Abstract
22q11.2 deletion syndrome (22q11DS) is a genetic disorder that conveys a significant risk for the development of social behavior disorders, including autism and schizophrenia. Also known as DiGeorge syndrome, 22q11DS is the second most common childhood genetic disorder and is characterized by an elevated risk for immune disorders, as 77% of individuals have an identifiable immune deficiency. We hypothesize that this immune dysfunction could contribute to the elevated risk of impaired social behavior seen in 22q11DS. The current study begins to elucidate these immune deficits and link them with the behavioral alterations associated with the disorder. Serum concentrations of a series of cytokines were examined, using a multiplex immunoassay, in sixteen individuals with 22q11DS and screened for autism-related behavior using the Autism Diagnostic Interview-Revised (ADI-R). This preliminary study examined correlations between specific immune proteins and each of the ADI-R algorithm scores (social, communication, and repetitive behavior). The inflammatory cytokine IL-1β, as well as the ratio between the inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10, were correlated with social scores (r = 0.851, p = 0.004; r = 0.580, p = 0.018). In addition, the inflammatory cytokines interferon gamma and IL-12p70 were correlated with repetitive behaviors (r = 0.795, p = 0.033; r = 0.774, p = 0.002). Interestingly, IL-12 has been reported to be increased in autistic children. These data show a positive relationship between severity of autism-related behaviors and level of serum concentrations of inflammatory cytokines in individuals with 22q11DS, providing a basis for further inquiry.
Keywords: DiGeorge syndrome, velo-cardio-facial syndrome, autism, repetitive behaviors, cytokines, immunity, inflammation, T-cell, schizophrenia
Introduction
22q11.2 deletion syndrome (22q11DS) is the second most common childhood genetic disorder, behind Down’s syndrome (~1:4000 births) (Oskarsdottir et al., 2004). 22q11DS, also known as DiGeorge syndrome and velo-cardio-facial syndrome, is characterized by a spontaneous 3MB hemizygous deletion on the long arm of chromosome 22 and results in a complex medical phenotype which may involve heart and palatal defects, facial dysmorphology, and thymic dysfunction (McDonald-McGinn et al., 1993).
22q11DS conveys a significant risk for the development of social behavior deficits, including autism-spectrum and schizophrenia-spectrum disorders. Research studies find that autism spectrum disorders occur in 14 to 45% of clinically-ascertained individuals with 22q11DS, as compared to >0.9% in the general population (McDonald-McGinn and Sullivan, 2011). Likewise, schizophrenia occurs in up to 33% of such individuals, which is 25 to 31 times higher than the general population (Drew et al., 2011). In fact, 22q11DS is the number one genetic risk factor for schizophrenia (Liu et al., 2002). Notably, schizophrenia disorder 4 is one of the genes deleted from chromosome 22 in the syndrome.
There is evidence that immune disruptions lead to alterations in behavioral function and the emergence of neurodevelopmental and psychiatric disorders. Schizophrenia has been strongly linked to immune dysfunction. In fact, some of the very first evidence linking prenatal immune function to neuropsychiatric outcomes was identified by schizophrenia researchers who found that the risk of disease was increased 7-fold for influenza exposure during the first trimester and that influenza exposure during early to mid-pregnancy increased the risk of schizophrenia 3-fold (Brown et al., 2004). Other maternal infections that have been linked to the development of schizophrenia include non-specific bacterial infections, Toxoplasma gondii, and herpes simplex virus type 2 (Sorensen et al., 2009, Mortensen et al., 2010, Pedersen et al., 2011). In addition, there is growing evidence for additional immune disturbances among schizophrenic patients. Higher concentrations of constitutively active and endotoxin-induced chemokines (i.e., monocyte chemotactic protein-1, macrophage inflammatory protein-1 alpha) and cytokines [i.e., interleukin (IL)-8, IL-18, and interferon gamma (INFγ)] are found in individuals with schizophrenia (Reale et al., 2011).
Similarly, in autism, there is evidence of altered immunity occurring both immediately after birth and throughout disease progression. There are multiple lines of evidence that suggest a role for immune dysfunction in autism, including neuroinflammation involving microglia, increased inflammatory cytokine and chemokine production in post-mortem brain tissue, and systemic immune activation of proteins and reduced immunoglobulin (Ig) antibody production (Onore et al., 2012). Immune function may also relate to social behavioral outcomes (Onore et al., 2012). For example, increased plasma levels of cytokines including IL-1β, IL-6, IL-8 and IL-12p40 were observed in autistic children compared with age-matched controls, and this cytokine production was associated with more aberrant behaviors, especially in individuals with developmental and behavioral regression (Ashwood et al., 2011a). Moreover, significantly reduced plasma levels of IgG and IgM were found in children with autism compared with age-matched typically developing children and children with developmental disabilities other than autism, suggesting an underlying defect in immune function. The reduction in specific Ig levels correlated with behavioral severity. Specifically, patients with the highest scores in behavioral dysfunction exhibited the greatest reduction in peripheral blood concentrations of IgG and IgM (Heuer et al., 2008). Another study found that a 10-point difference in IgG concentrations conferred an increased risk for autism (Grether et al., 2010). Another line of evidence relevant to 22q11DS suggests an increase in the Th1/Th2 ratio of individuals with autism (Li et al., 2009). This increased pro-inflammatory state was associated with greater impairments in the core features of autism (Ashwood et al., 2011b). Together these studies suggest immune system dysfunction in autism.
A prominent feature of the medical phenotype of 22q11DS includes elevated risk of immune disorders, as 77% of individuals have an identifiable immune dysfunction (McDonald-McGinn et al., 1993). The identified immune changes range from a primary T cell dysfunction to the presence of an autoimmune disease. Specifically, thymic hypoplasia in individuals with 22q11DS causes a reduced number of T cells at birth, and possibly the emergence of autoimmune disorders, allergies, and asthma, which can arise from an imbalance in T cell subsets. Of individuals with 22q11DS, 10% will develop autoimmune disorders (McDonald-McGinn and Sullivan, 2011), including Graves’ disease, diabetes, and celiac disease. Moreover, 12% of individuals with 22q11DS have IgA deficiency (McDonald-McGinn et al., 1993). In addition, polyarticular juvenile rheumatoid arthritis occurs in children with 22q11DS at a frequency 20 times that of the general population, with an age of onset ranging from 17 months to five years (McDonald-McGinn et al., 1993). The source of these immune disturbances are related to the deletion of several immune proteins whose DNA lies on the long end of chromosome 22, including interleukin 17 receptor A, immunoglobulin lambda-like polypeptide 1, and macrophage migration inhibitory factor. Despite multiple clinical studies that report a significant association between 22q11DS and immune-related disease, a systematic investigation of the immune profiles of individuals with 22q11DS, and their relationship with behavioral changes, has not previously been conducted.
In this preliminary study, we examined individuals with 22q11DS to identify genetically influenced immune disturbances. Cytokines that have been implicated in autism were measured in individuals with 22q11DS, using a multiplex immunoassay. Concentrations of the pro- inflammatory cytokines granulocyte macrophage colony-stimulating factor (GMcsf), INFγ , IL-12p70, IL-1β, IL-6, IL-8, and tumor necrosis factor-alpha (TNFα), and the anti-inflammatory cytokine IL-10 were examined. These immune proteins have not been studied in 22q11DS. In addition, autism-related behavior was examined as it relates to these cytokines.
Methods
Study Population
Research participants were recruited in reverse-age order from a case registry of individuals diagnosed with 22q11DS, which is maintained at Children’s Health Care of Atlanta. The current analysis is based on 16 individuals who participated in a study of neurocognitive and behavioral outcomes and who had also provided blood samples as part of a larger biological banking effort. All subjects had a confirmed diagnosis of 22q11DS by means of fluorescence in situ hybridization (FISH) for deletions on chromosome 22q11. The race/ethnicity distribution was as follows: 69% (n=11) Caucasian, 19% (n=3) African-American, 6% (n=1) Hispanic, and 6% (n=1) Asian. There were 9 (56%) males and 7 (44%) females, and the average age was 14 (SD = 1.98) with a range of 3 to 31 years.
Cytokine Assessments
Cytokines were assessed using a multiplex immunoassay format (Meso Scale Discovery, Gaithersburg, Maryland; MS6000 Human ProInflammatory Ultra-Sensitive Kit) (Breen et al., 2011, Dabitao et al., 2011). Serum from 16 individuals with 22q11DS was collected to examine concentrations of the autism-related cytokines: Gmcsf, INFγ , IL-12p70, IL-1β, IL-6, IL-8, TNFα and IL-10. Each sample was run in duplicate; including an 8-point standard curve, starting with 2500 pg/ml and continuing with a 4-fold dilution down to a zero concentration value. The lower limit of detection (LLOD) for each of these proteins is: 0.53, 1.4, 0.36, 0.27, 0.09, 0.50, and 0.21 pg/ml respectively. The recoveries of the assays were 98.8–102.5% for the measured cytokines. The calculated coefficient of variation (CV) for each protein was less than 15%; any duplicate that had a higher CV was removed from analysis.
Behavioral Assessments
Autism-related behavior was measured using the Autism Diagnostic Interview-Revised (ADI-R). This semi-structured interview, designed to identify individuals with a probable diagnosis of autism (Lord et al., 1994, Risi et al., 2006), relies on detailed parental report, rather than direct observation, and is considered one of the gold-standard assessments for evaluating autism-related behaviors. Each reported behavior or symptom is rated according to a three- or four-point scale (0 to 2, or 0 to 3), with higher scores reflecting greater severity of autism-related behaviors. Current and past symptoms of autism are recorded. The ADI-R yields diagnostic algorithm scores, comprised primarily of the past symptom ratings, across three domains: 1) the “social” domain, which assesses qualitative abnormalities in reciprocal social interaction, 2) the “communication” domain which assesses qualitative abnormalities in communication, with different algorithms for verbal and nonverbal individuals, and 3) the “repetitive behavior” domain which assesses restricted, repetitive, and stereotyped patterns of behavior. Higher scores indicate greater social impairments, greater communication impairments, or the presence of more severe or more frequent repetitive behavior. Although the ADI-R algorithm scores were originally designed to generate categorical diagnostic information, (Lord et al., 1994, Risi et al., 2006), these scores also provides reliable continuous metrics; which represent the severity of autism symptomatology and are frequently used in investigations of the biological and genetic basis of social and behavioral disability (Hernandez et al., 2009, Monk et al., 2009, McDuffie et al., 2010). In our study sample, only three individuals had ADI-R non-verbal communication scores and, therefore, were removed from the analysis involving the communication algorithm data. Within the study sample, four of 16 individuals met DSM-IV diagnostic criteria for an autism spectrum disorder (i.e., Autistic Disorder or Pervasive Developmental Disorder - Not Otherwise Specified, PDD-NOS) using information obtained from the ADI-R and the Autism Diagnostic Observation Schedule (ADOS), combined with clinical judgment.
Statistical Analysis
Correlation analysis was performed to investigate the association between ADI-R scores and the concentration of each of the cytokines: GMcsf, INFγ , IL-12p70, IL-1β, IL-2, IL-6, IL-8, TNFα, and IL-10. In addition, we also analyzed the ratio between the pro-inflammatory cytokines INFγ , IL-12p70, IL-1β, IL-6, IL-8, TNFα and the anti-inflammatory cytokine IL-10, using Pearson’s r. Natural log-transformation was performed on cytokines that had a right-skewed distribution. Proteins that had less than seven samples to analyze were removed from the study, and included GMcsf and IL-2. Multivariate linear regression model was then performed to evaluate the adjusted effects of the cytokines implicated in autism (i.e., INFγ , IL-12p70, IL-1β, IL-6, IL-8, TNFα, IL10, IL-6:IL-10, INFγ :IL-10, IL-12p70:IL-10, IL-1β:IL-10, IL-8:IL-10, and TNFα:IL-10) on the ADI-R scores, controlling for subject’s age at blood draw and gender. To maximize the number of values available for linear regression analysis, concentration measurements that were below the lower limit of detection (LLOD) were given a middle value between zero and the LLOD, for individual cytokines. In addition, outliers were identified for each cytokine as measurements that were at least three standard deviations above the mean level and were then replaced by the mean level plus three standard deviations. Natural log-transformation was performed on cytokines that had a right-skewed distribution. Any sample that had a CV higher than 15% was removed from analysis. Statistical analysis was performed using SAS (version 9.2; SAS Institute, Cary, NC) software. All statistical tests were two-sided and a p value of < 0.05 was considered statistically significant. Unadjusted p values are presented (Rothman, 1990).
Results
The cytokine concentrations (mean±SEM) were: GMcsf (3.69±1.34), IL-2 (1.36±0.67), INFγ (1.07±0.21), IL-12p70 (1.59±0.19), IL-1β (1.27±0.50), IL-6 (1.64±0.51), IL-8 (32.79±15.10), TNFα (12.69±3.22), IL-10 (2.35±0.42), IL-6:IL-10 (0.76±0.21), INFγ :IL-10 (0.68±0.18), IL-12p70:IL-10 (0.92±0.15), IL-1β:IL-10 (0.69±0.27), IL-8:IL-10 (21.46±9.23), and TNFα:IL-10 (6.91±1.62). Specifically, the inflammatory marker IL-1β, the ratio between the inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10 (Figure 1A), and the ratio between IL-1β and IL-10 were positively correlated with the ADI-R social score (r = 0.851, p = 0.004; r = 0.580, p = 0.018; r = 0.690, p = 0.040, respectively). In addition, the repetitive behavior score was positively correlated with the inflammatory cytokines IL-12p70 (Figure 1B) and INFγ , and the ratio between IL-12p70 and IL-10 (r = 0.774, p = 0.002; r = 0.795, p = 0.033; r = 0.563; p = 0.045, respectively). Correlations between the inflammatory cytokine concentrations and the communication score were not significant.
Figure 1. Pro-inflammatory cytokines positively correlate with autism-related behavior in individuals with 22q11DS.
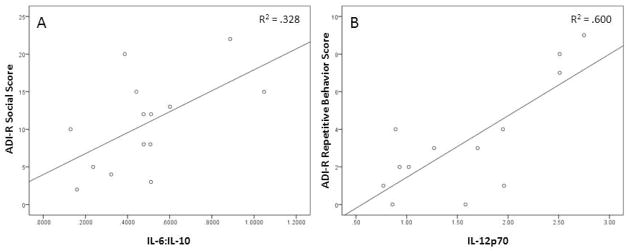
The ADI-R social score positively correlated with the ratio of pro-inflammatory cytokine IL-6 to anti-inflammatory cytokine IL-10 (A). A higher ADI-R score indicates more impaired social behavior. A positive correlation was observed for the repetitive behavior score on the ADI-R and IL-12p70 (B). A higher score reflects more frequent or severe repetitive behaviors.
Multivariate linear regression analyses also showed the concentration of cytokines implicated in autism have significant associations with social and repetitive behavior scores (Table 1B,C), after controlling for subjects age and gender. Specifically, the IL-6:IL-10 ratio was found to have a significant positive association with social interaction abnormalities (p=0.039; Table 1B). For repetitive behaviors, IL-12p70 had a significant positive association (p<0.0001), while IL-10 and age showed a significant negative association (p=0.047 and p=0.0003; Table 1C). Multivariate linear regression analysis was not performed using the ADI-R communication score; given that none of the correlations involving the communication score were significant.
Table 1. Correlation and Multivariate Linear Regression Analyses.
Correlations between ADI-R scores for the three domains: social behavior, communication, and repetitive behavior, and cytokine concentrations are shown in (A). The first row is the Pearson’s correlation coefficient, the second row is the p-value for the test of the Pearson’s r, and the third row is the number of observations. Higher ADI-R scores indicate greater social impairments, greater communication impairments, or the presence of more severe or more frequent repetitive behavior. Multivariate linear regression model of the ADI-R scores for social behavior (B) and repetitive behavior (C) are also shown. The ADI-R social score assesses qualitative abnormalities in reciprocal social interaction; while the repetitive score assesses restricted, repetitive, and stereotyped patterns of behavior.
| A. | |||||||
|---|---|---|---|---|---|---|---|
| ln IL-6 | IL-12p70 | ln INFγ | ln IL-1β | In IL-8 | ln TNFα | ln IL-10 | |
| Social | 0.408 | 0.296 | -0 170 | 0 851* | 0 441 | 0.466 | −0.180 |
| 0.117 | 0.325 | 0.661 | 0.004 | 0.088 | 0.069 | 0.504 | |
| 16 | 13 | 9 | 9 | 16 | 16 | 16 | |
| Repetitive | 0.2 | 0.774* | 0 795* | 0.336 | 0 380 | 0.369 | −0.1 S2 |
| 0.457 | 0.002 | 0.033 | 0.376 | 0.147 | 016 | 0499 | |
| 16 | 13 | 7 | 9 | 16 | 16 | 16 | |
| Communication | 0.323 | 0.477 | −0.773 | 0.663 | 0.235 | 0.23 | 0.031 |
| 0.751 | 0.117 | 0.125 | 0.151 | 0439 | 0.451 | 0.919 | |
| 13 | 12 | 5 | 6 | 13 | 13 | 13 | |
| ln IL-6:IL-10 | ln IL-12p:IL-10 | ln INFγ IL-10 | ln IL-1β: IL-10 | In IL-8:IL-10 | In TNFα:IL-10 | |
|---|---|---|---|---|---|---|
| Social | 0.580* | 0.305 | −0.125 | 0.690* | 0.492 | 0.486 |
| 0.018 | 0.312 | 0.749 | 0.040 | 0.053 | 0.056 | |
| 16 | 13 | 9 | 9 | 16 | 16 | |
| Repetitive | 0.351 | 0.563* | 0.48 | 0.305 | 0.433 | 0.43 |
| 0.183 | 0.045 | 0.191 | 0.425 | 0.094 | 0.097 | |
| 16 | 13 | 9 | 9 | 16 | 16 | |
| Communication | 0.376 | 0.217 | −0.146 | 0.109 | 0.171 | 0.111 |
| 0.205 | 0.499 | 0.755 | 0.837 | 0.718 | 0.718 | |
| 13 | 12 | 7 | 6 | 13 | 13 |
| B. Social Behavior Score on ADI-R
| |||
|---|---|---|---|
| Effect | Estimate | Standard Error | P value |
| Age (years) | 0.106 | 0.227 | 0.649 |
| Sex | −1.545 | 3.731 | 0.686 |
| ln IL-6:IL-10 | 4.52 | 1.954 | 0.039 |
| C. Repetitive Behavior Score on ADI-R
| |||
|---|---|---|---|
| Effect | Estimate | Standard Error | P value |
| Age (years) | −0.241 | 0.229 | 0.0003 |
| Sex | 0.811 | 0.61 | 0.22 |
| IL-12p70 | 3.237 | 0.406 | <.0001 |
| ln IL-10 | −1.182 | 0.502 | 0.0467 |
= p < 0.05
Discussion
These preliminary data represent a first step toward examining how cytokine levels relate to behavioral functioning in individuals with 22q11DS, a syndrome with increased risk for developing autism and schizophrenia (Meechan et al., 2009, Degos et al., 2010, Philip and Bassett, 2011). We found that inflammatory markers IL-6:IL-10, IL-1β, and IL-1β:IL-10 positively correlated with a measure of social impairment from the Autism Diagnostic Instrument-Revised (ADI-R), and that the IL-6:IL-10 inflammatory ratio was the strongest predictor of the ADI-R social impairment score, based on multivariate linear regression analysis. In addition, Th1-related inflammatory proteins, IL-12p70, INFγ , and the IL-12p70:IL-10 ratio, positively correlated with repetitive behavior, as measured by the ADI-R. Using linear regression, we found that IL-12p70 and IL-10 were associated with the ADI-R repetitive behavior score, with IL-12p70 being the strongest predictor. IL-12p70 concentration level was positively associated with the ADI-R repetitive behavior score; whereas, IL-10 had a negative association with this score. Collectively, this data supports our hypothesis that inflammatory markers are associated with higher levels of autism-related behavior (i.e., social impairments and repetitive behaviors) in 22q11DS. Conversely, we also found that the anti-inflammatory marker IL-10 was associated with lower levels of autism-related behavioral impairments.
The results of our study highlight the relation between autism-related behaviors and pro-inflammatory cytokine concentrations, including IL-12, IL-6, IL-1β, and INFγ . These findings complement other studies that have found increased cytokines levels in children with autism, as compared to age-matched controls (Singh, 1996, Jyonouchi et al., 2001, Ashwood et al., 2011a, Suzuki et al., 2011), and mirror the findings that increased cytokine concentration, including IL-12p40, IL-6, and IL-1β, is associated with more severe behavioral deficits in children with autism (Ashwood et al., 2011a).
Although the correlation and regression analyses used in this study limit our ability to speak to immune mechanisms and pathways in individuals with 22q11DS, our research is driven by the assumption that the immune environment, including the prenatal period, directly impacts brain function and structure, thereby influencing behavioral outcomes. For example, animal studies have found that prenatal immune activation, which results in the elevation of the pro-inflammatory cytokine IL-6 in brain tissue, causes behavioral changes in the offspring that mimic symptoms of autism (Smith et al., 2007, Boksa, 2010, Parker-Athill and Tan, 2010). IL-6 has shown to be involved in recognition memory, a specific type of learning that requires the ability to remember individual conspecifics. Similarly, increased expression of the cytokine IL-1β has been noted in the brains of offspring exposed to neonatal infection. This early-life infection creates a pro-inflammatory bias in the adult brain, where IL-1β is capable of suppressing BDNF and affecting brain development (Bilbo and Schwarz, 2009). Thus, the association between social behavior and IL-6:IL-10, IL-1β, and IL-1β:IL-10 identified in the current study could arise due to immune effects on brain structure and function.
In regards to Th1-related inflammatory proteins, it has long been known that IL-12 and INFγ are detrimental to learning and memory, when they exceed their physiological concentrations (Guerreiro et al., 2007, Derecki et al., 2010, Trollor et al., 2011). IL-12 has been indicated in cognitive function and is unique in that it is able to bridge the innate and adaptive immune responses by inducing the production of INFγ from natural killer and T cells and inducing the differentiation of naïve T cells into Th1 effectors. In our study, IL-12p70 and INFγ were associated with autism-related behavior. Consistent with our results, IL-12 has been shown to be increased in the plasma of individuals with autism, as compared with controls (Singh, 1996, Jyonouchi et al., 2005, Suzuki et al., 2011). In addition, elevated concentrations of maternal IFNγ , during mid-gestation, have been associated with a 50% increased risk of autism diagnosis (Goines et al., 2011). These results collectively suggest that IL-12 and IFNy may contribute to behavioral alterations in autism. Thus it is possible that IL-12 could play a role in 22q11DS psychiatric disease outcomes, such as autism or related behavioral impairments, either through its actions on the inflammatory cytokine INFκ or Th1 effector cells.
Our study results also suggest that the anti-inflammatory cytokine, IL-10, which is an inhibitor of IL-12 and its actions on INFγ , also contributes to behavioral functioning in 22q11DS. In our correlational analysis, we found that in individuals with 22q11DS, higher levels of IL-10 were associated with lower levels of autism-related behaviors. In addition, we showed that an inflammatory skew in the ratio of pro- to anti-inflammatory cytokines, as determined by IL-6:IL-10, was associated with social behavior deficits. This finding also has implications for how the immune changes inherent in 22q11DS could contribute to a susceptibility to autism. In 22q11DS, there is a significant decrease in FoxP3(+) regulatory T (Treg) cells (McLean-Tooke et al., 2008). Tregs are responsible for reducing inflammation and are down-regulated in autoimmune disorders. Therefore, the loss of Treg cells would drive an imbalance of cytokines towards a pro-inflammatory state, like we saw in our study. We hypothesize that the behavioral changes observed in 22q11DS, which are associated with increased inflammation, is secondary to alterations in the balance of pro- and anti-inflammatory T cell subsets, driven by this reduction in Treg. Collectively, our findings and prior studies suggest a complex interplay between prenatal factors and innate and adaptive immunity in regulating behavioral outcomes.
Overall, our study of individuals with 22q11DS yielded robust associations with inflammatory markers and autistic-related behaviors. However, due to this study's small sample size, replication of these findings is needed. In addition, longitudinal studies are also needed to examine how immune functioning relates to developmental trajectories, as psychiatric diagnoses are often related to an atypical pattern of development across time. Because our study was cross-sectional in nature and included a broad age range, we statistically controlled for age. Although some studies have reported age-related cytokine levels (Sack et al., 1998, Hartel et al., 2005, Hoffmann et al., 2005, Wiegering et al., 2009), age is not a moderator between cytokine and behavior in our data.
This preliminary study is the first to look at cytokines in the 22q11DS population and the first to associate immune status with behaviors that contribute to a psychiatric diagnosis. Conducting a larger study comparing cytokine levels in 22q11DS and other pediatric populations, and examining a broader range of behaviors (e.g., attention, impulsivity, social anxiety, or thought disturbances), is necessary to validate and expand these findings. This type of work could be instrumental in developing biomarkers preceding psychiatric illness in children with 22q11DS and more importantly identifying potential targets for medical interventions that could shift a child’s developmental trajectory towards more typical behavior.
A positive relationship was observed between autism-related behaviors and serum concentration of inflammatory cytokines in individuals with 22q11 deletion syndrome.
Acknowledgments
Financial Disclosures: This study was funded by the National Institute of General Medical Sciences IRACDA grant (K12 GM000680), the Simons Foundation, Brain and Behavior Research Foundation, and the Robert W. Woodruff Grant. We would like to thank the 22q families for their research participation and James Mullally for his technical support. The authors report no conflict of interest for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011a;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Altered T cell responses in children with autism. Brain Behav Immun. 2011b;25:840–849. doi: 10.1016/j.bbi.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, Dibben O, Margolick JB, Bream JH, Sambrano E, Martinez-Maza O, Sinclair E, Borrow P, Landay AL, Rinaldo CR, Norris PJ. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin Vaccine Immunol. 2011;18:1229–1242. doi: 10.1128/CVI.05032-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods. 2011;372:71–77. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degos V, Favrais G, Kaindl AM, Peineau S, Guerrot AM, Verney C, Gressens P. Inflammation processes in perinatal brain damage. J Neural Transm. 2010;117:1009–1017. doi: 10.1007/s00702-010-0411-x. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J Exp Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Crabtree GW, Markx S, Stark KL, Chaverneff F, Xu B, Mukai J, Fenelon K, Hsu PK, Gogos JA, Karayiorgou M. The 22q11.2 microdeletion: fifteen years of insights into the genetic and neural complexity of psychiatric disorders. Int J Dev Neurosci. 2011;29:259–281. doi: 10.1016/j.ijdevneu.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Mol Autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether JK, Croen LA, Anderson MC, Nelson KB, Yolken RH. Neonatally measured immunoglobulins and risk of autism. Autism Res. 2010;3:323–332. doi: 10.1002/aur.160. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Santana I, Bras JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer's disease and mild cognitive impairment. Neurodegener Dis. 2007;4:406–412. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- Hartel C, Adam N, Strunk T, Temming P, Muller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–453. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RN, Feinberg RL, Vaurio R, Passanante NM, Thompson RE, Kaufmann WE. Autism spectrum disorder in fragile X syndrome: a longitudinal evaluation. Am J Med Genet A. 2009;149A:1125–1137. doi: 10.1002/ajmg.a.32848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer L, Ashwood P, Schauer J, Goines P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1:275–283. doi: 10.1002/aur.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F, Albert MH, Arenz S, Bidlingmaier C, Berkowicz N, Sedlaczek S, Till H, Pawlita I, Renner ED, Weiss M, Belohradsky BH. Intracellular T-cell cytokine levels are age-dependent in healthy children and adults. Eur Cytokine Netw. 2005;16:283–288. [PubMed] [Google Scholar]
- Jyonouchi H, Geng L, Ruby A, Zimmerman-Bier B. Dysregulated innate immune responses in young children with autism spectrum disorders: their relationship to gastrointestinal symptoms and dietary intervention. Neuropsychobiology. 2005;51:77–85. doi: 10.1159/000084164. [DOI] [PubMed] [Google Scholar]
- Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T, Malik M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Abecasis GR, Heath SC, Knowles A, Demars S, Chen YJ, Roos JL, Rapoport JL, Gogos JA, Karayiorgou M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc Natl Acad Sci U S A. 2002;99:16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Emanuel BS, Zackai EH. 22q11.2 Deletion Syndrome. In: Pagon RA, et al., editors. GeneReviews. Seattle (WA): 1993. [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) Medicine (Baltimore) 2011;90:1–18. doi: 10.1097/MD.0b013e3182060469. [DOI] [PubMed] [Google Scholar]
- McDuffie A, Abbeduto L, Lewis P, Kover S, Kim JS, Weber A, Brown WT. Autism spectrum disorder in children and adolescents with fragile X syndrome: within-syndrome differences and age-related changes. Am J Intellect Dev Disabil. 2010;115:307–326. doi: 10.1352/1944-7558-115.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean-Tooke A, Barge D, Spickett GP, Gennery AR. Immunologic defects in 22q11.2 deletion syndrome. J Allergy Clin Immunol. 2008;122:362–367. 367 e361–364. doi: 10.1016/j.jaci.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB, Pedersen CB, Hougaard DM, Norgaard-Petersen B, Mors O, Borglum AD, Yolken RH. A Danish National Birth Cohort study of maternal HSV–2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr Res. 2010;122:257–263. doi: 10.1016/j.schres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89:148–151. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker-Athill EC, Tan J. Maternal immune activation and autism spectrum disorder: interleukin-6 signaling as a key mechanistic pathway. Neurosignals. 2010;18:113–128. doi: 10.1159/000319828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MG, Stevens H, Pedersen CB, Norgaard-Pedersen B, Mortensen PB. Toxoplasma infection and later development of schizophrenia in mothers. Am J Psychiatry. 2011;168:814–821. doi: 10.1176/appi.ajp.2011.10091351. [DOI] [PubMed] [Google Scholar]
- Philip N, Bassett A. Cognitive, behavioural and psychiatric phenotype in 22q11.2 deletion syndrome. Behav Genet. 2011;41:403–412. doi: 10.1007/s10519-011-9468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale M, Patruno A, De Lutiis MA, Pesce M, Felaco M, Di Giannantonio M, Di Nicola M, Grilli A. Dysregulation of chemo-cytokine production in schizophrenic patients versus healthy controls. BMC Neurosci. 2011;12:13. doi: 10.1186/1471-2202-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, Cook EH, Jr, Leventhal BL, Pickles A. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Sack U, Burkhardt U, Borte M, Schadlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5:28–32. doi: 10.1128/cdli.5.1.28-32.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK. Plasma increase of interleukin-12 and interferon-gamma. Pathological significance in autism. J Neuroimmunol. 1996;66:143–145. doi: 10.1016/0165-5728(96)00014-8. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophr Bull. 2009;35:631–637. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, Yoshihara Y, Wakuda T, Takebayashi K, Takagai S, Matsumoto K, Tsuchiya KJ, Iwata Y, Nakamura K, Tsujii M, Sugiyama T, Mori N. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PLoS One. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Agars E, Kuan SA, Baune BT, Campbell L, Samaras K, Crawford J, Lux O, Kochan NA, Brodaty H, Sachdev P. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age (Dordr) 2011 doi: 10.1007/s11357-011-9301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegering V, Eyrich M, Wunder C, Gunther H, Schlegel PG, Winkler B. Age-related changes in intracellular cytokine expression in healthy children. Eur Cytokine Netw. 2009;20:75–80. doi: 10.1684/ecn.2009.0149. [DOI] [PubMed] [Google Scholar]


