Abstract
Introduction and objectives
Most studies based on state and nation-wide registries evaluating perioperative outcome after carotid endarterectomy (CEA) rely on hospital discharge data only. Therefore, the true 30-day complication risk after carotid revascularization may be underestimated.
Methods
We used the National Surgical Quality Improvement Program (NSQIP) database 2005–2010 to assess the in-hospital and post discharge rate of any stroke, death, cardiac event (new Q-wave MI or cardiac arrest), combined stroke/death and combined adverse outcome (S/D/CE) at 30 days following CEA. Multivariable analyses were used to identify predictors for in-hospital and post discharge events separately, and in particular, those that predict post discharge events distinctly.
Results
A total of 35,916 patients who underwent CEA during 2005–2010 were identified in the NSQIP database. 59% were male (median age 72 years) and 44% had a previous neurologic event. Thirty-day stroke rate was 1.6% (n=591), death rate was 0.8% (n=272), cardiac event rate was 1.0% (n=350), stroke or death rate was 2.2% (n=794) and combined S/D/CE rate was 2.9% (n=1043). 33% of strokes, 53% of deaths, 32% of cardiac events, 40% of combined stroke/death and 38% of combined S/D/CE took place after hospital discharge. Patients with a prior stroke or TIA had similar proportions of post discharge events as compared to patients without prior symptoms. Independent predictors for post discharge events, but not for in-hospital events were female gender (stroke [OR 1.6, 95% CI 1.2–2.1] and stroke/death [OR 1.4, 95% CI 1.1–1.7]), renal failure (stroke [OR 3.0, 95% CI 1.4–6.2]) and COPD (stroke/death [OR 1.8, 95% CI 1.4–2.4] and S/D/CE [OR 1.8, 95% CI 1.4–2.3]).
Conclusions
With 38% of perioperative adverse events after CEA happening post hospitalization, regardless of symptoms status, we need to be alert to the ongoing risks after discharge particularly in women, patients with renal failure, or a history of COPD. This emphasizes the need for reporting and comparing 30-day adverse event rates when evaluating outcomes for CEA, or comparing carotid stenting to CEA.
INTRODUCTION
The benefit of carotid endarterectomy (CEA) and carotid angioplasty and stenting (CAS) is highly influenced by the rate of perioperative adverse events, defined as stroke, myocardial infarction (MI), or mortality up to 30-days after the procedure. Many studies reporting and comparing perioperative complication rates following carotid revascularization rely on state and nation-wide registries, which only include in-hospital data.1–3 However, procedure related complications and mortality after revascularization procedures might also take place after hospital discharge. Results from the Society for Vascular Surgery Vascular Registry suggest that in-hospital events do not reflect the full procedural event rate after CAS and CEA, as an additional 31% and 22% of combined adverse events, respectively, occurred after discharge from the hospital.4 However, in that analysis less than 50% of the total patients completed 30-day follow up, and thus these estimates may under- or overestimate the true event rates. Others have suggested that 10–37% of strokes took place after discharge, but these studies are limited by small study size or incomplete follow-up.5, 6 Also, these analyses did not include adverse outcomes after CEA other than stroke. In order to compare and evaluate outcomes of CEA and CAS, it seems crucial to report 30-day outcome. For patients, it is important to understand the true operative risk they are facing when deciding whether to undergo CEA. Those patients who are at high risk to develop procedural related events after discharge might benefit from closer surveillance after discharge and possibly changes in management. Different preoperative patient characteristics may be related to the timing of events. Our objective was to assess the in-hospital and post discharge rate of adverse events following CEA in a 100% follow-up cohort at 30 days and to identify independent predictors for the timing of these events.
METHODS
Database
Data were obtained from medical records of patients undergoing CEA between 2005–2010 in the American College of Surgeon’s (ACS) National Surgical Quality Improvement Program (NSQIP) database. The NSQIP is a multicenter, prospective quality-improvement registry that includes academic and private U.S. hospitals. In 2005, 37 institutions participated in the program, and the number has increased to 258 by 2010. Demographics, preoperative risk factors, intraoperative variables, and 30-day postoperative mortality and morbidity outcomes are collected, validated, and submitted by a trained and audited surgical clinical nurse-reviewer designated by the ACS. No specific procedural information on CEA (such as reconstruction technique, shunt use, type of artery closure or neurologic monitoring) is captured by the current iteration of NSQIP. Postsurgical data are obtained for the entire 30-day time period, regardless of whether the patient is discharged to the outpatient setting before this time. A detailed description of the NSQIP study methods has been previously published and validated.7 The NSQIP data are subject to annual auditing and the reliability of accurate data acquisition has improved with each year.8
Patient selection
The NSQIP database was queried to identify patients undergoing CEA between 2005 and 2010 using the Current Procedural Terminology (CPT) codes 35301 and 35390. Cases were selected in which CEA was the primary procedure. Patients undergoing concurrent cardiac surgery were excluded. The remaining procedure data were searched to ensure that no other major procedure was included. Indication for surgery (symptom status and degree of stenosis) is not available in the database. Therefore, we were not able to formally stratify patients by symptom status. However, NSQIP captures a history of a previous neurologic event (stroke, TIA) and hemiplegia, without the timing and laterality of these events. This variable was used to distinguish patients who were clearly asymptomatic (ASX) from those who had previous neurological symptoms (SXS). Recent work from our group showed that those with prior SXS were most likely to be symptomatic.9
Endpoints and Measurements
Our primary endpoint was the development of stroke, death, or a cardiac event within 30 days after CEA. Stroke was defined as the development of an embolic, thrombotic, or hemorrhagic vascular accident or stroke with motor, sensory, or cognitive dysfunction (e.g. hemiplegia, hemiparesis, aphasia, sensory deficit, impaired memory) that persists for 24 or more hours. A cardiac event was defined as a new Q-wave myocardial infarction on ECG or cardiac arrest that necessitated cardiopulmonary resuscitation. Our secondary endpoint was wound infection, defined as either involving the carotid artery, deep (involving deep soft tissues e.g., fascia and muscle layers of the incision) or a superficial surgical site infection (limited to skin and subcutaneous).
Results were stratified for the in-hospital period (intra-operative or pre-discharge) and the post discharge period through 30-days after surgery. Timing to adverse event was recorded per day, starting from the day of surgery (day 0). The proportion of post discharge events was analyzed for both patients with a history of neurologic symptoms and patients without a history of neurologic symptoms. For in-hospital analysis, patients with post discharge events were excluded. Likewise, for post discharge events, patients with in-hospital events were excluded. If patients suffered both in hospital and post discharge events, the in-hospital event was counted for analysis. Predictor variables for the primary outcome included demographics and preoperative variables. Continuous variables were categorized for the purpose of this study. Detailed definitions of these variables are listed in the Appendix.
Statistical Analysis
Bivariate analysis was carried out to assess the relation of the preoperative variables with the primary outcome (stroke, death, cardiac event or a composite of stroke/death and S/D/CE) at the different time points (in-hospital, post-discharge, 30-day) using Pearson χ2 test and Fisher’s exact test. Initial bivariate analysis included 29 preoperative demographic and comorbidity variables. Multivariable logistic regression was used to assess independent risk factors for outcome events at each of the above specified time points. Demographics and preoperative variables were entered into the multivariable regression analysis if P < .2 in bivariate analysis. Associations were calculated using backward elimination procedure, in which all variables were entered in the first step and removed stepwise based on the highest non-significant P-value (P ≥0.05). After carrying out this iterative process, covariates were included in the final model if predictive of primary outcome events in any of the three specified clinical time intervals with this model demonstrating the contribution of each covariate to timing of events. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. Hosmer and Lemeshow test was used to test the goodness of fit in each model. Statistical analysis was performed using SPSS version 19.0 statistical software (SPSS Inc, Chicago Illinois, USA).
RESULTS
A total of 35,916 patients undergoing CEA between 2005 and 2010 in the NSQIP database were identified and included for analysis. The median age was 72 years (Interquartile Range [IQR] 13), 59.1% were men, and 44.1% had a history of stroke, TIA or hemiplegia. Demographics, clinical characteristics and operative details are shown in Table I.
Table I.
Demographics and clinical characteristics of 35,916 patients undergoing carotid endarterectomy
| Variable | N or Median | % or (IQR) |
|---|---|---|
| Demographics | ||
| Age, y | 72 | (13) |
| Age | ||
| < 60 y | 5123 | 14.4 |
| 60 – 69 y | 11283 | 31.8 |
| 70 – 80 y | 13226 | 37.3 |
| > 80 y | 5871 | 16.5 |
| Gender | ||
| Male | 21184 | 59.1 |
| Female | 14657 | 40.9 |
| Race, non-white | 3046 | 8.5 |
| Clinical characteristics | ||
| Redo CEA | 82 | 0.2 |
| Hx stroke/ hemiplegia | 8936 | 24.9 |
| Hx of TIA | 9954 | 27.7 |
| Hx of Angina or MI | 1364 | 3.8 |
| Congestive Heart Failure | 371 | 1.0 |
| Previous PCI | 6710 | 18.7 |
| Previous Cardiac Surgery | 8196 | 22.8 |
| Diabetes Mellitus | 9984 | 27.8 |
| Hypertension | 30658 | 85.4 |
| Current smoker | 10033 | 27.9 |
| Hx of smoking | ||
| < 10 pack-y | 11161 | 32.3 |
| 10 – 29 pack-y | 4939 | 13.8 |
| 30 – 49 pack-y | 3726 | 10.4 |
| 50 – 70 pack-y | 3517 | 9.8 |
| > 70 pack-y | 2921 | 8.1 |
| Alcohol use > 2 eh/day | 1557 | 4.3 |
| BMI | ||
| < 18.5 | 537 | 1.5 |
| 18.5 – 24.9 | 9507 | 26.5 |
| 25 – 29.9 | 13814 | 38.5 |
| 30 – 34.9 | 7470 | 20.8 |
| 35 – 40 | 2687 | 7.5 |
| ≥ 40 | 1154 | 3.2 |
| Height | ||
| < 64 inch | 12040 | 34.0 |
| 64 – 70 inch | 14750 | 41.6 |
| > 70 inch | 8636 | 24.4 |
| Renal failure | 476 | 1.3 |
| Hx of COPD | 3792 | 10.6 |
| Dyspnea | 6847 | 19.1 |
| Steroid use | 747 | 2.1 |
| Hx of revascularization for PVD | 3499 | 9.7 |
| Restpain/gangrene | 344 | 1.0 |
| Functional status | ||
| Independent | 33952 | 94.5 |
| Dependent | 1961 | 5.5 |
| Emergency procedure | 614 | 1.7 |
| ASA class > 3 | 4753 | 13.2 |
| General anesthesia | 30184 | 84.1 |
| Vascular surgeon | 34019 | 94.7 |
| PGY level ≤ 3 | 4152 | 11.6 |
Hx, history, TIA, transient ischemic attack, MI, myocardial infarction, PCI, percutaneous coronary intervention, BMI, Body Mass Index; COPD, chronic obstructive pulmonary disease, PVD, peripheral vascular disease, ASA, American Society of Anesthesiologists; PGY, post-graduate year of resident
Stroke rate at 30-days was 1.6% (n=591, prior neurologic SXS: 2.4%, ASX 1.1%, P <.001, OR 2.25 95% CI 1.89–2.66), death rate was 0.8% (n=272, prior neurologic SXS 1.1%, ASX 0.5%, P<.001, OR 2.03 95% CI 1.59–2.59) and cardiac event rate was 1.0% (n=350, prior neurologic SXS 1.1%, ASX 0.8%, P = .003, OR 1.38 95% CI 1.12–1.70). Combined stroke/death rate was 2.2% (n= 794, prior neurologic SXS: 3.1% vs. ASX 1.5%, P<.001, OR 2.16 95% CI 1.87–2.50) and combined S/D/CE rate was 2.9% (n=1043, prior neurologic SXS 3.9%, ASX 2.1%, P <.001, OR 1.86, 95% CI 1.64–2.11). The median length of hospital stay was 1 day (IQR 1).
Timing of events
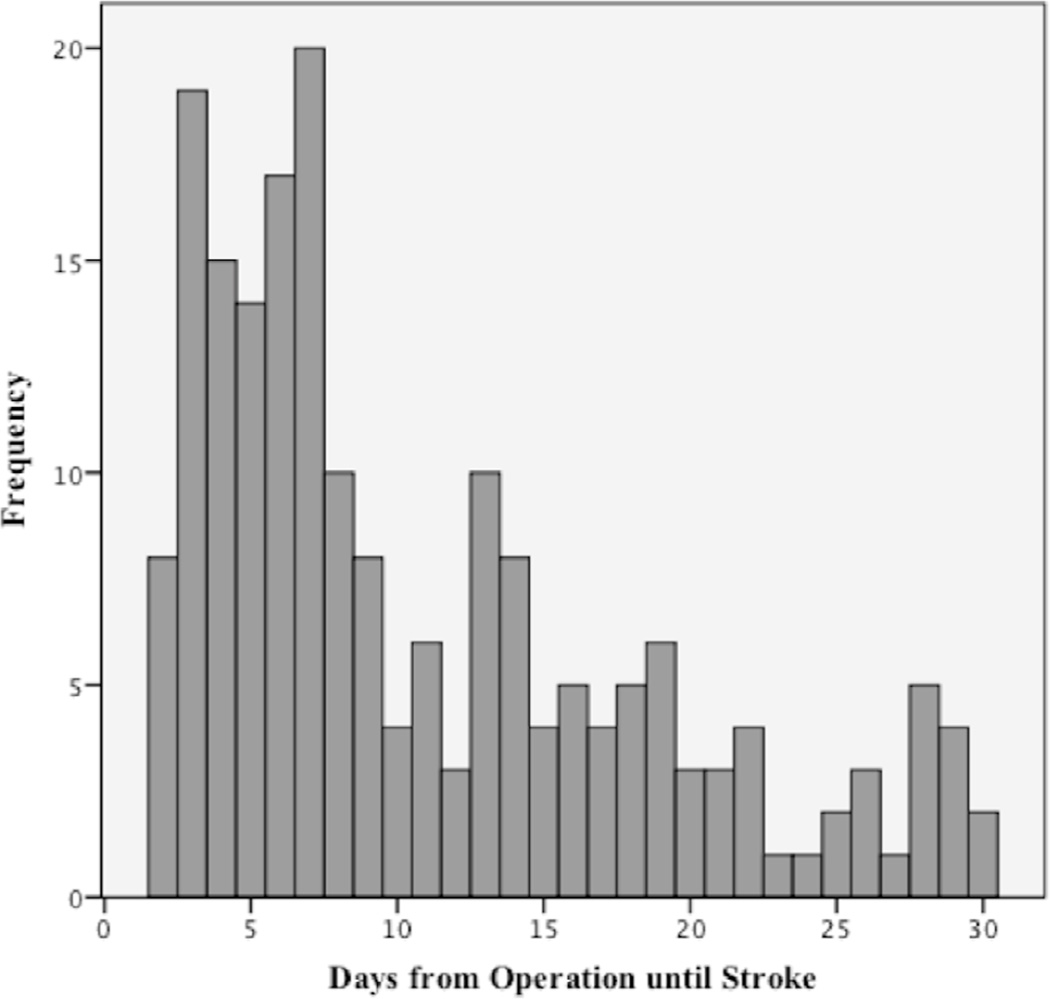
In-hospital S/D/CE occurred in 656 patients (1.8%). After discharge, an additional 38% of S/D/CE (n=399, 1.1%) occurred: 33% of strokes (n=195, 0.5%), 53% of deaths (n=144, 0.4%), 32% of cardiac events (n=122, 0.3%) and 40% of stroke/death (n=320, 0.9%) occurred after discharge. The proportion of combined S/D/CE after discharge was similar in patients with prior neurological symptoms versus those without (39% and 38%, respectively). Post discharge, stroke happened in 34% of patients with prior neurologic symptoms, and in 32% of ASX patients. 52% of deaths, 31% of cardiac events and 40% of stroke/death occurred after discharge in patients with prior neurologic symptoms, versus 55% of deaths, 33% of cardiac events and 40% of stroke/death in ASX patients. (Table II) In-hospital adverse events happened at a median of 1 day (IQR 1). These patients were discharged from the hospital at a median of 7 days (IQR 8) postoperatively. Patients who experienced post discharge events were discharged at day one (median) post operatively (IQR 1). Post discharge stroke occurred at a median of 8 days after the operation (IQR 11). (Figure 1) MI or cardiac arrest (cardiac events) after discharge took place at a median of 6 days (IQR 17) and 11 days (IQR 19), respectively. Patients who survived to discharge but did not survive the post discharge period, died at a median interval of 11 days (IQR 15).
Table II.
Outcome and timing of in-hospital, post discharge and 30-day events of 35,916 patients undergoing carotid endarterectomy
| All patients | Time to event, days | In-hospital event rate | Post discharge event rate | 30-Day event rate | Proportion of events that occurred after discharge |
|
|---|---|---|---|---|---|---|
| Median, IQR | Mean ± SD | N (%) | N (%) | N (%) | ||
| Stroke | 1, 6 | 4.5 ± 6.6 | 396 (1.1) | 195 (0.5) | 591 (1.6) | 33% |
| Death | 8.5, 14 | 11.2 ± 8.6 | 128 (0.4) | 144 (0.4) | 272 (0.8) | 53% |
| Cardiac event | NA | NA | 238 (0.7) | 112 (0.3) | 350 (1.0) | 32% |
| Stroke/Death | NA | NA | 480 (1.3) | 320 (0.9) a | 794 (2.2) | 40% |
| Stroke/Death/Cardiac event | NA | NA | 656 (1.8) | 399 (1.1) b | 1043 (2.9) | 38% |
| Patients with a prior neurological event | ||||||
| Stroke | 2, 6 | 4.6 ± 6.4 | 249 (1.6) | 127 (0.8) | 376 (2.4) | 34% |
| Death | 9, 15 | 11.8 ± 8.7 | 81 (0.5) | 86 (0.5) | 167 (1.1) | 52% |
| Cardiac event | NA | NA | 126 (0.8) | 56 (0.4) | 182 (1.1) | 31% |
| Stroke/Death | NA | NA | 302 (1.9) | 201 (1.3) | 497 (3.1) | 40% |
| Stroke/Death/Cardiac event | NA | NA | 387 (2.4) | 238 (1.5) | 616 (3.9) | 39% |
| Asymptomatic patients | ||||||
| Stroke | 1, 6 | 4.5 ± 6.9 | 147 (0.7) | 68 (0.3) | 215 (1.1) | 32% |
| Death | 8, 8 | 10.5 ±8.2 | 47 (0.2) | 58 (0.3) | 105 (0.5) | 55% |
| Cardiac event | NA | NA | 112 (0.6) | 56 (0.3) | 168 (0.8) | 33% |
| Stroke/Death | NA | NA | 178 (0.9) | 119 (0.6) | 297 (1.5) | 40% |
| Stroke/Death/Cardiac event | NA | NA | 269 (1.3) | 161 (0.8) | 427 (2.1) | 38% |
IQR, Interquartile range, SD, Standard deviation, N, Number of patients experiencing the events, Na, data not available
6 patient died post discharge after having an in-hospital stroke
12 patients experienced a secondary post discharge event, after having an in-hospital stroke (N=6) or cardiac event (N=6)
Figure 1.
Days from operation until post discharge stroke after carotid endarterectomy
Thirty-day wound infection rate was 0.5% (N=197). The majority of wound infections took place after discharge; 94% of superficial wound infection (N=141, 0.4%), 94% of deep wound infection (N=47, 0.1%) and 89% of carotid infection (N=9, 0.03%).
Predictors for stroke
In-hospital
Independent predictors for in-hospital stroke were redo-CEA, a history of stroke/hemiplegia, history of TIA, history of angina/MI, underweight (versus normal weight) and obesity class II (versus normal weight), functional dependent status (versus independent), emergency procedures and ASA class >3. (Table III)
Table III.
Independent preoperative predictors for in-hospital, post discharge and 30-day stroke
| In-hospital | Post discharge | 30 – Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Female | 1.0 | 0.8 – 1.3 | 0.9 | 1.6 | 1.2 – 2.1 | <.01 | 1.2 | 0.99 – 1.4 | 0.06 |
| Age <60 years a | 1.2 | 0.9 – 1.7 | 0.1 | 1.5 | 0.99 – 2.2 | 0.07 | 1.3 | 1.04 – 1.7 | 0.03 |
| Redo CEA | 3.3 | 1.03 – 10.6 | 0.05 | 2.4 | 0.3 – 17.2 | 0.4 | 3.0 | 1.1 – 8.2 | 0.04 |
| Hx of stroke/hemiplegia | 1.9 | 1.5 – 2.3 | <.001 | 2.3 | 1.7 – 3.1 | <.001 | 2.0 | 1.7 – 2.4 | <.001 |
| Hx of TIA | 1.4 | 1.2 – 1.8 | <.01 | 1.5 | 1.1 – 2.1 | <.01 | 1.5 | 1.2 – 1.7 | <.001 |
| Hx of angina/MI | 1.6 | 1.04 – 2.4 | 0.03 | 1.7 | 1.0 – 3.1 | 0.06 | 1.6 | 1.2 – 2.3 | <.01 |
| Renal failure | 1.0 | 0.4 – 2.2 | 0.9 | 3.0 | 1.4 – 6.2 | <.01 | 1.5 | 0.9 – 2.7 | 0.1 |
| Underweight b | 2.3 | 1.3 – 4.1 | <.01 | 0.9 | 0.3 – 2.9 | 0.8 | 1.8 | 1.1 – 3.0 | 0.03 |
| Obesity class II b | 1.5 | 1.01 – 2.1 | 0.05 | 0.9 | 0.5 – 2.0 | 0.6 | 1.3 | 0.9 – 1.7 | 0.2 |
| Functional status, dependent c | 1.6 | 1.2 – 3.2 | <.01 | 1.4 | 0.9 – 2.3 | 0.2 | 1.7 | 1.3 – 2.2 | <.001 |
| Emergency procedure | 1.9 | 1.1 – 3.2 | 0.01 | 0.9 | 0.3 – 2.4 | 0.8 | 1.5 | 0.99 – 2.5 | 0.05 |
| ASA class > 3 | 1.4 | 1.1 – 1.8 | 0.02 | 1.2 | 0.8 – 1.7 | 0.4 | 1.3 | 1.1 – 1.6 | 0.02 |
OR, Odds Ratio, CI, Confidence Interval, CEA, carotid endarterectomy, Hx, history, TIA, transient ischemic attack, MI, myocardial infarction, ASA, American Society of Anesthesiology
vs. 60–70 yr
vs. normal weight
vs. independent
Post discharge
History of stoke/hemiplegia, history of TIA, renal failure and female gender were associated with increased risk of post discharge stroke on multivariable analyses. (Table III) Women were more likely to have a post discharge stroke than men (38.1% vs. 29.0%, OR 1.57, 95% CI 1.18 – 2.09, P =0.002). In patients with a previous neurological event, a significantly higher stroke rate was seen in women compared to men (1.0% vs. 0.7% P = 0.02, OR 1.5, 95% CI 1.1 – 2.1). In ASX patients, the stroke rate was again higher in women with a similar odds ratio, however this did not reach statistical significance (0.4% vs. 0.3%, P=0.08, OR 1.6, 95% CI 0.97 – 2.5). Stroke in woman took place at a median of 2 days (IQR 6), compared to 1 day (IQR 6) in men (P =0.4). Stroke in patients with renal failure took place at a median of 7 days (IQR 9) after discharge, compared to 1 day (IQR 6) in patients without renal failure (P<.001). Female gender and renal failure were both predictive for post discharge stroke in multivariable analysis, but not for in-hospital stroke. (Table III)
Predictors for stroke or death
In-hospital
In multivariable analysis, age >80 year, history of stroke/hemiplegia, history of TIA, history of angina/MI, renal failure, history of revascularization for peripheral vascular disease (PVD), dependent functional status, emergency procedures and ASA class >3 were independent predictors for stroke or death. (Table IV)
Table IV.
Independent preoperative predictors for in-hospital, post discharge and 30-day stroke or death
| In-hospital | Post discharge | 30 – Day | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Female | 1.1 | 0.9 – 1.3 | 0.4 | 1.4 | 1.1 – 1.7 | <.01 | 1.2 | 1.03 – 1.4 | 0.02 |
| Age < 60 a | 1.1 | 0.9 – 1.6 | 0.2 | 1.4 | 0.96 – 1.9 | 0.09 | 1.3 | 1.01 – 1.6 | 0.05 |
| Age > 80 a | 1.4 | 1.1 – 1.8 | 0.01 | 1.3 | 0.9 – 1.9 | 0.09 | 1.4 | 1.1 – 1.7 | <.01 |
| Hx of stroke/hemiplegia | 1.8 | 1.5 – 2.2 | <.001 | 2.0 | 1.5 – 2.5 | <.001 | 1.9 | 1.6 – 2.2 | <.001 |
| Hx of TIA | 1.3 | 1.04 – 1.5 | 0.02 | 1.3 | 0.99 – 1.6 | 0.05 | 1.3 | 1.1 – 1.5 | <.01 |
| Hx of Angina/MI | 1.9 | 1.4 – 2.7 | <.001 | 1.6 | 1.1 – 2.6 | 0.03 | 1.8 | 1.4 – 2.4 | <.001 |
| Renal failure | 2.1 | 1.3 – 3.4 | <.01 | 3.2 | 1.9 – 5.5 | <.001 | 2.5 | 1.7 – 3.6 | <.001 |
| Hx of COPD | 1.2 | 0.9 – 1.5 | 0.3 | 1.8 | 1.4 – 2.4 | <.001 | 1.4 | 1.2 – 1.7 | <.01 |
| Hx of PVD | 1.5 | 1.1 – 2.0 | <.01 | 1.3 | 0.9 – 1.9 | 0.1 | 1.4 | 1.2 – 1.8 | <.001 |
| Functional status, dependent b | 2.2 | 1.7 – 2.9 | <.001 | 1.6 | 1.2 – 2.4 | <.01 | 2.0 | 1.6 – 2.5 | <.001 |
| Emergency procedure | 2.7 | 1.8 – 3.9 | <.001 | 0.6 | 0.2 – 1.5 | 0.3 | 1.9 | 1.3 – 2.7 | <.01 |
| ASA class > 3 | 1.6 | 1.3 – 2.0 | <.001 | 1.3 | 0.99 – 1.8 | 0.06 | 1.5 | 1.3 – 1.8 | <.001 |
OR, Odds Ratio, CI, Confidence Interval, Hx, history, TIA, transient ischemic attack, MI, myocardial infarction, COPD, chronic obstructive pulmonary disease, PVD, peripheral vascular disease, ASA, American Society of Anesthesiology
vs. 60–70 yr
vs. independent
Post discharge
Female gender, history of stroke/hemiplegia, history of angina/MI, renal failure, COPD and dependent functional status were independently associated with post discharge stroke or death. Female gender and COPD were predictive for post discharge stroke/death, but not for in-hospital events. (Table IV)
Predictors for other adverse events
A history of COPD or dyspnea was predictive for post discharge and 30-day death, but not for in-hospital death. (Table V, available online) For cardiac events, no differential predictors were identified in the post discharge time period compared to in-hospital time frame. (Table VI, available online) As was seen with death and stroke/death, patients with a history of COPD were at increased risk for post discharge and 30-day combined S/D/CE, but not for in-hospital adverse events. (Table VII, available online). Although the risk factors identified for post discharge events (but not for in-hospital outcome) predicted different endpoints, the cumulative effect of these risk factors (female gender, renal failure and COPD) is shown in table VIII (online appendix) for all different time points. Patients undergoing emergency procedures were at increased risk for all in-hospital events, but not for post discharge events. All independent predictors for death (Table V), cardiac events (Table VI) and combined S/D/CE (Table VII) with respect to the different time intervals are available as an online supplement.
DISCUSSION
In a large number of patients among both community and academic institutions in the United States, carotid endarterectomy was performed with very low complication rates for stroke, death or cardiac events (MI or cardiac arrest). Approximately one third of procedural related events occur after discharge from the hospital. This was true for both patients with prior neurologic symptoms and for those who were asymptomatic. In this study we identified predictors for post discharge events, which have not previously been reported. We found that independent predictors for post discharge events, but not for in-hospital events were female gender (stroke and stroke/death), renal failure (stroke) and COPD (death, stroke/death and S/D/CE). Previously, Sidawy et al.4 described the occurrence of adverse events happening after discharge but within 30-days of revascularization. For CAS they found that 31% of combined strokes/deaths or MI’s were not captured during hospital admission; for CEA 28% of events were missed when only analyzing in-hospital data. Although less than half of patients in that analysis had 30-day follow-up, our results confirm these estimates in a 100% follow-up cohort. Most administrative vascular registries do not include post discharge events. The results of this study indicate that this may be a confounding feature for many studies based on such datasets.1–3, 10 It is well known that that hospital administrative data are not reliable to estimate non-fatal operative complication rates for surgical procedures in general.11 Recently, the reliability of administrative data to determine outcomes specifically for carotid revascularization procedures was questioned.9, 12
Consistent entry of data beyond the in-hospital period seems to be not only important for true perioperative event risk estimation, but also to identify patients at risk for adverse perioperative events. Registries such as the NSQIP are critical to evaluate rare events such as postoperative stroke after CEA since single surgeon or single center experience are typically underpowered to evaluate procedures with low event rates. Our results demonstrate that in a subgroup of patients adverse events are more likely to happen after discharge, possibly influencing preoperative counseling and perioperative management. The timing of strokes suggests that some may be due to hyperperfusion and subsequent intracerebral hemorrhage.13, 14 Intracerebral hemorrhage occurs at unpredictable intervals in the postoperative course and its mechanism remains unclear. Previous analyses identified high-grade stenosis and severe intra- or postoperative hypertension as possible risk factors.13 Better blood pressure control and perhaps selective transcranial Doppler monitoring might benefit these patients.15 ‘Late’ stroke might also occur due to thrombo-embolism13 in patients who do not respond to anti-platelet therapy. Preoperative testing for antiplatelet responsiveness may identify subgroups at risk that may benefit from additional antiplatelet medication. Unfortunately, the type and laterality of post-operative stroke is not captured in the NSQIP. Future research efforts should evaluate the mechanism of post-operative stroke to guide further changes in perioperative management.
In our study we found that stroke in women seems to happen more frequently after discharge. Studies based on in-hospital results did not find differences in stroke and death rates after CAS and CEA in relation to gender.10, 16 However, several others have also identified women as a subgroup of patients at higher risk for 30-day adverse outcome after CEA.6, 17 Especially for asymptomatic women, the benefit of surgery may be less than that for men.18, 19 In our analyses, the difference between men and woman in post discharge events was identified for both those with, and without a previous neurological event, although this did not quite reach statistical significance for asymptomatic patients. Gender differences in outcome of CEA are still not well understood and merit further investigation.20, 21 Renal failure was also an independent predictor for post discharge stroke, but not for in-hospital or 30-day stroke. Two studies based on NSQIP data6, 22 found that impaired renal function was an independent risk factor for mortality and cardiac and pulmonary morbidity after CEA, but was not associated with increased risk of neurologic complications at 30-days, which was consistent with our results. Also other reports have suggested that renal failure is a risk factor for increased stroke risk and a marker for advanced atherosclerotic disease causing morbidity and mortality.23–25
Several authors have reported risk factors associated with adverse outcome after CEA in order to identify high-risk groups and optimize management of patients with carotid artery disease.5, 6, 18, 26, 27 Similar to prior reports we found that symptom status was a consistent predictor for adverse events (both in-hospital and post discharge), and that a history of preoperative stroke was more predictive than a history of TIA.27, 28 Among other risk factors for only in-hospital or both in-hospital and post discharge outcome, we identified several patient characteristics previously described by others, including diabetes26, 27 and age >8027, 29. Interestingly, we found that patients with redo-CEA had increased risk for in-hospital stroke, whereas others did not30, 31 or only identified increased risk for local complications such as cranial nerve injury.32 However, these studies might not have detected a difference due to low event rates and small sample sizes. Adequately powered studies are needed to define optimal treatment in these patients. Under- and overweight patients had increased risk for stroke, suggesting that obesity is not only a risk factor for mortality,33, 34 but also for morbidity after CEA. This obesity paradox has been previously identified with vascular surgery procedures with a reverse J-shaped relation of BMI and adverse outcome, with the highest risk in the underweight and morbidly obese extremes, and the lowest rates in the overweight and mildly obese patients.33–36 Not surprisingly and consistent with previous literature,5, 27, 37 emergent procedures were predictive for all in-hospital adverse outcomes. This increased risk was, however, not persistent after discharge. This is understandable as most emergent procedures would be presumed to be performed for either stroke-in-evolution or crescendo TIA.28, 38
This study has several limitations. NSQIP does not define preoperative symptom status in the same manner as most clinical trials.6 Although a recent report from our group showed that NSQIP does identify symptomatic patients with a high sensitivity, the number of false positives was about 25% (due to stroke or TIA occurring > 6 months prior to surgery or contralateral to the CEA).9 Therefore, we were only able to stratify the analysis regarding timing of events for patients with and without a previous neurological event and accounted for these symptoms individually in multivariable prediction models. However, importantly, we did not find a difference in the occurrence of post-discharge stroke in those who were clearly asymptomatic compared to a group who had pre-operative neurologic events, the vast majority of which were likely within 6 months of and ipsilateral to their CEA. Another limitation inherent to this database is the lack of anatomical preoperative factors such as history of previous neck radiation, degree of stenosis, or radical neck dissection. Also, the retrospective nature of the data may introduce a selection bias, which might have influenced the results. Because non-fatal cardiac events proved to have a strong effect on patient survival, we included cardiac events as one of our primary outcome measures. Our definition of a cardiac event will capture both cardiac arrest and new Q-wave MI on ECG, but is somewhat limited by the NSQIP database because patients with ST-elevation MI (troponin leak) will be missed. Lastly, CAS procedures are not yet included in the NSQIP, but will be in the future allowing comparison of the two procedures.
Conclusion
With 38% of perioperative adverse events after CEA happening post hospitalization, regardless of symptom status, surgeons should be alert to the ongoing risks after discharge particularly in women and patients with renal failure or a history of COPD. For research and quality improvement purposes, the full 30-day adverse event rates should be reported and compared when evaluating CEA or comparing CAS and CEA.
Supplementary Material
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734
Appendix: Definitions of preoperative variables
| Variable | Definition |
|---|---|
| Hx of stroke/hemiplegia | A history of a cerebrovascular accident (embolic, thrombotic, or hemorrhagic) with or without persistent residual motor, sensory, or cognitive dysfunction. Acute or chronic neuromuscular injury resulting in total or partial paralysis or paresis (weakness) of one side of the body. |
| Hx of TIA | A history of focal neurologic deficits (e.g. numbness of an arm or amaurosis fugax) of sudden onset and brief duration (usually <30 minutes) that usually reflects dysfunction in a cerebral vascular distribution. |
| Hx of Angina or MI | A history of angina (pain or discomfort between the diaphragm and the mandible resulting from myocardial ischemia) or myocardial infarction (MI, a non-Q wave or a Q wave infarct in the six months prior to surgery) For patients on anti-anginal medications, only patients who had angina within one month prior to surgery are included. |
| Congestive Heart Failure | Newly diagnosed CHF within the previous 30 days or a diagnosis of chronic CHF with new signs or symptoms in the 30 days prior to surgery |
| Previous PCI | A percutaneous coronary intervention (PCI) at any time (including any attempted PCI). This includes either balloon dilatation or stent placement. |
| Previous Cardiac Surgery | Any major cardiac surgical procedures, includes coronary artery bypass graft surgery, valve replacement or repair, repair of atrial or ventricular septal defects, great thoracic vessel repair, cardiac transplant, left ventricular aneurysmectomy, insertion of left ventricular assist devices (LVAD), etc. Not include are pacemaker insertions or automatic implantable cardioverter defibrillator (AICD) insertions. |
| Diabetes Mellitus | Diabetes mellitus with oral agents or insulin. The treatment regimen of the patient’s chronic, long-term management. |
| Hypertension | Persistent elevation of systolic blood pressure > 140 mm Hg or a diastolic blood pressure > 90 mm Hg or requires an antihypertensive treatment (e.g., diuretics, beta blockers, ACE inhibitors, calcium channel blockers) at the time the patient is being considered as a candidate for surgery (which should be no longer than 30 days prior to surgery). |
| Current smoker | Current smoker (cigarettes) within one year. |
| Hx of smoking | If the patient has ever been a smoker, the total number of pack/years (number of packs of cigarettes smoked per day times the number of years the patient has smoked) of smoking for this patient is provided. |
| Alcohol use > 2 eh/day | The patient admits to drinking >2 ounces of hard liquor or > two 12 oz. cans of beer or > two 6 oz. glasses of wine per day in the two weeks prior to admission. |
| BMI | Body mass index, calculated as weight (kg) divided by height (m)2 <18.5 underweight, 18.5–24.9 normal weight, 25–29.9 overweight, 30–34.9 obese class I, 35–40 obese class II, ≥40 obese class III |
| Renal failure | Acute (steadily increasing azotemia [increase in BUN] and a rising creatinine of above 3 mg/dl < 24 h prior to surgery) or chronic renal failure requiring treatment with peritoneal dialysis, hemodialysis, hemofiltration, hemodiafiltration, or ultrafiltration within 2 weeks prior to surgery. |
| Hx of COPD | History of severe chronic obstructive pulmonary disease resulting in any one or more of the following: -Functional disability from COPD (e.g., dyspnea, inability to perform ADLs) -Hospitalization in the past for treatment of COPD -Requires chronic bronchodilator therapy with oral or inhaled agents. -An FEV1 of <75% of predicted on pulmonary function testing. Patients are not included whose only pulmonary disease is asthma, an acute and chronic inflammatory disease of the airways resulting in bronchospasm. Patients are not included with diffuse interstitial fibrosis or sarcoidosis. |
| Dyspnea | Difficult, painful, or labored breathing <30 days of surgery. Dyspnea may be symptomatic of numerous disorders that interfere with adequate ventilation or perfusion of the blood with oxygen |
| Steroid use | If a patient requires regular administration of oral or parenteral corticosteroid medications (e.g., Prednisone, Decadron) in the 30 days prior to surgery for a chronic medical condition (e.g., COPD, asthma, rheumatologic disease, rheumatoid arthritis, inflammatory bowel disease). Patients who only receive short course steroids (duration 10 days or less) in the 30 days prior to surgery are not included |
| Hx of revascularization for PVD | History of revascularization/amputation for peripheral vascular disease (PVD): any type of angioplasty (including stent placement) or revascularization procedure for atherosclerotic PVD (e.g., aorta-femoral, femoral-femoral, femoral-popliteal) or a patient who has had any type of amputation procedure for PVD (e.g., toe amputations, transmetatarsal amputations, below the knee or above the knee amputations). Patients who have had amputation for trauma or a resection of abdominal aortic aneurysms should not be included. |
| Restpain/gangrene | Rest pain is a more severe form of ischemic pain due to occlusive disease, which occurs at rest and is manifested as a severe, unrelenting pain aggravated by elevation and often preventing sleep. Gangrene is a marked skin discoloration and disruption indicative of death and decay of tissues in the extremities due to severe and prolonged ischemia. Patients included with ischemic ulceration and/or tissue loss related to peripheral vascular disease. Fournier’s gangrene are not included. |
| Functional status | Functional health status prior to surgery <30days. This variable focuses on the patient’s abilities to perform activities of daily living. |
| Emergency procedure | An emergency case is usually performed as soon as possible and no later than 12 hours after the patient has been admitted to the hospital or after the onset of related preoperative symptomatology. |
| ASA class > 3 | The American Society of Anesthesiology (ASA) Physical Status Classification of the patient�s present physical condition on a scale from 1–5 as it appears on the anesthesia record. ASA 1 -Normal healthy patient ASA 2 -Patient with mild systemic disease ASA 3 -Patient with severe systemic disease ASA 4 -Patient with severe systemic disease that is a constant threat to life ASA 5 -Moribund patient who is not expected to survive without the operation. |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures: M.L. Schermerhorn is a consultant for Endologix, Medtronic, and Boston Scientific. For all other authors none were disclosed.
This manuscript will be presented at the of the New England Society for Vascular Surgery Annual Meeting, Boston, MA, September 21–23, 2012; oral presentation
References
- 1.Nolan BW, De Martino RR, Goodney PP, Schanzer A, Stone DH, Butzel D, et al. Comparison of carotid endarterectomy and stenting in real world practice using a regional quality improvement registry. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.03.009. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eslami MH, McPhee JT, Simons JP, Schanzer A, Messina LM. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307–315. doi: 10.1016/j.jvs.2010.08.080. [DOI] [PubMed] [Google Scholar]
- 3.Vogel TR, Dombrovskiy VY, Haser PB, Scheirer JC, Graham AM. Outcomes of carotid artery stenting and endarterectomy in the United States. J Vasc Surg. 2009;49:325–330. doi: 10.1016/j.jvs.2008.08.112. [DOI] [PubMed] [Google Scholar]
- 4.Sidawy AN, Zwolak RM, White RA, Siami FS, Schermerhorn ML, Sicard GA. Risk-adjusted 30-day outcomes of carotid stenting and endarterectomy: Results from the SVS Vascular Registry. J Vasc Surg. 2009;49:71–79. doi: 10.1016/j.jvs.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Goodney PP, Likosky DS, Cronenwett JL. Factors associated with stroke or death after carotid endarterectomy in Northern New England. J Vasc Surg. 2008;48:1139–1145. doi: 10.1016/j.jvs.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Kang JL, Chung TK, Lancaster RT, Lamuraglia GM, Conrad MF, Cambria RP. Outcomes after carotid endarterectomy: is there a high-risk population? A National Surgical Quality Improvement Program report. J Vasc Surg. 2009;49:331–338. doi: 10.1016/j.jvs.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Fink AS, Campbell DA, Jr, Mentzer RM, Jr, Henderson WG, Daley J, Bannister J, et al. The National Surgical Quality Improvement Program in non-veterans administration hospitals: initial demonstration of feasibility. Ann Surg. 2002;236:344–353. doi: 10.1097/00000658-200209000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiloach M, Frencher SK, Jr, Steeger JE, Rowell KS, Bartzokis K, Tomeh MG, et al. Toward Robust Information: Data Quality and Inter-Rater Reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2009;210:6–16. doi: 10.1016/j.jamcollsurg.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Bensley RP, Yoshida S, Lo RC, Fokkema M, Darling JD, Handam AD, et al. Accuracy of administrative versus clinical data to evaluate carotid endarterectomy and caritid stenting. Manuscript under revision J Vasc Surg. 2012. doi: 10.1016/j.jvs.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rockman CB, Garg K, Jacobowitz GR, Berger JS, Mussa FF, Cayne NS, et al. Outcome of carotid artery interventions among female patients, 2004 to 2005. J Vasc Surg. 2011;53:1457–1464. doi: 10.1016/j.jvs.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Best WR, Khuri SF, Phelan M, Hur K, Henderson WG, Demakis JG, et al. Identifying patient preoperative risk factors and postoperative adverse events in administrative databases: results from the department of veterans affairs national surgical quality improvement program. J Am Coll Surg. 2002;194:257–266. doi: 10.1016/s1072-7515(01)01183-8. [DOI] [PubMed] [Google Scholar]
- 12.Hertzer NR. The Nationwide Inpatient Sample may contain inaccurate data for carotid endarterectomy and carotid stenting. J Vasc Surg. 2011;55:263–266. doi: 10.1016/j.jvs.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 13.Riles TS, Imparato AM, Jacobowitz GR, Lamparello PJ, Giangola G, Adelman MA, et al. The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg. 1994;19:206–216. doi: 10.1016/s0741-5214(94)70096-6. [DOI] [PubMed] [Google Scholar]
- 14.de Borst GJ, Moll FL, van de Pavoordt HD, Mauser HW, Kelder JC, Ackerstaf RG. Stroke from carotid endarterectomy: when and how to reduce perioperative stroke rate? Eur J Vasc Endovasc Surg. 2001;21:484–489. doi: 10.1053/ejvs.2001.1360. [DOI] [PubMed] [Google Scholar]
- 15.Pennekamp CW, Tromp SC, Ackerstaff RG, Bots ML, Immink RV, Spiering W, et al. Prediction of cerebral hyperperfusion after carotid endarterectomy with transcranial Doppler. Eur J Vasc Endovasc Surg. 2012;43:371–376. doi: 10.1016/j.ejvs.2011.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Akbari CM, Pulling MC, Pomposelli FB, Jr, Gibbons GW, Campbell DR, LoGerfo FW. Gender and carotid endarterectomy: Does it matter? J Vasc Surg. 2000;31:1103–1109. doi: 10.1067/mva.2000.106490. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. The Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 18.Calvillo-King L, Xuan L, Zhang S, Tuhrim S, Halm EA. Predicting Risk of Perioperative Death and Stroke After Carotid Endarterectomy in Asymptomatic Patients. Stroke. 2010;41:2786–2794. doi: 10.1161/STROKEAHA.110.599019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothwell PM, Goldstein LB. Carotid Endarterectomy for Asymptomatic Carotid Stenosis. Stroke. 2004;35:2425–2427. doi: 10.1161/01.STR.0000141706.50170.a7. [DOI] [PubMed] [Google Scholar]
- 20.den Hartog AG, Algra A, Moll FL, de Borst GJ. Mechanisms of gender-related outcome differences after carotid endarterectomy. J Vasc Surg. 2010;52:1062–1071. e6. doi: 10.1016/j.jvs.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 21.Bensley RP, Lo RC, Hurks R, Chaikof EL, Hamdan AD, Wyers MC, et al. Gender differences in presentation of patients undergoing CEA and CAS in VSGNE. Manuscript in progress. 2012 [Google Scholar]
- 22.Sidawy AN, Aidinian G, Johnson Iii ON, White PW, DeZee KJ, Henderson WG. Effect of chronic renal insufficiency on outcomes of carotid endarterectomy. J Vasc Surg. 2008;48:1423–1430. doi: 10.1016/j.jvs.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Kretz B, Abello N, Brenot R, Steinmetz E. The impact of renal insufficiency on the outcome of carotid surgery is influenced by the definition used. J Vasc Surg. 2010;51:43–50. doi: 10.1016/j.jvs.2009.08.070. [DOI] [PubMed] [Google Scholar]
- 24.Hamdan AD, Pomposelli FB, Jr, Gibbons GW, Campbell DR, LoGerfo FW. Renal insufficiency and altered postoperative risk in carotid endarterectomy. J Vasc Surg. 1999;29:1006–1011. doi: 10.1016/s0741-5214(99)70241-7. [DOI] [PubMed] [Google Scholar]
- 25.Stoner MC, Abbott WM, Wong DR, Hua HT, Lamuraglia GM, Kwolek CJ, et al. Defining the high-risk patient for carotid endarterectomy: an analysis of the prospective National Surgical Quality Improvement Program database. J Vasc Surg. 2006;43:285–295. doi: 10.1016/j.jvs.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 26.Tu JV, Wang H, Bowyer B, Green L, Fang J, Kucey D, et al. Risk Factors for Death or Stroke After Carotid Endarterectomy. Stroke. 2003;34:2568–2573. doi: 10.1161/01.STR.0000092491.45227.0F. [DOI] [PubMed] [Google Scholar]
- 27.Halm EA, Tuhrim S, Wang JJ, Rockman C, Riles TS, Chassin MR. Risk Factors for Perioperative Death and Stroke After Carotid Endarterectomy. Stroke. 2009;40:221–229. doi: 10.1161/STROKEAHA.108.524785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bond R, Rerkasem K, Rothwell PM. Systematic review of the risks of carotid endarterectomy in relation to the clinical indication for and timing of surgery. Stroke. 2003;34:2290–2301. doi: 10.1161/01.STR.0000087785.01407.CC. [DOI] [PubMed] [Google Scholar]
- 29.Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Sternbergh WC, et al. Age and Outcomes After Carotid Stenting and Endarterectomy. Stroke. 2011;42:3484–3490. doi: 10.1161/STROKEAHA.111.624155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Borst GJ, Zanen P, de Vries J-PP, van de Pavoordt ED, Ackerstaff RG, Moll FL. Durability of surgery for restenosis after carotid endarterectomy. J Vasc Surg. 2008;47:363–371. doi: 10.1016/j.jvs.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Hobson RW, 2nd, Goldstein JE, Jamil Z, Lee BC, Padberg FT, Jr, Hanna AK, et al. Carotid restenosis: operative and endovascular management. J Vasc Surg. 1999;29:228–235. doi: 10.1016/s0741-5214(99)70376-9. discussion 35-8. [DOI] [PubMed] [Google Scholar]
- 32.AbuRahma AF, Abu-Halimah S, Bensenhaver J, Nanjundappa A, Stone PA, Dean LS, et al. Primary carotid artery stenting versus carotid artery stenting for postcarotid endarterectomy stenosis. J Vasc Surg. 2009;50:1031–1039. doi: 10.1016/j.jvs.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 33.Davenport DL, Xenos ES, Hosokawa P, Radford J, Henderson WG, Endean ED. The influence of body mass index obesity status on vascular surgery 30-day morbidity and mortality. J Vasc Surg. 2009;49:140–147. e1. doi: 10.1016/j.jvs.2008.08.052. [DOI] [PubMed] [Google Scholar]
- 34.Mullen JT, Moorman DW, Davenport DL. The Obesity Paradox: Body Mass Index and Outcomes in Patients Undergoing Nonbariatric General Surgery. Ann Surg. 2009;250:166–172. doi: 10.1097/SLA.0b013e3181ad8935. [DOI] [PubMed] [Google Scholar]
- 35.Reeves JG, Kasirajan K, Veeraswamy RK, Ricotta Ii JJ, Salam AA, Dodson TF, et al. Characterization of resident surgeon participation during carotid endarterectomy and impact on perioperative outcomes. J Vasc Surg. 2011;55:268–273. doi: 10.1016/j.jvs.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 36.Giles KA, Wyers MC, Pomposelli FB, Hamdan AD, Ching YA, Schermerhorn ML. The impact of body mass index on perioperative outcomes of open and endovascular abdominal aortic aneurysm repair from the National Surgical Quality Improvement Program, 2005–2007. J Vasc Surg. 2010;52:1471–1477. doi: 10.1016/j.jvs.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Musser DJ, Nicholas GG, Reed JF., 3rd Death and adverse cardiac events after carotid endarterectomy. J Vasc Surg. 1994;19:615–622. doi: 10.1016/s0741-5214(94)70034-6. [DOI] [PubMed] [Google Scholar]
- 38.Rerkasem K, Rothwell PM. Systematic Review of the Operative Risks of Carotid Endarterectomy for Recently Symptomatic Stenosis in Relation to the Timing of Surgery. Stroke. 2009;40:e564–e572. doi: 10.1161/STROKEAHA.109.558528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.