Abstract
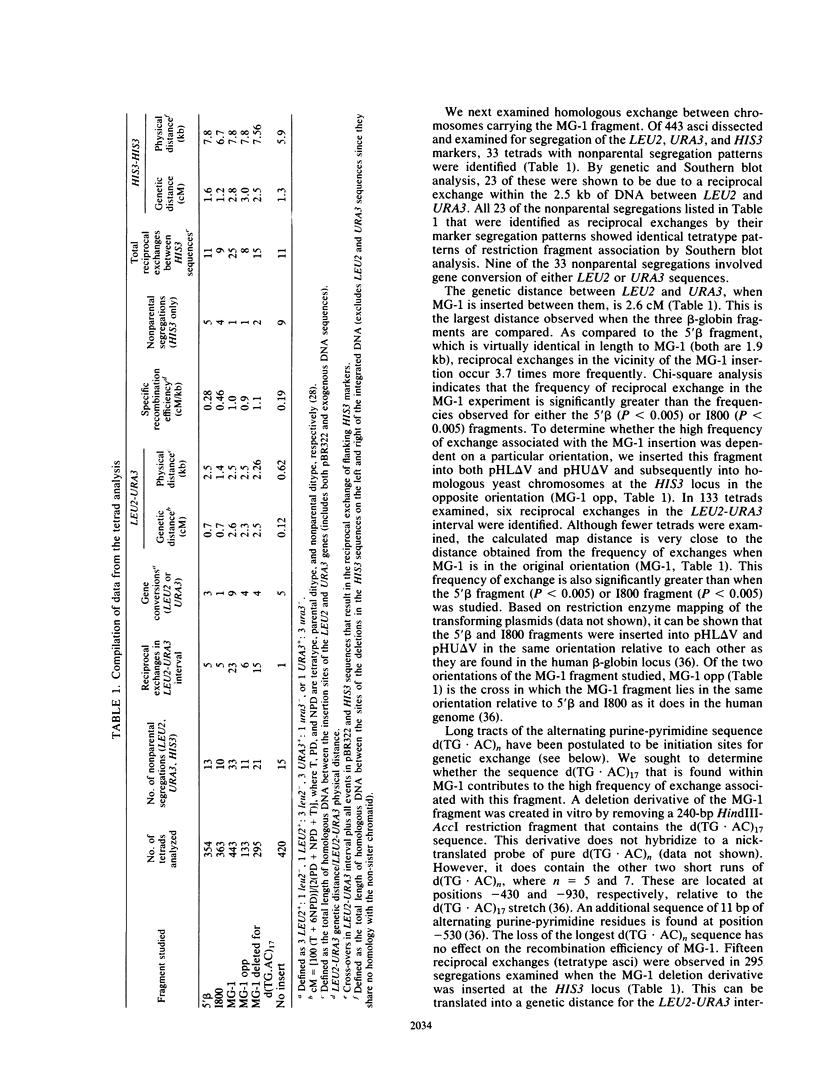
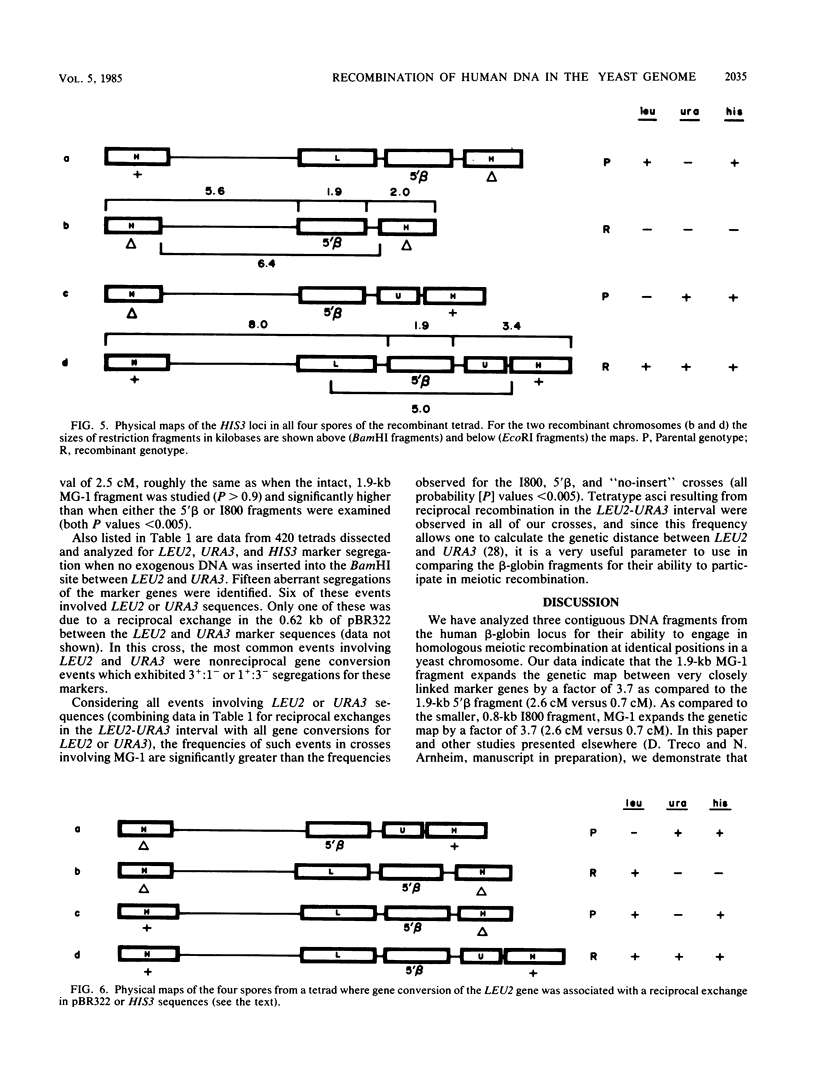
We describe a novel system for the analysis of sequence-specific meiotic recombination in Saccharomyces cerevisiae. A comparison of three adjacent restriction fragments from the human beta-globin locus revealed that one of them, previously hypothesized to contain a relative hot spot for genetic recombination, engages in reciprocal exchange during yeast meiosis significantly more frequently than either of the other two fragments. Removal of the longest of four potential Z-DNA-forming regions from this fragment does not affect the high frequency of genetic recombination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Boehm C. D., Serjeant G. R., Theisen C. E., Dover G. J., Kazazian H. H., Jr Origin of the beta S-globin gene in blacks: the contribution of recurrent mutation or gene conversion or both. Proc Natl Acad Sci U S A. 1984 Feb;81(3):853–856. doi: 10.1073/pnas.81.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E., Orkin S. H., Kazazian H. H., Jr, Goff S. C., Boehm C. D., Waber P. G., Sexton J. P., Ostrer H., Fairbanks V. F., Chakravarti A. Evidence for multiple origins of the beta E-globin gene in Southeast Asia. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6608–6611. doi: 10.1073/pnas.79.21.6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Borts R. H., Lichten M., Hearn M., Davidow L. S., Haber J. E. Physical monitoring of meiotic recombination in Saccharomyces cerevisiae. Cold Spring Harb Symp Quant Biol. 1984;49:67–76. doi: 10.1101/sqb.1984.049.01.010. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Hicks J. B. Replication and recombination functions associated with the yeast plasmid, 2 mu circle. Cell. 1980 Sep;21(2):501–508. doi: 10.1016/0092-8674(80)90487-0. [DOI] [PubMed] [Google Scholar]
- Chakravarti A., Buetow K. H., Antonarakis S. E., Waber P. G., Boehm C. D., Kazazian H. H. Nonuniform recombination within the human beta-globin gene cluster. Am J Hum Genet. 1984 Nov;36(6):1239–1258. [PMC free article] [PubMed] [Google Scholar]
- Dunn B., Szauter P., Pardue M. L., Szostak J. W. Transfer of yeast telomeres to linear plasmids by recombination. Cell. 1984 Nov;39(1):191–201. doi: 10.1016/0092-8674(84)90205-8. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Lefranc M. P., Rabbitts T. H. Mechanisms of divergence and convergence of the human immunoglobulin alpha 1 and alpha 2 constant region gene sequences. Cell. 1984 Mar;36(3):681–688. doi: 10.1016/0092-8674(84)90348-9. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Hamada H., Petrino M. G., Kakunaga T. A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6465–6469. doi: 10.1073/pnas.79.21.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerberg C., Klein J. Linkage disequilibrium between H-2 and t complexes in chromosome 17 of the mouse. Nature. 1975 Nov 27;258(5533):296–299. doi: 10.1038/258296a0. [DOI] [PubMed] [Google Scholar]
- Hammerberg G., Klein J. Linkage relationships of markers on chromosome 17 of the house mouse. Genet Res. 1975 Oct;26(2):203–211. doi: 10.1017/s0016672300015998. [DOI] [PubMed] [Google Scholar]
- Hamza H., Haedens V., Mekki-Berrada A., Rossignol J. L. Hybrid DNA formation during meiotic recombination. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7648–7651. doi: 10.1073/pnas.78.12.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Hinnen A., Fink G. R. Properties of yeast transformation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1305–1313. doi: 10.1101/sqb.1979.043.01.149. [DOI] [PubMed] [Google Scholar]
- Hsu Y. P., Kohlhaw G. B. Overproduction and control of the LEU2 gene product, beta-isopropylmalate dehydrogenase, in transformed yeast strains. J Biol Chem. 1982 Jan 10;257(1):39–41. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Antonarakis S. E., Sexton J. P., Boehm C. D., Goff S. C., Waber P. G. Molecular characterization of seven beta-thalassemia mutations in Asian Indians. EMBO J. 1984 Mar;3(3):593–596. doi: 10.1002/j.1460-2075.1984.tb01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M. W., Klysik J., Singleton C. K., Zarling D. A., Jovin T. M., Hanau L. H., Erlanger B. F., Wells R. D. Intervening sequences in human fetal globin genes adopt left-handed Z helices. J Biol Chem. 1984 Jun 10;259(11):7268–7274. [PubMed] [Google Scholar]
- Kobori J. A., Winoto A., McNicholas J., Hood L. Molecular characterization of the recombination region of six murine major histocompatibility complex (MHC) I-region recombinants. J Mol Cell Immunol. 1984;1(2):125–135. [PubMed] [Google Scholar]
- Lichten M., Fox M. S. Effects of nonhomology on bacteriophage lambda recombination. Genetics. 1983 Jan;103(1):5–22. doi: 10.1093/genetics/103.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R., Krystal M., Arnheim N. A member of a new repeated sequence family which is conserved throughout eucaryotic evolution is found between the human delta and beta globin genes. Nucleic Acids Res. 1981 Nov 25;9(22):5931–5947. doi: 10.1093/nar/9.22.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in Saccharomyces. Genetics. 1966 Jan;53(1):165–173. doi: 10.1093/genetics/53.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Flavell R. A. The DNA sequence of the 5' flanking region of the human beta-globin gene: evolutionary conservation and polymorphic differences. Nucleic Acids Res. 1982 Mar 25;10(6):2109–2120. doi: 10.1093/nar/10.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordheim A., Rich A. The sequence (dC-dA)n X (dG-dT)n forms left-handed Z-DNA in negatively supercoiled plasmids. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1821–1825. doi: 10.1073/pnas.80.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Polymorphism and molecular pathology of the human beta-globin gene. Prog Hematol. 1983;13:49–73. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petes T. D., Botstein D. Simple Mendelian inheritance of the reiterated ribosomal DNA of yeast. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5091–5095. doi: 10.1073/pnas.74.11.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Schwartz E., Ballantine M., Surrey S. Nucleotide sequence analysis of the delta beta-globin gene region in humans. J Biol Chem. 1983 Oct 10;258(19):11599–11609. [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Radding C. M. The mechanism of conversion of deletions and insertions. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1315–1316. doi: 10.1101/sqb.1979.043.01.150. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Carbon J. Functional expression of cloned yeast DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1977 Feb;74(2):487–491. doi: 10.1073/pnas.74.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose M., Casadaban M. J., Botstein D. Yeast genes fused to beta-galactosidase in Escherichia coli can be expressed normally in yeast. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2460–2464. doi: 10.1073/pnas.78.4.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton C. K., Kilpatrick M. W., Wells R. D. S1 nuclease recognizes DNA conformational junctions between left-handed helical (dT-dG n. dC-dA)n and contiguous right-handed sequences. J Biol Chem. 1984 Feb 10;259(3):1963–1967. [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stahl F. W. Symposium on DNA replication and recombination. Summary. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1353–1356. doi: 10.1101/sqb.1979.043.01.154. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Production of a functional eukaryotic enzyme in Escherichia coli: cloning and expression of the yeast structural gene for imidazole-glycerolphosphate dehydratase (his3). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5255–5259. doi: 10.1073/pnas.74.12.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N., Szostak J. W. Recombination between sequences in nonhomologous positions. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5675–5679. doi: 10.1073/pnas.80.18.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]