Abstract
The lysosome is a key subcellular organelle that receives and degrades macromolecules from endocytic, secretory and autophagic pathways. Lysosomal function is thus critical for an efficient autophagic process. However, the molecular mechanisms mediating lysosomal function upon autophagic induction are largely unknown. Our laboratory recently discovered that upon autophagy activation, the lysosome is activated, and this functional activation is dependent on MTORC1 suppression, suggesting that MTORC1 exerts a suppressive effect on lysosomal function. Therefore, data from our study demonstrate that MTORC1 exerts a dual inhibitory effect on autophagy, blocking autophagy not only at the initiation stage via suppression of the ULK1 complex, but also at the degradation stage via inhibition of lysosomal function. We think that understanding the negative regulatory effect of MTORC1 on lysosomal function expands the functional scope of MTORC1 in autophagy regulation, and offers new clues for developing novel interventional strategies in autophagy- and lysosome-related diseases.
Keywords: autophagy, lysosome, MTORC1, autophagosome, fusion
Autophagy is an evolutionarily conserved process in which intracellular proteins and organelles are sequestered in autophagosomes and subsequently degraded by lysosomal enzymes, to preserve and generate energy. Thus, the late stage of autophagy relies on the cooperation of two organelles, autophagosomes and lysosomes, and the lysosome plays a critical role in an efficient autophagic degradation process. Recent studies have revealed the coordinated lysosomal expression and regulation (CLEAR) gene network and transcription factor EB (TFEB) as master regulator of lysosome biogenesis. However, the question of how a lysosome adapts itself to an optimal condition to digest the inner membranes and lumenal contents after fusing with an autophagosome (with neutral interior pH) remains. We thus aimed to understand the functional activation of lysosomes in the course of autophagy and the underlying regulatory mechanisms.
One major finding from our study is that MTORC1 is a negative regulator of lysosomal function. We first demonstrated that lysosomes are activated in cells treated with various autophagic inducers, including EBSS (starvation condition) and two catalytic MTOR inhibitors (PP242 and Torin1), based on parameters such as enhanced lysosomal acidification, cathepsin enzyme activities and rate of proteolysis. Next, we established the causative role of MTORC1 suppression in lysosomal activation, based on the following observations: (i) In Tsc2 KO MEFs, only PP242, but not starvation, is able to inhibit MTORC1 and activate lysosomal function; (ii) supplementation with leucine + IGF1 in cells under starvation conditions restores MTORC1 activity and thus suppresses lysosomal function, whereas such supplements are ineffective on both MTORC1 and lysosome activity in cells treated with PP242; (iii) increased intracellular amino acid concentration resulting from cycloheximide treatment restores MTORC1 activity and suppresses lysosomal function in cells under starvation conditions, but not in cells treated with PP242 or Torin1; and (iv) trehalose, a MTOR-independent autophagic inducer, fails to block MTORC1 and has no activating effect on lysosomes. Taken together, these observations establish the negative regulatory role of MTORC1 in lysosomal function.
One interesting question raised by our findings is how MTORC1 exerts its inhibitory effect on lysosomes. To gain insight into the molecular mechanism underlying the correlation between MTORC1 and lysosomes, we tested two possibilities. First, whether MTORC1 lysosomal localization is associated with its inhibitory effect on lysosomes. Second, whether MTORC1-mediated lysosome function depends on TFEB. In principle, lysosomal translocation is required for MTORC1 activation in response to amino acid stimulation. In our study, we observed that starvation reduces the amount of MTORC1 in the lysosomal fraction, whereas MTOR inhibitors (rapamycin and PP242) enhance MTORC1 localization to lysosomes. When we utilized a genetic approach to block MTORC1 translocation to lysosomes, we found that PP242 is still able to increase lysosomal enzyme activity, whereas EBSS fails to do so. Thus, our data indicate that it is the MTORC1 activity, not the MTORC1 lysosomal localization per se, which regulates lysosomal function.
MTORC1 is a key upstream kinase that directly phosphorylates and inhibits TFEB. In this study, we first demonstrate that TFEB is an important mediator in lysosomal activation following suppression of MTORC1 function based on the results that TFEB is activated by MTORC1 suppression, and knockdown of TFEB abates lysosomal activation in cells under starvation conditions or treated with PP242. Intriguingly, there are some interesting observations suggesting that lysosomal activation induced by MTORC1 suppression is not all mediated via TFEB activation. For instance, increased lysosomal acidification and cathepsin enzyme activity in starved cells occurs earlier than the transcriptional activation of TFEB. More importantly, we observed a similar level of TEFB activation induced by starvation in the cells deficient for ATG5 or ATG7 in which no lysosomal activation is observed. Therefore, it is thought that TFEB activation following MTORC1 inhibition is necessary, but not sufficient, for lysosomal activation.
In addition to the involvement of MTORC1 in the regulation of lysosomal function, another key finding from this study is that lysosomal activation in autophagy depends on the presence of key ATG proteins in the cell. Such a conclusion is supported by the facts that no significant lysosomal activation could be found in cells with deficient amounts of ATG5 or ATG7. Since starvation or PP242 have similar inhibitory effects on MTORC1 in wild-type and Atg5 or Atg7 knockout cells, and ATG5 and ATG7 are essential for the formation of autophagosomes, we reasoned that lysosomal activation in the course of autophagy requires the formation of autophagosomes. In order to establish the link between autophagosome formation and lysosomal activation, we then tested whether autophagosome-lysosome fusion is relevant to enhanced lysosomal function following MTORC1 suppression. To do this, we manipulated the autophagosome-lysosome fusion process by using both genetic and pharmacological approaches, which allowed us to test the importance of autolysosome formation in lysosome activation. Notably, the blockage of autophagosome-lysosome fusion abolishes the lysosomal activation induced by starvation. This finding supports our hypothesis that autophagosome-lysosome fusion is required for lysosomal activation mediated by MTORC1 suppression. Therefore, it is likely that the increased acidification and lysosomal enzyme activity more likely originated from autolysosomes, instead of lysosomes per se. At present, the remaining challenge is to identify the molecular targets of MTORC1 that regulate lysosomal function, and such targets could be either located at the lysosome or in the cytosol.
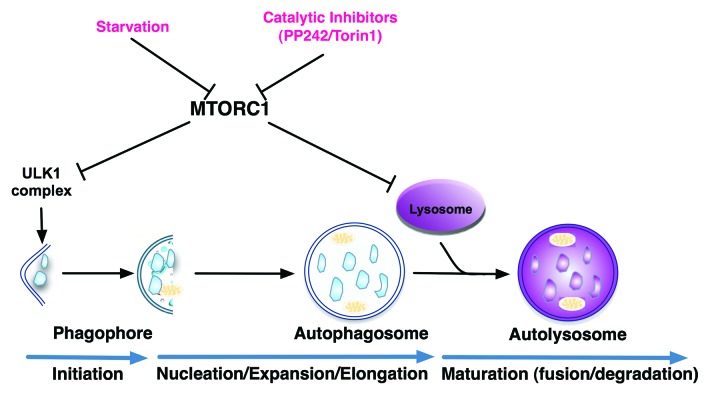
Overall, this study answers a fundamental question regarding how lysosome function is regulated in the course of autophagy. Data from our study clearly demonstrate that lysosomal function is actively upregulated in the course of autophagy induced by nutrient starvation or MTOR inhibition, and the functional activation of lysosomes requires both MTORC1 suppression and autophagosome-lysosome fusion. As illustrated in Figure 1, MTORC1 has been well established as a critical negative regulator of autophagy via suppression of the ULK1 complex at the initiation stage of the autophagic process. This study thus reveals a new regulatory function of MTORC1 in autophagy via suppression of lysosomal function, and blockage of maturation and the degradation stage of autophagy.
Figure 1. MTORC1 exerts a dual inhibitory effect on autophagy; blocking autophagy at the initiation stage via suppression of the ULK1 complex, and at the degradation stage via inhibition of lysosomal function.
Glossary
Abbreviations:
- MTORC1
mechanistic target of rapamycin complex 1
- TFEB
transcription factor EB
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/23965