Abstract
Chronic inflammation and oxidative stress have been implicated in the pathophysiology of Major Depressive Disorder (MDD), as well as in a number of chronic medical conditions. The aim of this study was to examine the relationship between peripheral inflammatory and oxidative stress markers in un-medicated subjects with MDD compared to non-depressed healthy controls and compared to subjects with MDD after antidepressant treatment. We examined the relationships between IL-6, IL-10, and the IL-6/IL-10 inflammatory ratio vs. F2-isoprostanes (F2-IsoP), a marker of oxidative stress, in un-medicated MDD patients (n = 20) before and after 8 weeks of open-label sertraline treatment (n = 17), compared to healthy non-depressed controls (n = 20). Among the un-medicated MDD subjects, F2-IsoP concentrations were positively correlated with IL-6 concentrations (p < 0.05) and were negatively correlated with IL-10 concentrations (p < 0.01). Accordingly, F2-IsoP concentrations were positively correlated with the ratio of IL-6/IL-10 (p < 0.01). In contrast, in the control group, there were no significant correlations between F2-IsoPs and either cytokine or their ratio. After MDD subjects were treated with sertraline for 8 weeks, F2-IsoPs were no longer significantly correlated with IL-6, IL-10 or the IL-6/IL-10 ratio. These data suggest oxidative stress and inflammatory processes are positively associated in untreated MDD. Our findings are consistent with the hypothesis that the homeostatic buffering mechanisms regulating oxidation and inflammation in healthy individuals become dysregulated in untreated MDD, and may be improved with antidepressant treatment. These findings may help explain the increased risk of comorbid medical illnesses in MDD.
Keywords: Inflammation, Oxidation, Oxidative stress, IL-6, IL-10, F2-isoprostane, Depression, Sertraline
1. Introduction
Chronic inflammation and oxidative stress have been implicated in the pathophysiology of Major Depressive Disorder (MDD) (Berk et al., 2011; Capuron and Miller, 2011; Chauhan and Chauhan, 2006; Croonenberghs et al., 2002; Forlenza et al., 2007; Khanzode et al., 2003; Maes, 2011a,b, 1999; Miller, 2010; Raison and Miller, 2011; Wolkowitz et al., 2008) as well as a number of serious medical conditions (Maes et al., 2011b), including cardiovascular disease and atherosclerosis (Krishnan, 2010; Lakshmi et al., 2009; Tousoulis et al., 2008; Uno and Nicholls, 2010), chronic renal disease (Cottone et al., 2008), pulmonary disease (Jelic and Le Jemtel, 2008), rheumatoid arthritis (Stamp et al., 2012), certain cancers (Khansari et al., 2009; Maes et al., 2011b; Reuter et al., 2010), metabolic syndrome, obesity and diabetes (Agrawal et al., 2007; Assumpcao et al., 2008; Ferder et al., 2006; Guerrero-Romero and Rodriguez-Moran, 2006), and in the normal physiology of cellular aging and immuno-senescence (Cannizzo et al., 2011; De la Fuente and Miquel, 2009). While inflammation and oxidation have generally been studied separately in these conditions, the interplay between them has been less well-studied, despite mounting evidence that their interaction plays a major role in the pathogenesis of many diseases (Ambade and Mandrekar, 2012; Forlenza and Miller, 2006; Maes et al., 2011e, 2007; Rahman, 2003; Sarandol et al., 2007a). Examining the interplay between chronic inflammatory states and oxidative stress is likely to deepen our understanding of the pathophysiology of MDD and other diseases, offer greater insight into the associations between MDD and co-morbid medical illnesses with inflammatory or oxidative associations (such as diabetes, arthritis, dementia, metabolic syndrome and cardiovascular disease), and potentially guide novel approaches to the treatment of depression and its comorbid systemic diseases (Maes et al., 2011c; Nemeroff and Goldschmidt-Clermont, 2012; Wolkowitz et al., 2011b).
A large body of evidence suggests that MDD is accompanied by activation of inflammatory pathways, reflected by an increased levels of inflammatory cytokines, such as Interleukin (IL)-1β, IL-2, IL-6, interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α) (Anisman et al., 1999a,b; Maes, 1999; Nassberger and Traskman-Bendz, 1993). Two recent meta-analyses showed significantly higher IL-6 concentrations among depressed versus non-depressed patients but no significant difference in IL-10 concentrations (Dowlati et al., 2010; Hiles et al., 2012). A causal relationship between inflammation and depression is suggested by observational studies of patients undergoing immunotherapy for hepatitis C or cancer who developed depressed mood and neurovegetative accompanying these treatments (Capuron et al., 2001; Dieperink et al., 2000; Kelley et al., 2003; Valentine and Meyers, 1995; Zdilar et al., 2000). Other small-scale studies also suggest antidepressant effects of anti-inflammatory medications (Muller et al., 2006; Nery et al., 2008) as well as anti-inflammatory effects of antidepressants (Abbasi et al., 2012; Felger et al., 2012; Hannestad et al., 2011).
A smaller but growing body of evidence also points to increased oxidative stress in MDD. Elevated plasma and/or urine oxidative stress markers (e.g., increased F2-isoprostanes [IsoPs] or 8-hydroxydeoxyguanosine [8-OHdG], along with decreased anti-oxidant compounds, such as Vitamins C and E) have been reported in individuals with MDD and in those with chronic psychological stress (Forlenza and Miller, 2006; Irie et al., 2001a,b, 2005; Maes et al., 2000), and the concentration of peripheral oxidative stress markers is positively correlated with the severity and chronicity of depression (Forlenza and Miller, 2006; Irie et al., 2003, 2002; Maes et al., 2011a; Miyaoka et al., 2005; Tsuboi et al., 2004; Yager et al., 2010). Antioxidant compounds are also being considered as possible antidepressant treatments (Gibson et al., 2012; Khanzode et al., 2003; Scapagnini et al., 2012; Wolkowitz et al., 2011b).
Inflammation and oxidative stress are inextricably connected in physiologic as well as disease states; they have even been termed “essential partners” in certain diseases (Ambade and Mandrekar, 2012). Under normal physiologic conditions, oxidative stress and activation of the immune system are generally short-lived due to intrinsic negative feedback mechanisms, such as increased production of anti-oxidant compounds or of anti-inflammatory cytokines. In certain chronic disease states, however, both of these systems remain activated and may, indeed, form a positive self-sustaining feedback loop, or a “co-activation” state (Jesmin et al., 2010). Over time, such co-activation may lead to a higher risk of disease and to more serious disease (Ambade and Mandrekar, 2012; Il’yasova et al., 2008; Jesmin et al., 2010; Khansari et al., 2009; Kotani and Taniguchi, 2012; Kregel and Zhang, 2007; Martinon, 2010; Rahman, 2003; Skalicky et al., 2008; Terlecky et al., 2012; Tschopp and Schroder, 2010).
The aim of the current study was to examine the relationship between inflammation and oxidative stress in the plasma of unmedicated subjects with MDD before and after antidepressant treatment, and in un-medicated subjects with MDD compared to non-depressed healthy controls. We hypothesized that un-medicated subjects with MDD would show stronger positive correlations between oxidative stress and inflammatory markers than the same subjects after antidepressant treatment and than healthy non-depressed controls.
2. Methods
2.1. Subjects
Twenty subjects with MDD, diagnosed with the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 2002), and 20 matched healthy controls (matched by sex, ethnicity and age ±3 yr) were enrolled. Subjects were recruited by fliers, craigslist postings (http://sfbay.craigslist.org), and newspaper advertisements and, in the case of depressed subjects, clinical referrals. Subjects gave written informed consent to participate in this study, which was approved by the University of California, San Francisco (UCSF) Committee on Human Research and were paid for their participation. SCID interviews were conducted by an experienced clinical psychologist and were clinically verified by a separate psychiatric interview with a Board-certified psychiatrist. Depressed subjects with psychosis, post-traumatic stress disorder or bipolar histories were excluded, although other co-morbid anxiety disorders were allowed when the depressive diagnosis was determined to be the primary diagnosis. Seven of the depressed subjects had one or more secondary co-morbid psychiatric diagnoses as follows: three with generalized anxiety disorder, two with obsessive compulsive disorder, two with binge eating disorder (one of whom was in remission) and one with social anxiety disorder. Healthy controls were also screened with the SCID, and were required to have no present or past history of any DSM-IV Axis I or Axis II diagnosis. Potential subjects were excluded if they met SCID criteria for alcohol or substance abuse within 6 months of entering the study. Subjects in both groups were medically healthy (assessed by physical examination, review of systems and screening routine laboratory tests), had no acute illnesses or infections, and had not had any vaccinations within 6 weeks of entering the study. All subjects (depressed and control) were free of any psychotropic medications, including antidepressants, antipsychotics and mood stabilizers, as well as any hormone supplements, steroid-containing birth control or other interfering medications or Vitamin supplements above the U.S. Recommended Daily Allowances (e.g. Vitamin C, 90 mg/day), for a minimum of 6 weeks before entry into the study (with the exception of short-acting sedative-hypnotics, as needed, up to a maximum of 3 times per week, but none within 1 week prior to testing). The sample described in the present report incorporates the smaller sample previously reported on, which was used to test a different set of hypotheses (Dhabhar et al., 2009).
2.2. Procedures
Subjects were admitted as outpatients to the UCSF Clinical and Translational Science Institute’s Clinical Research Center at 8:00 am, having fasted (except water) since 10:00 pm the night before. Before proceeding with testing, all subjects were required to test negative on a urine toxicology screen (measuring the presence of abused drugs) and, in women of childbearing capacity, a urine pregnancy test. After the subjects had sat quietly for 45 min, blood samples were obtained for the assay of serum IL-6, IL-10 and for the oxidative metabolite, plasma F2-IsoP. Severity of depression in the depressed subjects was ascertained with the observer-rated 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1967). Depressed and control subjects were also rated with the Inventory of Depressive Symptomatology, 30-item self-rated version (IDS) for purposes of comparing the two groups, since the HDRS-17 is not intended for use in non-depressed populations, and since the IDS shows greater sensitivity at lower levels of depressive severity (Rush et al., 1996). The IDS was also used in analyses in which depressive severity was utilized as an independent variable across groups. Scores on the IDS range from 0 to 84 and are highly correlated with scores on the HDRS-17 (Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS), 2012). Ratings and blood assays were performed blind to each other.
Following these baseline behavioral and biochemical tests, the MDD subjects were treated in an open-label manner with sertraline for 8 weeks, after which blood was re-drawn for F2-IsoP and cytokine assays, and depression ratings repeated. Sertraline dosing was based on clinicians’ judgment, based on efficacy and tolerability. Sertraline dosing began with 50 mg per day, increasing to a maximum of 200 mg per day, as tolerated and as warranted by clinical response. In two cases, the beginning dose was initially lowered to 25 mg per day because of transient side effects. Medication compliance was monitored by pill counts and by plasma antidepressant levels at week 4 and week 8 of treatment. The mean plasma concentration of (sertraline + N-desmethylsertraline) at week 4 was 46 ± 23 ng ml−1; range: 10–97 ng ml−1, and at week 8 was 67 ± 37 ng ml−1; range: 10–146 ng ml−1. All individuals had plasma concentrations within the range of published steady state concentrations for sertraline at therapeutic doses (Mauri et al., 2002), indicating good compliance with medication treatment. One depressed subject prematurely dropped out of the study due to clinical worsening while on sertraline, and two were excluded due to developing new exclusionary criteria (periodontitis and elevated fasting blood sugar) during the 8 week follow-up period, leaving 17 treated MDD subjects. Control subjects were not reevaluated after their initial baseline visit.
2.3. Assays
2.3.1. Blood collection
Blood for the plasma F2-IsoP assay was collected into EDTA tubes with no vacuum, and blood for the serum IL-6 and IL-10 assays was collected into serum separator tubes. Plasma and sera were frozen and stored at −80 C until assay.
2.3.2. Oxidative stress assay
F2-IsoPs are prostaglandin-like compounds formed in vivo via a non-enzymatic mechanism involving the free radical-initiated peroxidation of arachidonic acid. F2-IsoPs are very accurate measures of lipid peroxidation and are important markers of oxidative stress (Milne et al., 2005). F2-IsoPs were measured by the Eicosanoid Laboratory at Vanderbilt University. F2-IsoPs were extracted and purified with solid phase extraction and thin layer liquid chromatography and then converted to trimethylsilyl ether derivatives and analyzed by gas chromatography–mass spectrometry (GC–MS) as described previously (Milne et al., 2007; Morrow and Roberts, 2002). F2-IsoP values were not available for one MDD subject at baseline and for three MDD subjects at Week 8 due to poor chromatography, and for one MDD subject at Week 8 due to a value below the level of detection.
2.3.3. Inflammatory cytokine assays
IL-6 is a prototypic pro-inflammatory cytokine (although it also has some anti-inflammatory effects, especially during acute inflammation) (Scheller et al., 2011; Xing et al., 1998). It exerts stimulatory effects on T- and B-cells, and thus generally favors chronic inflammatory responses. IL-10 is an anti-inflammatory/immuno-modulating cytokine, and the IL-6/IL-10 ratio is considered an index of net pro-inflammatory activity (Dhabhar et al., 2009; Fredericks et al., 2010). A high sensitivity enzyme-linked immunosorbent assay was used to quantify IL-6 and IL-10 concentrations (R&D Systems, Minneapolis, MN). The IL-6 assay intra- and interassay coefficients of variation are 7% and 8% respectively. For IL- 10, intra- and inter-assay coefficients of variation are 8% and 11% respectively. Each sample was analyzed in duplicate according to manufacturer protocol. Cytokine assays were performed in the lab of Dr. Firdaus Dhabhar at Stanford University. One healthy control was an extreme outlier with serum IL-6 and IL-10 concentrations > 16 and > 10 SD’s (respectively) above the mean, which were not normalized by standard transformation methods; this subject’s cytokine data were excluded from analysis. In several cases (7 controls and 7 MDD subjects at baseline and 7 MDD subjects at Week 8), IL-10 concentrations were below the level of detection of the assay; in such cases, IL-10 values were conservatively set to just below the minimum observed value in either group, specifically 0.031 pg/ml.
Due to lack of assay data or the lack of Week 8 treatment visits for certain subjects, the final samples sizes for F2-IsoPs were: 20 controls, 19 MDD subjects at baseline and 13 MDD subjects at Week 8; for IL-6 were: 19 controls, 20 MDD subjects at baseline and 17 MDD subjects at Week 8; and for IL-10 were: 19 controls, 19 MDD subjects at baseline and 17 MDD subjects at Week 8.
2.4. Statistics
Distributions were examined for normality, and non-normal distributions were natural log transformed. Accordingly, each of the major dependent variables, IL-6, IL-10, IL-6/IL-10 ratio and F2-IsoPs, was transformed to its natural log prior to analysis. As mentioned, one healthy control subject had extremely elevated cytokine levels (10–16 SD’s above the mean), which could not be normalized by log or square root transformation, and that subject’s cytokine data were not included. We assessed the impact of age, sex, body mass index, and current tobacco use as potential confounds of these biochemical markers. Between-group comparison of the demographic variables was by independent sample t-tests, Chi-square tests, and univariate analysis of covariance (ANCOVA), controlling for the significant demographic confounders where indicated. Within-group changes with treatment were assessed with paired t-tests. Correlations between the biochemical measures (e.g., IL-6 vs. F2-IsoPs) were assessed by partial correlations, controlling for significant covariates: IL-6 and F2-IsoPs were significantly associated with BMI (r = 0.32, p = 0.05; p = 0.38, p = 0.02, respectively), and IL-10 and IL-6/IL-10 with current tobacco use (t = −3.43, p < 0.01; t = 3.32, p < 0.01, respectively). For these analyses, independent sample tests were utilized rather than paired tests, since the total sample sizes for most analyses were unequal after taking missing data into account. Next, we tested whether the relationship between oxidative (F2-IsoPs) and immunologic markers were significantly different in subjects depending on presence and degree of depressive symptoms with linear regression modeling and interaction analyses. First, we tested the dichotomous group affiliation (MDD vs. controls) interaction with F2-IsoPs predicting IL-6, IL-10, and IL-6/IL-10. Then, we tested an interaction between the IDS and F2-IsoPs predicting IL-6, IL-10, and IL-6/IL-10. We followed moderation techniques as outlined by Cohen et al. (2003), Hayes and Matthes (2009), and Preacher et al. (2006), and used the modprobe macro for SPSS at http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html. All tests were two-tailed with an alpha = 0.05.
3. Results
3.1. Demographics
As shown in Table 1, age, sex distribution, ethnicity distribution, BMI and current tobacco use did not significantly differ between the MDD and control groups. The mean IDS ratings in the control and MDD groups at baseline were 4.95 ± 4.89 versus 37.21 ± 9.59, respectively (p < 0.003) (Table 1).
Table 1.
Characteristics of the control and MDD groups.
| Controls | n | MDD | n | t, F, Chi | p | |
|---|---|---|---|---|---|---|
| Age (yr) | 36.42 ± 12.13 | 19 | 37.00 ± 10.77 | 20 | −1.58 | 0.88 |
| Sex (% male) | 37.00% | 19 | 35.00% | 20 | 0.12 | 0.91 |
| BMI | 24.48 ± 3.73 | 19 | 24.78 ± 4.29 | 20 | −0.23 | 0.82 |
| Ethnicity | 19 | 20 | 0.84 | 0.84 | ||
| Caucasian | 73.68% | 70.00% | ||||
| African-American | 15.79% | 10.00% | ||||
| Asian | 5.26% | 10.00% | ||||
| Other or mixed | 5.26% | 10.00% | ||||
| Current tobacco use (%) | 19 | 20 | 2.30 | 0.32 | ||
| None | 89.47% | 75.00% | ||||
| Some days | 10.53% | 15.00% | ||||
| Daily | 0.00% | 10.00% | ||||
| Lifetime tobacco use (%) | 42.11% | 19 | 50.00% | 20 | 0.48 | 0.63 |
| IDS | 4.95 ± 4.89 | 19 | 37.21 ± 9.59 | 14 | −11.53 | 0.00 |
| F2-IsoP (ng/ml) | 0.044 ± 0.015 | 19 | 0.043 ± 0.016 | 19 | 0.11 | 0.75 |
| IL-6 (pg/ml) | 0.73 ± 0.37 | 18 | 0.84 ± 0.823 | 20 | 0.75 | 0.39 |
| IL-10 (pg/ml) | 0.55 ± 0.61 | 18 | 0.40 ± 0.66 | 19 | 0.03 | 0.87 |
| IL-6/IL-10 | 8.63 ± 12.21 | 18 | 11.68 ± 14.20 | 19 | 0.00 | 0.95 |
Data are means ± SD of raw data. p values for IL-6, IL-10, IL-6/IL-10, and F2-IsoP were derived from natural log-transformed data. Between-group means comparisons were assessed by univariate ANCOVA for F2-isoP’s and IL-6, adjusted for BMI, and IL-10 and IL-6/IL-10, adjusted for current tobacco use. The sample sizes above were not identical across all variables where data were missing.
3.2. Cytokine and isoprostane concentrations in depressed and control subjects
At study entry, the depressed and control groups did not significantly differ in concentrations of IL-6, IL-10, IL-6/IL-10, or F2-IsoPs, (Table 1), although this study was not intended to test differences in baseline cytokine levels, since that was tested previously in a subset of the current sample (Dhabhar et al. 2009). Notably, anxiety levels as measured by the Hamilton Anxiety Rating Scale were also not found to correlate significantly with cytokine levels or F2-IsoPs.
3.3. Effects of sertraline treatment
With sertraline treatment, depressed subjects showed highly significant declines in depressive symptoms. HDRS-17 ratings decreased from 18.71 ± 3.22 to 10.24 ± 6.32 (p < 0.001). Mean IL-6 concentration increased from 0.96 to 1.31 pg/mL (p = 0.03). However, F2-IsoPs, IL-10, and the IL-6/IL-10 ratio did not significantly change with treatment (Table 2).
Table 2.
Baseline measurements vs. Week 8 of sertraline treatment for MDD group.
| Baseline | Week 8 | n | Paired-t | p | |
|---|---|---|---|---|---|
| HDRS | 18.71 ± 3.22 | 10.24 ± 6.32 | 17 | 5.23 | <.001 |
| F2-IsoP (ng/ml) | 0.047 ± 0.016 | 0.048 ± 0.019 | 13 | 0.07 | 0.94 |
| IL-6 (pg/ml) | 0.96 ± 0.84 | 1.31 ± 0.98 | 17 | −2.37 | 0.03 |
| IL-10 (pg/ml) | 0.27 ± 0.35 | 0.37 ± 0.62 | 17 | −0.19 | 0.85 |
| IL-6/IL-10 | 12.92 ± 14.52 | 16.06 ± 16.19 | 17 | −1.62 | 0.13 |
Data are means ± SD of raw data. p values were calculated using paired-sample t-tests of log-transformed F2-IsoP and cytokine values. Certain sample sizes and baseline values for the MDD’s in this table differ from those in Table 1, because only MDD’s with both baseline and Week 8 data are included in Table 2.
3.4. Correlation between inflammation and oxidation markers as a function of MDD diagnosis
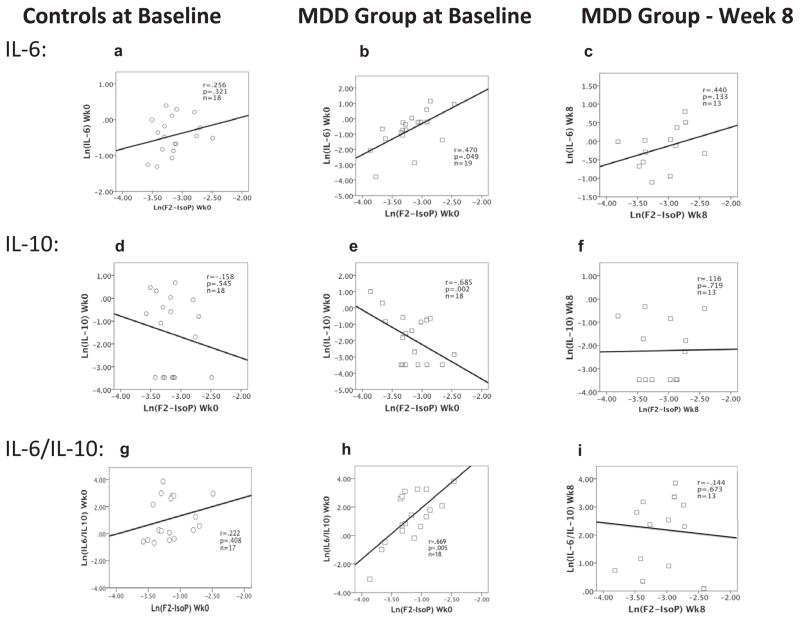
Among the depressed subjects, F2-IsoP concentrations were significantly positively correlated with IL-6 concentrations (r = 0.47, p < 0.05) (Fig. 1b) and significantly negatively correlated with IL-10 concentrations (r =−.69, p = 0.002) (Fig. 1e). Accordingly, F2-IsoP concentrations were strongly positively correlated with the ratio of IL-6/IL-10 (r = 0.70, p = 0.005) (Fig. 1h). No significant correlations between F2-IsoPs and both cytokines and their ratio were seen in the control group (Fig. 1a, d and g). Following 8 weeks of sertraline treatment in the MDD group, the correlations between F2-IsoPs and IL-6 (r = 0.44, p = 0.13), IL-10 (r = 0.12, p = 0.72), and the IL-6/IL-10 ratio (r = −0.14, p = 0.67) were no longer statistically significant (Fig 1c, f and i).
Fig. 1.
Correlations between F2-IsoPs and IL-6, IL-10 and the IL-6/IL-10 ratio among the control group at baseline (1a, d and g, respectively), and among the MDD group at baseline (1b, e and h, respectively) and after 8 weeks treatment with sertraline (1c, f and i, respectively). Figures portray raw data; correlation coefficients and p values are determined by partial correlation tests, adjusted for covariates BMI and tobacco when significantly correlated.
3.5. Differential correlation between inflammation and oxidation markers as a function of depressive symptom severity
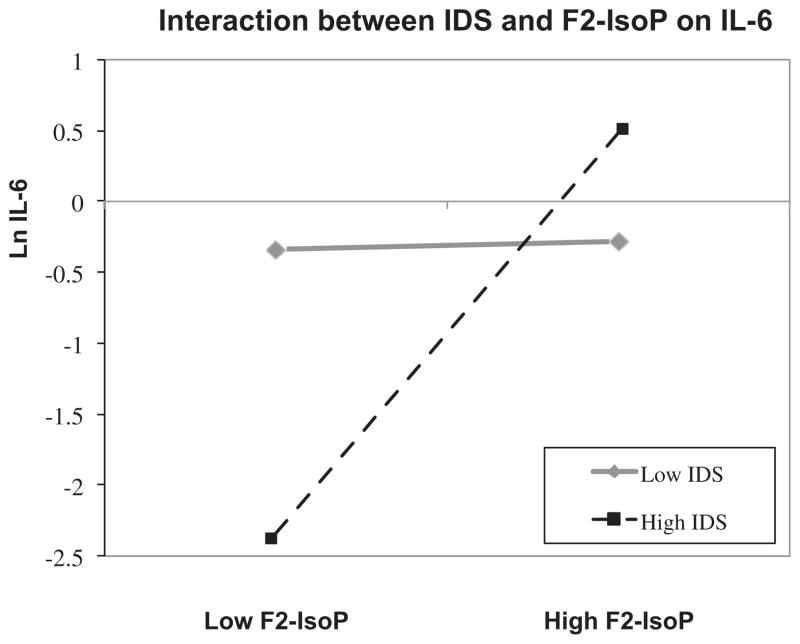
In a final set of analyses, we tested whether subjects with greater or lesser severity ratings of depression (across diagnostic groups) would show different degrees of association between cytokines (IL-6, IL-10, and IL-6/IL-10) and F2-IsoPs, as described in the Methods section above. Results indicated that group affiliation (MDD vs. control) did not significantly interact with F2-IsoPs to predict IL-6. On the other hand, IDS did moderate the relationship between F2-IsoPs and IL-6 (β = 0.059, SE = 0.028, p < 0.05). These results indicate that, although the relationship between oxidative and inflammation markers did not significantly differ by group affiliation, they did differ along the continuum of depressive symptom severity across groups. Specifically, oxidative and inflammatory markers were significantly positively correlated as depressive symptom severity increased. We employed the Johnson– Neyman statistical approach to examine the significant intervals at which depression significantly moderated the relationship between oxidative stress and inflammation (Preacher et al., 2006). At IDS ratings at or above 17.5 (which corresponds to a HDRS-17 score of 22, indicating “severe” depression), F2-IsoPs were significantly correlated with IL-6. However, at values below 17.5, F2-IsoPs were not significantly correlated with IL-6 (see Fig. 2). There were no significant interaction effects between IDS ratings and F2-IsoPs in predicting IL-10 or the IL-6/IL-10 ratio.
Fig. 2.
Two-way interaction plot illustrating the differential relationship between F2-IsoPs and IL-6, as a function of high versus low depression scores on the IDS. High and low are defined as 1 SD above or below the mean value for both F2-IsoPs and the IDS, based on the approach described by Hayes and Matthes (2009) and the corresponding modprobe macro in SPSS. In subjects with low depression scores, F2- IsoP and IL-6 concentrations are not significantly related. But in subjects with high depression ratings, progressively higher F2-IsoP concentrations are associated with higher IL-6 concentrations.
4. Discussion
The major aim of this study was to assess the relationship between oxidative stress and inflammatory markers in un-medicated MDD. As hypothesized, oxidation and inflammation were significantly positively correlated in un-medicated MDD subjects but not in healthy controls. Interaction analyses of baseline data demonstrated that this differential interplay between inflammation and oxidative stress was observed with increased levels of depression (IDS ratings ≥ 17.5). Oxidative stress in un-medicated MDD subjects was positively correlated with the pro-inflammatory cytokine IL-6, negatively with the anti-inflammatory cytokine IL-10, and hence positively correlated with the inflammatory index IL-6/IL-10. After MDDs were treated with sertraline for 8 weeks, the partial correlation coefficient between IL-6 and F2-IsoPs dropped from 0.47 to 0.44 and became non-significant, likely due to the reduced sample size at week 8. However, F2-IsoP’s strong negative association with IL-10 and strong positive association with IL-6/IL-10 observed at baseline were no longer observed after 8 weeks treatment with sertraline. To our knowledge, this study is the first to show in un-medicated depressed subjects that there is a significant positive association between inflammatory and oxidative stress markers, which is not present in healthy non-depressed controls, suggesting that an important biological characteristic of major depression may be the absence of a counter-regulatory mechanism buffering the relationship between these two systems.
As discussed above, theories regarding the roles of inflammation and oxidation in the pathophysiology of MDD are gaining empirical support, although few, if any, studies have previously assessed the interplay between these pathologic processes in MDD. Our results are consistent with the growing literature of positive correlations between oxidative and inflammatory processes in several serious chronic medical conditions (such as cardiovascular, renal and pulmonary diseases, diabetes and metabolic syndrome, etc., reviewed above (Agrawal et al., 2007; Cottone et al., 2006; Ferder et al., 2006; Guerrero-Romero and Rodriguez-Moran, 2006; Jelic and Le Jemtel, 2008; Krishnan, 2010; Lakshmi et al., 2009). The direct correlation between oxidation and inflammation seen in MDD might help explain the increased risk of certain serious medical co-morbid illnesses that have oxidative and/or inflammatory underpinnings (Forlenza and Miller, 2006; Leuner et al., 2012; Maes et al., 2011b; Nemeroff and Goldschmidt-Clermont, 2012; Tousoulis et al., 2008; Uno and Nicholls, 2010). Of course, in the absence of prospective longitudinal data, it is impossible to gauge whether the oxidative/immune interplay we observed is causally related to depression or medical comorbidities and, if so, which antedates the other. Notably, all of the subjects in the present study were medically healthy, with no comorbid medical conditions or medications that would be expected to alter oxidative or inflammatory markers, showing that the presence of medical illness is not necessary for this relationship. Further, BMI and tobacco use, which can affect immune and oxidative markers, did not differ between groups, and analyses were controlled for BMI and tobacco use when needed, making BMI and tobacco use unlikely explanations of our findings.
In normal physiologic conditions, inflammatory and oxidative processes assist in adaptive responses to stressors and defense against pathogens. Restoration to a healthy state, however, depends upon these processes diminishing when no longer needed. Elaborate systems have evolved to keep these systems in check under physiologic conditions through negative feedback mechanisms, such as increased enzymatic and non-enzymatic antioxidant defenses (e.g. superoxide dismutase, glutathione peroxidase, catalase, GSH, vitamin C and vitamin E, etc.) (De la Fuente and Miquel, 2009) as well as anti-inflammatory cytokines (e.g. IL-10, IL-4, IL-13 (Isomaki and Punnonen, 1997)) and negative acute phase reactants (APR’s) (e.g., albumin, transferrin, transthyretin, etc.) (Gruys et al., 2005). For example, reactive oxygen species (ROS) release can stimulate production of antioxidant enzymes through the redox responsive transcription factor Nrf2 to counteract oxidative stress (Singh et al., 2010). In response to acute inflammation, alpha-1 antitrypsin, an APR, suppresses TNF-α expression and modulates endothelial cell activation (Subramaniyam et al., 2008), while haptoglobin and albumin, other APRs, can bind ROS (Leonard and Maes, 2012). Similarly, adenosine triphosphate (ATP) appears to act as a redox-signaling molecule that buffers oxidation and inflammation through downregulation of TNF-α and upregulation of IL-10 (Kaur et al., 2006; Swennen et al., 2006), thereby dampening ROS-induced nuclear factor-kappa B (NF-κB) stimulation and inflammatory-driven ROS production. Under prolonged pathologic conditions, however, such counter-regulatory effects may be attenuated.
Oxidative stress occurs when the synthesis of oxygen free radicals exceeds the body’s ability to offset them with antioxidant molecules (e.g., Vitamins C and E, glutathione, glutathione peroxidase, superoxide dismutase enzymes, catalase, etc.) and sets in motion multiple pathways capable of stimulating inflammatory activity (Khansari et al., 2009; Khanzode et al., 2003; Sarandol et al., 2007b; Spooner and Yilmaz, 2011). ROS activate inflammasomes such as NLRP3, a cytoplasmic protein complex that modulates innate immune function by activating caspase-1, which increases pro-inflammatory cytokines such as IL-1β (Jin and Flavell, 2010; Martinon, 2010; Tschopp and Schroder, 2010; Zhou et al., 2010). ROS also increase inflammation by activating certain stress-activated kinases such as ERK, JNK, and p38 (Closa and Folch-Puy, 2004; Perricone et al., 2009). Another major mechanism by which ROS regulate inflammatory processes involves stimulation of transcription factors, e.g. NF-κB and activator protein-1 (AP-1) (Closa and Folch-Puy, 2004; Hayley et al., 2005; Rahman, 2003; Reuter et al., 2010), which have multiple downstream effects including: (a) synthesis of pro-inflammatory cytokines, (b) activation of mitogen-activated protein (MAP) kinase pathways (which activate both pro- and anti-inflammatory gene transcription), (c) activation of stress-response protective genes (e.g., manganese superoxide dismutase [MnSOD] and glutamylcysteine synthetase), and (d) histone/chromatin modifications (viz., increased acetylation and decreased deacetylation [HDAC2]) that lead to an increase in pro-inflammatory gene expression (Rahman, 2003). Increases in pro-inflammatory cytokine release provoke an inflammatory cascade, recruiting additional inflammatory cells and amplifying leukocyte infiltration (Caballero and Coto-Montes, 2012; Rahman, 2003; Tschopp and Schroder, 2010). In the CNS, IFN-γ has been implicated in stimulating ROS release from microglia (Pawate et al., 2004). Also, various pro-inflammatory cytokines in the CNS activate indoleamine 2,3-dioxygenase (IDO), a rate-limiting enzyme involved in catabolism of tryptophan, a serotonin precursor. IDO upregulation results in a depletion of tryptophan and a concomitant increase in metabolites, 3-hydroxy-kynurenine and quinolonic acid, which then provoke ROS production (Maes et al., 2011d; Miller et al., 2009; Wichers and Maes, 2004).
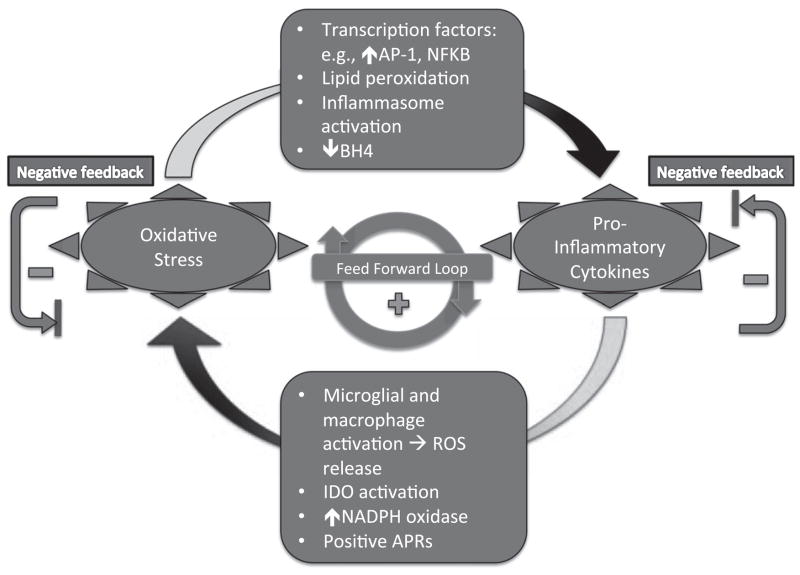
Acute inflammation may result in activation of guanosine-tri-phosphate-cyclohydrolase-1 (GTP-CH1), which is necessary for tetrahydrobiopterin (BH4) synthesis, and subsequently for aromatic amino acid and biogenic amine (norpeinephrine, epinephrine and serotonin) synthesis. However, chronic inflammation together with increased ROS production results in shunting biopterin synthesis to inactive neopterin, while the cofactor tetrahydrobiopterin (BH4), necessary for aromatic amino acid hydroxylase activities, is reduced and destroyed by ROS, resulting in reduced concentrations of biogenic amines. Such a situation has been proposed and reported in the elderly, and may be associated with increased susceptibility to depression in this population (Capuron et al., 2011). As illustrated in Fig. 3, the complex interactions between the oxidative and inflammatory pathways contain mechanisms for both mutual amplification (positive feedback or a “vicious cycle”) and for negative feedback homeostasis.
Fig. 3.
Complex interactions between the oxidative and inflammatory pathways contain mechanisms for both mutual amplification (positive feedback or a “vicious cycle”) and for negative feedback homeostasis. In this Figure, some of the specific mediators of positive feedback are portrayed. For example, reactive oxygen species (ROS) activate inflammasomes such as NLRP3, which increase pro-inflammatory cytokines such as IL-1β. ROS also increase inflammation by activating certain stress-activated kinases such as ERK, JNK, and p38. Also, ROS can stimulate transcription factors, e.g. NF-κB and activator protein-1 (AP-1), to stimulate pro-inflammatory cytokine expression. Conversely, pro-inflammatory cytokines can indirectly provoke oxidative stress by activating microglia and macrophages, which release ROS; activating indoleamine 2,3-dioxygenase (IDO), which produces byproducts, 3-hydroxy-kynurenine and quinolonic acid; and stimulating nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase), which generates free radicals. Just as negative acute phase reactants (APRs) can have antioxidant effects, some positive APRs, such as C-reactive protein (CRP), have been linked to increased oxidative stress (Il’yasova et al., 2008).
We infer from our data and previous literature on this subject, that certain counter-regulatory processes may be disturbed in severe MDD and in certain chronic medical illnesses, converting a homeostatic negative feedback system into a positive feed-forward one (Dhabhar et al., 2009; Sternberg et al., 1992). Such a vicious cycle of inflammation and oxidation would predictably have adverse physiological consequences (Biswal et al., 2012; Khansari et al., 2009; Rahman, 2003). An analogous process of dysregulated feedback was previously described within the inflammatory system itself (Dhabhar et al., 2009). Other examples of defective feedback regulation in MDD, which may or may not bear any relationship to that shown here, include the hypothalamic–pituitary–adrenal (HPA) axis glucocorticoid receptor negative feedback dysregulation (Modell et al., 1997), the TNF-α-HPA axis feedback system (Himmerich et al., 2006), the relationship between IL-6 and IL-10 concentrations (Dhabhar et al., 2009) and the relationship between noradrenergic system and CRH/glucocorticoid system regulation (Wong et al., 2000).
Surprisingly, the MDD subjects showed a significant increase in IL-6 concentrations after 8 weeks of sertraline treatment. Although one study similarly found an increase in IL-6 concentrations after treatment with duloxetine, an SNRI (Fornaro et al., 2011), a recent meta-analysis of 22 studies showed that IL-6 concentrations non-significantly decreased with antidepressant treatment (Hannestad et al., 2011). In our study the IL-6/IL-10 ratio, which may be a more accurate reflection of the inflammatory milieu, did not change with treatment.
Among the strengths of this study were our use of medically healthy, rigorously screened and diagnosed subjects who were free of psychotropic and other medications, our use of closely matched samples (by age, sex, and ethnicity), our ascertainment of possible covariates such as BMI and tobacco use, and a naturalistic 8 week antidepressant treatment arm, verified with plasma antidepressant concentration assessments. Among the study’s weaknesses, our sample size was small, especially with several subjects’ assay results not available. Due to low circulating levels of IL-10, several subjects’ values were below the level of detection. Hence imputed values (set conservatively just below the lowest observed value in both groups) were used, although this would tend to bias towards the null hypotheses. It is possible that the lack of a significant inflammation-oxidation correlation in the MDD subjects after 8 weeks of treatment is secondary to the smaller sample size that that time point. This would be especially concerning if the subjects who failed to complete the treatment trial were less severely depressed at baseline, as severity predicted positive inter-correlations between inflammation and oxidation. We assessed this possibility and found no significant difference in baseline depression ratings in the subjects who dropped out compared to the remainder of the MDD subjects. Also, our biochemical data are based on single time-point blood samplings, and, since blood samples were all obtained in a rested state in the morning, it is unknown whether similar relationships between oxidation and inflammation markers would be seen at other times in the day or under states of acute or chronic stress. We also were not able to control for extraneous variables that may have impacted our findings, such as differences in diet, exercise, sleep, etc. Although age was not significantly correlated with any of our variables, our subjects’ age range was rather limited (our subjects had an average age of 37 yr old, and only two were over the age of 60, when cellular senescence and decreased buffering capacity for oxidative stress and inflammation are more likely to become apparent (Caballero and Coto-Montes, 2012). In addition, several of our subjects had comorbid anxiety diagnoses, which could have influenced the biochemical data, although in each case the anxiety diagnosis was considered secondary to the MDD diagnosis, and we found no significant correlations between anxiety ratings and cytokines or F2- IsoPs. Another limitation is that control subjects were not re-assessed at a Week 8 time point, so changes observed with 8 weeks of sertraline treatment in the MDD subjects cannot definitively exclude effects of time. Finally, our blood-based measurements of oxidative/inflammatory markers were taken peripherally, from which we cannot infer local tissue or CNS levels.
Our understanding of the intricacies of how inflammation and oxidation are co-regulated in depression is in its infancy; however, evidence is growing that inflammation and oxidation may form part of a vicious and self-reinforcing signaling cycle in a number of illnesses, including depression. Oxidative stress and inflammation may be among the clues we have to understanding the increased co-morbidity between depression and numerous physical illnesses that are also characterized by inflammation and oxidation. For example, we previously reported in subjects with MDD that peripheral blood mononuclear cell telomere length (an index of cell aging and a predictor of poor health outcomes and early mortality (Cawthon et al., 2003; Epel et al., 2004; Wolkowitz et al., 2010; Wolkowitz et al., 2011a) is inversely correlated with markers of inflammation as well as oxidation (Wolkowitz et al., 2011a). Elucidation of the complex interactions between the multiple pathways and networks linking inflammation and oxidative stress will require additional research, including identifying population subgroups and system biology approaches (Jesmin et al., 2010). Determining the mechanisms responsible for the apparent loss of counter-regulatory control between the oxidative and inflammatory systems may lead to new insights into the underlying pathophysiology of MDD and may suggest novel treatment targets for depression and its associated medical comorbidities.
References
- Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J Affect Disord. 2012 doi: 10.1016/j.jad.2012.03.033. http://dx.doi.org/10.1016/j.jad.2012.03.033. [DOI] [PubMed]
- Agrawal NK, Maiti R, Dash D, Pandey BL. Cilostazol reduces inflammatory burden and oxidative stress in hypertensive type 2 diabetes mellitus patients. Pharmacol Res. 2007;56(2):118–123. doi: 10.1016/j.phrs.2007.04.007. http://dx.doi.org/10.1016/j.phrs.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol. 2012;2012:853175. doi: 10.1155/2012/853175. http://dx.doi.org/10.1155/2012/853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry. 1999a;4 (2):182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Interleukin-1 beta production in dysthymia before and after pharmacotherapy. Biol Psychiatry. 1999b;46 (12):1649–1655. doi: 10.1016/s0006-3223(99)00211-5. [DOI] [PubMed] [Google Scholar]
- Assumpcao CR, Brunini TM, Matsuura C, Resende AC, Mendes-Riberio AC. Impact of the L-arginine-nitric oxide pathway and oxidative stress on the pathogenesis of the metabolic syndrome. Open Biochem J. 2008;2:108–115. doi: 10.2174/1874091X00802010108. http://dx.doi.org/10.2174/1874091X00802010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, Yucel M, Gama CS, Dodd S, Dean B, Magalhaes PV, Amminger P, McGorry P, Malhi GS. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804–817. doi: 10.1016/j.neubiorev.2010.10.001. http://dx.doi.org/10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Biswal S, Thimmulappa RK, Harvey CJ. Experimental therapeutics of Nrf2 as a target for prevention of bacterial exacerbations in COPD. Proc Am Thorac Soc. 2012;9(2):47–51. doi: 10.1513/pats.201201-009MS. http://dx.doi.org/10.1513/pats.201201-009MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero BC, Coto-Montes A. An insight into the role of autophagy in cell responses in the aging and neurodegenerative brain. Histol Histopathol. 2012;27:263–275. doi: 10.14670/HH-27.263. [DOI] [PubMed] [Google Scholar]
- Cannizzo ES, Clement CC, Sahu R, Follo C, Santambrogio L. Oxidative stress, inflamm-aging and immunosenescence. J Proteomics. 2011;74(11):2313–2323. doi: 10.1016/j.jprot.2011.06.005. http://dx.doi.org/10.1016/j.jprot.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226– 238. doi: 10.1016/j.pharmthera.2011.01.014. http://dx.doi.org/10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Gualde N, Bosmans E, Dantzer R, Maes M, Neveu PJ. Association between immune activation and early depressive symptoms in cancer patients treated with interleukin-2-based therapy. Psychoneuroendocrinology. 2001;26 (8):797–808. doi: 10.1016/s0306-4530(01)00030-0. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70(2):175–182. doi: 10.1016/j.biopsych.2010.12.006. http://dx.doi.org/10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. http://dx.doi.org/10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13(3):171–181. doi: 10.1016/j.pathophys.2006.05.007. http://dx.doi.org/10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Closa D, Folch-Puy E. Oxygen free radicals and the systemic inflammatory response. IUBMB Life. 2004;56(4):185–191. doi: 10.1080/15216540410001701642. http://dx.doi.org/10.1080/15216540410001701642. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis in the behavioral sciences. 3. Erlbaum; Mahwah, NJ: 2003. [Google Scholar]
- Cottone S, Palermo A, Vaccaro F, Vadala A, Buscemi B, Cerasola G. Oxidative stress and inflammation in long-term renal transplanted hypertensives. Clin Nephrol. 2006;66 (1):32–38. doi: 10.5414/cnp66032. [DOI] [PubMed] [Google Scholar]
- Cottone S, Lorito MC, Riccobene R, Nardi E, Mulè G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008;21 (2):175–179. [PubMed] [Google Scholar]
- Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45 (1):1–6. doi: 10.1159/000048665. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15 (26):3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res. 2009;43(11):962–969. doi: 10.1016/j.jpsychires.2009.05.010. http://dx.doi.org/10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dieperink E, Willenbring M, Ho SB. Neuropsychiatric symptoms associated with hepatitis C and interferon alpha: a review. Am J Psychiatry. 2000;157 (6):867–876. doi: 10.1176/appi.ajp.157.6.867. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. http://dx.doi.org/10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. http://dx.doi.org/10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Cole SW, Pace TW, Hu F, Woolwine BJ, Doho GH, Raison CL, Miller AH. Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychol Med. 2012;42(8):1591–1603. doi: 10.1017/S0033291711002868. http://dx.doi.org/10.1017/s0033291711002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep. 2006;8 (3):191–198. doi: 10.1007/s11906-006-0050-7. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IVTR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York State Psychiatric Institute Biometrics Research; 2002. [Google Scholar]
- Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2′-deoxyguanosine in clinical depression. Psychosom Med. 2006;68(1):1–7. doi: 10.1097/01.psy.0000195780.37277.2a. http://dx.doi.org/10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Schaeffer EL, Gattaz WF. The role of phospholipase A2 in neuronal homeostasis and memory formation: implications for the pathogenesis of Alzheimer’s disease. J Neural Transm. 2007;114(2):231–238. doi: 10.1007/s00702-006-0597-0. http://dx.doi.org/10.1007/s00702-006-0597-0. [DOI] [PubMed] [Google Scholar]
- Fornaro M, Martino M, Battaglia F, Colicchio S, Perugi G. Increase in IL-6 levels among major depressive disorder patients after a 6 week treatment with duloxetine 60 mg/day: a preliminary observation. Neuropsychiatr Dis Treat. 2011;7:51–56. doi: 10.2147/NDT.S16382. http://dx.doi.org/10.2147/NDT.S16382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericks CA, Drabant EM, Edge MD, Tillie JM, Hallmayer J, Ramel W, Kuo JR, Mackey S, Gross JJ, Dhabhar FS. Healthy young women with serotonin transporter SS polymorphism show a pro-inflammatory bias under resting and stress conditions. Brain Behav Immun. 2010;24(3):350–357. doi: 10.1016/j.bbi.2009.10.014. http://dx.doi.org/10.1016/j.bbi.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SA, Korade Z, Shelton RC. Oxidative stress and glutathione response in tissue cultures from persons with major depression. J Psychiatr Res. 2012 doi: 10.1016/j.jpsychires.2012.06.008. http://dx.doi.org/10.1016/j.jpsychires.2012.06.008. [DOI] [PMC free article] [PubMed]
- Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6(11):1045–1056. doi: 10.1631/jzus.2005.B1045. http://dx.doi.org/10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Romero F, Rodriguez-Moran M. Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev. 2006;22(6):471–476. doi: 10.1002/dmrr.644. http://dx.doi.org/10.1002/dmrr.644. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6 (4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a metaanalysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. http://dx.doi.org/10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hayley S, Poulter MO, Merali Z, Anisman H. The pathogenesis of clinical depression: stressor- and cytokine-induced alterations of neuroplasticity. Neuroscience. 2005;135(3):659–678. doi: 10.1016/j.neuroscience.2005.03.051. http://dx.doi.org/10.1016/j.neuroscience.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.06.001. http://dx.doi.org/10.1016/j.bbi.2012.06.001. [DOI] [PubMed]
- Himmerich H, Binder EB, Kunzel HE, Schuld A, Lucae S, Uhr M, Pollmacher T, Holsboer F, Ising M. Successful antidepressant therapy restores the disturbed interplay between TNF-alpha system and HPA axis. Biol Psychiatry. 2006;60(8):882–888. doi: 10.1016/j.biopsych.2006.03.075. http://dx.doi.org/10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Il’yasova D, Ivanova A, Morrow JD, Cesari M, Pahor M. Correlation between two markers of inflammation, serum C-reactive protein and interleukin 6, and indices of oxidative stress in patients with high risk of cardiovascular disease. Biomarkers. 2008;13(1):41–51. doi: 10.1080/13547500701617708. http://dx.doi.org/10.1080/13547500701617708. [DOI] [PubMed] [Google Scholar]
- Inventory of Depressive Symptomatology (IDS) and Quick Inventory of Depressive Symptomatology (QIDS) 2012. Retrieved University of Pittsburgh Epidemiology Data Center, 7/17/2012. [Google Scholar]
- Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311 (4):1014–1018. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Ikeda M, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jpn J Cancer Res. 2001a;92 (3):367–376. doi: 10.1111/j.1349-7006.2001.tb01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. Int Arch Occup Environ Health. 2001b;74 (2):153–157. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Miyata M, Kasai H. Psychological mediation of a type of oxidative DNA damage, 8-hydroxydeoxyguanosine, in peripheral blood leukocytes of non-smoking and non-drinking workers. Psychother Psychosom. 2002;71 (2):90–96. doi: 10.1159/000049351. [DOI] [PubMed] [Google Scholar]
- Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39 (6):553–560. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Isomaki P, Punnonen J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann Med. 1997;29 (6):499–507. doi: 10.3109/07853899709007474. [DOI] [PubMed] [Google Scholar]
- Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008;18(7):253– 260. doi: 10.1016/j.tcm.2008.11.008. http://dx.doi.org/10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Jesmin J, Rashid MS, Jamil H, Hontecillas R, Bassaganya-Riera J. Gene regulatory network reveals oxidative stress as the underlying molecular mechanism of type 2 diabetes and hypertension. BMC Med Genomics. 2010;3(1):45. doi: 10.1186/1755-8794-3-45. http://dx.doi.org/10.1186/1755-8794-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Flavell RA. Inflammasome activation. The missing link: how the inflammasome senses oxidative stress. Immunol Cell Biol. 2010;88(5):510–512. doi: 10.1038/icb.2010.56. http://dx.doi.org/10.1038/icb.2010.56. [DOI] [PubMed] [Google Scholar]
- Kaur K, Sharma AK, Dhingra S, Singal PK. Interplay of TNF-alpha and IL- 10 in regulating oxidative stress in isolated adult cardiac myocytes. J Mol Cell Cardiol. 2006;41(6):1023–1030. doi: 10.1016/j.yjmcc.2006.08.005. http://dx.doi.org/10.1016/j.yjmcc.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17 (Suppl 1):S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3 (1):73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8(6):365–370. doi: 10.1179/135100003225003393. http://dx.doi.org/10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- Kotani K, Taniguchi N. Correlation between high-sensitivity C-reactive protein and reactive oxygen metabolites during a one year period among asymptomatic subjects. J Clin Med Res. 2012;4(1):52–55. doi: 10.4021/jocmr755w. http://dx.doi.org/10.4021/jocmr755w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R18–R36. doi: 10.1152/ajpregu.00327.2006. http://dx.doi.org/10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 2010;49(7):1229–1238. doi: 10.1093/rheumatology/keq037. http://dx.doi.org/10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- Lakshmi SV, Padmaja G, Kuppusamy P, Kutala VK. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009;46 (6):421–440. [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. http://dx.doi.org/10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Leuner K, Muller WE, Reichert AS. From mitochondrial dysfunction to amyloid beta formation: novel insights into the pathogenesis of Alzheimer’s disease. Mol Neurobiol. 2012 doi: 10.1007/s12035-012-8307-4. http://dx.doi.org/10.1007/s12035-012-8307-4. [DOI] [PubMed]
- Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. http://dx.doi.org/10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011a;35(3):664–675. doi: 10.1016/j.pnpbp.2010.06.014. http://dx.doi.org/10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Maes M. An intriguing and hitherto unexplained co-occurrence. Depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Prog Neuropsychopharmacol Biol Psychiatry. 2011b;35(3):784–794. doi: 10.1016/j.pnpbp.2010.06.023. http://dx.doi.org/10.1016/j.pnpbp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Maes M, De Vos N, Pioli R, Demedts P, Wauters A, Neels H, Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58 (3):241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- Maes M, DeVos N, Wauters A, Demedts P, Maurits VW, Neels H, Bosmans E, Altamura C, Lin A, Song C, Vandenbroucke M, Scharpe S. Inflammatory markers in younger vs elderly normal volunteers and in patients with Alzheimer’s disease. J Psychiatr Res. 1999;33 (5):397–405. doi: 10.1016/s0022-3956(99)00016-3. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011a;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. http://dx.doi.org/10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, Obuchowiczwa E, Goehler L, Brzeszcz J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocrinol Lett. 2011b;32 (1):7–24. [PubMed] [Google Scholar]
- Maes M, Leonard B, Fernandez A, Kubera M, Nowak G, Veerhuis R, Gardner A, Ruckoanich P, Geffard M, Altamura C, Galecki P, Berk M. (Neuro)inflammation and neuroprogression as new pathways and drug targets in depression: from antioxidants to kinase inhibitors. Prog Neuropsychopharmacol Biol Psychiatry. 2011c;35(3):659–663. doi: 10.1016/j.pnpbp.2011.02.019. http://dx.doi.org/10.1016/j.pnpbp.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011d;35(3):702–721. doi: 10.1016/j.pnpbp.2010.12.017. http://dx.doi.org/10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Leunis JC, Geffard M. IgM-mediated autoimmune responses directed against multiple neoepitopes in depression: new pathways that underpin the inflammatory and neuroprogressive pathophysiology. J Affect Disord. 2011e;135(1–3):414–418. doi: 10.1016/j.jad.2011.08.023. http://dx.doi.org/10.1016/j.jad.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression - and other conditions characterized by tryptophan depletion induced by inflammation. Neuroendocrinol Lett. 2007;28 (6):826–831. [PubMed] [Google Scholar]
- Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40(3):616–619. doi: 10.1002/eji.200940168. http://dx.doi.org/10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- Mauri MC, Laini V, Cerveri G, Scalvini ME, Volonteri LS, Regispani F, Malvini L, Manfre S, Boscati L, Panza G. Clinical outcome and tolerability of sertraline in major depression: a study with plasma levels. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26 (3):597–601. doi: 10.1016/s0278-5846(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. http://dx.doi.org/10.1016/j.bbi.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. http://dx.doi.org/10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10(Suppl 1):S10–S23. doi: 10.1080/13547500500216546. http://dx.doi.org/10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L, 2nd, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. http://dx.doi.org/10.1016/s0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, Inagaki T, Horiguchi J. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15(3):249–252. doi: 10.1016/j.euroneuro.2004.11.002. http://dx.doi.org/10.1016/j.euroneuro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Modell S, Yassouridis A, Huber J, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;65 (3):216– 222. doi: 10.1159/000127275. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. http://dx.doi.org/10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680–684. doi: 10.1038/sj.mp.4001805. http://dx.doi.org/10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- Nassberger L, Traskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatr Scand. 1993;88 (1):48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Goldschmidt-Clermont PJ. Heartache and heartbreak-the link between depression and cardiovascular disease. Nat Rev Cardiol. 2012 doi: 10.1038/nrcardio.2012.91. http://dx.doi.org/10.1038/nrcardio.2012.91. [DOI] [PubMed]
- Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008;23(2):87–94. doi: 10.1002/hup.912. http://dx.doi.org/10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77(4):540–551. doi: 10.1002/jnr.20180. http://dx.doi.org/10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Perricone C, De Carolis C, Perricone R. Glutathione: a key player in autoimmunity. Autoimmun Rev. 2009;8(8):697–701. doi: 10.1016/j.autrev.2009.02.020. http://dx.doi.org/10.1016/j.autrev.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31 (4):437. [Google Scholar]
- Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol. 2003;36 (1):95–109. doi: 10.5483/bmbrep.2003.36.1.095. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–475. doi: 10.1007/s11920-011-0232-0. http://dx.doi.org/10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radical Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. http://dx.doi.org/10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Sarandol A, Kirli S, Akkaya C, Altin A, Demirci M, Sarandol E. Oxidative–antioxidative systems and their relation with serum S100 B levels in patients with schizophrenia: effects of short term antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2007a;31(6):1164–1169. doi: 10.1016/j.pnpbp.2007.03.008. http://dx.doi.org/10.1016/j.pnpbp.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative–antioxidative systems. Hum Psychopharmacol. 2007b;22(2):67–73. doi: 10.1002/hup.829. http://dx.doi.org/10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants: fact or fiction? CNS Drugs. 2012;26(6):477–490. doi: 10.2165/11633190-000000000-00000. http://dx.doi.org/10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–888. doi: 10.1016/j.bbamcr.2011.01.034. http://dx.doi.org/10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Singh S, Vrishni S, Singh BK, Rahman I, Kakkar P. Nrf2-ARE stress response mechanism: a control point in oxidative stress-mediated dysfunctions and chronic inflammatory diseases. Free Radical Res. 2010;44(11):1267–1288. 507670. doi: 10.3109/10715762.2010.507670. http://dx.doi.org/10(3109/10715762) [DOI] [PubMed] [Google Scholar]
- Skalicky J, Muzakova V, Kandar R, Meloun M, Rousar T, Palicka V. Evaluation of oxidative stress and inflammation in obese adults with metabolic syndrome. Clin Chem Lab Med. 2008;46(4):499–505. doi: 10.1515/CCLM.2008.096. http://dx.doi.org/10.1515/CCLM.2008.096. [DOI] [PubMed] [Google Scholar]
- Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int J Mol Sci. 2011;12(1):334–352. doi: 10.3390/ijms12010334. http://dx.doi.org/10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, Winyard PG, Kettle AJ. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford) 2012 doi: 10.1093/rheumatology/kes193. http://dx.doi.org/10.1093/rheumatology/kes193. [DOI] [PubMed]
- Sternberg EM, Chrousos GP, Wilder RL, Gold PW. The stress response and the regulation of inflammatory disease. Ann Intern Med. 1992;117 (10):854– 866. doi: 10.7326/0003-4819-117-10-854. [DOI] [PubMed] [Google Scholar]
- Subramaniyam D, Virtala R, Pawlowski K, Clausen IG, Warkentin S, Stevens T, Janciauskiene S. TNF-alpha-induced self expression in human lung endothelial cells is inhibited by native and oxidized alpha1-antitrypsin. Int J Biochem Cell Biol. 2008;40(2):258–271. doi: 10.1016/j.biocel.2007.07.016. http://dx.doi.org/10.1016/j.biocel.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Swennen EL, Dagnelie PC, Bast A. ATP inhibits hydroxyl radical formation and the inflammatory response of stimulated whole blood even under circumstances of severe oxidative stress. Free Radical Res. 2006;40(1):53–58. doi: 10.1080/10715760500364298. http://dx.doi.org/10.1080/10715760500364298. [DOI] [PubMed] [Google Scholar]
- Terlecky SR, Terlecky LJ, Giordano CR. Peroxisomes, oxidative stress, and inflammation. World J Biol Chem. 2012;3(5):93–97. doi: 10.4331/wjbc.v3.i5.93. http://dx.doi.org/10.4331/wjbc.v3.i5.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tousoulis D, Andreou I, Antoniades C, Tentolouris C, Stefanadis C. Role of inflammation and oxidative stress in endothelial progenitor cell function and mobilization: therapeutic implications for cardiovascular diseases. Atherosclerosis. 2008;201(2):236–247. doi: 10.1016/j.atherosclerosis.2008.05.034. http://dx.doi.org/10.1016/j.atherosclerosis.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10(3):210–215. doi: 10.1038/nri2725. http://dx.doi.org/10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Tsuboi H, Shimoi K, Kinae N, Oguni I, Hori R, Kobayashi F. Depressive symptoms are independently correlated with lipid peroxidation in a female population: comparison with vitamins and carotenoids. J Psychosom Res. 2004;56(1):53–58. doi: 10.1016/S0022-3999(03)00567-1. http://dx.doi.org/10.1016/S0022-3999(03)00567-1. [DOI] [PubMed] [Google Scholar]
- Uno K, Nicholls SJ. Biomarkers of inflammation and oxidative stress in atherosclerosis. Biomark Med. 2010;4(3):361–373. doi: 10.2217/bmm.10.57. http://dx.doi.org/10.2217/bmm.10.57. [DOI] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA. Successful treatment of interferon-alpha-induced mood disorder with nortriptyline. Psychosomatics. 1995;36 (4):418–419. doi: 10.1016/s0033-3182(95)71658-9. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Maes M. The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-alpha-induced depression. J Psychiatry Neurosci. 2004;29 (1):11–17. [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: do stress and depression accelerate cell aging? World J Biol Psychiatry. 2008;9(1):2–5. doi: 10.1080/15622970701875601. http://dx.doi.org/10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. http://dx.doi.org/10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress-preliminary findings. PLoS ONE. 2011a;6(3):e17837. doi: 10.1371/journal.pone.0017837. http://dx.doi.org/10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011b;13 (1):25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA. 2000;97 (1):325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei XF, Achong MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101(2):311–320. doi: 10.1172/JCI1368. http://dx.doi.org/10.1172/jci1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35(9):1356–1362. doi: 10.1016/j.psyneuen.2010.03.010. http://dx.doi.org/10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31(6):1207–1211. doi: 10.1053/jhep.2000.7880. http://dx.doi.org/10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. doi: 10.1038/ni.1831. http://dx.doi.org/10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]