Abstract
Oral vaccines are safe and easy to administer and convenient for all ages. They have been successfully developed to protect from many infectious diseases acquired through oral transmission. We recently found in animal models that formulation of oral vaccines in a nanoparticle-releasing microparticle delivery system is a viable approach for selectively inducing large intestinal protective immunity against infections at rectal and genital mucosae. These large-intestine targeted oral vaccines are a potential substitute for the intracolorectal immunization, which has been found to be effective against rectogenital infections but is not feasible for mass vaccination. Moreover, the newly developed delivery system can be modified to selectively target either the small or large intestine for immunization and accordingly revealed a regionalized immune system in the gut. Future applications and research endeavors suggested by the findings are discussed.
Keywords: oral vaccine, nanoparticle, mucosa, intestine, T cell, rectogenital infection
Introduction
Induction of mucosal immunity is essential to stop person-to-person transmission of pathogenic microorganisms and to limit their multiplication within the mucosal tissue. Vaccination through a mucosal route is shown to offer advantages for enhanced mucosal immune responses that result in better local protection. Oral delivery of vaccines represents the most attractive mode of administration over other routes of delivery due to the fact that the oral vaccination is noninvasive, safe and simple to execute, showing good patient compliance and clinical practicality (Table 1). The oral polio vaccine, which consists of live attenuated polioviruses, is a clear demonstration of the fact that oral vaccination against a highly contagious human enterovirus has succeeded in eradicating this virus in almost all countries.1 The reason for the few countries still with endemic polio is only the lack of access of portions of the population to the vaccine. The polio vaccine is known to mimic the humoral immune response induced by wild strains of poliovirus orally transmitted. It protects the individual against paralytic poliomyelitis by preventing the virus from disseminating to the nervous system through the blood stream. The significant property of the vaccine is actually the ability to inhibit invading viruses from propagating in the mucosal tissue of the small intestine and, hence, to effectively control the virus from spreading from mucosal linings to other tissues or being shed. Another oral vaccine that is effective primarily against the small intestine infection is the rotavirus oral vaccine. Rotarix and RotaTeq are the two currently used vaccines that confer protection against rotavirus gastroenteritis as effectively as 70%, and the protection can reach 85% to 100% to prevent severe rotavirus gastroenteritis.2,3
Table 1. Route of immunization against rectogenital infection in animal models.
| Route | Advantages | Disadvantages | Feasibility |
|---|---|---|---|
| Oral |
Easy to administer, convenient mode of remote immunization |
No to low levels of rectogenital responses induced and risk of inducing oral tolerance |
Practical for mass vaccination and preferred |
| Intranasal |
Easy to administer, convenient mode of remote immunization |
Inducing modest levels of cellular and humoral responses and weak protection, pre-existing immunity to some delivery vehicles, may exacerbate nasal or respiratory inflammation; risk of transport through olfactory nerve to brain. |
Practical for mass vaccination |
| Intracolorectal |
Local immunization inducing both cellular and humoral rectal and vaginal responses |
Vaccination requiring facility and a trained professional, discomfort and accidental trauma may occur |
Feasible for special treatment but impractical for mass vaccination |
| Intravaginal |
Local immunization inducing humoral vaginal responses |
Vaccination efficacy is influenced by the menstrual cycle and no responses induced in the large intestine |
Feasible for special treatment but impractical for mass vaccination |
| Targeted oral | Easy to administer, passing through small intestine and selectively targeting large intestine to induce immune protective immunity comparable to intracolorectal immunization | Extra manufacturing procedures required but not significant | Practical for mass vaccination and preferred |
Although oral delivery is potentially preferred for many reasons for vaccination against enteroviruses, oral vaccines have not been developed specifically to target the large intestine mucosa against virus infection. Advantages of inducing immunity from the colorectal mucosa lie in the facts that it is the most effective route to induce immunity in the colorectal area against virus transmission and that vaginal immune responses could be induced at the same time.4 However, simple oral delivery is ineffective at inducing protective immunity at either rectal or genital mucosa.5 A three-pronged barrier interferes. First, a vaccine, especially in the form of protein or peptide, is subject to many obstacles while traveling through the GI tract. These include the low gastric pH, digestive enzymes and bile salts, which can result in deactivation and degradation of the vaccine. Without safe passage through these harsh environments, the vaccine cannot be effectively absorbed by the epithelial layer. Second, the vaccine components must be delivered preferentially to the large intestine, to mimic intrarectal delivery and not be taken up in the small intestine. Third, the surviving vaccine needs to cross the epithelial layer through a number of mechanisms and enter the gut-associated lymphoid tissue, where the mucosal immune responses may be induced. Formulating technology that enables oral vaccines to work efficiently in the distal gut still remains a substantial challenge.
Various recombinant or attenuated viral or bacterial strains have been developed for oral delivery of vaccines, but there is an immunological obstacle that needs to be overcome, pre-existing humoral or cellular immunity to the vector and newly developed anti-vector immune responses against the next vaccination. Peptides, used in epitope vaccines, are usually not immunogenic by themselves and should not be prematurely eliminated by the immune system, but would be very sensitive to enzymatic degradation. When combined with immune adjuvants, such vaccines are proven effective at inducing cellular immune responses for infections.6-8
Based on the current understanding of mucosal immunity, particulates containing vaccines may be formulated to achieve effective delivery to mucosal surfaces as well as transport across the epithelium for local vaccination. To penetrate the epithelium, vaccines need to target the epithelial cells including M cells and enterocytes. M cells are specialized epithelial cells predominantly residing in the follicular-associated epithelium of Peyer’s patches, which are abundant in number in the distal small intestine. Lumenal antigens or particulates up to 1 μm in diameter are taken up by M cells mainly through transcytosis9 or captured by dendritic cells (DCs) extending processes into the lumen.10 Enterocytes dramatically outnumber M cells and play an important role in the uptake and transport of lumenal antigens into the gut-associated lymphoid tissues.
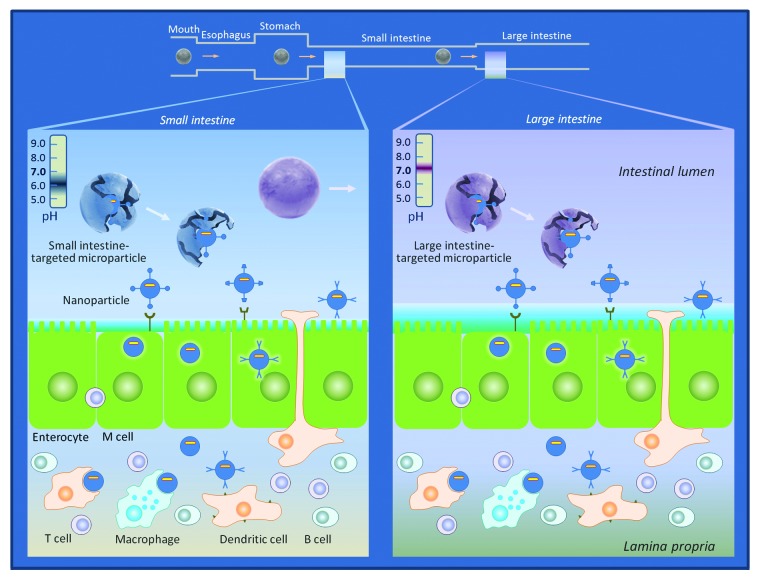
In our recent study,11 we developed a nanoparticle-releasing microparticle vaccine delivery system for site-specific immunization at the large intestine. We encapsulated peptide vaccines in biodegradable PLGA nanoparticles and then coated the particle surface with an acid resistant polymer Eudragit F30D to form micro-sized particles, which dissolve only at pH over 7. This device allows microparticles to release the nanoparticles containing vaccines selectively after passing through the small intestine where the pH is lower. The microparticles were intentionally designed to be too large to be taken up by epithelial cells or DCs, so that uptake could not occur until the nanoparticles are released from the Eudragit FS30D microparticles in the large intestine, allowing selective delivery to the immune system where desired. We indeed found that nanoparticle uptake from the FS30D microparticles occurred mainly at the mucosal surface of the large intestine, instead of in the small intestine. Therefore, this device was successfully built to protect the vaccine from being deactivated or degraded prior to the large intestine. When we coated the vaccine nanoparticles with another Eudragit formulation, namely L100–55, which can dissolve at pH 5.5 as found in the small intestine, following oral delivery the uptake was detected mainly in the small intestine and barely in the large intestine, as expected. Therefore, the concept of this delivery system was actually proven correct. Thus, by taking advantage of the differential pH sensitivity of the different Eudragit formulations, the transit time through the gut relative to the time required for release of the nanoparticles and the size of the microparticles that prevented premature uptake, we for the first time have been able to deliver the vaccines selectively to either the small or large intestine and induce the immune responses at the corresponding site.
Evading Roadblocks in the Gut Lumen
Secretory IgA (sIgA) present in the intestinal lumen acts as the first line of defense in innate immunity against pathogen invasion and enterotoxin insults. SIgA blocks pathogens or enterotoxins from attacking by binding epitopes on their surfaces and causing the agglutination of microbial pathogens, thereby interfering with their adherence to and penetration across mucosal surfaces.12 This immune exclusion process is enhanced by the secretory component, which mediates anchoring of sIgA to the mucus layer, resulting in efficient mucosal protection.13 Such natural or prior humoral mucosal immunity is a major hurdle for oral vaccination with microbial vectors or proteins.14 Children who have maternal sIgA antibodies from breast milk appear to experience reduced effectiveness of rotavirus vaccines.15 Immunogenic vaccines encapsulated in biosynthetic materials could evade antibody-mediated recognition and exclusion and thus gain better access to epithelial cells for uptake and the following transcytotic transport through the epithelial barrier.
Delivering Vaccines beyond the Small Intestine: Directed Safe Passage to the Front Line of Defense
Most live attenuated or inactivated viral vaccines are considered safe, but live oral viral vaccines have often shown greater efficacy. As they may maintain the ability to revert toward virulence and there is the probability of contamination with intact viruses, live vaccines sometimes can cause vaccine-induced diseases.16 New cases of vaccine-associated paralytic poliomyelitis, although not common, have been repeatedly discovered in many countries. There are also concerns about post-vaccination complications. For example, one type of oral rotavirus vaccine has increased the risk of intussusception in children and those who previously had intussusception cannot be vaccinated for this reason. To achieve high standards of safety and efficacy, researchers have made great efforts in developing non-infectious agents such as peptides, proteins and nucleic acids for oral vaccines. Protection of these agents against the hostile gut environment is, however, required for the oral delivery. Various biocompatible materials have been utilized to encapsulate vaccines to form either micro- or nano-sized particles, including polymers, lipid-based vesicles, starch, gelatin, viral-like particles and ISCOMS, which are primarily dissolved and/or absorbed in the small intestinal mucosa and elicit immune responses either locally or peripherally. Large intestinal immune responses were virtually never induced,11 or at least have not been fully investigated by most of the studies.
Both the size and controlled release capability are the key factors that influence the delivery of vaccine particles.17 For vaccine delivery beyond the small intestine, it is essential to use microparticles with a pH-dependent dissolution profile. The microparticle size should be considerably larger than the range for M cell-mediated uptake or phagocytosis by macrophages or DCs, such that particles, if they remain undissolved, are not taken up before arriving at the destination. Microparticles resistant to a lower pH are not dissolved in the small intestine and able to deliver vaccines undamaged through the terminal ileum, from which the increased pH facilitates dissolution, allowing release of enclosed nanoparticles for subsequent uptake by the large intestinal mucosa. Uptake of nanoparticle vaccines subsequently results in induction of large intestinal immune responses. Equally importantly, vaginal immune responses are elicited by the vaccine delivered to the large intestinal mucosa. This makes large intestine targeted oral vaccines more applicable for mass vaccination to prevent sexually transmitted infections (STIs)
In contrast to the large intestine-targeted microparticles, microparticles soluble at a lower pH value can break down in the small intestine. In this case, vaccines are taken up primarily in the small intestine and induce immune responses therein. Neither uptake nor immune responses are significant in the large intestine. Therefore, the newly developed nanoparticle-releasing microparticle delivery system should be able to target individual segments of the intestine to achieve site-specific mucosal vaccination.
A Vaccine Strategy to Study Immune Compartmentalization
The gut is divided into a number of segments with physiologically different functions. The small intestine and the large intestine appear to differ from each other in immune responses to a vaccine. As we recently discovered, induction of T cell immune responses in the small intestine does not lead to significant immune responses in the large intestine and vice versa, so there is clear compartmentalization,11 and even within the small intestine, antigen delivery at different levels differentially affects the antibody isotypes induced in the serum, although local mucosal antibody responses in different segments of the small intestine were not examined.18 Thus, vaccine responses in each anatomical segment may vary over the entire length of the intestine. How these segments respond differently to a vaccine has been a subject of great interest for decades and has been studied from the early days of mucosal immunology, but the only techniques available were cumbersome and invasive, possibly affecting the outcome. Intraloop19 and intralumenal20 immunization of experimental animals performed by laparotomy are the procedures commonly used to study intestinal immune responses. Such intestinal models provide an effective approach to the longitudinal measurement of segmental immune responses to dissect the mechanisms involved in uptake and recognition of lumenal antigens but may cause significant disturbance of the tissues involved and therefore influence the results. Site-specific oral vaccine delivery by the nanoparticle-releasing microparticle system provides a vaccine safe and physiological access to the mucosal site of interest, representing a novel alternative method to study vaccine-induced immunity at different segments of the intestine, through a non-invasive approach that avoids these drawbacks. Following the same vein, the new delivery system can also be developed for segmental challenge of various pathogenic agents in addition to the ligated intestinal loop method. A comprehensive approach is likely to greatly improve our understanding of the intestinal immunity against pathogen invasion in natural vs. experimental settings.
We previously demonstrated that immunization locally in the large intestine induced robust immune responses at the site of immunization and immunization via a distant mucosal site proved to be less effective.4,21,22 Antigen challenge through the nose of animals pre-sensitized to the same antigen induced eosinophilia in the lung but not in the intestine.23 As shown in our recent study,11 oral immunization with nanoparticles that target the large intestinal mucosa did not induce immune responses in the small intestine while immunization targeting the small intestine failed to elicit the large intestinal immune responses as well. These discriminations may result from the differences in specialized anatomical and immunological features of the intestinal wall24 or the presence of and gut interaction with commensal bacteria in the lumen.25 It has been well established that immune cells, including dendritic cells, T helper cells, T regulatory cells and intraepithelial CD4+ and CD8+ T cells, are compositionally, phenotypically and functionally different between the small and large intestines.26-29 Regulation of immune cell recruitment appears also to be different between the two intestinal segments. Integrin α4β7/MAdCAM-1 and CCR9/CCL25 are engaged in homing of T cells to the small intestine but not so much (especially for CCR9) to the large intestine, where ligands for CCR9 and integrin α4β7 are weakly expressed.30,31 T cells recruitment does not, however, follow the same mechanisms every place even within the small intestine. Expression of CCL25 is much lower in the terminal ileum than that in the proximal part and homing of T cells to the ileum is relatively independent of the CCR9/CCL25 pathway.24 Instead, activated CD4+ T cells have markedly upregulated expression of CCR2 in Crohn’s disease and CCR2/CCL2 interactions promote T cell migration to the inflamed ileum.32
Pathogen recognition receptors are expressed at various levels by intestinal epithelial cells. TLR2 and TLR4 are significantly expressed in the proximal and distal colon, respectively, while TLR3 is expressed in both small and large intestines.33 NOD-like receptor (NOD) 1 is constitutively expressed in colon epithelial cells, in contrast to NOD2 which is primarily located in epithelial cells of the terminal ileum.34 A recent study shows that NOD1 and NOD2 are critical for induction of a Th17-mediated response primarily in the cecum of mice.35 Therefore, due to the discrepancies in molecular and cellular characteristics between the small and large intestines and among segments of the small intestine, immune responses to a vaccine would be expected to be different from one segment to another in terms of magnitude, duration, activation vs. tolerance, cell-mediated vs. antibody-mediated immunity, etc. The site-specific vaccine delivery system will allow more comprehensive characterization of mucosal immunity of each gut segment in a physiological, non-invasive way and, as a result, enable us to develop more precisely targeted and more effective oral vaccines.
Adaptation for Other Oral Vaccination Strategies
The working mechanism for the nanoparticle vaccine delivery system is size-dependent particle uptake through phagocytosis by DCs and macrophages after nonspecific transcytosis through M cells and enterocytes into the mucosal tissue or by DCs that directly sample the gut lumen. However, on the apical surface of enterocytes and M cells, there exist various common or special receptors such as adhesion molecules β1 integrins,36 carcinoembryonic antigen-related cell adhesion molecule (CEACAM)-1,37 chemokine receptors CCR1 through 8 and CXCR4,38,39 neonatal Fc receptor (FcRn),40 many of which are utilized for uptake and transcytosis of lumenal macromolecules and microorganisms. Glycoprotein 2 (GP2), expressed on the M cell apical surface, has recently been identified as a receptor for FimH from the outer membrane of type-I-piliated bacteria.41 GP2-dependent transcytosis of S. Typhimurium through M cells results in antigen-specific immune responses in Peyer's patches. Pattern recognition molecules also play a critical role in the recognition and transcytosis of bacteria across the epithelium.42 M cells have specific markers that can be targeted by antibodies43 and nanoparticle delivery of vaccines to the dome of Peyer’s patches could be achieved if antibodies are coupled. Antibodies against the lumenal surface of enterocytes, if developed, may potentially be used as well. These receptor-dependent transcytotic pathways are promising targets for the development of nanoparticle oral vaccines. A ligand for its receptor expressed on the surface of either enterocytes or M cells can be conjugated on the nanoparticle surface to specifically target to the receptor for enhanced vaccine uptake and transport to the mucosal immune system.44 Integration of site-specific nanoparticle releasing technology and strategies on optimizing vaccine uptake and transepithelial transport efficacy as well as intestinal DCs (ref45) would greatly improve oral vaccine delivery system in accuracy and efficacy (Fig. 1).
Figure 1. Selective targeting of epithelial cells and dendritic cells by nanoparticle-releasing microparticle oral vaccines for enhanced biomaterial uptake and transport. Conventional enterocytes and specialized epithelial cells overlie the intestinal epithelium. Some underlying dendritic cells extend their dendrites to sample lumen antigen. Cell-specific targeting of these cells may enhance oral vaccine efficacy by improving mucosal uptake, transepithelial transport and subsequent antigen processing and presentation.
Due to the immunological and physiological differences between the small and large intestine as well as interspecies differences in intestinal immunity, cautions need to be taken when extrapolating from animal models to humans. M cells are more common in the terminal ileum than in other segments of small intestine and colorectum. As human M cells are only half as numerous as in mice and express specific thin surface glycocalyx (sialyl Lewis A antigen)46 and β1 integrin,36 vaccines specifically targeting human M cells may have to be more potent. The pattern of FcRn expression differs between humans and rodents; human adult FcRn capable of transcytosing IgG from the basolateral surface to the intestinal lumen for antigen binding can recycle antigen-bound IgG across the epithelium back to the lamina propria.40,47 In addition, FcRn is expressed by lamina propria antigen presenting cells. Thus, an IgG-antigen immune complex may be designed in human vaccines allowing FcRn-mediated reverse transcytosis by enterocytes as well as direct antigen acquisition by intestinal DCs. It has been found that activation through TLR2 in mice upregulates effector T cell responses as a result of transient loss of regulatory T cells (Tregs) suppression activity.48 However, TLR2 stimulation in humans decreases adaptive immune responses by enhancing the Treg suppressive function,49 suggesting that TLR2-targeted immune adjuvants should be reevaluated for efficacy in humans, although one must use caution in considering all TLR2 responses as equivalent. We have previously found that TLR2/6 ligands were better adjuvants than TLR2/1 ligands, whereas others have found more suppressive activity from TLR2/1, so function of the two heterodimers is not equivalent.6 As compared with mice, the frequency intraepithelial γδ T cells is much lower in the small intestine (50% vs. 10%) but significantly higher in the colon of humans (10% vs. 40%), and the number of CD8αα T cells is variable (5–37%).50 Of note, the mucus thickness varies along the length of the gut; colon mucus is a few times thicker than that in the small intestine. It would be more challenging for vaccine design to target human large intestine as mucus therein is ten times thicker than that in mice. Therefore, the differences between species as well as among different GI segments may imply an unpredictable impact on vaccine delivery and anticipated immune responses that should be investigated in preliminary studies and taken into account in any future clinical study.
Conclusion
The nanoparticle-releasing microparticle system not only makes oral administration of vaccines capable of selectively targeting mucosal surfaces of the small intestine or large intestine, but potentially is a useful means to study basic immunological mechanisms of the gut and its immune compartmentalization. Many novel applications may be explored such as combinations with other vaccine strategies to enhance vaccine’s specificity and efficacy. Optimization of this technology for human use merits further investigation.
Acknowledgment
This work was supported in part by the Intramural Research Program of NIH, the National Cancer Institute, the Center for Cancer Research and the Intramural AIDS Targeted Antiviral Program, National Natural Science Foundation of China (31170872) and Beijing Natural Science Foundation (5131002).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/gutmicrobes/article/24197
References
- 1.Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine. 2011;29(Suppl 4):D80–5. doi: 10.1016/j.vaccine.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Human Rotavirus Vaccine Study Group Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 3.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Rotavirus Efficacy and Safety Trial (REST) Study Team Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Q, Thomson CW, Rosenthal KL, McDermott MR, Collins SM, Gauldie J. Immunization with adenovirus at the large intestinal mucosa as an effective vaccination strategy against sexually transmitted viral infection. Mucosal Immunol. 2008;1:78–88. doi: 10.1038/mi.2007.3. [DOI] [PubMed] [Google Scholar]
- 5.McConnell EL, Basit AW, Murdan S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, and serum IgG compared to oral administration. Vaccine. 2008;26:639–46. doi: 10.1016/j.vaccine.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Q, Egelston C, Vivekanandhan A, Uematsu S, Akira S, Klinman DM, et al. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: implications for vaccines. Proc Natl Acad Sci U S A. 2008;105:16260–5. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Q, Egelston C, Gagnon S, Sui Y, Belyakov IM, Klinman DM, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–16. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, et al. Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination. Proc Natl Acad Sci U S A. 2011;108:E989–97. doi: 10.1073/pnas.1104264108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838–45. doi: 10.1023/A:1016085108889. [DOI] [PubMed] [Google Scholar]
- 10.Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel JP. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142:592–601, e3. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Talton J, Zhang G, Cunningham T, Wang Z, Waters RC, et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat Med. 2012;18:1291–7. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mestecky J, Russell MW. Specific antibody activity, glycan heterogeneity and polyreactivity contribute to the protective activity of S-IgA at mucosal surfaces. Immunol Lett. 2009;124:57–62. doi: 10.1016/j.imlet.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthésy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–15. doi: 10.1016/S1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 14.Barington T, Kristensen K, Henrichsen J, Heilmann C. Influence of prevaccination immunity on the human B-lymphocyte response to a Haemophilus influenzae type b conjugate vaccine. Infect Immun. 1991;59:1057–64. doi: 10.1128/iai.59.3.1057-1064.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serazin AC, Shackelton LA, Wilson C, Bhan MK. Improving the performance of enteric vaccines in the developing world. Nat Immunol. 2010;11:769–73. doi: 10.1038/ni0910-769. [DOI] [PubMed] [Google Scholar]
- 16.Hanley KA. The double-edged sword: How evolution can make or break a live-attenuated virus vaccine. Evolution (N Y) 2011;4:635–43. doi: 10.1007/s12052-011-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Cronkhite RI, Michael JG. Sub-compartmentalization of the gastrointestinal (GI) immune system determined with microbeads that differ in release properties. Vaccine. 2004;22:2106–15. doi: 10.1016/j.vaccine.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Lange S, Holmgren J. Protective antitoxic cholera immunity in mice: influence of route and number of immunizations and mode of action of protective antibodies. Acta Pathol Microbiol Scand C. 1978;86C:145–52. doi: 10.1111/j.1699-0463.1978.tb02572.x. [DOI] [PubMed] [Google Scholar]
- 20.Snoeck V, Verfaillie T, Verdonck F, Goddeeris BM, Cox E. The jejunal Peyer’s patches are the major inductive sites of the F4-specific immune response following intestinal immunisation of pigs with F4 (K88) fimbriae. Vaccine. 2006;24:3812–20. doi: 10.1016/j.vaccine.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–6. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 22.Belyakov IM, Isakov D, Zhu Q, Dzutsev A, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against simian/human immunodeficiency viral depletion of mucosal CD4+ T cells. J Immunol. 2007;178:7211–21. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Q, Thomson CW, Zhang G, Stämpfli M, McDermott MR, Collins SM, et al. Eosinophilia is induced in the colon of Th2-sensitized mice upon exposure to locally expressed antigen. Am J Physiol Gastrointest Liver Physiol. 2007;293:G383–90. doi: 10.1152/ajpgi.00341.2006. [DOI] [PubMed] [Google Scholar]
- 24.Stenstad H, Svensson M, Cucak H, Kotarsky K, Agace WW. Differential homing mechanisms regulate regionalized effector CD8alphabeta+ T cell accumulation within the small intestine. Proc Natl Acad Sci U S A. 2007;104:10122–7. doi: 10.1073/pnas.0700269104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4607–14. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundqvist C, Baranov V, Hammarström S, Athlin L, Hammarström ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–87. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H. Differences in intraepithelial lymphocytes in the proximal, middle, distal parts of small intestine, cecum, and colon of mice. Immunol Invest. 2009;38:780–96. doi: 10.3109/08820130903258800. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara D, Chen L, Wei B, Braun J. Small intestine CD11c+ CD8+ T cells suppress CD4+ T cell-induced immune colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G939–47. doi: 10.1152/ajpgi.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff MJ, Leung JM, Davenport M, Poles MA, Cho I, Loke P. TH17, TH22 and Treg cells are enriched in the healthy human cecum. PLoS One. 2012;7:e41373. doi: 10.1371/journal.pone.0041373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–8. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassani B, Villablanca EJ, Quintana FJ, Love PE, Lacy-Hulbert A, Blaner WS, et al. Gut-tropic T cells that express integrin α4β7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology. 2011;141:2109–18. doi: 10.1053/j.gastro.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor SJ, Paraskevopoulos N, Newman R, Cuan N, Hampartzoumian T, Lloyd AR, et al. CCR2 expressing CD4+ T lymphocytes are preferentially recruited to the ileum in Crohn’s disease. Gut. 2004;53:1287–94. doi: 10.1136/gut.2003.028225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furrie E, Macfarlane S, Thomson G, Macfarlane GT, Microbiology & Gut Biology Group. Tayside Tissue & Tumour Bank Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–74. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut. 2003;52:1591–7. doi: 10.1136/gut.52.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nat Med. 2011;17:837–44. doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 36.Schulte R, Kerneis S, Klinke S, Bartels H, Preger S, Kraehenbuhl JP, et al. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell Microbiol. 2000;2:173–85. doi: 10.1046/j.1462-5822.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 37.Baranov V, Hammarström S. Carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1), apically expressed on human colonic M cells, are potential receptors for microbial adhesion. Histochem Cell Biol. 2004;121:83–9. doi: 10.1007/s00418-003-0613-5. [DOI] [PubMed] [Google Scholar]
- 38.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–67. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 39.Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP. Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci U S A. 2002;99:9410–4. doi: 10.1073/pnas.142586899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida M, Claypool SM, Wagner JS, Mizoguchi E, Mizoguchi A, Roopenian DC, et al. Human neonatal Fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–83. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, et al. Uptake through glycoprotein 2 of FimH(+) bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–30. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 42.Tyrer PC, Ruth Foxwell A, Kyd JM, Otczyk DC, Cripps AW. Receptor mediated targeting of M-cells. Vaccine. 2007;25:3204–9. doi: 10.1016/j.vaccine.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007;204:2789–96. doi: 10.1084/jem.20070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devriendt B, De Geest BG, Goddeeris BM, Cox E. Crossing the barrier: Targeting epithelial receptors for enhanced oral vaccine delivery. J Control Release. 2012;160:431–9. doi: 10.1016/j.jconrel.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Devriendt B, De Geest BG, Cox E. Designing oral vaccines targeting intestinal dendritic cells. Expert Opin Drug Deliv. 2011;8:467–83. doi: 10.1517/17425247.2011.561312. [DOI] [PubMed] [Google Scholar]
- 46.Kraehenbuhl JP, Neutra MR. Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol. 2000;16:301–32. doi: 10.1146/annurev.cellbio.16.1.301. [DOI] [PubMed] [Google Scholar]
- 47.Baker K, Qiao SW, Kuo T, Kobayashi K, Yoshida M, Lencer WI, et al. Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Semin Immunopathol. 2009;31:223–36. doi: 10.1007/s00281-009-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci U S A. 2006;103:7048–53. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–32. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Gibbons DL, Spencer J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 2011;4:148–57. doi: 10.1038/mi.2010.85. [DOI] [PubMed] [Google Scholar]