Summary
T-cell receptor (TCR) signaling driven by interaction of the TCR with specific complexes of self-peptide and the major histocompatibility complex, determines T cell fate in thymic development. However, the signaling pathway through which TCR signal strength regulates distinct T cell lineages remains unknown. Here we have used mice lacking the endoplasmic reticulum Ca2+ sensors STIM1 and STIM2 to show that STIM-induced store-operated Ca2+ entry is not essential for thymic development of conventional TCRαβ+ T cells, but is specifically required for the development of agonist-selected T cells (regulatory T cells, invariant natural killer T cells and TCRαβ+ CD8αα+ intestinal intraepithelial lymphocytes). The severe impairment of agonist-selected T cell development is mainly due to a defect in interleukin-2 (IL-2) or IL-15 signaling. Thus, STIM1 and STIM2-mediated store-operated Ca2+ influx, leading to efficient activation of NFAT (nuclear factor of activated T-cells), is critical for the post-selection maturation of agonist-selected T cells.
Introduction
Thymic selection depends on the affinity of T-cell receptor-peptide major histocompatibility complex glycoprotein (TCR-pMHC) interactions. In conventional TCRαβ+ T cell development, weak or absent TCR-pMHC interactions cannot support thymocyte survival, leading to death by neglect. Moderate-affinity TCR-pMHC interactions lead to the development of functional T cells through positive selection. High-affinity TCR-pMHC interactions normally trigger apoptosis of self-reactive thymocytes through negative selection. However, some self-reactive thymocytes mature into unconventional T-lineage cells through an alternative selection process defined as agonist selection (Baldwin et al., 2004; Stritesky et al., 2012). Agonist-selected unconventional T-cell subsets are thought to have a regulatory role in immune system and are classified into three main cell types; forkhead box P3 (Foxp3)+ regulatory T (Treg) cells, invariant natural killer T (iNKT) cells and TCRαβ+ CD8αα+ intestinal intraepithelial lymphocytes (IELs) (Hsieh et al., 2012; Kronenberg and Gapin, 2002; Lambolez et al., 2007). It has been proposed that these T cells require relatively strong and sustained TCR signals for their development (Baldwin et al., 2004). Although this affinity model is acknowledged, there remains a longstanding question concerning how the TCR signal strength and duration regulates the development of these distinct T cell subsets.
Engagement of TCR-pMHC activates several protein tyrosine kinases and ultimately phospholipase C (PLC)-γ1. Activated PLC-γ1 hydrolyzes phosphatidylinositol 4,5-bisphosphate into diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3), which induces the release of Ca2+ from the endoplasmic reticulum (ER). In turn ER store depletion triggers store-operated Ca2+ entry, the predominant mechanism for sustained increase of intracellular free Ca2+ ([Ca2+]i) downstream of the TCR. Store-operated Ca2+ entry leads to activation of the phosphatase calcineurin, which in turn activates the transcription factor NFAT (Feske, 2007; Hogan et al., 2010). The induction of store-operated Ca2+ entry is controlled by two major molecules, the ER Ca2+ sensor stromal interaction molecule (STIM)1 (Liou et al., 2005; Roos et al., 2005) and calcium release-activated calcium (CRAC) channels ORAI1 (Feske et al., 2006; Vig et al., 2006; Zhang et al., 2006). STIM1 is an acknowledged positive regulator of store-operated CRAC channels. Loss of STIM1 abrogates TCR-induced store-operated Ca2+ entry and NFAT activation, resulting in impaired proliferation and cytokine production by peripheral human and mouse T cells (McCarl et al., 2010; Oh-Hora et al., 2008; Picard et al., 2009). The related protein STIM2 regulates sustenance of calcium entry and NFAT activation in mouse CD4+ T cells (Oh-Hora et al., 2008), but also regulates basal concentration of [Ca2+ ]i in Hela cells (Brandman et al., 2007).
In thymocytes, TCR signal strength well correlates with magnitude and duration of Ca2+ influx. An in vitro study demonstrated that a strong TCR signal elicited by peptides promoting negative selection sustained a high concentration of [Ca2+]i with large Ca2+ influx, whereas a weak TCR signal by peptides promoting positive selection induces a small Ca2+ influx and increased [Ca2+]i concentration gradually (Daniels et al., 2006; Nakayama et al., 1992). In contrast, an ex vivo study showed that thymocytes undergoing positive selection showed a substantial increase of [Ca2+]i through sustained Ca2+ oscillations (Bhakta et al., 2005). Since store-operated Ca2+ entry provides both large and sustained Ca2+ influx with T cells, store-operated Ca2+ entry has long been thought to be a critical Ca2+ entry pathway in T cell development. However, there is no direct evidence for this assumption.
To elucidate the role of Ca2+ influx during T-cell ontogeny, we analyzed mice in which STIM1 and its homologue STIM2 were deleted in T cells or hematopoietic cells. We found that STIM-dependent store-operated Ca2+ entry is not essential for the development or positive selection of conventional TCRαβ+ T cell, but specifically regulates the development of agonist-selected T cells. The ablation of STIM1 and STIM2 significantly compromised the cytokine-driven expansion and functional maturation of agonist-selected precursors. Absence of store-operated Ca2+ entry resulted in substantially decreased expression of NFAT target genes, which led to impaired upregulation of Il2rb in iNKT cells and TCRαβ+ CD8αα+ IELs. The administration of agonist complexes of IL-2 and anti-IL-2 rescued Treg cell proliferation but not suppressive function, whereas administration of IL-15 partially rescued differentiation into TCRαβ+ CD8αα+ IELs. These results suggest that post-selection maturation of all agonist-selected precursors requires continuous high activity of NFAT by TCR-mediated store-operated Ca2+ entry, to induce efficient expression of downstream genes of NFAT and NFAT-target transcription factors.
Results
Store-operated Ca2+ entry is dispensable for the development of conventional TCRαβ+ T cells
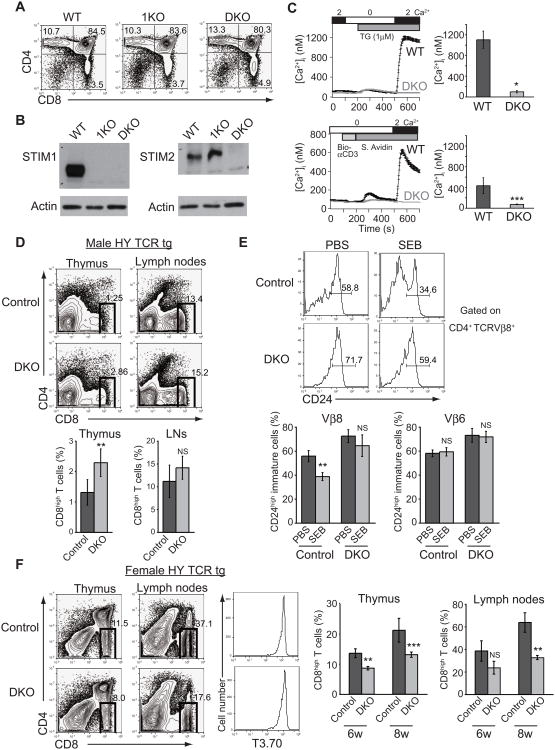
In both humans and mice, deficiency of STIM1 alone does not influence the number of conventional TCRαβ+ T cells in the periphery (Beyersdorf et al., 2009; Picard et al., 2009) (Oh-hora et al., unpublished observations), suggesting that STIM2 may compensate for Ca2+ influx in thymocytes. To examine the role of STIM-dependent store-operated Ca2+ entry in thymocyte development, we first generated chimeric mice by transplanting fetal liver cells derived from Stim1fl/fl Cmv-Cre (Stim1−/−) mice or Stim1fl/flStim2fl/fl Cmv-Cre (Stim1−/−Stim2−/−) mice into CD45.1 + Rag2-deficient mice. Surprisingly, the resulting chimeric mice displayed normal development of conventional TCRαβ T cells (Figure 1A), in contrast to the reported phenotype of mice with a disruption of Cnb1, encoding a regulatory subunit of the Ca2+-dependent phosphatase calcineurin (Neilson et al., 2004). We confirmed that CD4+CD8+ double-positive (DP) thymocytes from Stim1−/− Stim2−/− mice did not express STIM1 and STIM2 proteins, and showed no store-operated Ca2+ entry after stimulation with thapsigargin or anti-CD3 (Figure 1B and 1C). To assess the role of STIM proteins in thymocyte development in more detail, we established Stim1fl/flStim2fl/fl Vaν-iCre mice. Again, store-operated Ca2+ entry was abrogated in stimulated CD4-CD8- double-negative (DN) and DP thymocytes from Stim1fl/flStim2fl/fl Vaν-iCre mice (Figures S1A and S1B). Despite this defect, thymic cell numbers and expression of the cell surface markers CD4 and CD8 were comparable to those in control mice (Figures S1C and S1D). Thus although store-operated Ca2+ entry exists in a functional form in DP thymocytes, it is not absolutely essential for thymic development of conventional TCRαβ+ T cells.
Figure 1. STIM-dependent store-operated Ca2+ entry is dispensable for thymic selection of T cells.
(A) Conventional T cell development in mice transplanted with fetal liver cells isolated from Stim1−/− (1KO), Stim1−/− Stim2−/− (DKO) and wild-type (WT, Stim1+/+Stim2+/+) littermate control embryos at embryonic day 14.5. (B) Protein expression of STIM1 (left) and STIM2 (right) in DP thymocytes from (A). (C) Ca2+ influx in response to 1 μM thapsigargin (TG) (top, n=5) or anti-CD3 crosslinking (bottom, n=3) in littermate wild-type control (black) and Stim1−/− Stim2−/− (grey) DP thymocytes from (A). (D) Flow cytometric analysis of negative selection in male Stim1fl/flStim2fl/fl Lck-Cre Rag2−/− HY TCR-transgenic (tg) mice (top) and frequency of CD8high T cells in the thymus or lymph nodes (bottom). Control, Stim1+/+Stim2+/+ Lck-Cre Rag2−/− HY TCR; DKO, Stim1fl/flStim2fl/fl Lck-Cre Rag2−/− HY TCR. 3 of six weeks old mice were analyzed in each case. (E) SEB-induced negative selection in Stim1fl/flStim2fl/fl Vaν-iCre mice. Frequencies of SEB-sensitive CD24hiCD4+TCRVβ8+ and SEB-insensitive CD24hiCD4+TCRVβ6+ immature thymocytes were analyzed 2 days after the administration of 200 μg SEB. Control, Stim1fl/flStim2fl/fl; DKO, Stim1fl/flStim2fl/fl Vaν-iCre. 4 mice were analyzed in each case. (F) Flow cytometric analysis of positive selection in female Stim1fl/flStim2fl/fl Lck-Cre Rag2−/− HY TCR-transgenic (tg) mice (left and center) and frequency of CD8high T cells in the thymus or lymph nodes (right). Control, Stim1+/+Stim2+/+ Lck-Cre Rag2−/− HY TCR; DKO, Stim1fl/flStim2fl/flLck-Cre Rag2−/− HY TCR. 3 mice were analyzed in each case. Data are representative of two (A, B, C and E) and more than three (D and F) independent experiments. Error bars (C, D, E and F) denote mean ± SEM. *, P<0.001; **, P<0.03; ***, P<0.05; NS, not significant. See also Figure S1.
Sustained oscillations of [Ca2+]i are essential for positive selection of conventional TCRαβ+ T cells in the thymus (Bhakta et al., 2005). To examine the function of STIM proteins in positive and negative selection of thymocytes in vivo, we crossed Stim1fl/flStim2fl/fl Lck-Cre mice to HY TCR-transgenic (TCR-tg) Rag2−/− mice (Kisielow et al., 1988). In male STIM-deficient HY TCR-tg mice, there was a moderate increase in the number of CD8+ T cells in the thymus compared to littermate controls (Figure 1D), indicating that loss of store-operated Ca2+ entry might affect the efficiency of negative selection. To further assess the contribution of store-operated Ca2+ entry to positive and negative selection in vivo, we injected staphylococcal enterotoxin B (SEB) intraperitoneally into littermate control and Stim1fl/flStim2fl/fl Vaν-iCre mice (Kishimoto et al., 1998). In contrast to littermate control mice, Stim1fl/flStim2fl/fl Vaν-iCre mice displayed a higher frequency of TCRVβ8+CD4+CD24high immature thymocytes (58.8% versus 71%; Figure 1E), but no difference of CD24lo mature CD4+ T cells in the periphery (Figure S1E), suggesting delayed positive selection of immature thymocytes after positive selection. After SEB injection, the corresponding numbers of CD24high immature thymocytes were 34.6% in littermate controls versus 59.4% in Stim1fl/flStim2fl/fl Vaν-iCre mice (Figure 1E), suggesting that loss of store-operated Ca2+ entry causes a mild impairment of negative selection. Positive selection was essentially normal in female STIM-deficient HY TCR-tg mice (Figure 1F), as judged by unaltered TCR repertoires and normal numbers of TCRint CD69int thymocytes undergoing selection (Starr et al., 2003) (Figures S1F to S1H). However, the frequencies of CD8high T cells in the thymus and lymph nodes of female STIM-deficient HY TCR-tg mice were slightly lower compared to those in control mice. Overall, these results demonstrate that store-operated Ca2+ entry contributes to negative selection, but is not absolutely essential for positive selection during the development of conventional TCRαβ+ T cells
Absence of Store-operated Ca2+ entry results in impaired development of agonist-selected T cells
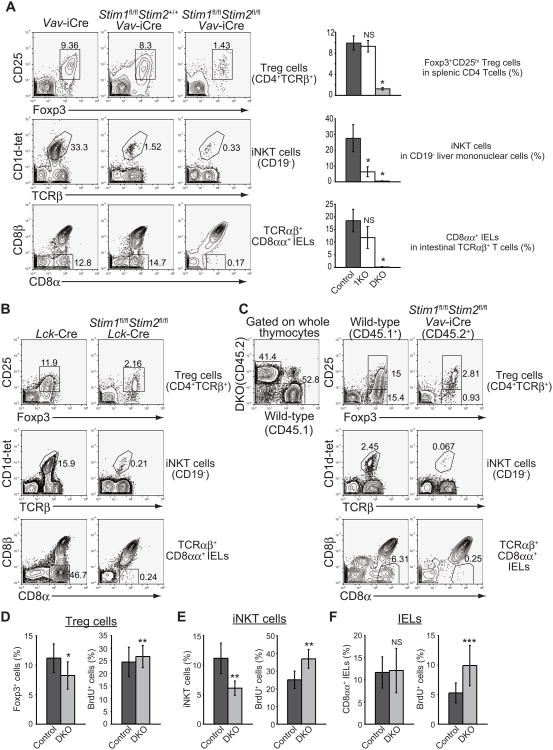
The observed partial impairment of negative selection, with no effect on positive selection, suggested that store-operated Ca2+ entry might preferentially regulate the differentiation of self-reactive thymocytes. We therefore asked whether Stim deficiency influenced the development of agonist-selected T cells. In contrast to conventional TCRαβ+ T cells, the frequencies of Foxp3+ Treg cells, iNKT cells and TCRαβ+ CD8αα+ IELs were significantly reduced in Stim1fl/flStim2fl/fl Vaν-iCre and Stim1fl/flStim2fl/fl Lck-Cre mice (Figures 2A and 2B). Notably, the three different lineages of self-reactive T cells were differentially affected in Stim1fl/flStim2+/+ Vaν-iCre and Stim1fl/flStim2fl/fl Vaν-iCre mice (Figure 2A). Loss of STIM1 alone caused a substantial reduction in the numbers of iNKT cells, to <5% of control, whereas loss of both STIM1 and STIM2 was required to observe a major reduction in the numbers of Foxp3+ Treg cells and TCRαβ+ CD8αα+ IELs (Figure 2A), as we previously showed for Foxp3+ Treg cells (Oh-Hora et al., 2008). Consistent with this observation, a recent report also demonstrated that STIM1 deficiency alone causes a severe reduction of iNKT cells in the periphery in humans (Fuchs et al., 2012). Thus these three agonist-selected subsets of T cells show different hierarchies of dependence on store-operated Ca2+ entry. Moreover, the requirement for store-operated Ca2+ entry is cell-intrinsic: In competitive bone marrow chimeras generated by transplantation of a 1:1 mixture of Stim1fl/flStim2fl/fl Vaν-iCre (CD45.2) bone marrow and wild-type (CD45.1) bone marrow into irradiated Rag1−/− mice, STIM-deficient bone marrow gave rise to fewer numbers of all three agonist-selected subsets of T-lineage cells (Figure 2C).
Figure 2. STIM-deficient mice show a significant decrease of regulatory T cells, iNKT cells and TCRαβ+ CD8αα+ IELs in the periphery.
(A) Flow cytometric analysis of Foxp3+ regulatory T cells (top), CD1d-tetramer+ iNKT cells (middle) and TCRαβ+ CD8αα+ IELs (bottom) in Stim1+/+Stim2+/+ Vaν-iCre (left), Stim1fl/flStim2+/+ Vaν-iCre (center) and Stim1fl/flStim2fl/fl Vaν-iCre (right) mice. Right, average frequencies of each population. Control (dark grey bar); 1KO (open bar); DKO (light grey bar). n=4 or 5. *, P<0.01. (B) Flow cytometric analysis of spleen, liver mononuclear cells and intraepithelial cells as in (A). Stim1+/+Stim2+/+ Lck-Cre (left), Stim1fl/flStim2fl/fl Lck-Cre (right). (C) Flow cytometric analysis of Foxp3+ regulatory T cells (top), CD1d-tetramer+ iNKT cells (middle) and TCRαβ+ CD8αα+ IELs (bottom) in competitive mixed bone marrow chimeras. (D to F) Frequencies of Foxp3+ regulatory T cells in splenic CD4+TCRβ+ cells (D), CD1d-tetramer+ iNKT cells in CD19- liver mononuclear cells (E) and CD8αα+ IELs in intestinal TCRαβ+ T cells (F) (left) from the BrdU-treated, poly IC-injected mice, and the frequencies of BrdU-retaining cells in Foxp3+ cells, iNKT cells and TCRαβ+ CD8αα+ IELs (right). n=4-6. *, P<0.005; **, P<0 05; ***, P=0.067; NS, not significant. Data are representative of two (C to H) and more than three (A and B) independent experiments. Error bars (A, D, E and F) denote mean ± SEM. See also Figure S2.
The reduced number of peripheral agonist-selected T cells is not due to impaired cell survival
To examine whether STIM proteins regulate the survival of agonist-selected T cells in the periphery, we carried out in vivo 5-bromo-2′-deoxyuridine (BrdU) labeling experiments in Stim1fl/flStim2fl/fl Mx1-Cre mice in which the Stim1 and Stim2 genes were acutely deleted by administration of polyinosinic-polycytidylic acid (poly IC). Mice were given BrdU in their drinking water for 10 days, injected with poly IC over the course of the next 4 days, and analyzed 1 month later (Figure S2A). We confirmed that the administration of poly IC efficiently deleted both the Stim1 gene and the Stim2 gene in T cells (Figure S2B). Although both the splenic Foxp3+ Treg population (Figures 2D and S2C) and the fraction of iNKT cells in liver mononuclear cells (Figures 2E and S2D) showed a moderate decrease in Stim1fl/flStim2fl/fl Mx1-Cre mice a month after poly IC treatment, perhaps due to decreased homing or new generation of these cells, the frequency of BrdU-labeled cells was not altered. In TCRαβ+ CD8αα+ IELs from Stim1fl/flStim2fl/fl Mx1-Cre mice, the percentages of BrdU-retaining cells were also increased compared to those from littermate controls (Figures 2F and S2E). Thus these results suggest that store-operated Ca2+ entry regulates agonist-selected T cell development in the thymus rather than the expansion and survival of these agonist-selected T cells in the periphery.
Impaired post-selection maturation of agonist-selected T cells in mice lacking STIM1 and STIM2
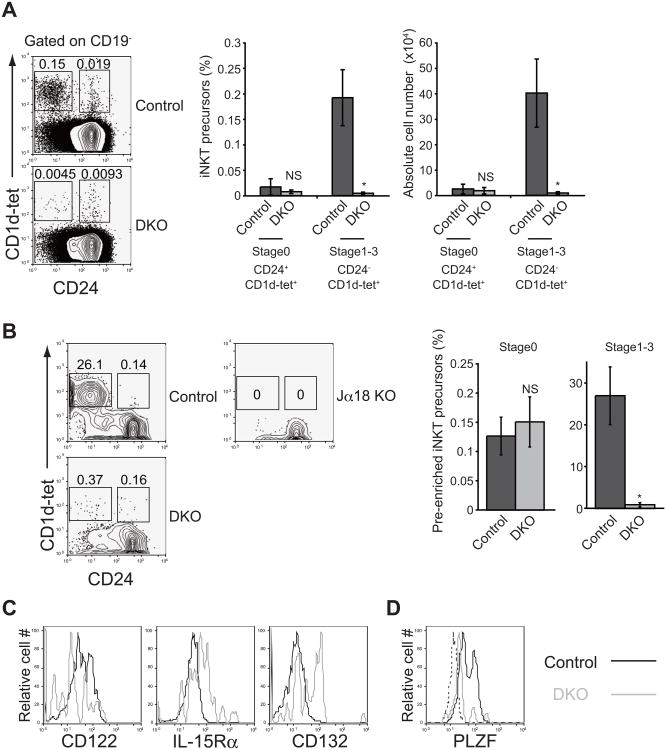
We next examined the effect of store-operated Ca2+ entry on the thymic development of these agonist-selected T cells. iNKT cells develop from DP thymocytes and are selected by lipid antigens presented by MHC class I-like molecule CD1d. After agonist selection, iNKT cell precursors express promyelocytic leukemia zinc finger (PLZF) encoded by Zbtb16 (Kovalovsky et al., 2008; Savage et al., 2008), a master regulator of iNKT cell development, and then expand and mature in response to IL-15 signaling in association with upregulation of CD122, the common β chain of the IL-2 and IL-15 receptors (Godfrey and Berzins, 2007). Since the population of iNKT cells in the thymus is very small, we investigated their development in whole thymocytes as well as in a pre-enriched population defined by staining with CD1d tetramer loaded with α-galactosylceramide (αGalCer). In Stim1fl/flStim2fl/fl Vaν-iCre mice, we detected slightly reduced frequencies and numbers of CD1d tetramer loaded with aGalCer-binding CD24+ iNKT cell precursors (Figure 3A and 3B, Stage 0), the earliest post-agonist selection subset of iNKT cell precursors (Godfrey and Berzins, 2007). By contrast, more differentiated αGalCer-binding CD24- iNKT cell precursors (Stages 1, 2, 3) were all abolished in the absence of store-operated Ca2+ entry (Figure 3A and 3B), suggesting that differentiation of iNKT cell precursors after agonist selection is severely impaired by the loss of store-operated Ca2+ entry. Indeed, the expression of CD122 expression was substantially impaired in residual STIM-deficient iNKT cell precursors (Figure 3C). Furthermore, expression of PLZF was also markedly reduced by the loss of store-operated Ca2+ entry (Figure 3D). These results indicate that the post-selection expansion and functional maturation of iNKT cells also requires store-operated Ca2+ entry, and potentially IL-15 signals as well.
Figure 3. Loss of store-operated Ca2+ entry results in impaired iNKT cell development due to reduced expression of CD122 and PLZF.
(A) Flow cytometric analysis of α-galactosylceramide-loaded CD1d tetramer-positive iNKT cell precursors in the thymus isolated from control and DKO mice (left), frequency of iNKT cell precursors in CD19- thymocytes (center) and absolute cell number (right) of iNKT cell precursors in the thymus. Control, Stim1fl/flStim2fl/fl or Stim1+/+Stim2+/+ Vaν-iCre; DKO, Stimffl/flStim2fl/fl Vaν-iCre. n=5. (B) Flow cytometric analysis (left) and frequencies (right) of pre-enriched thymic iNKT precursors by α-galactosylceramide-loaded CD1d tetramer. Tcraj-18−/− mice (Jα18 KO) were used as a negative control. Control, Stim1fl/flStim2fl/fl or Stim1+/+Stim2+/+ Vaν-iCre; DKO, Stim1fl/flStim2fl/fl Vaν-iCre. n=5. (C) Expression of IL-15 receptor components in iNKT cell precursors from control (black lines) and DKO (grey lines) mice. (D) PLZF expression in thymic iNKT cells at stage1-3. Control: black line, DKO: grey line, Isotype control: dashed line. *, P<0.01; NS, not significant. Data are representative of two (B to D) and four (A) independent experiments. Error bars (A and B) denote mean ± SEM.
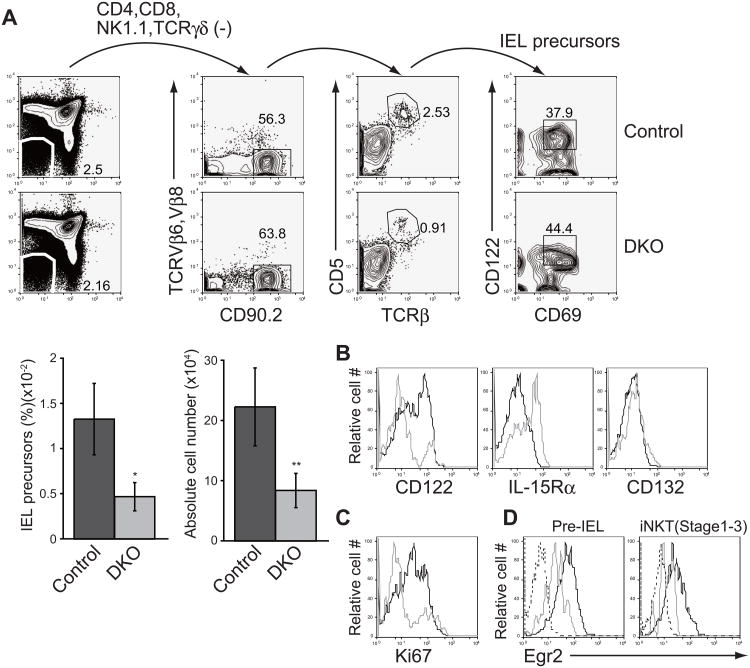
It is generally thought that TCRαβ+ CD8αα+ IELs arise in the thymus (Leishman et al., 2002), however, the molecular mechanism underlying their development is poorly understood. Although the thymic precursors of TCRαβ+ CD8αα+ IELs after agonist selection were defined as DN TCRβ+ CD5+ cells (Gangadharan et al., 2006), this population contains NKT cells and mucosal-associated invariant T (MAIT) cells expressing invariant Vα19 TCR coupled preferentially with TCRVβ6 and TCRVβ8 (Treiner et al., 2003). Therefore we refer here to DN TCRβ+ CD5+ cells as “rough pre-IELs”, and define “pre-IELs” as DN CD90.2+ CD5+ CD69+ CD122+ TCRβ+ NK1.1- TCRγδ- TCRVβ6- and TCRVβ8- cells. The number of pre-IELs was substantially decreased but not abolished in Stim1fl/flStim2fl/fl Vaν-iCre mice (Figure 4A), suggesting that agonist selection of IELs occurs to some degree in the absence of store-operated Ca2+ entry. Proliferation of DN TCRβ+ CD5+ “rough pre-IEL” cells in Stim1fl/flStim2fl/fl Vaν-iCre mice, which contain true pre-IELs, was substantially diminished relative to littermate controls due to impaired upregulation of CD122 (Figures 4B and 4C), another marker of pre-IELs (Lambolez et al., 2007), suggesting that store-operated Ca2+ entry might influence IEL development by modulating cytokine receptor signaling. We further analyzed the gene expression pattern of DN TCRβ+ CD5+ “rough pre-IEL” cells. In addition to CD122, we found that expression of the early growth factor 2 (Egr2) and Egr3 genes, well known NFAT target genes, was reduced at the mRNA level by the loss of store-operated Ca2+ entry (Table S1). Indeed, not only rough pre-IELs but also iNKT cell precursors in Stim1fl/flStim2fl/fl Vaν-iCre mice showed substantial reduction of Egr2 protein expression (Figure 4D). This result is consistent with a recent report that Egr2 regulates the expression of CD122 and PLZF in iNKT cell precursors (Seiler et al., 2012). These data suggest that the NFAT-Egr2 signaling pathway regulates TCRαβ+ CD8αα+ IEL development as well as iNKT cell development.
Figure 4. STIM-deficient mice have a smaller population of thymic TCRαβ+ CD8αα+ IEL precursors.
(A) Flow cytometric analysis of TCRαβ+ CD8αα+ IEL precursors (pre-IELs) among CD4- CD8- thymocytes from control mice and VavDKO mice and frequency in total thymocytes (left) and absolute cell number (right) of pre-IELs. Data are representative of three independent experiments. Error bars denote mean ± SEM. n=5. *, P<0.01, **, P<0.05. (B) Expression of IL-15 receptor components in DN CD5+TCRβ+ thymocytes from control (black lines) and DKO (grey lines) mice. Data are representative of three independent experiments. (C) Proliferation of DN CD5+TCRβ+ thymocytes from control (black lines) and DKO (grey lines) mice was assessed by Ki-67 staining. Data are representative of three independent experiments. (D) Egr2 expression in DN CD5+TCRβ+ thymocytes and iNKT cell precursors. Control: black line, DKO: grey line, Isotype control: dashed line. Data are representative of two (D) and more than three (A to C) independent experiments. See also Table S1.
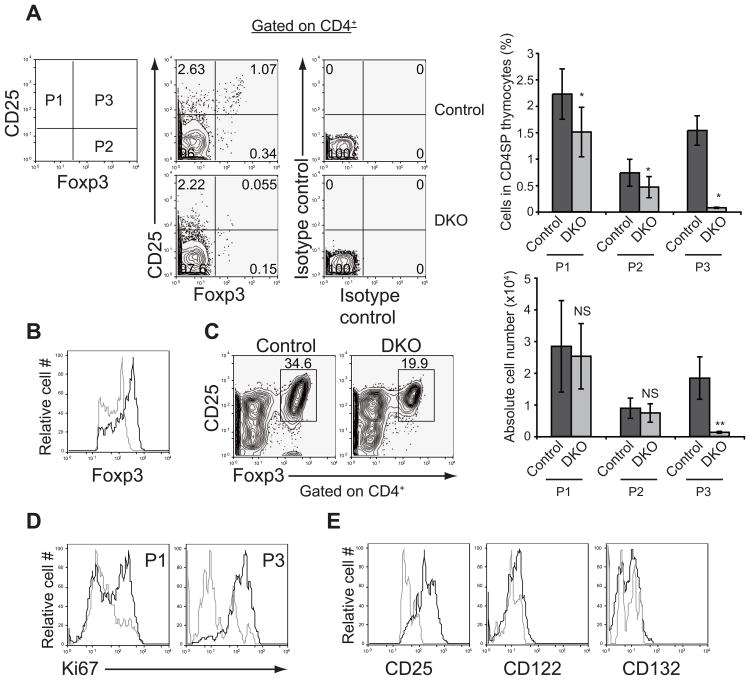
Finally, we interrogated the early stages of Foxp3+ Treg cell development in detail. We found that the frequencies and cell numbers of the mature Foxp3+CD25+ Treg cells (population 3) were significantly decreased in Stim1fl/flStim2fl/fl Vaν-iCre neonates on postnatal day 2, whereas those of the immature Foxp3-CD25+ cells (population 1) and Foxp3+CD25- cells (population 2) were only modestly decreased (Figure 5A). Interestingly, the amount of Foxp3 expression in Stim1fl/flStim2fl/fl Vaν-iCre mice is lower than that of control mice (Figure 5B), suggesting that maximum expression of Foxp3 requires store-operated Ca2+ entry. Foxp3-CD25hi cells are thought to be a major population containing Treg cell precursors (Lio and Hsieh, 2008). Indeed, STIM-deficient Foxp3-CD25+ cells differentiated into Foxp3+CD25+ Treg cells in vitro (Figure 5C), suggesting that Treg cell precursors can be generated in the absence of store-operated Ca2+ entry. Thus, these findings indicate that agonist selection of Treg cells is intact in the absence of store-operated Ca2+ entry. The decreased frequency of Treg cells in Stim1fl/flStim2fl/fl Vaν-iCre mice reflected decreased proliferation rather than impaired expression of the prosurvival molecule, Bcl-2 (Figures 5D and S3). CD25 upregulation was substantially decreased in STIM-deficient Foxp3+CD25+ Treg cells, whereas CD122 expression was reduced mildly (Figure 5E).
Figure 5. Absence of store-operated Ca2+ entry blocks Treg cell development.
(A) Staining of Foxp3 versus CD25 on CD4-positive thymocytes in neonates at day 2 after birth (left) and frequency (upper right) and absolute cell number (lower right) of each population. Error bars denote mean ± SEM. n=6. *, P<0.002, **, P<0.01; NS, not significant. (B) Foxp3 expression in Foxp3+CD25+ cells (population 3) from control (black lines) and DKO (grey lines) mice. (C) In vitro differentiation of Treg cell precursors into Foxp3+CD25high Treg cells. (D) Proliferation of Foxp3+CD25+ cells from control (black lines) and DKO (grey lines) mice was assessed by Ki-67 staining. (E) Expression of IL-2 receptor components in Foxp3+CD25+ from control (black lines) and DKO (grey lines) mice. Data are representative of two (A, C and E) and more than three (B and D) independent experiments. See also Figure S3.
Collectively, these data demonstrate that store-operated Ca2+ entry via the STIM-ORAI pathway is critical for the differentiation of all three agonist-selected T cells after agonist selection mediated by the TCR. Notably, developing agonist-selected T cells all showed impaired upregulation of cytokine receptor subunits, suggesting that the impaired maturation of these T cell subsets might be due to weakened IL-2 or IL-15 signaling.
Cytokine administration facilitates differentiation of post-selection precursors into mature cells, but does not restore their effector functions
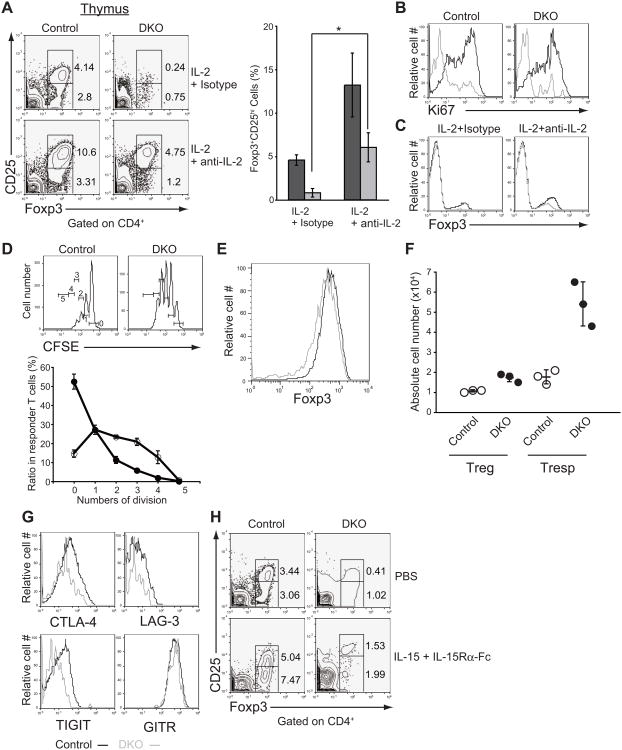
Both IL-2 and IL-15 signaling facilitate the differentiation of agonist-selected T cell precursors into mature cells (Gangadharan et al., 2006; Lio and Hsieh, 2008). We asked whether we could restore functionally mature Treg cells in STIM-deficient mice by injection with immune complexes of either IL-2 and anti-IL-2 (Boyman et al., 2006) or IL-15 and IL-15Rα-Fc (Elpek et al., 2010) to provide sustained IL-2 and IL-15 signaling respectively. In Stim1fl/flStim2fl/fl Vaν-iCre mice treated with IL-2-anti-IL-2 immune complexes, the number of CD25+Foxp3+ Treg cells was restored to that observed in untreated littermate control mice (4-5% of CD4+ T cells) (Figures 6A and S4A). Moreover, proliferation of Foxp3+ Treg cells was also remarkably enhanced (Figures 6B and S4B). The Foxp3+ Treg cells recovered from Stim1fl/flStim2fl/fl Vaν-iCre mice treated with IL-2-anti-IL-2 immune complexes were Helios-positive, indicating that they are derived from thymic Treg cells rather than peripheral naïve CD4+ T cells (Thornton et al., 2010) (Figure S4C). Foxp3 expression was slightly augmented by treatment of Stim1fl/flStim2fl/fl Vaν-iCre mice with IL-2-anti-IL-2 immune complexes (Figures 6C and S4D) but these STIM-deficient Foxp3+ Treg cells nevertheless failed to inhibit the proliferation of responder T cells in vitro (Figure 6D). The impaired suppressive activity cells was not due to downregulation of Foxp3 expression or decreased survival (Figures 6E and 6F), but rather to lower expression of inhibitory molecules such as CTLA-4, LAG-3 and TIGIT (Figure 6G). Consistent with our previous data (Wu et al., 2006), these results collectively indicate that STIM-ORAI-mediated store-operated Ca2+ entry is crucial for IL-2-driven differentiation or proliferation and the functional maturation of Treg cells by modulating NFAT activity.
Figure 6. Store-operated Ca2+ entry controls proliferation and functional maturation of Treg cell precursors.
(A) Flow cytometric analysis of thymic CD4+ Foxp3+CD25+ Treg cells in mice treated with IL-2-immune complexes (left) and frequency of Foxp3+CD25+ Treg cells in CD4SP thymocytes (right). Control, Stim1fl/flStim2fl/fl (dark grey bar); DKO, Stim1fl/flStim2fl/fl Vaν-iCre (light grey bar). Error bars denote mean ± SEM. n=3. *, P<0.05. (B) Enhanced proliferation of Foxp3+CD25+ Treg cells following injection of IL-2-anti-IL-2 immune complexes (black lines) but not immune complexes of IL-2 and isotype control monoclonal antibody (grey lines), in the same experiment as that shown in (A). (C) Amounts of Foxp3 expression in control (black lines) and DKO (grey lines) thymocytes following injection of IL-2-anti-IL-2 immune complexes. (D) Impaired suppressive function of the recovered Treg cells. Carboxyfluorescein succinimidyl ester (CFSE)-labeled responder T cells were cocultured with Treg cells treated with IL-2-anti-IL-2 immune complexes. Control, Stim1fl/flStim2fl/fl Foxp3hCD2 or Stim1+/+Stim2+/+ Foxp3hCD2 (closed circle); DKO, Stim1fl/flStim2fl/fl CD4-Cre Foxp3hCD2 (open circle). Error bars denote mean ± SEM. n=3. (E) Amounts of Foxp3 expression on the recovered control (black lines) and DKO (grey lines) Treg cells. (F) Cell numbers of each population after 3 days of coculture. Control. Open circle; DKO, closed circle. (G) Flow cytometric analysis of the expression of inhibitory molecules on thymic Foxp3+CD25+ Treg cells in control (black lines) and DKO (grey lines), in the same experiment as that shown in (D). (H) Flow cytometric analysis of thymic Foxp3+CD25+ Treg cells in mice treated with IL-15-immune complexes. Control, Stim1fl/flStim2fl/fl; DKO, Stim1fl/flStim2fl/fl Vaν-iCre. Data are representative of two (B to H) and more than three (A) independent experiments. See also Figure S4.
In contrast to IL-2-anti-IL-2 immune complexes, IL-15-immune complexes did not efficiently rescue Treg cell numbers (Figure 6H). Treatment with IL-15-immune complexes did, however, induce the expansion of thymic DN TCRβ+ CD5+ “rough pre-IEL” cells in vivo; however, the numbers of mature TCRαβ+ CD8αα+ IELs, the numbers of thymic iNKT precursor cells and peripheral mature iNKT cells, all remained suppressed (Figures S4E and S4F). Notably, in vitro culture of DN TCRβ+ CD5+ “rough pre-IEL” cells in IL-15 resulted in partial recovery of TCRαβ+ CD8αα+ IELs (Figure S4G), demonstrating that STIM-deficient pre-IELs possess the ability to differentiate into TCRαβ+ CD8αα+ IELs in the presence of IL-15. These data raise the possibility that the impaired development of IELs in the absence of STIM may be due to their inability to properly localize to IL-15-rich tissues such as cryptopatches.
NFAT activity is required for efficient development of agonist-selected T cells
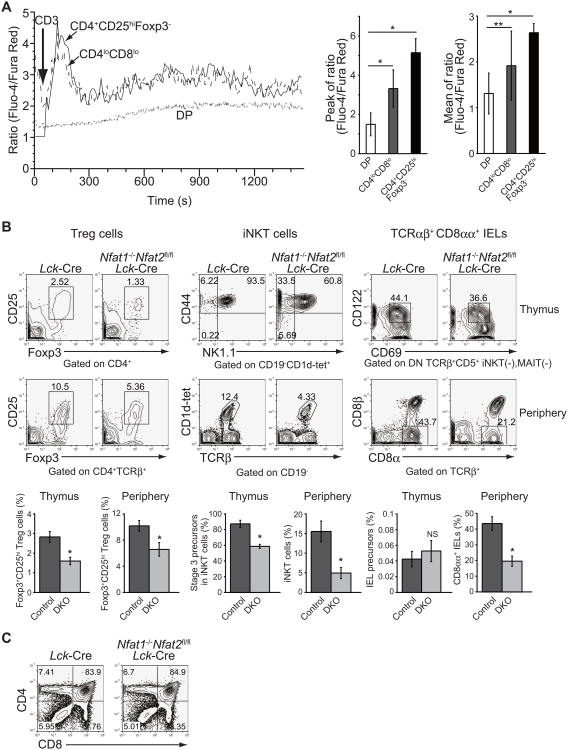
The above results strongly suggest that the maturation of agonist-selected T cells requires sustained large Ca2+ influx. Since the natural ligands of Treg cells, IELs and conventional thymocytes undergoing positive selection remain unknown, we stimulated whole thymocytes with anti-CD3 antibody and measured Ca2+ influx by flow cytometry. Wild-type immature Foxp3-CD25+ cells as well as CD4loCD8lo DP cells containing iNKT precursors and pre-IELs showed larger and more sustained Ca2+ influx in response to the stimulation with anti-CD3 antibody, while STIM-deficient immature Foxp3-CD25+ cells showed little Ca2+ influx (Figure 7A and S5). Lastly, we examined the effect of the Ca2+-responsive transcription factor NFAT on agonist-selected T cell development. NFAT consists of a family of four transcription factors (NFAT1-4, also known as NFATc1-c4), each of which is preferentially expressed in different subsets of T cells (Hogan et al., 2003). Since specific deletion of NFAT4 in T cells causes a partial block in positive selection of conventional TCRαβ+ T cells (Cante-Barrett et al., 2007), we examined mice in which NFAT1 and NFAT2 were deleted in T cells to avoid the effect of NFAT4 deficiency on T cell development. In Nfat1−/−Nfat2fl/fl Lck-Cre mice, the development of Treg cells and iNKT cells, but not conventional TCRαβ+ T cells, was partially blocked in the thymus, whereas the post-thymic development of all agonist-selected T cells was substantially impaired (Figure 7B and 7C). Given the predominant expression of NFAT4 in DP thymocytes, NFAT4 may also contribute to the development of agonist-selected T cells. Together with the data from STIM-deficient mice, these data indicate that STIM-mediated store-operated Ca2+ entry regulates the post-selection maturation of agonist-selected T cells through activation of all NFATs expressed in T cells.
Figure 7. Efficient agonist-T cell development requires full activation of NFATs.
(A) Ca2+ influx in response to anti-CD3 crosslinking (n=3) in wild-type CD4+CD25hiFoxp3- (Treg precursors, solid line), CD4loCD8lo (Population including iNKT and pre-IELs) and DP thymocytes (conventional T cells). Error bars denote mean ± SEM. n=3. *, P<0.05; **, P=0.07. (B and C) Flow cytometric analysis of agonist-selected T-lineage cells (B) and conventional T cell development (C) in NFAT1 and NFAT2-doubly deficient mice. Frequencies represent Foxp3+CD25hi Treg cells in CD4SP thymocytes or in splenic CD4+TCRβ+ cells, iNKT cells in CD19- liver mononuclear cells, IEL precursors in total thymocytes or CD8αα+ IELs in intestinal TCRαβ+ T cells. Data are representative of two (B and C) and three (A) independent experiments. Error bars denote mean ± SEM. n=4. *, P<0.05; NS, not significant. See also Figure S5.
Discussion
We demonstrate here that agonist-selected T cells require store-operated Ca2+ entry for their development. Loss of store-operated Ca2+ entry results in severe impairment of post-selection proliferation and functional maturation of agonist-selected T cells, by modulating the expression of cytokine receptors and effector molecules. In contrast, development of conventional TCRαβ+ T cells either does not require store-operated Ca2+ entry or can be compensated for by non-store-operated pathways of Ca2+ influx. These data suggest that sustained store-operated Ca2+ entry, full NFAT activation and efficient expression of NFAT-driven genes are important factors in agonist-selected T cell development. Importantly, our data in the STIM-deficient mouse model are consistent with phenotypes observed in human patients with non-functional mutations or point mutations in the STIM1 gene, who show specific reduction of peripheral agonist-selected T cells including Foxp3+ Treg cells and iNKT cells (Fuchs et al., 2012; Picard et al., 2009).
Previous studies have suggested that sustained TCR signals allow agonist-selected T cell precursors to mature (Baldwin et al., 2004), however, little is known about the molecular pathways responsible for their development. Self-reactive thymocytes show high and rapid Ca2+ influx through the interaction of self-peptides with TCRs. Given that STIM-ORAI signaling induces rapid large Ca2+ influxes but also maintains high concentrations of [Ca2+]i, which supports long-term consequences of Ca2+ signaling such as gene expression, store-operated Ca2+ entry seems to fulfill both modes of Ca2+ influx during thymic development of agonist-selected T cells. Indeed, our results have shown that lack of store-operated Ca2+ entry influences post-selection maturation of agonist-selected T cells rather than agonist selection itself. We speculate that differentiation of these cells after agonist selection in response to prolonged Ca2+ influx via store-operated Ca2+ entry involves efficient expression of NFAT target genes, e.g. Cd25 and Egr2, or sustained cooperation of NFAT with other transcription factors (Chen et al., 1998; Wu et al., 2006). Consistent with this idea, hyperactive TCR signaling enables higher Egr2 expression, which allows thymocytes to differentiate into iNKT cells (Seiler et al., 2012).
Even a complete lack of store-operated Ca2+ entry caused only a mild impairment of negative selection in our STIM-deficient mice. A more severe impairment is observed in LAT(Y136F) mutant mice and T cell-specific Plcg1−/− mice) in which Ca2+ influx downstream of TCR activation is abolished (Fu et al., 2010; Sommers et al., 2005). The expression of Bim, a proapoptotic protein that plays a critical role in negative selection (Bouillet et al., 2002), can be induced by Ca2+-responsive protein kinase C as well as by treatment with phorbol myristate acetate (PMA), indicating that pathways other than Ca2+ signaling are involved in negative selection (Cante-Barrett et al., 2006). Thus, the stronger impairment of negative selection observed in LAT(Y136F) mutant and Plcg1−/− mice compared to STIM-deficient mice reflects the fact that both the LAT and PLC-γ1 mutations interfere with additional, non-Ca2+-dependent (i.e. DAG-dependent) signaling pathways downstream of PLC-γ1 activation.
In contrast to negative selection, several studies have reported that the store-operated Ca2+ entry-calcineurin-NFAT pathway is essential for positive selection of conventional TCRαβ+ T cells. Calcineurin-deficient mice show a complete block of positive selection in vivo (Neilson et al., 2004), and genetic disruption of NFAT4 activity partially impairs positive selection due to increased cell death (Cante-Barrett et al., 2007). Another study has shown that positively-selecting thymocytes display characteristic sustained Ca2+ oscillations, which allows thymocytes to form stable interactions with stromal cells (Bhakta et al., 2005). In contrast, although STIM-regulated store-operated Ca2+ entry is a major Ca2+ influx pathway in T cells, our results demonstrate that the loss of store-operated Ca2+ entry does not affect positive selection.
Why is positive selection normal in the absence of store-operated Ca2+ entry? Potentially, DP thymocytes could use one or more non- store-operated Ca2+ channels during positive selection to compensate for the absence of store-operated Ca2+ entry. Indeed, CRAC channel inhibitors did not inhibit the Ca2+ oscillations elicited by TCR stimulation in thymocytes undergoing positive selection (Bhakta et al., 2005), suggesting that Ca2+ influx might be independent of the STIM-ORAI pathway. DP thymocytes express many diverse Ca2+-permeable channels (Oh-hora et al unpublished data). Among these, transient receptor potential cation channel M7 (TRPM7) is highly expressed in thymocytes and mediates the permeation of both Mg2+ and Ca2+. Mice with a targeted deletion of the Trpm7 gene in T cells show a partial developmental block in the DN to DP thymocyte transition without altering Mg2+ homeostasis because of impaired differentiation of thymic medullary epithelial cells (Jin et al., 2008), suggesting that immature thymocytes utilize TRPM7 to take up Ca2+ from the extracellular space. The L-type voltage-dependent Ca2+ channel Cav1.4 may also compensate for the loss of store-operated Ca2+ entry because Cav1.4-deficient mice show a subtle but clear reduction of mature SP thymocytes (Omilusik et al., 2011). Finally, other pathways may contribute to Ca2+ entry during positive selection: for instance, a very small number of IP3 receptors in the plasma membrane (estimated at 1-2 receptors) accounts for as much as 50% of total Ca2+ influx in B cells (Dellis et al., 2006).
A “gauntlet” model proposes that thymocytes mature by receiving continuous weak stimulation from multiple contacts with peptide-expressing stromal cells, which allows them to acquire positively selecting signals without making stable contact with thymic stromal cells (Ebert et al., 2008). Consistent with this model, peripheral primary T cells serially encounter antigen-presenting dendritic cells over hours and accumulate incremental activation signals until T cells reach a threshold that triggers stable interactions with dendritic cells (Mempel et al., 2004). Since positive selection occurs over a period of 3-5 days as thymocytes migrate from the outer cortex to the medulla (Huesmann et al., 1991; Shortman et al., 1991), multiple contacts with thymic stromal cells, which generate small Ca2+ signals induced by STIM-independent Ca2+ influx, might allow DP thymocytes to differentiate into CD4 or CD8 single-positive (SP) thymocytes after accumulating signals reach a threshold required to positive selection. Indeed, we observed delayed maturation of conventional STIM-deficient T cells compared to control. In this scenario, store-operated Ca2+ entry might be essential to reach a higher threshold required for agonist-selected T cell development.
In addition to TCR signaling, cytokine signaling is another key process that regulates development of agonist-selected T cells. IL-2 and IL-15 are critical for terminal differentiation of all agonist-selected T cells (Godfrey and Berzins, 2007; Lambolez et al., 2007; Lio and Hsieh, 2008). Our data show that IL-2 or IL-15 signaling alone restores the expansion of Treg cell precursors but not the suppressive function of mature Treg cells, suggesting that acquisition of mature effector function further requires both TCR signaling and IL-2 or IL-15 signaling. Indeed, both Treg cells (Oh-Hora et al., 2008) and iNKT cells (Zullo et al., 2007) need NFAT activity downstream of the TCR for their effector functions.
In addition to IL-2 and IL-15, transforming growth factor (TGF)-β is also essential for agonist-selected T cell development. Mice in which TGF-β signaling is disrupted show a significant decrease of agonist-selected T cell populations (Doisne et al., 2009; Konkel et al., 2011; Li et al., 2006; Liu et al., 2008; Marie et al., 2006). However, in contrast to STIM-deficient mice, these mice show expansion of Treg cells in the thymus of adult mice and upregulation of CD122 in iNKT cell precursors and pre-IELs. Moreover, lack of TGF-β signaling exacerbates negative selection of conventional TCRαβ+ T cells (Ouyang et al., 2010). Hence, it is unlikely that defects in TGF-β signaling underlie the impaired development of agonist-selected T cells in STIM-deficient mice.
In contrast to Treg cells and iNKT cells, the mechanism of TCRαβ+ CD8αα+ IEL development is still enigmatic. Although Ca2+ signaling seems to be central to the development of TCRαβ+ CD8αα+ IELs, our studies did not fully elucidate the role of NFAT in pre-IELs. In the absence of store-operated Ca2+ entry, the expression of Egr2, an NFAT target gene, was substantially downregulated in pre-IELs, and this was accompanied by decreased expression of CD122 and PLZF. Since PLZF-deficient mice show normal frequency of TCRαβ+ CD8αα+ IELs in the periphery (Kreslavsky et al., 2009), CD122 is a preferential target of NFAT-Egr2 pathway. However, CD122 expression seems to be only part of the program that regulates the terminal differentiation of TCRαβ+ CD8αα+ IELs in vivo. Exogenous IL-15 administration rescued TCRαβ+ CD8αα+ IELs differentiation of STIM-deficient pre-IELs in vitro but not in vivo, suggesting that NFAT might regulate recruitment of pre-IELs to IL-15-rich tissues in the small intestine. Since CCL25 is rich in small intestine, its receptor CCR9 seems to be essential for the recruitment of pre-IELs to the gut. Although the expression of CCR9 is regulated by the cooperation of NFAT1 with retinoic acid receptor-retinoid X receptor complex in T cells (Ohoka et al., 2011), genetic disruption of CCR9 in mice results in an increased frequency of TCRαβ+ CD8αα+ IELs (Uehara et al., 2002; Wurbel et al., 2001). Thus it is likely that NFAT regulates other chemokine receptors or adhesion molecules. Alternatively, NFAT might induce or cooperate with an uncharacterized master regulator of TCRαβ+ CD8αα+ IELs. Further studies are needed to elucidate the molecular identity and mechanism of these cells. In conclusion, our findings suggest a common TCR signaling pathway of agonist-selected T cells in which Foxp3+ Treg cells, iNKT cells and TCRαβ+ CD8αα+ IELs require strong and sustained Ca2+ influx by store-operated Ca2+ entry for their post-selection maturation.
Experimental Procedures
Mice
All mice were maintained in specific pathogen-free barrier facilities at Harvard Medical School or Tokyo Medical and Dental University and were used in accordance with protocols approved by the Center for Animal Resources and Comparative Medicine of Harvard Medical School or the Institutional Animal Care and Use Committee of Tokyo Medical and Dental University and conformed to relevant guidelines and laws.
Supplementary Material
Acknowledgments
We thank K. Kitamura (The University of Tokyo) for providing Plat-E packaging cell lines, as well as K. Okamoto, M. Guerrini, A. Terashima, Y. Muratani and T. Kato for discussion and assistance. This work was supported in part by Grant-in-Aid for Scientific Research on Priority Areas from the Japan Society for the Promotion of Science (JSPS), PRESTO from the Japan Science and Technology Agency (JST) (to M.O.), Grants-in-Aid for GCOE Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to M.O. and H.T.), ERATO, Takayanagi Osteonetwork Project from the JST (to H.T.) and NIH grants to S. F. (AI 066128) and A. R. (AI40127 and AI84167). M.O. was also supported by grants from Takeda Life Science Foundation and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. N. K. is supported by JSPS Research Fellowships for Young Scientists.
Footnotes
Supplemental Information. Supplemental information includes five figures, one table and Supplemental Procedures and can be found with this article online at
Competing interests statement. The authors declare that they have competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–6520. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- Beyersdorf N, Braun A, Vogtle T, Varga-Szabo D, Galdos RR, Kissler S, Kerkau T, Nieswandt B. STIM1 -independent T cell development and effector function in vivo. J Immunol. 2009;182:3390–3397. doi: 10.4049/jimmunol.0802888. [DOI] [PubMed] [Google Scholar]
- Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131:1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim. J Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- Cante-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179:103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- Chen L, Glover JN, Hogan PG, Rao A, Harrison SC. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature. 1998;392:42–48. doi: 10.1038/32100. [DOI] [PubMed] [Google Scholar]
- Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- Dellis O, Dedos SG, Tovey SC, Taufiq Ur R, Dubel SJ, Taylor CW. Ca2+ entry through plasma membrane IP3 receptors. Science. 2006;313:229–233. doi: 10.1126/science.1125203. [DOI] [PubMed] [Google Scholar]
- Doisne JM, Bartholin L, Yan KP, Garcia CN, Duarte N, Le Luduec JB, Vincent D, Cyprian F, Horvat B, Martel S, et al. iNKT cell development is orchestrated by different branches of TGF-β signaling. J Exp Med. 2009;206:1365–1378. doi: 10.1084/jem.20090127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert PJ, Ehrlich LI, Davis MM. Low ligand requirement for deletion and lack of synapses in positive selection enforce the gauntlet of thymic T cell maturation. Immunity. 2008;29:734–745. doi: 10.1016/j.immuni.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elpek KG, Rubinstein MP, Bellemare-Pelletier A, Goldrath AW, Turley SJ. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Rα complexes. Proc Natl Acad Sci U S A. 2010;107:21647–21652. doi: 10.1073/pnas.1012128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE, et al. Phospholipase Cγ1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Rensing-Ehl A, Speckmann C, Bengsch B, Schmitt-Graeff A, Bondzio I, Maul-Pavicic A, Bass T, Vraetz T, Strahm B, et al. Antiviral and regulatory T cell immunity in a patient with stromal interaction molecule 1 deficiency. J Immunol. 2012;188:1523–1533. doi: 10.4049/jimmunol.1102507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRαβ+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- Huesmann M, Scott B, Kisielow P, von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991;66:533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto H, Surh CD, Sprent J. A role for Fas in negative selection of thymocytes in vivo. J Exp Med. 1998;187:1427–1438. doi: 10.1084/jem.187.9.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, Chen W. Control of the development of CD8αα+ intestinal intraepithelial lymphocytes by TGF-β. Nat Immunol. 2011;12:312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of γδ T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Lambolez F, Kronenberg M, Cheroutre H. Thymic differentiation of TCRαβ+ CD8αα+ IELs. Immunol Rev. 2007;215:178–188. doi: 10.1111/j.1600-065X.2006.00488.x. [DOI] [PubMed] [Google Scholar]
- Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- McCarl CA, Khalil S, Ma J, Oh-hora M, Yamashita M, Roether J, Kawasaki T, Jairaman A, Sasaki Y, Prakriya M, et al. Store-operated Ca2+ entry through ORAI1 is critical for T cell-mediated autoimmunity and allograft rejection. J Immunol. 2010;185:5845–5858. doi: 10.4049/jimmunol.1001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Ueda Y, Yamada H, Shores EW, Singer A, June CH. In vivo calcium elevations in thymocytes with T cell receptors that are specific for self ligands. Science. 1992;257:96–99. doi: 10.1126/science.1621102. [DOI] [PubMed] [Google Scholar]
- Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka Y, Yokota A, Takeuchi H, Maeda N, Iwata M. Retinoic acid-induced CCR9 expression requires transient TCR stimulation and cooperativity between NFATc2 and the retinoic acid receptor/retinoid × receptor complex. J Immunol. 2011;186:733–744. doi: 10.4049/jimmunol.1000913. [DOI] [PubMed] [Google Scholar]
- Omilusik K, Priatel JJ, Chen X, Wang YT, Xu H, Choi KB, Gopaul R, McIntyre-Smith A, Teh HS, Tan R, et al. The Cav1.4 calcium channel is a critical regulator of T cell receptor signaling and naive T cell homeostasis. Immunity. 2011;35:349–360. doi: 10.1016/j.immuni.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360:1971–1980. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MP, Mathew R, Liszewski MK, Spooner C, Barr K, Meng F, Singh H, Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Vremec D, Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+32+ thymocytes as post-selection intermediates leading to mature T cells. J Exp Med. 1991;173:323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, El-Khoury D, Fuller CL, Shores EW, Love PE, Samelson LE. Mutation of the phospholipase C-γ1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu Rev Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionary conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T-and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo AJ, Benlagha K, Bendelac A, Taparowsky EJ. Sensitivity of NK1.1-negative NKT cells to transgenic BATF defines a role for activator protein-1 in the expansion and maturation of immature NKT cells in the thymus. J Immunol. 2007;178:58–66. doi: 10.4049/jimmunol.178.1.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.