Abstract
Earlier studies have shown that macroautophagy is not a constitutively activated process, however, the mechanism of activation is not fully understood. Here, we report that autophagy is a dynamic process in cancer cells in response to glucose starvation. In addition, we determined that FOXO1 turnover is involved in the regulation of this dynamic process. X-box binding protein 1u (XBP1u) plays a critical role in FOXO1 degradation by recruiting FOXO1 to the 20S proteasome. Moreover, the phosphorylation of XBP1u by mitogen-activated protein kinases 1 and 3 (MAPK1/3, also known as ERK2/1) on serine residues 61 and 176 was found to be essential for the enhancement of the interaction between XBP1u and FOXO1. Thus, our findings support the hypothesis that the turnover of FOXO1 induced by MAPK1/3 and XBP1u is a critical factor regulating the autophagic process.
Keywords: FOXO1, XBP1u, ERK, autophagy, cancer
Autophagy is a process of intracellular degradation that delivers cytoplasmic constituents to the lysosome for the maintenance of homeostasis and bioenergetics in mammalian cells. Through the induction of autophagy, mammalian cells are able to obtain an influx of free amino acids and other essential nutrients. However, autophagy is rarely persistently activated in response to stress, but instead is activated in a dynamic manner to avoid autophagy-induced cell death. For example, autophagy commonly occurs during the first few hours of serum starvation, where it provides an initial burst of free amino acids for the synthesis of essential proteins; however, this autophagic process is less observed after the first 4–8 h of serum starvation in normal rat kidney cells or mouse fibroblasts.
Several regulators have been reported to be important for the induction of autophagy, including BECN1, mechanistic target of rapamycin (MTOR) and the FOXO family members. In particular, recent reports have also raised the possibility that FOXO family members are involved in the induction of autophagy via both transcription-dependent and -independent pathways. Loss of FOXO proteins is also responsible for the attenuation of autophagy in Drosophila, mouse muscle cells and several cancer cell lines. Since FOXO family proteins are key regulators of multiple important biological processes, factors that influence the fate of FOXO1 are of great interest to researchers.
In our recent study, we found that FOXO1 turnover is highly associated with the dynamic autophagy process. In addition, we also identified XBP1u as an important mediator of FOXO1 degradation, thus indicating that XBP1 is a critical protein for the regulation of autophagy by controlling FOXO1 degradation.
FOXO1 Degradation is Associated with Autophagy Dynamics
To set up a long-term autophagic model, we used glutamine-deficient medium to starve human cancer cells. In response to glutamine starvation, autophagy reaches a peak at 24 h and gradually decreases to normal levels by 36 h post-treatment. Meanwhile, we found that FOXO1 levels are gradually decreased in response to glutamine starvation. Interestingly, the FOXO1 acetylation level increases after glutamine starvation and also reaches a peak by 24 h after treatment. These changes in FOXO1 acetylation are highly correlated with its interaction with ATG7 and the autophagic changes observed in the HCT116 cells induced by glutamine starvation. Thus, these results suggest that the dynamic autophagic process is likely to be associated with the changes in FOXO1 acetylation.
Next we found that FOXO1 is degraded in a ubiquitin-independent, but proteasome-dependent, pathway in response to glutamine starvation. By using an in vitro protein degradation assay, we further confirmed that FOXO1 is degraded by the 20S proteasome.
XBP1u is Required for the Glutamine Starvation-Induced FOXO1 Degradation
To further determine which protein is associated with FOXO1 degradation in response to glutamine starvation, we designed several siRNA fragments and finally determined that XBP1 is responsible. Since there are two forms of the XBP1 protein, XBP1s (the spliced form) and XBP1u (the unspliced form), we next transfected plasmids encoding XBP1u or XBP1s into HCT116 cells and found that the FOXO1 protein level is markedly reduced by overexpression of XBP1u, but not XBP1s. In addition, FOXO1’s interaction with XBP1u is increased after glutamine starvation. By using the in vitro protein degradation assay, we also found that FOXO1 degradation by the 20S proteasome is enhanced by co-incubation with the XBP1u protein. Moreover, the addition of in vitro translated full-length XBP1u, but not N-terminal XBP1u, which is not able to bind FOXO1, facilitates the FOXO1-20S proteasome interaction. Taken together, these data suggest that upon glutamine starvation, XBP1u mediates FOXO1 degradation possibly by facilitating FOXO1 entry into the channel of the 20S proteasome.
Phosphorylation of XBP1u by MAPK1/3 is Critical for the Degradation of FOXO1
It has been reported that different stress stimuli could activate the MAPK1/3 signaling pathway, which plays a crucial role in the regulation of many cellular biological processes. In our study, we found that MAPK1/3 also plays a critical role in FOXO1 degradation upon glutamine starvation. In addition, our data indicate that MAPK1/3-induced XBP1u phosphorylation at Ser61 and Ser176, but not FOXO1’s phosphorylation, is required for the degradation of FOXO1. Overexpression of XBP1u (wild type), but not the XBP1u mutant (S61,176A), induces a decrease in FOXO1 protein levels. The dephosphorylation of XBP1u promotes its translocation from the cytoplasm to the nucleus and thus attenuates interactions with the 20S proteasome, which leads to a decrease in FOXO1 degradation.
XBP1u induces Degradation of FOXO1 and Decreases Autophagy
Because FOXO1 is essential for the induction of autophagy in response to serum starvation or oxidative stress, we tested whether XBP1u suppresses autophagy via induction of FOXO1 degradation. We observed that the number of LC3 puncta significantly decreases when glutamine-starved HCT116 cells are transfected with XBP1u, but not with XBP1s (Fig. 1). Additionally, the glutamine starvation-induced autophagic level is markedly reduced in XBP1-knockdown HCT116 cells compared with control cells. In contrast, in FOXO1 XBP1 double-knockdown HCT116 cells, glutamine starvation does not induce autophagy. These data indicate that downregulation of XBP1 promotes autophagy, which requires FOXO1.

Figure 1. HCT116 cells were transfected with a control plasmid or a plasmid encoding flag-XBP1u or flag-XBP1s. Twenty-four hours after transfection, the cells were subjected to glutamine starvation for 24 h, and the formation of GFP-LC3 punctate signals was observed.
It is well known that autophagy is a nonapoptotic form of programmed cell death. Our study suggests that autophagy is a protective mechanism under starvation conditions, as long as the autophagic process is not persistently activated. Once autophagy becomes constitutively activated, it induces cell death, which is most likely the outcome of excessive degradation of essential cellular components. Consistently, we found that after glutamine starvation the maintenance of FOXO1 activity by knocking down XBP1u is sufficient to decrease cell viability in an autophagy-dependent manner.
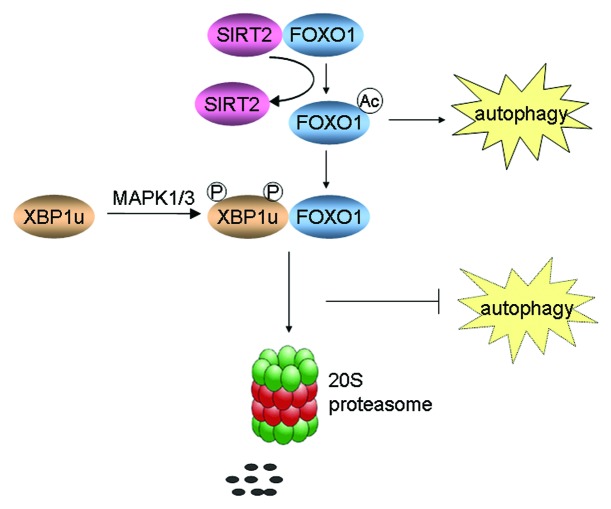
In summary, the axis of MAPK1/3-XBP1u-FOXO1 may be an essential pathway for the induction of autophagy, which is executed by cascades of several proteins. Various stresses, at least in the condition of glutamine starvation, lead to FOXO1-modulated autophagy. However, longer glutamine starvation also induces activation of MAPK1/3, which phosphorylates XBP1u. Phosphorylated XBP1u in turn binds with FOXO1 and brings it into the proteasome for degradation, which leads to the interruption of autophagy (Fig. 2).
Figure 2. A schematic model showing the relationship between autophagy and XBP1u-induced FOXO1 degradation.
Acknowledgments
This study was supported by grants from the Ministry of Science and Technology of China (2011CB910100), Program for New Century Excellent Talents in University, and the National Natural Science Foundation of China (Grants 81222028).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/23918