Abstract
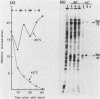
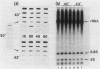
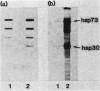
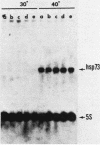
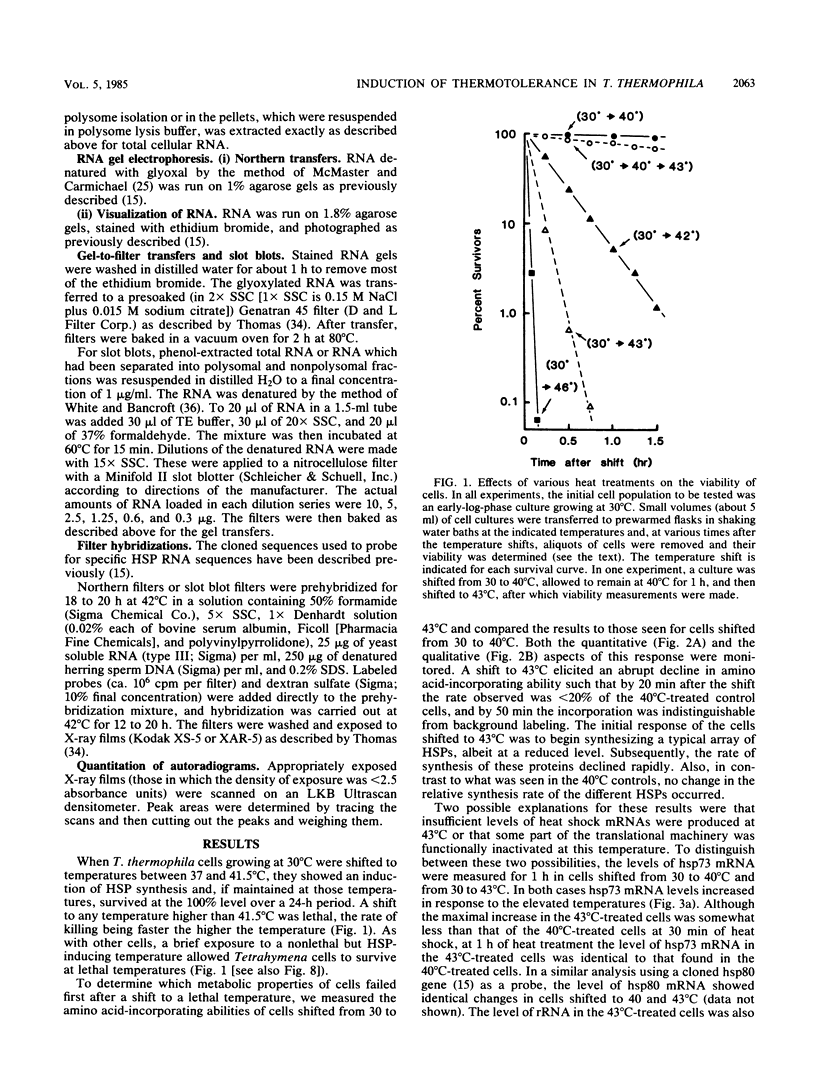
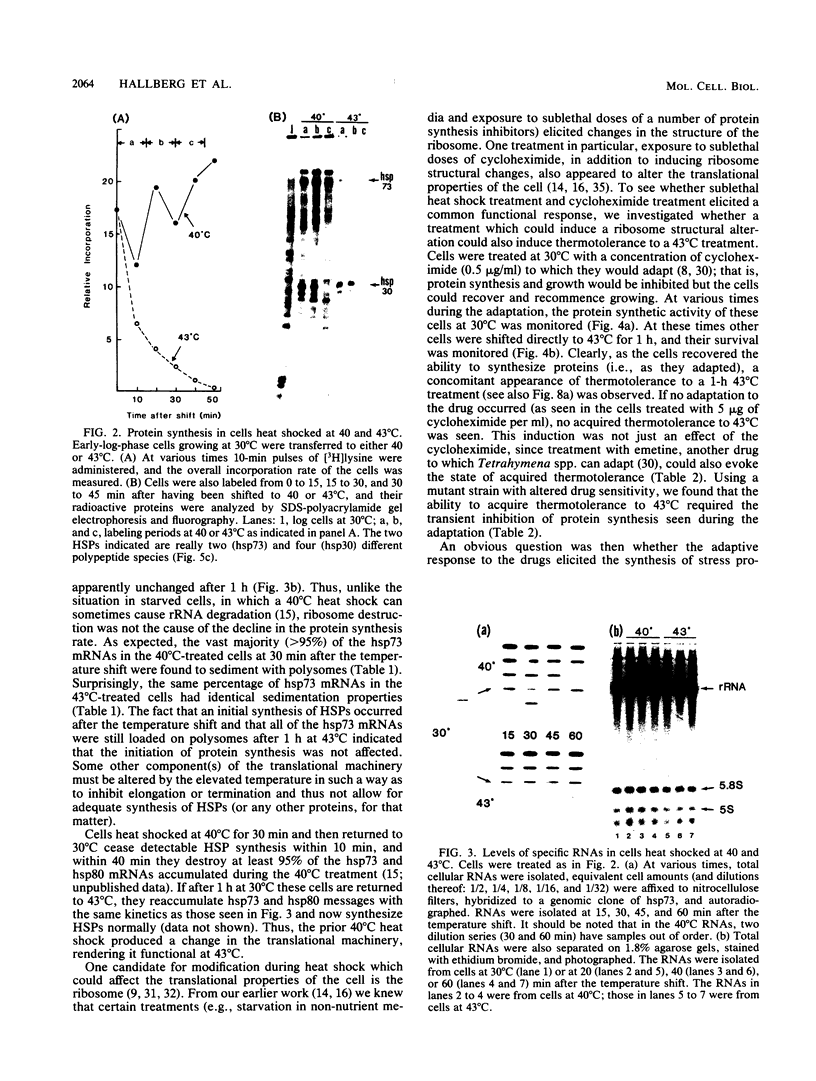
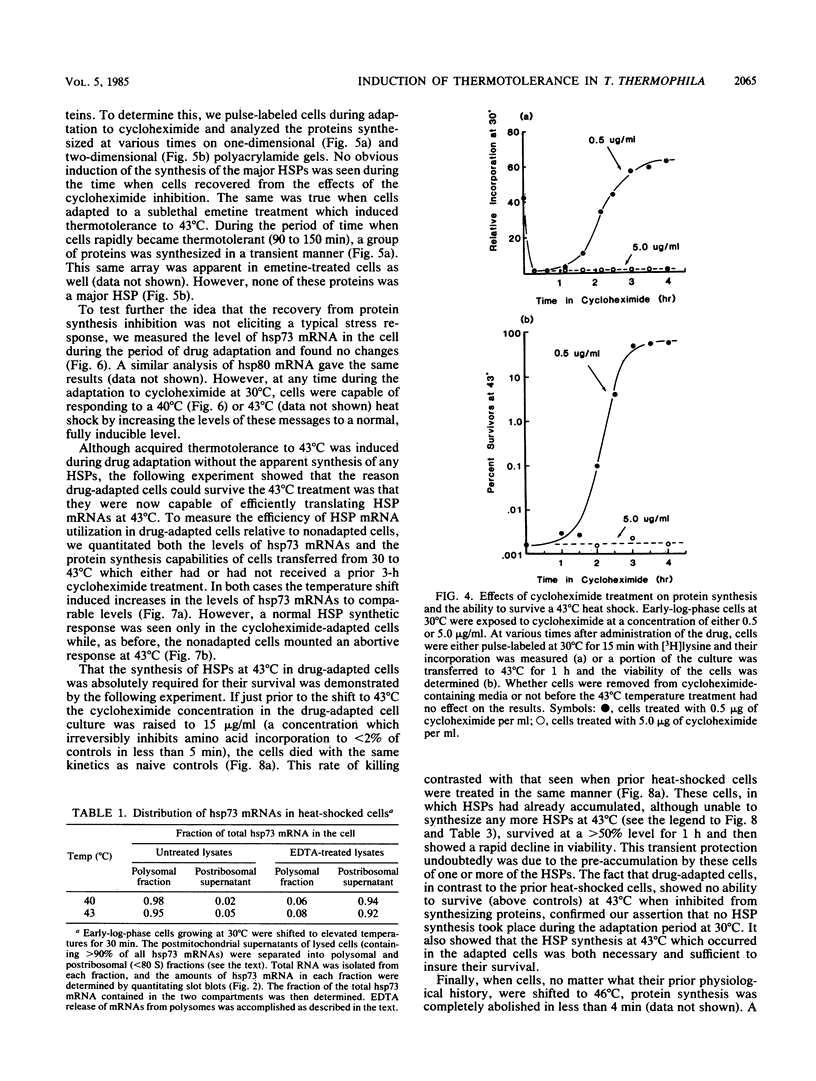
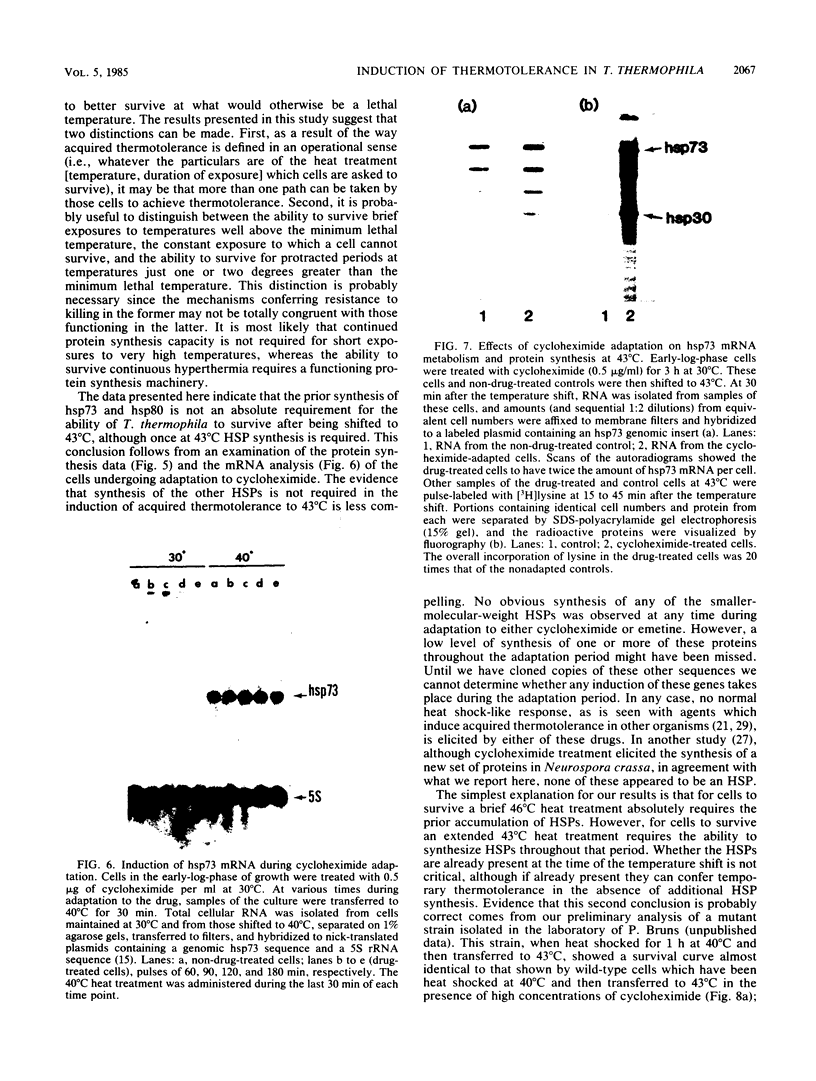
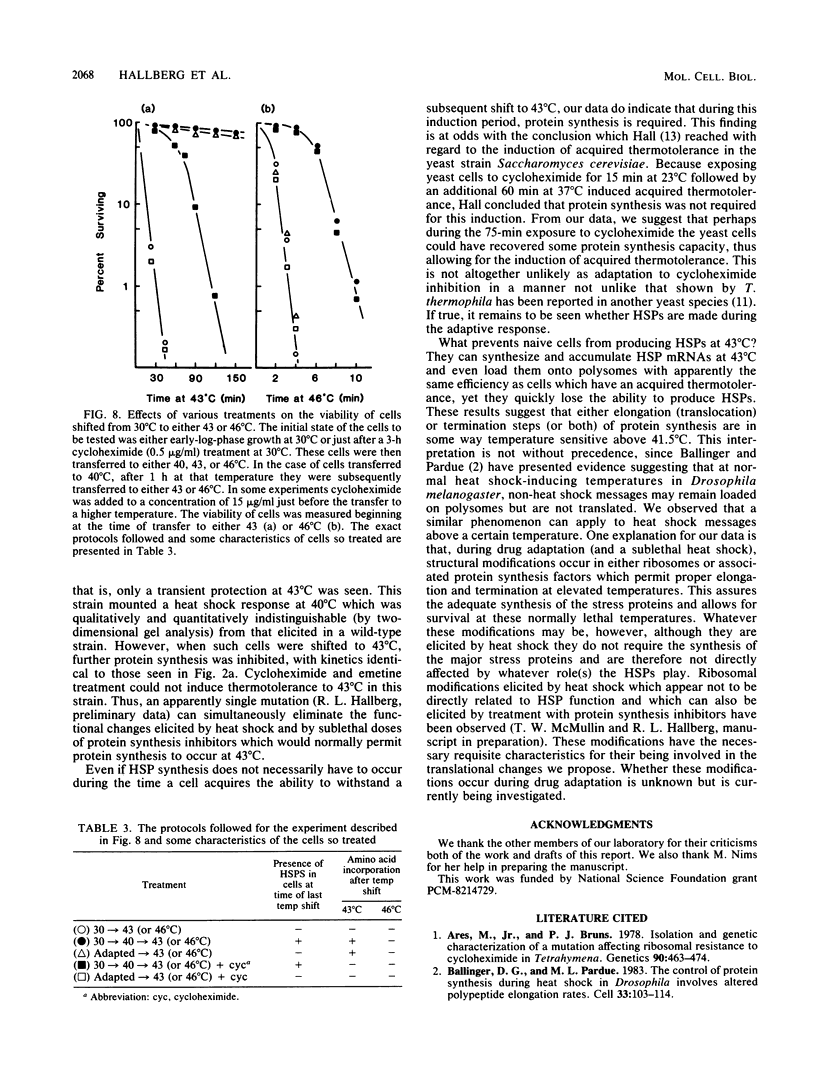
When Tetrahymena thermophila cells growing at 30 degrees C are shifted to either 40 or 43 degrees C, the kinetics and extent of induction of heat shock mRNAs in both cases are virtually indistinguishable. However, the cells shifted to 40 degrees C show a typical induction of heat shock protein (HSP) synthesis and survive indefinitely (100% after 24 h), whereas those at 43 degrees C show an abortive synthesis of HSPs and die (less than 0.01% survivors) within 1 h. Cells treated at 30 degrees C with the drugs cycloheximide or emetine, at concentrations which are initially inhibitory to protein synthesis and cell growth but from which cells can eventually recover and resume growth, are after this recovery able to survive a direct shift from 30 to 43 degrees C (ca. 70% survival after 1 h). This induction of thermotolerance by these drugs is as efficient in providing thermoprotection to cells as is a prior sublethal heat treatment which elicits the synthesis of HSPs. However, during the period when drug-treated cells recover their protein synthesis ability and simultaneously acquire the ability to subsequently survive a shift to 43 degrees C, none of the major HSPs are synthesized. The ability to survive a 1-h, 43 degrees C heat treatment, therefore, does not absolutely require the prior synthesis of HSPs. But, as extended survival at 43 degrees Celsius depends absolutely on the ability of cells to continually synthesize HSPs, it appears that a prior heat shock as well as the recovery from protein synthesis inhibition elicits a change in the protein synthetic machinery which allows the translation of HSP mRNAs at what would otherwise be a nonpermissive temperature for protein synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ares M., Jr, Bruns P. J. Isolation and genetic characterization of a mutation affecting ribosomal resistance to cycloheximide in Tetrahymena. Genetics. 1978 Nov;90(3):463–474. doi: 10.1093/genetics/90.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Banerji S. S., Theodorakis N. G., Morimoto R. I. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol. 1984 Nov;4(11):2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E. M., Woodward M. P. Small heat shock proteins in Drosophila may confer thermal tolerance. Exp Cell Res. 1983 Sep;147(2):437–442. doi: 10.1016/0014-4827(83)90225-2. [DOI] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Jacobsen K. Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature sensitive growth. Cell. 1984 Oct;38(3):841–849. doi: 10.1016/0092-8674(84)90279-4. [DOI] [PubMed] [Google Scholar]
- Frankel J. An analysis of the recovery of tetrahymena from effects of cycloheximide. J Cell Physiol. 1970 Aug;76(1):55–63. doi: 10.1002/jcp.1040760109. [DOI] [PubMed] [Google Scholar]
- GUNDERSEN K., WADSTEIN T. Morphological changes and resistance induced in Saccharomyces pastorianus by the antibiotic cycloheximide. J Gen Microbiol. 1962 Jun;28:325–332. doi: 10.1099/00221287-28-2-325. [DOI] [PubMed] [Google Scholar]
- Glover C. V. Heat shock induces rapid dephosphorylation of a ribosomal protein in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1781–1785. doi: 10.1073/pnas.79.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hall B. G. Yeast thermotolerance does not require protein synthesis. J Bacteriol. 1983 Dec;156(3):1363–1365. doi: 10.1128/jb.156.3.1363-1365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Hallberg E. M. Characterization of a cycloheximide-resistant Tetrahymena thermophila mutant which also displays altered growth properties. Mol Cell Biol. 1983 Apr;3(4):503–510. doi: 10.1128/mcb.3.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Kraus K. W., Findly R. C. Starved Tetrahymena thermophila cells that are unable to mount an effective heat shock response selectively degrade their rRNA. Mol Cell Biol. 1984 Oct;4(10):2170–2179. doi: 10.1128/mcb.4.10.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg R. L., Wilson P. G., Sutton C. Regulation of ribosome phosphorylation and antibiotic sensitivity in Tetrahymena thermophila: A correlation. Cell. 1981 Oct;26(1 Pt 1):47–56. doi: 10.1016/0092-8674(81)90032-5. [DOI] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman J., Feldman J. F. Cycloheximide and heat shock induce new polypeptide synthesis in Neurospora crassa. Mol Cell Biol. 1982 Oct;2(10):1167–1173. doi: 10.1128/mcb.2.10.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesset J., Palm C., McLaughlin C. S. Induction of heat shock proteins and thermotolerance by ethanol in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1340–1345. doi: 10.1016/0006-291x(82)92147-7. [DOI] [PubMed] [Google Scholar]
- Roberts C. T., Jr, Orias E. On the mechanism of adaptation to protein synthesis inhibitors by Tetrahymena. Facilitation, cross adaptation, and resensitization. J Cell Biol. 1974 Sep;62(3):707–716. doi: 10.1083/jcb.62.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Hooper A. B. Adaptation to cycloheximide of macromolecular synthesis in Tetrahymena. J Cell Physiol. 1978 Apr;95(1):1–11. doi: 10.1002/jcp.1040950102. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]