Abstract
Objective
To compare the motor relearning effect of a surface peroneal nerve stimulator (PNS) versus usual care on lower limb motor impairment, activity limitation, and quality of life among chronic stroke survivors.
Design
Single-blinded randomized controlled trial
Setting
Teaching hospital of academic medical center
Participants
110 chronic stroke survivors (> 12-wks post-stroke) with unilateral hemiparesis and dorsiflexion strength of ≤ 4/5 on the Medical Research Council scale
Interventions
Subjects were stratified by motor impairment level and then randomized to ambulation training with either a surface PNS device or usual care (ankle foot orthosis or no device) intervention. Subjects were treated for 12-wks and followed for 6-months post-treatment.
Main Outcome Measures
Lower limb portion of the Fugl-Meyer (FM) Assessment (motor impairment), the Modified Emory Functional Ambulation Profile (mEFAP) performed without a device (functional ambulation), and the Stroke Specific Quality of Life (SSQOL) scale.
Results
There was no significant treatment group main effect or treatment group by time interaction effect on FM, mEFAP, or SSQOL raw scores (p>0.05). The time effect was significant for the three raw scores (p<0.05). However, when comparing average change scores from baseline (T1) to end of treatment, (T2, 12-wks), and at 12-wks (T3) and 24-wks (T4) after end of treatment, significant differences were noted only for the mEFAP and SSQOL scores. The change in the average scores for both mEFAP and SSQOL occurred between T1 and T2, followed by relative stability thereafter.
Conclusions
There was no evidence of a motor relearning effect on lower limb motor impairment in either the PNS or usual care groups. However, both PNS and usual care groups demonstrated significant improvements in functional mobility and quality of life during the treatment period, which were maintained at 6-months follow-up.
Keywords: Motor relearning, hemiparesis, peroneal nerve stimulation, gait
Stroke is a leading cause of motor impairment and disability with an incidence of 795,000/year and prevalence of approximately 6.4 million in the U.S.1 Mobility limitations associated with walking may affect up to 75% of the individuals who sustain a stroke each year.2 Footdrop, the decreased ability to dorsiflex the ankle during the swing phase of gait, is an important post-stroke lower extremity (LE) motor impairment which contributes to mobility related disability. The rehabilitation intervention considered usual care for treatment of moderate to severe post-stroke dorsiflexion weakness during gait is an ankle foot orthosis (AFO); patients with less severe dorsiflexion weakness are generally prescribed ankle strengthening and gait training exercises only. A peroneal nerve stimulator (PNS), which dorsiflexes the ankle during the swing phase of gait, has been proposed as an alternative to an AFO.3–5 A PNS appears to be superior to no device in improving ambulation function.6 However, data on superiority to an AFO are inconsistent.6–10
Emerging data suggest that functionally relevant, active repetitive movement strategies facilitate motor relearning following stroke. 11 Motor relearning is defined as the reacquisition of motor skills or the reduction of motor impairment following damage to the central nervous system.12 Thus, in addition to dorsiflexing the ankle during functional ambulation, daily use of a PNS may facilitate motor relearning of the lower limb4–5, 13–21 such that in the long-term, neither an AFO nor a PNS is needed. In contrast, ambulation with an AFO could limit active repetitive movements at the ankle and inhibit motor relearning.22–23 To date, however, the comparative effect of a surface PNS versus usual care, including an AFO, on post-stroke motor relearning has not been evaluated in a randomized controlled trial.
The primary objective of this study was to compare the effects of a PNS and usual care on lower limb motor impairment among chronic stroke survivors. The secondary objective was to compare the effect of a PNS and usual care on lower limb activity limitation and overall quality of life. The demonstration of a surface PNS as an effective therapeutic intervention to facilitate motor relearning as measured on standard clinical scales could have significant impact on post-stroke motor recovery, and potentially establish a new standard of care for stroke rehabilitation.
METHODS
Study Design
A randomized controlled trial was performed comparing ambulation training with a surface PNS device (PNS group) to usual care (UC group). Chronic hemiparetic stroke subjects were treated for 12-wks (Device Usage period) and followed for a total of 6-months post-treatment. Outcome assessments were performed at baseline (T1), end of the Device Usage period (T2), and at 12-wks (T3) and 24-wks (T4) post-treatment.
Subjects
Subjects were recruited from a stroke rehabilitation outpatient program within a multi-hospital academic medical center. The institutional review boards of the involved hospitals approved the study protocol, and all participants signed informed consent. Inclusion criteria were age ≥ 18 years, ≥ 12-wks post-stroke with unilateral hemiparesis, and ankle dorsiflexion strength of ≤ 4/5 on the Medical Research Council (MRC) scale. Subjects were required to ambulate ≥ 30-ft without an AFO, score ≥ 24 on the Berg Balance Scale, and demonstrate correction of footdrop using a PNS without evidence of knee hyperextension during stance. Subjects were excluded for LE edema, skin breakdown, or absent sensation; serious cardiac arrhythmias, pacemakers or other implanted electronic systems; pregnancy; uncontrolled seizure disorder; concomitant lower motor neuron dysfunction and non-stroke upper motor neuron dysfunction; uncompensated hemineglect; sensory or motor peripheral neuropathy; fixed ankle plantarflexor contracture; or LE botulinum toxin injection within the 3-months prior to enrollment.
Randomization Procedure
As post-stroke motor outcomes may be affected by baseline motor function24–25, eligible subjects were first stratified on the basis of presence or absence of volitional ankle dorsiflexion prior to being randomized to the PNS or UC group. The randomization sequence was concealed in consecutively numbered envelopes which were allocated once eligibility was determined.
Devices
The PNS device was the Odstock Dropped-Foot Stimulator (ODFS)a, 3, 5, 16, a single-channel surface stimulator which detects heel rise at pre-swing via a 3-mm insole pressure sensing footswitch. The AFO was a custom molded hinged AFO with plantarflexion block which was fabricated using conventional techniquesb.
Intervention
The 12-wk Device Usage Period consisted of a Functional Training phase (two 1-hr sessions per wk × 5-wks) and a Post-Functional Training phase (three 1-hr sessions over 7 wks). During the Functional Training phase, subjects were trained to use their devices for home and community mobility with assistive device as needed. Standard Physical Therapy interventions were used and individualized based on the baseline functional status of each subject. Activities included passive and active range of motion (ROM) exercises, lower extremity strengthening (supine and standing), standing balance activities, weight-shifting activities to the affected limb using parallel bars with transition to least restrictive assistive device, and refinement of a reciprocal gait pattern (visual and manual cues were given). Exercises were done with multiple repetitions with increase in difficulty and decrease in cues, with and without the assigned device, as appropriate. A focus of the research therapy sessions was on higher level gait activities including functionally relevant movement tasks such as, stair climbing, walking on various surfaces (tile, carpet, ramps), negotiation of obstacles, community stepping (curbs), and treadmill training, as appropriate.
Subjects independently used their devices up to 8 hours per day during the Device Usage Period once device safety was demonstrated. At each of the Post-Functional training phase sessions, device function, application, and usage guidelines were reviewed with each subject to maximize device compliance. At completion of the Device Usage Period, all subjects discontinued use of the assigned device.
Outcome Assessments
The primary outcome measure was the lower extremity (LE) portion of the Fugl-Meyer Assessment (FMA),26–28 a valid measure of post-stroke motor impairment. The FM assesses LE reflexes, flexor and extensor synergy patterns, volitional movement, and coordination and speed through a series of movement tasks for a maximum score of 34. Secondary outcome measures were the modified Emory Functional Ambulation Profile (mEFAP) and the Stroke Specific Quality of Life (SSQOL) score. Activity limitation was assessed with the mEFAP,29–30 a functional mobility test that measures the time (seconds) to ambulate through five common environmental terrains (floor, carpet, up-and-go, obstacles, and stairs). The mEFAP score used for analysis was the sum of the five timed performance subscores. The SSQOL31 is a valid, reliable measurement which assesses health-related quality of life in stroke subjects and consists of a 49-item scale (each scored 1–5) which represents 12 domains for a maximum score of 245. All subjects were assessed while not wearing the treatment device.
Statistical Analysis
The study was originally designed as a 2 × 2 factorial design with treatment group (PNS vs. usual care) and dorsiflexion status (present vs. absent) as between subjects factors. Based on anticipated effect sizes on the FM derived from prior studies32–34 and alpha of 0.05, a sample size of 32 per cell or total of 128 subjects was calculated to detect the anticipated differences between cells with 80% power. However, during subject accrual, we experienced uneven recruitment with only 26% of subjects assigned to the dorsiflexion absent group. Thus, the study was converted to a single factor design (PNS vs. usual care) with anticipated difference in FM between groups of 5 points (0.83 S.D), which increases the power of the study to 99%. Even if the difference is as small as 3 points, the design has an 80% power to detect this difference. The stratification on dorsiflexion status was maintained during randomization to ensure even distribution of baseline motor function. Thus, we believe this change maintains a fair comparison that is not confounded by differences in subgroups.
All analyses were performed as intent-to-treat. Wilcoxon rank sum test or Fisher’s exact test was performed to evaluate participant baseline characteristics and baseline FM, mEFAP, and SSQOL scores between-group differences. Each outcome was modeled using a linear mixed effects approach to evaluate the mean change in primary and secondary outcome measures with treatment group. Time was considered discrete, since measurements were made at the four time periods (0, 12, 24, and 36-wks). However, since there was some variation in the exact date that individual measurements took place, we allowed for different growth rates for individuals by including a random intercept and slope in the models. Mixed effects models are well-suited for handling correlated repeated measurements, missing data and dropouts in longitudinal studies.35 In this study, the models yielded estimates of the treatment group, time, and treatment group by time effects while permitting us to control for potential confounders. We adjusted for age, sex, interval post-stroke, involved hemisphere, and stroke etiology.
In order to assess the motor relearning effect of PNS on lower limb motor impairment and impact on activity limitation, and quality of life over time, of primary interest was the two-way interaction between treatment group and time. We used an unstructured covariance structure, which made no assumptions about the variances and covariances, and allowed for differences in variability of the measurements at each time point. Model estimation was performed via Restricted Maximum Likelihood (REML) using PROC MIXED in SAS Version 9.2.36 A p-value of 0.05 was defined as the level of significance.
Since FM and SSQOL both have more than 11 distinct points, we approximated an interval scale in our models37. This approach is justified based on accepted statistical methodology and may in fact be conservative.38–39 However, we also modeled all three outcomes using a more robust estimator for the standard error, the EMPIRICAL option in PROC MIXED40, which is an asymptotically consistent “sandwich” estimator, in case parametric assumptions were violated. Further, we tested for differences between the treatment groups at each time point using the nonparametric Wilcoxon rank sum test with Bonferroni’s correction.
RESULTS
Participants and Baseline Characteristics
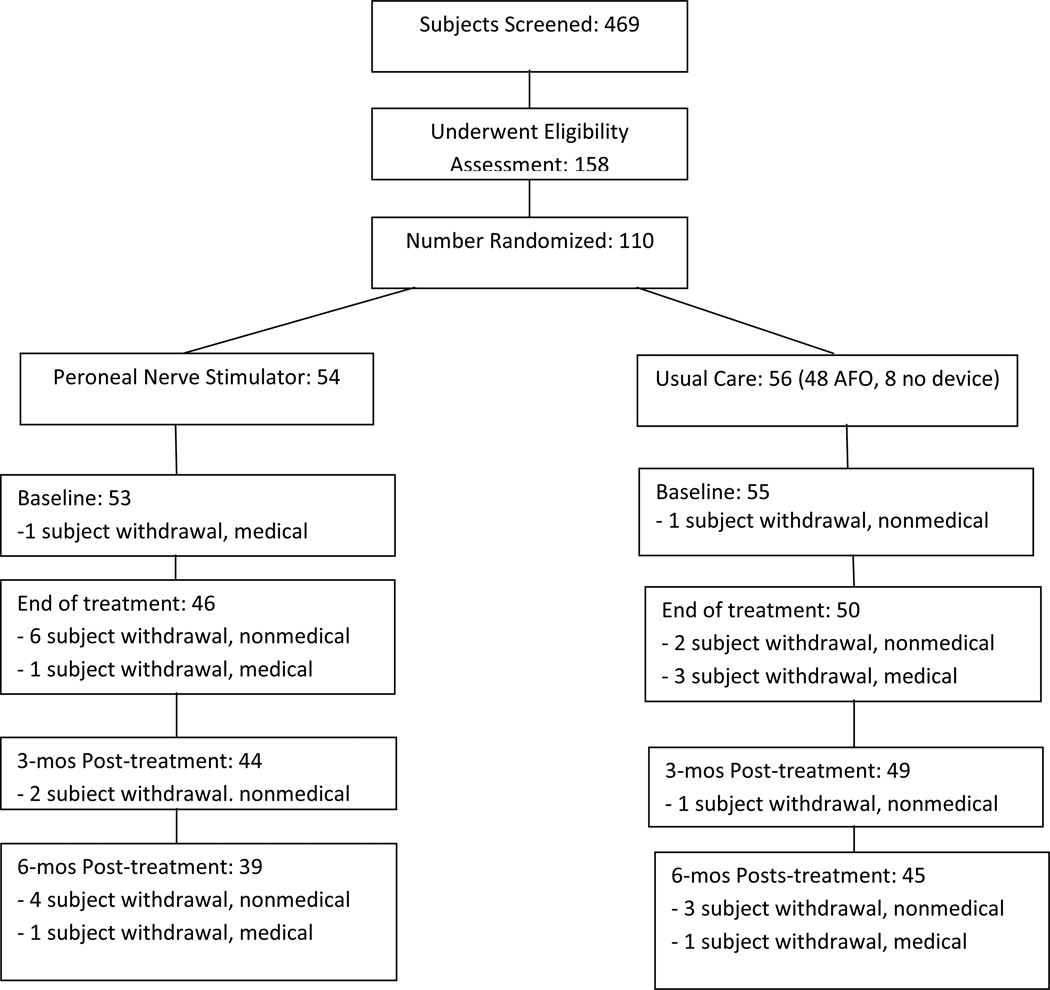
Figure 1 shows the participant flow diagram. A total of 110 or 23% of screened stroke survivors satisfied inclusion criteria and enrolled in the study. We experienced a lower than expected recruitment rate, and thus we did not reach our target enrollment of 128. Nevertheless, with the conversion of the study from a 2 × 2 factorial design to a single factor design, 110 subjects still translates to a power of 99% to detect the anticipated difference in FM scores between groups.
Figure 1.
Participant Flow Diagram. AFO: ankle foot orthosis.
Baseline characteristics of participants are shown in Table I. Forty-eight subjects (86%) randomized to usual care were treated with an AFO; eight subjects (14%) were treated with no device. Subject drop-out rates at T1, T2, T3 and T4 were 2%, 13%, 15% and 24%, respectively. The reasons for study drop-out were elective subject withdrawal due to nonmedical reason (12 subjects), medical issue unrelated to study device (7 subjects), subject lost to follow-up (5 subjects), and other (2 subjects).
Table I.
Baseline characteristics of treatment groups including means ± standard deviations and/or medians with 1st and 3rd quartiles. PNS: Peroneal nerve stimulation; UC: usual care; DF: dorsiflexion; mEFAP: Modified Emory Functional Ambulation Profile; SSQOL: Stroke Specific Quality of Life; T1: first outcome assessment (baseline).
| PNS n= 54 (mean ± SD) |
Median (1st quartile, 3rd quartile) |
UC n = 56 (mean ± SD) |
Median (1st quartile, 3rd quartile) |
p-value | |
|---|---|---|---|---|---|
| Age (yrs) | 52.8 ± 12.2 | 52.5 (44, 60) | 53.2 ± 10.1 | 54 (46.5, 59.5) | 0.84 |
| Male: female | 30:24 | 37:19 | 0.33 | ||
| Interval post-CVA (mos) | 44.7 ± 97.5 | 11 (6, 49) | 44.9 ± 79.2 | 18.5 (8, 45.5) | 0.27 |
| Etiology | 0.70 | ||||
| Embolic | 13 | 12 | |||
| Thrombotic | 17 | 23 | |||
| Lacunar | 9 | 6 | |||
| Hemorrhagic | 15 | 15 | |||
| Hemisphere | .09 | ||||
| Right | 35 | 27 | |||
| Left | 19 | 29 | |||
| DF absent: DF present | 14:40 | 15:41 | 0.70 | ||
| Fugl-Meyer (T1) | 20.1 ± 5.9 | 20 (16, 25) | 20.3 ± 6.0 | 21 (17, 24) | 0.73 |
| mEFAP (T1) | 121.5 ± 86.6 | 92.6 (59.0, 138.3) | 118.4 ± 74.1 | 90.7 (66.1, 154.4) | 0.80 |
| SSQOL (T1) | 179.1 ± 35.7 | 180.5 (150.5, 205.5) | 175.3 ± 40.7 | 180.0 (146, 210) | 0.81 |
Fugl-Meyer Assessment
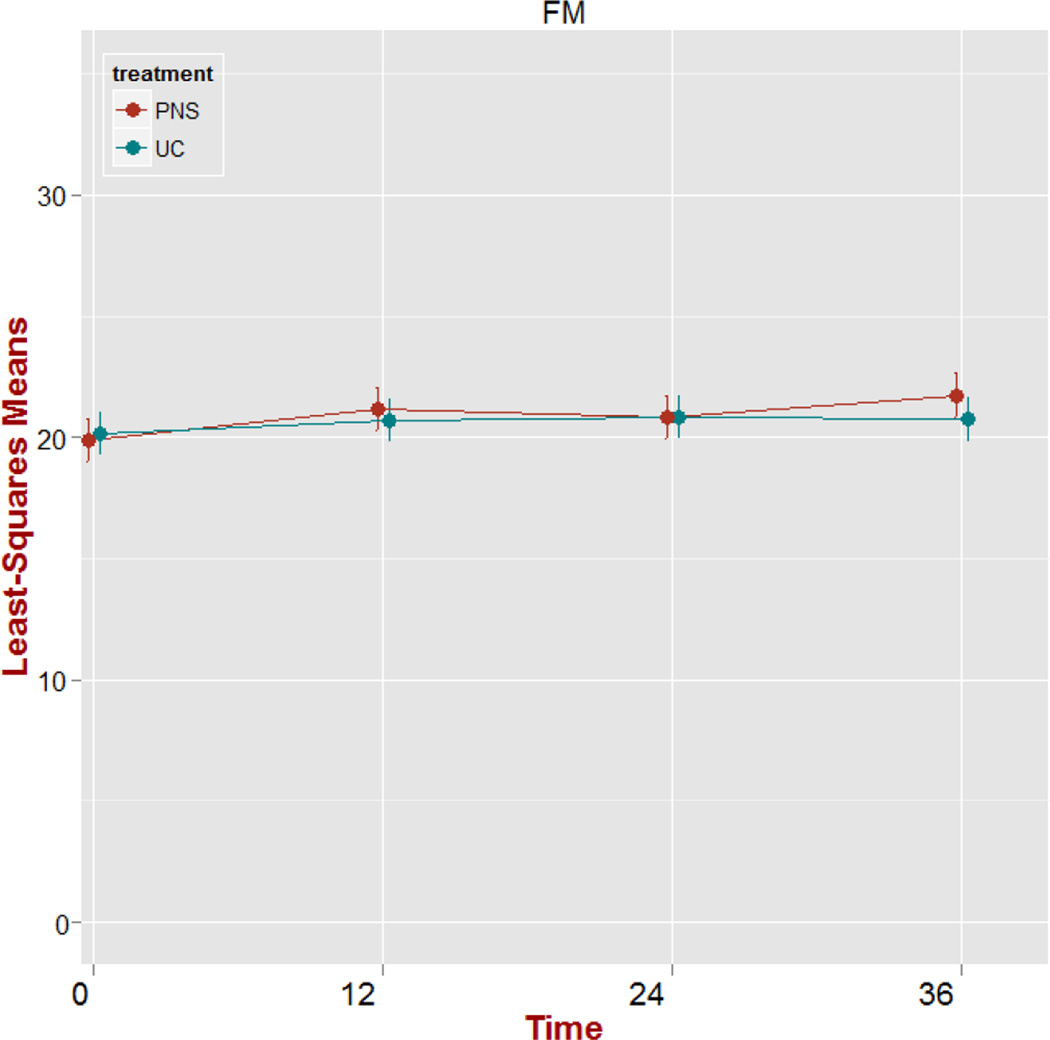
There was no significant treatment group main effect (p=0.797) or treatment group by time interaction effect (p=0.321) on FM raw scores. The time effect was significant (p=0.007). However, we observed no significant changes (p>0.05) in FM score trajectories from baseline to each time point. Shown in Figure 2 is a plot of adjusted means over time including standard error bars for FM scores. We use Least-Squares (LS) Means to provide a correction of the mean for missing data, estimating the marginal means for a balanced population, while adjusting for the confounders in the model.36 The plot displays the relatively flat time effect from T1 to each subsequent time periods indicating no significant change from baseline.
Figure 2.
Plot of adjusted means over time (weeks) for Fugl-Meyer (FM) scores including standard error bars. PNS: peroneal nerve stimulation; UC: usual care.
Modified Emory Functional Ambulation Profile
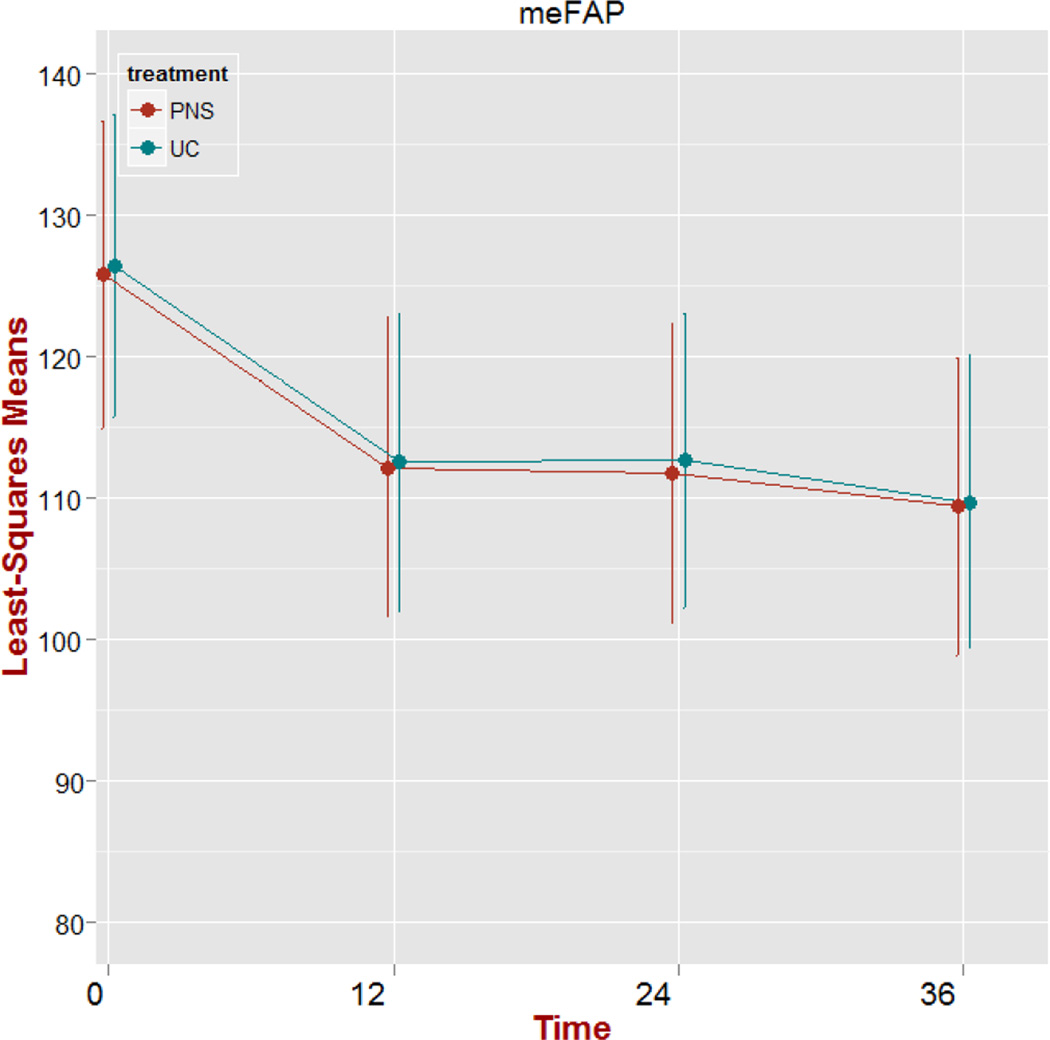
There was no significant treatment group main effect (p=0.968) or treatment group by time interaction effect (p> 0.999) on mEFAP raw scores. The time effect was significant (p<0.001). Model parameter estimates of the time effect at T2, T3, and T4 were all significantly lower than the baseline (T1) estimate. Shown in Figure 3 is the plot of adjusted means over time for the mEFAP scores including standard error bars. Both treatment groups follow the same trajectory of a negative average change in mEFAP scores during treatment, which level off during the post-treatment period.
Figure 3.
Plot of adjusted means over time (weeks) for the Modified Emory Functional Ambulation (mEFAP) scores (secs) including standard error bars. PNS: peroneal nerve stimulation; UC: usual care.
Stroke Specific Quality of Life
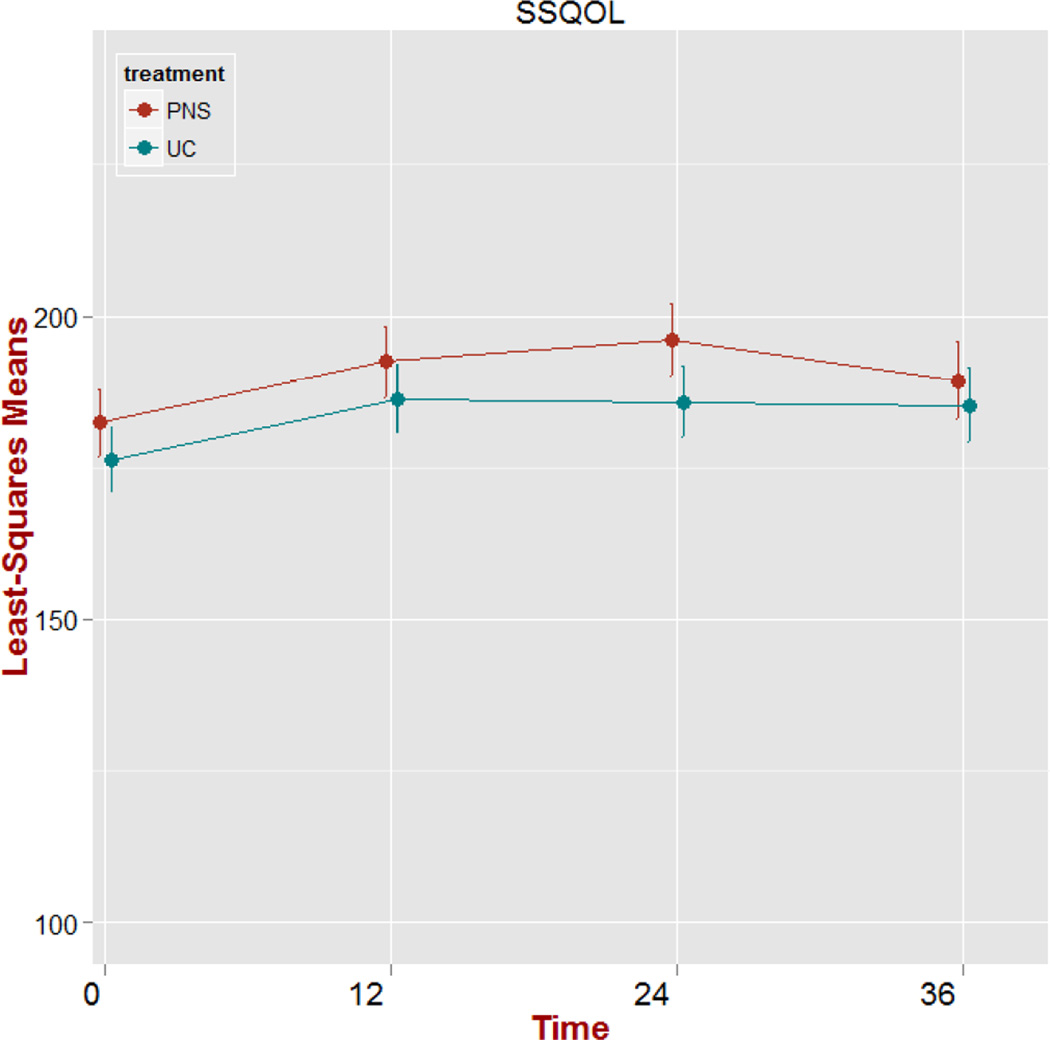
There was no significant treatment group main effect (p=0.360) or treatment group by time interaction effect (p=0.627) on SSQOL raw scores. The time effect was significant (p<0.001). Model parameter estimates of the time effect at T2-T4 were significantly higher than the baseline (T1) estimate. Shown in Figure 4 is the plot of adjusted means over time for SSQOL scores including standard error bars. Both treatment groups follow the same trajectory of a positive average change in SSQOL scores during treatment, which level off during the post-treatment period.
Figure 4.
Plot of adjusted means over time (weeks) for the Stroke Specific Quality of Life (SSQOL) scores including standard error bars. PNS: peroneal nerve stimulation; UC: usual care.
Table II shows the model parameter estimates, 95% Confidence Interval (CI), and p-value of time effect during treatment for FM, mEFAP, and SSQOL.
Table II.
Model Parameter Estimates, 95% Confidence Interval (CI) and p-value of Time Effect during Treatment. FM:Fugl-Meyer; mEFAP: Modified Emory Functional Ambulation Profile; SSQOL: Stroke Specific Quality of Life.
| Outcome | Estimate | 95% CI | p-value |
|---|---|---|---|
| FM | 0.525 | (−0.345, 1.396) | 0.238 |
| meFAP | −13.864 | (−21.256, −6.473) | <0.001 |
| SSQOL | 9.910 | (3.724, 16.096) | 0.002 |
Robust Statistical Methods
We came to the same conclusions as reported above using more robust standard errors in our models, in case parametric assumptions are violated. Further, none of the p-values were significant for the Wilcoxon rank sum tests for any of the three outcomes at any time point, even prior to performing Bonferroni’s correction, verifying that there are no significant treatment group differences at any time point.
DISCUSSION
The primary finding of this study was that the use of a surface PNS and usual care were not associated with improvements in motor relearning among chronic stroke survivors as measured by the LE FM. In addition, the use of a PNS was no more effective than usual care in improving ambulation function (mEFAP) or quality of life (SSQOL). However, both groups experienced significant improvements in ambulation function and quality of life during the treatment phase, which was maintained at 6-months.
In the context of the present study, the use of a PNS and usual care among chronic stroke survivors did not facilitate LE motor relearning as indicated by change in the FM motor impairment score. The literature suggests that activity dependent neuroplasticity requires tasks that are novel (challenging to perform), highly repetitive, functionally relevant, and cognitive engaging.11 Data also suggest that earlier intervention is more effective than later intervention.41 While ambulation with a PNS may be functionally relevant, the intervention specific task of ankle dorsiflexion may not be sufficiently novel and cognitively engaging. While subjects likely experienced high number of task repetitions, we do not know the actual number of dorsiflexion repetitions. A PNS usage monitor, now routinely incorporated in commercial PNS devices, was not available at study onset. Lastly, the potential for motor relearning may be more limited in chronic stroke survivors than subacute stroke survivors.
The secondary finding of this trial was that a PNS was no more effective than usual care on functional mobility (activity limitation). However, subjects in both groups exhibited significant improvement in functional mobility during the treatment period, which was sustained throughout the follow-up period. Given that subjects were enrolled an average of 45-months post-stroke and FM scores did not improve throughout the trial, it is highly unlikely that natural recovery or intervention mediated reduction in motor impairment contributed to this clinical improvement. Nevertheless, it is possible that the use of a PNS conveyed focal effects not detectable by a global impairment measure such as the FM. Other PNS studies have demonstrated changes in isometric dorsiflexion/plantar flexion strength,17 dorsiflexion torque,18–19 maximum root mean square of the tibialis anterior (TA) muscle,21 TA EMG activity, 17–19 and EMG co-contraction ratios.19 However, there would be no reason to expect similar changes in the usual care group. Thus, it is unlikely that theoretical focal impairment changes, not measurable by FM score, contributed to the functional improvements. An alternative explanation is that compensatory strategies, not specific to the treatment intervention, may have been acquired during the physical therapy ambulation training and treatment period, resulting in sustained improved functional mobility. Post-stroke functional improvements in the absence of clear changes in neurophysiologic parameters suggest the probable role of compensatory strategies.42–43 In a study of post-stroke gait recovery,43 functional gait improvements, which were not associated with change in coordination patterns of muscle activation, were proposed to be related to compensatory strategies and biomechanical changes of the nonparetic lower extremity. It is possible that both the PNS and usual care intervention induced the same global outcomes in terms of functional ambulation and quality of life, but did so by triggering different strategies for motor re-learning or different compensatory behaviors. If this is the case, one of the two treatments could produce a more effective response to facilitate long-term motor recovery. While our trial did not formally assess for compensatory strategies, all subjects additionally underwent quantitative gait analysis at each outcome assessment, and future analysis of these data will allow testing of this hypothesis.
The final finding of this trial was that although a PNS was no more effective than usual care in improving stroke specific quality of life, all subjects exhibited improved quality of life, irrespective of the study intervention. This effect was noted during the treatment period and was sustained for the duration of the trial. Given a trajectory that parallels the improvement in functional mobility, a reasonable explanation for this observation is the improvement in functional mobility. This concomitant improvement in quality of life suggests that the change in functional mobility observed in this study was clinically relevant. This finding is consistent with prior studies which have shown that multiple factors, including level of independence in ADLs and functional mobility, contribute significantly to quality of life following stroke.44–49
The present study failed to demonstrate the superiority of PNS over usual care in reducing LE motor impairment and activities limitation and improving quality of life of chronic stroke survivors. However, an important finding of the study is that a time-limited gait rehabilitation intervention implemented on average 45-months post-stroke can lead to clinically important changes in ambulation function that are maintained for at least 6-months after end of treatment. This study adds to the growing evidence in the literature50–51 that rehabilitation interventions in the chronic phase of stroke can be effective and clinically relevant. The study results also contribute to the ongoing debate52 regarding specificity of treatment on post-stroke gait intervention treatment effects.
Study Limitations
The study has a number of limitations. First, the study was changed from a 2 × 2 factorial to a single factor design. Thus, we were not able to determine the role of baseline dorsiflexion function on study outcomes. Second, we did not reach our target recruitment. The difference of 5-points in LE Fugl-Meyer between groups may be too large. A smaller difference may be clinically significant, but the study lacked the power to detect this smaller effect. This concern is mitigated by the fact that while a difference of 3–4 points may be clinically important, Figure 2 shows that the actual difference was on the order of 1–2 points. Third, the FM may not be sensitive to detect clinically important changes in motor impairment as discussed above. Fourth, we do not have accurate device usage data to compare PNS to usual care device usage. Our original study design included specific monitoring of device usage using a clinical step recorder embedded into the AFO and the device usage monitor capability of the PNS device. Unfortunately, the early iteration of the PNS devices which were purchased at initiation of this trial did not have usage monitoring capability. Future studies will need to incorporate reliable usage monitors to assess compliance and community performance. Fifth, treatment duration beyond 12-wks and/or device application in the subacute post-stroke period may translate into clinically important differences between groups. Finally, we experienced a relatively large drop-out rate of 24%, which may have compromised internal validity.
CONCLUSIONS
There was no evidence of a motor relearning effect on lower limb motor impairment in either the PNS or usual care groups as measured by the FM. However, even in the chronic phase of stroke, both PNS and usual care groups demonstrated significant improvements in functional mobility and quality of life that were sustained at 6-months.
Acknowledgments
Funded by the National Institute for Child Health and Human Development grants R01HD44816 (PI: Chae) and K23HD060689 (PI:Sheffler) , and the National Center for Research Resource grant UL1TR 000439.
The authors certify that one author has an affiliation with or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants and patents received or pending, royalties) with an organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the manuscript AND all such affiliations and involvements are disclosed on this title page of the manuscript. Paul N. Taylor is the co-inventor of the PNS device evaluated in this study and is named on two patents for the device. The patents are assigned to Salisbury NHS Foundation Trust. He also holds shares in Odstock Medical Limited.
I certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on me or on any organization with which I am associated AND, if applicable, I certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript. (Sheffler, Gunzler, Buurke, IJzerman, Chae)
The authors certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified above on the title page of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Suppliers
Odstock Medical Limited, The National Clinical FES Centre, Salisbury District Hospital, Salisbury Wiltshire, SP2 8BJ, United Kingdom +44(0)1722 439540 Contact: Paul Taylor PhD
G A Guilford & Son Ltd Orthotics and Prosthetic Center, 13515 Brookpark Rd Cleveland, OH 44142 216-362-1350 Contact: Skip Guilford
Contributor Information
Lynne R. Sheffler, Dept of Physical Medicine and Rehabilitation (PM&R), Case Western Reserve University (CWRU); Cleveland Veterans Affairs (VA) Functional Electrical Stimulation (FES) Center of Excellence; Dept of PM&R, MetroHealth Rehabilitation Institute of Ohio, MetroHealth Medical Center, 4229 Pearl Road, N5-524, Cleveland, Ohio 44109; Ph 216.957.3570/ FAX 216.957.2884, lsheffler@metrohealth.org
Paul N. Taylor, The National Clinical FES Center, Salisbury District Hospital, Salisbury, UK; p.taylor@salisburyfes.com.
Douglas D. Gunzler, Center for Health Policy Research, MetroHealth Medical Center, Cleveland, Ohio; dgunzler@metrohealth.org.
Jaap H. Buurke, Roessingh Research & Development, Enschede, The Netherlands; j.buurke@rrd.nl.
Maarten J. IJzerman, Department of Health Technology and Services Research, University of Twente, Enschede, The Netherlands; m.j.ijzerman@utwente.nl.
John Chae, Depts of PM&R and Biomedical Engineering, CWRU; Cleveland VA FES Center of Excellence; Dept of PM&R, MetroHealth Rehabilitation Institute of Ohio, MetroHealth Medical Center; jchae@metrohealth.org.
REFERENCES
- 1.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Zorowitz R, Bates B, et al. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36:e100–e143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- 3.Burridge JH, Taylor PN, Hagan SA, Wood DE, Swain ID. The effects of common peroneal stimulation on the effort and speed of walking: a randomized controlled trial with chronic hemiplegic patients. Clin Rehabil. 1997;11:201–210. doi: 10.1177/026921559701100303. [DOI] [PubMed] [Google Scholar]
- 4.Stein RB, Everaert DG, Thompson AK, et al. Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair. 2010;24:152–167. doi: 10.1177/1545968309347681. [DOI] [PubMed] [Google Scholar]
- 5.Taylor PN, Burridge JH, Dunkerley AL, et al. Clinical use of the Odstock dropped foot stimulator: its effect on the speed and effort of walking. Arch Phys Med Rehabil. 1999;80:1577–1583. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- 6.Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, MJ IJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. 2004;28:577–586. doi: 10.1111/j.1525-1594.2004.07310.x. [DOI] [PubMed] [Google Scholar]
- 7.van Swigchem R, Vloothuis J, den Boer J, Weerdesteyn V, Geurts AC. Is transcutaneous peroneal stimulation beneficial to patients with chronic stroke using an ankle-foot orthosis? A within-subjects study of patients' satisfaction, walking speed and physical activity level. J Rehabil Med. 2010;42:117–121. doi: 10.2340/16501977-0489. [DOI] [PubMed] [Google Scholar]
- 8.Sheffler LR, Hennessey MT, Naples GG, Chae J. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair. 2006;20:355–360. doi: 10.1177/1545968306287925. [DOI] [PubMed] [Google Scholar]
- 9.Kottink AI, Hermens HJ, Nene AV, et al. A randomized controlled trial of an implantable 2- channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil. 2007;88:971–978. doi: 10.1016/j.apmr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Ring H, Treger I, Gruendlinger L, Hausdorff JM. Neuroprosthesis for footdrop compared with an ankle-foot orthosis: effects on postural control during walking. J Stroke Cerebrovasc Dis. 2009;18:41–47. doi: 10.1016/j.jstrokecerebrovasdis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14:S57–S76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 12.Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can J Neurol Sci. 1995;22:257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- 13.Sheffler LR, Hennessey MT, Naples GG, Chae J. Improvement in functional ambulation as a therapeutic effect of peroneal nerve stimulation in hemiplegia: two case reports. Neurorehabil Neural Repair. 2007;21:366–369. doi: 10.1177/1545968306297869. [DOI] [PubMed] [Google Scholar]
- 14.Sabut SK, Lenka PK, Kumar R, Mahadevappa M. Effect of functional electrical stimulation on the effort and walking speed, surface electromyography activity, and metabolic responses in stroke subjects. J Electromyogr Kinesiol. 2010;20:1170–1177. doi: 10.1016/j.jelekin.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sabut SK, Sikdar C, Mondal R, Kumar R, Mahadevappa M. Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil. 2010;32:1594–1603. doi: 10.3109/09638281003599596. [DOI] [PubMed] [Google Scholar]
- 16.Wright PAMG, Swain ID. Training effects of electrical stimulation and the conventional ankle foot orthosis in the correction of drop foot following stroke. Paper presented at: The First Annual Conference of FESnet 2002; Glasgow, Scotland. 2002. [Google Scholar]
- 17.Carnstam B, Larsson LE, Prevec TS. Improvement of gait following functional electrical stimulation. I. Investigations on changes in voluntary strength and proprioceptive reflexes. Scand J Rehabil Med. 1977;9:7–13. [PubMed] [Google Scholar]
- 18.Merletti R, Zelaschi F, Latella D, Galli M, Angeli S, Sessa MB. A control study of muscle force recovery in hemiparetic patients during treatment with functional electrical stimulation. Scand J Rehabil Med. 1978;10:147–154. [PubMed] [Google Scholar]
- 19.Yan T, Hui-Chan CW, Li LS. Functional electrical stimulation improves motor recovery of the lower extremity and walking ability of subjects with first acute stroke: a randomized placebo-controlled trial. Stroke. 2005;36:80–85. doi: 10.1161/01.STR.0000149623.24906.63. [DOI] [PubMed] [Google Scholar]
- 20.Burridge J, Taylor P, Hagan S, Swain I. Experience of clinical use of the Odstock dropped foot stimulator. Artif Organs. 1997;21:254–260. doi: 10.1111/j.1525-1594.1997.tb04662.x. [DOI] [PubMed] [Google Scholar]
- 21.Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, Groothuis-Oudshoorn CG, MJ IJ. Therapeutic effect of an implantable peroneal nerve stimulator in subjects with chronic stroke and footdrop: a randomized controlled trial. Phys Ther. 2008;88:437–448. doi: 10.2522/ptj.20070035. [DOI] [PubMed] [Google Scholar]
- 22.Hesse S, Werner C, Matthias K, Stephen K, Berteanu M. Non-velocity-related effects of a rigid double-stopped ankle-foot orthosis on gait and lower limb muscle activity of hemiparetic subjects with an equinovarus deformity. Stroke. 1999;30:1855–1861. doi: 10.1161/01.str.30.9.1855. [DOI] [PubMed] [Google Scholar]
- 23.Romkes J, Hell AK, Brunner R. Changes in muscle activity in children with hemiplegic cerebral palsy while walking with and without ankle-foot orthoses. Gait Posture. 2006;24:467–474. doi: 10.1016/j.gaitpost.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Lin KC, Huang YH, Hsieh YW, Wu CY. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23:336–342. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Wolf SL, Blanton S, Winstein C, Nichols-Larsen DS. The EXCITE Trial: Predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22:486–493. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 27.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 28.Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. Reliability of the Fugl-Meyer assessment for testing motor performance in patients following stroke. Phys Ther. 1993;73:447–454. doi: 10.1093/ptj/73.7.447. [DOI] [PubMed] [Google Scholar]
- 29.Baer HR, Wolf SL. Modified emory functional ambulation profile: an outcome measure for the rehabilitation of poststroke gait dysfunction. Stroke. 2001;32:973–979. doi: 10.1161/01.str.32.4.973. [DOI] [PubMed] [Google Scholar]
- 30.Liaw LJ, Hsieh CL, Lo SK, Lee S, Huang MH, Lin JH. Psychometric properties of the modified Emory Functional Ambulation Profile in stroke patients. Clin Rehabil. 2006;20:429–437. doi: 10.1191/0269215506cr950oa. [DOI] [PubMed] [Google Scholar]
- 31.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–1369. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 32.Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–979. doi: 10.1161/01.str.29.5.975. [DOI] [PubMed] [Google Scholar]
- 33.Francisco G, Chae J, Chawla H, et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79:570–575. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 34.Duncan PW, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar DB. Similar motor recovery of upper and lower extremities after stroke. Stroke. 1994;25:1181–1188. doi: 10.1161/01.str.25.6.1181. [DOI] [PubMed] [Google Scholar]
- 35.Fitzmaurice GMLN, Ware JH. Applied Longitudinal Analysis. New York: John Wiley and Sons; 2004. [Google Scholar]
- 36.Enhancements in SAS/STAT 9.2 Software. Cary, North Carolina: SAS Institute Inc; 2008. [Google Scholar]
- 37.Nunnally J, Berstein I. Psychometric Theory. Third ed. McGraw-Hill Series in Psychology; 1994. [Google Scholar]
- 38.Johnson DRCJ. Ordinal measures in multiple indicator models: a simulation study of categorization error. American Sociological Review. 1983;48:398–407. [Google Scholar]
- 39.Zumbo BDZD. Is the selection of statistical methods governed by level of measurement? Canadian Psychology. 1993;34:390–400. [Google Scholar]
- 40.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. Oxford: Oxford Science; 1994. [Google Scholar]
- 41.Biernaskie J, Chernenko G, Corbett D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J Neurosci. 2004;24:1245–1254. doi: 10.1523/JNEUROSCI.3834-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geurts AC, de Haart M, van Nes IJ, Duysens J. A review of standing balance recovery from stroke. Gait Posture. 2005;22:267–281. doi: 10.1016/j.gaitpost.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Buurke JH, Nene AV, Kwakkel G, Erren-Wolters V, Ijzerman MJ, Hermens HJ. Recovery of gait after stroke: what changes? Neurorehabil Neural Repair. 2008;22:676–683. doi: 10.1177/1545968308317972. [DOI] [PubMed] [Google Scholar]
- 44.Nichols-Larsen DS, Clark PC, Zeringue A, Greenspan A, Blanton S. Factors influencing stroke survivors' quality of life during subacute recovery. Stroke. 2005;36:1480–1484. doi: 10.1161/01.STR.0000170706.13595.4f. [DOI] [PubMed] [Google Scholar]
- 45.Leach MJ, Gall SL, Dewey HM, Macdonell RA, Thrift AG. Factors associated with quality of life in 7-year survivors of stroke. J Neurol Neurosurg Psychiatry. 2011;82:1365–1371. doi: 10.1136/jnnp.2010.234765. [DOI] [PubMed] [Google Scholar]
- 46.Owolabi MO. What are the consistent predictors of generic and specific post-stroke health- related quality of life? Cerebrovasc Dis. 2010;29:105–110. doi: 10.1159/000262305. [DOI] [PubMed] [Google Scholar]
- 47.Lynch EB, Butt Z, Heinemann A, et al. A qualitative study of quality of life after stroke: the importance of social relationships. J Rehabil Med. 2008;40:518–523. doi: 10.2340/16501977-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel MD, McKevitt C, Lawrence E, Rudd AG, Wolfe CD. Clinical determinants of long-term quality of life after stroke. Age Ageing. 2007;36:316–322. doi: 10.1093/ageing/afm014. [DOI] [PubMed] [Google Scholar]
- 49.Choi-Kwon S, Choi JM, Kwon SU, Kang DW, Kim JS. Factors that Affect the Quality of Life at 3 Years Post-Stroke. J Clin Neurol. 2006;2:34–41. doi: 10.3988/jcn.2006.2.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolf SL, Thompson PA, Winstein CJ, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–2315. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hornby TG, Straube DS, Kinnaird CR, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Top Stroke Rehabil. 2011;18:293–307. doi: 10.1310/tsr1804-293. [DOI] [PubMed] [Google Scholar]