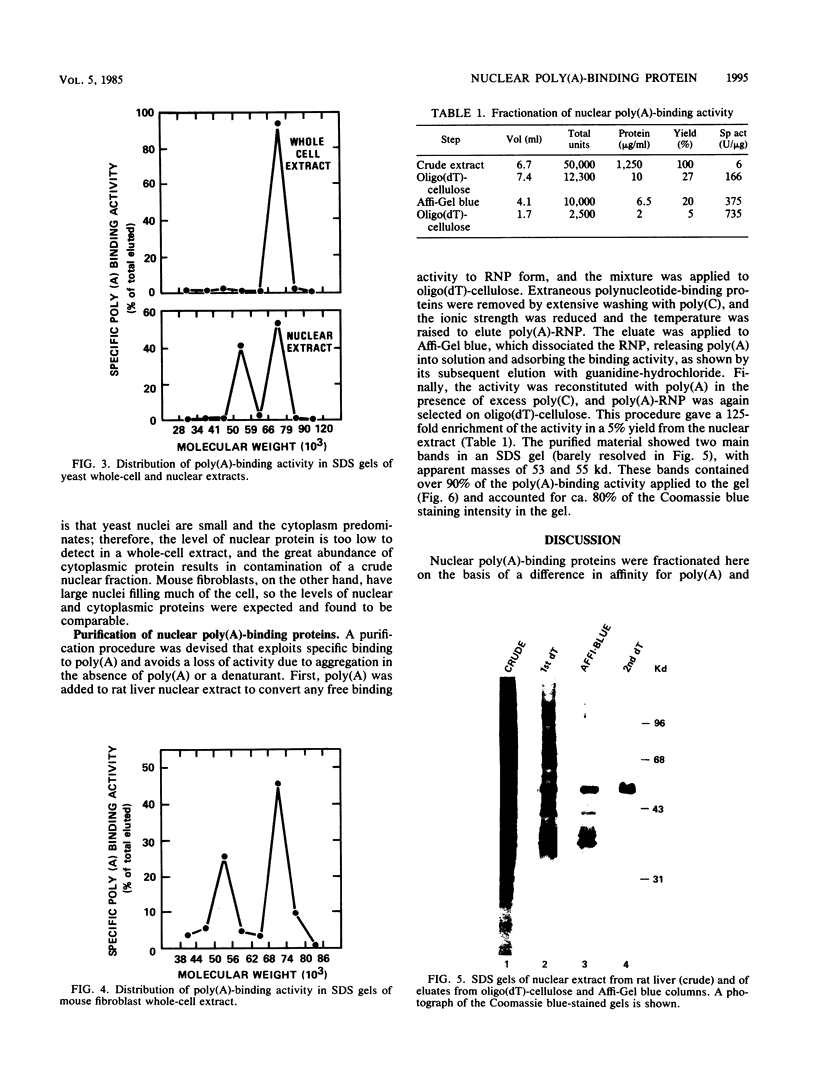
Polyadenylate-binding activity can be detected in eluates from sodium dodecyl sulfate gels by a nitrocellulose filter-binding assay. Nuclear extracts from rat liver show a single peak of binding activity at 50 to 55 kilodaltons; cytoplasmic extracts show a single peak at 70 to 80 kilodaltons, corresponding to a 75-kilodalton protein previously described. Similar results are obtained with yeast and mouse fibroblasts, indicating a high degree of conservation of both nuclear and cytoplasmic polyadenylate-binding proteins. The activity from rat liver nuclei has been purified 125-fold on the basis of specific binding to polyadenylate and shows two main bands in sodium dodecyl sulfate gels at 53 and 55 kilodaltons.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer B. W., Kornberg R. D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram R. J., Kornberg R. D. Specific protein binding to far upstream activating sequences in polymerase II promoters. Proc Natl Acad Sci U S A. 1985 Jan;82(1):43–47. doi: 10.1073/pnas.82.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. R. Ultraviolet light-induced crosslinking of mRNA to proteins. Nucleic Acids Res. 1979 Feb;6(2):715–732. doi: 10.1093/nar/6.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Pluskal M. G., Sarkar S. Thermal chromatography of eukaryotic messenger ribonucleoprotein particles on oligo (dT)-cellulose. Evidence for common mRNA-associated proteins in various cell types. FEBS Lett. 1979 Jan 1;97(1):84–90. doi: 10.1016/0014-5793(79)80058-7. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Ribonucleoprotein organization of polyadenylate sequences in HeLa cell heterogeneous nuclear RNA. J Mol Biol. 1975 Jun 25;95(2):227–238. doi: 10.1016/0022-2836(75)90392-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeStourgeon W. M., Beyer A. L., Christensen M. E., Walker B. W., Poupore S. M., Daniels L. P. The packaging proteins of core hnRNP particles and the maintenance of proliferative cell states. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):885–898. doi: 10.1101/sqb.1978.042.01.090. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Pullman J., Stevens B., Kinniburgh A. Substructure of nuclear ribonucleoprotein complexes. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):899–909. doi: 10.1101/sqb.1978.042.01.091. [DOI] [PubMed] [Google Scholar]
- Munroe S. H., Pederson T. Messenger RNA sequences in nuclear ribonucleoprotein particles are complexed with protein as shown by nuclease protection. J Mol Biol. 1981 Apr 15;147(3):437–449. doi: 10.1016/0022-2836(81)90494-0. [DOI] [PubMed] [Google Scholar]
- Quinlan T. J., Billings P. B., Martin T. E. Nuclear ribonucleoprotein complexes containing polyadenylate from mouse ascites cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2632–2636. doi: 10.1073/pnas.71.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Lau A. S., Munro H. N., Baliga B. S., Sarkar S. Release of in vitro-synthesized poly(A)-containing RNA from isolated rat liver nuclei: characterization of the ribonucleoprotein particles involved. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1751–1755. doi: 10.1073/pnas.76.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Setyono B., Greenberg J. R. Proteins associated with poly(A) and other regions of mRNA and hnRNA molecules as investigated by crosslinking. Cell. 1981 Jun;24(3):775–783. doi: 10.1016/0092-8674(81)90103-3. [DOI] [PubMed] [Google Scholar]
- Sperling R., Sperling J., Levine A. D., Spann P., Stark G. R., Kornberg R. D. Abundant nuclear ribonucleoprotein form of CAD RNA. Mol Cell Biol. 1985 Mar;5(3):569–575. doi: 10.1128/mcb.5.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomcsányi T., Molnár J., Tigyi A. Structural characterization of nuclear poly(A)-protein particles in rat liver. Eur J Biochem. 1983 Mar 15;131(2):283–288. doi: 10.1111/j.1432-1033.1983.tb07261.x. [DOI] [PubMed] [Google Scholar]