Abstract
Cytokine induced killer (CIK) cells are in clinical testing against various tumor types including multiple myeloma. Here, we show that CIK cells have activity against subcutaneous and disseminated models of human myeloma (KAS-6/1) which can be enhanced by infecting the CIK cells with an oncolytic measles virus (MV) or by pre-treating the myeloma cells with ionizing radiation (XRT). KAS-6/1 cells were killed by co-culture with CIK or MV-infected CIK (CIK/MV) cells and addition of an anti-NKG2D antibody inhibited cytolysis by 50%. Human bone marrow stromal cells can however reduce CIK and CIK/MV mediated killing of myeloma cells (RPMI 8226, JJN-3 and MM1). In vivo, CIK and CIK/MV prolonged the survival of mice with systemic myeloma, although CIK/MV showed enhanced antitumor activity compared to CIK. Irradiation of the KAS-6/1 cells induced mRNA and protein expression of NKG2D ligands, MICA and MICB, in a dose dependent manner and enhanced delivery of CIK/MV to the irradiated tumors. In both subcutaneous and disseminated myeloma models, XRT at 2 Gy resulted in superior prolongation of the survival of mice given CIK/MV therapy compared to CIK/MV with no XRT. This study demonstrates the potential of CIK against myeloma and that combination of virotherapy with radiation could be used to further enhance therapeutic outcome using CIK cells.
Keywords: cytokine induced killer cells, virotherapy, myeloma, irradiation, NKG2D, MICA/B
INTRODUCTION
Multiple myeloma (MM) is a disseminated cancer consisting of malignant monoclonal plasma cells that reside in the bone marrow [1, 2]. It is estimated that about 21,700 incident cases occur annually in the USA, and about 10,710 subjects are reported to die from the disease each year [3]. Despite a variety of therapeutic options including alkylator-based chemotherapy, corticosteroids, autologous stem cell transplantation, immunomodulatory drugs such as lenalidomide, and the proteasome inhibitor, bortezomib, outcomes remain are poor [4, 5].
Cytokine-induced killer (CIK) cells are a heterogeneous subset of ex-vivo expanded T lymphocytes that present phenotypic and functional properties of both natural killer (NK) and T cells, and have major histocompatibility complex (MHC)-unrestricted antitumor activity against both solid tumors and hematologic malignancies [4, 6]. The tumor cell killing activity of CIK cells is mediated via the natural killer group 2 D (NKG2D) molecule on the CIK cell membrane interacting with non MHC ligands on the tumor cell surface [7]. The main NKG2D ligands include the major histocompatibility complex (MHC) class I-related chain A and B (MICA and MICB) and the family of UL16-binding proteins (ULBP-1, 2, and 3) [8–10]. In humans, NKG2D ligands are transcriptionally induced by various types of cellular stress such as heat shock, oxidative stress, ionizing radiation and viral infection [11, 12]. CIK cells have been shown to target a variety of solid tumors and hematologic malignancies [13–16]. Several clinical trials are ongoing testing the antitumor activity of CIK therapy in human subjects and CIK infusions have been well-tolerated at doses of 106 to 1010 cells [14, 16]. However, clinical activity has been modest to date, limited by suboptimal cellular trafficking and tumor cell killing [16].
Tumor selective replication competent viruses (oncolytic viruses) have shown promising anticancer activity in preclinical animal models and several viruses are currently being evaluated in clinical trials against a variety of cancers [17–19]. We have been developing a live attenuated strain of measles virus as an oncolytic agent for cancer therapy [20]. Measles is a negative strand RNA virus of the family Paramyxoviridae. Its entry into target cells is mediated by the measles hemagglutinin (H) attachment glycoprotein which binds to cellular receptors, CD46, signaling lymphocyte activating molecule (SLAM) or nectin-4, leading to fusion of the viral and cell membranes mediated by the viral fusion glycoprotein [21–24]. Later, each MV infected cell fuses with surrounding uninfected cells to form larger syncytia which are non-viable and undergo apoptotic cell death [25];[26]. A recombinant oncolytic MV encoding the human thyroidal sodium-iodide symporter (MV-NIS) is currently undergoing Phase I clinical evaluation (intravenous administration) in patients with relapsed multiple myeloma at Mayo Clinic [27, 28]
In the current study, we combined CIK therapy with oncolytic measles virus therapy to evaluate if MV infection could enhance the CIK antitumor killing activity. The working hypothesis was that the MV infected CIK cells should traffick to the tumor deposits and kill some of the tumor cells, as well as transferring the virus to the tumors to initiate viral induced oncolysis. The impact of stromal cells on CIK activity was also investigated by co-culture of myeloma cells with a human bone marrow stromal cell line HS-5. Since myeloma is highly radiosensitive, we also investigated the idea that ionizing radiation could enhance the therapeutic outcome of MV-CIK therapy in both subcutaneous and disseminated models of human myeloma.
MATERIALS AND METHODS
Cell Culture and Virus Propagation
Vero African green monkey kidney cells purchased from the American Type Culture Collection (ATCC, Manassas, VA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 100 μg/ml penicillin, and 100 μg/ml of streptomycin. MM1, KAS-6/1, RPMI 8226 and JJN3 (MM1 and KAS6/1 are kind gifts from R. Fonseca and D.F. Jelinek, Mayo Clinic) were cultured in 10% FBS Roswell Park Memorial Institute (RPMI)-1640 medium. Culture medium of KAS-6/was supplemented with 1 ng/ml recombinant human interleukin-6 (R&D Systems, Minneapolis, MN). Measles virus expressing green fluorescent protein (MV-GFP) [29] or sodium iodide symporter (MV-NIS) [28] was propagated and titrated on Vero cells as previously described [30].
Generation of Cytokine Induced Killer Cells
Human peripheral blood lymphocytes (PBL) were isolated from healthy human volunteers. To generate CIK cells, the PBL were cultured in complete medium consisting RPMI-1640, 10% FBS, 2mM L-glutamine, 100 U/ml penicillin, 100μg/ml streptomycin, 50 μM 2-mercaptoethanol and 1000 U/ml recombinant human interferon-gamma (Peprotech, Rocky Hill, NJ) on day 1 [31]. After 24 hours, 50 ng/ml monoclonal antibody against CD3 and 300 IU/ml recombinant human interleukin-2 (IL-2) (BD Biosciences, San Jose, CA) were added. Fresh complete medium containing 300 IU/ml IL-2 was added every 2–3 days. Cells were harvested after 14 days and characterized for standard CIK cell markers by flow cytometry.
Flow Cytomery Analysis
CIK cells were characterized using FITC-conjugated or PE-conjugated anti-CD3 (BD Pharmingen,San Diego, CA), anti-CD4 (eBioscience,San Diego, CA), anti-CD8 (eBioscience,San Diego, CA) or anti-CD56 (eBioscience,San Diego, CA), anti-CD46 (BD Pharmingen, San Diego, CA ) or anti-CD150 (BioLegend, San Diego, CA), and APC-conjugated anti-NKG2D (BD Pharmingen, San Diego, CA).
CIK Cell Cytotoxicity Assay
Target cells, KAS-6/1-Luc cells expressing firefly luciferase [26], were plated into 96-well plates at 104 cells/well. CIK were infected with MV-NIS at MOI of 0.5, 1.0 or 2.0 for 2 hours and extensively washed. Effector cells, CIK cells or MV-infected CIK cells, were added at specified effector:target cell ratios (1.25:1, 2.5:1, 5:1, 10:1) and co-cultured for 24 or 48 hours. The numbers of viable of KAS-6/1-Luc cells was determined by assaying for luciferase activity using the Promega Luciferase assay system (E1501) according to manufacturer’s instructions (Promega, Madison, WI) and measuring the relative light units (RLU) on the Top Count NXT Scintillation and Luminescence Counter (Packard, Meriden, CT) in black 96-well plates [26].
For co-culture experiments, cells were plated at specified HS-5 to myeloma cell ratio (0:1, 1:5, 1:2, 1:1, 2:1, 5:1). Myeloma cells used were RPMI8226-Luc, JJN-3-Luc and MM1-Luc cells. Plates were incubated for 24 hours after which the co-cultures were incubated with either medium alone (controls), MV-NIS alone at MOI of 1.0, CIK or CIK/MV at MOI of 1 (CIK:myeloma at 1:1 ratio). Viability of luciferase positive myeloma cells were measured as relative luciferase unit using the Promega luciferase assay system as described above.
Virus Infection Assays
CIK cells were infected with MV-GFP at MOI of 0.5, 1.0, or 2.0 for 2 hours at 37°C. After the virus inoculum was removed, the cells were cultured for 24, 48, and 72 hours in the presence of a fusion inhibitory peptide (FIP, Z-D-Phe-Phe-Gly-OH; Bachem, Americas Inc., Torrance CA). The percentage of green fluorescent MV-GFP–infected cells was determined by flow cytometry.
MV-Luc Replication in CIK cells
CIK cells were infected with MV-Luc at MOI of 1.0, for 2 hours at 37°C. After the virus inoculum was removed, the cells were cultured for 24, 48, 72 and 96 hours. Cell-associated viruses were released by two freeze-thaw cycles as previously described [32]. Viral titers were determined by TCID50 titration on Vero cells.
Reverse transcription polymerase chain reaction (RT-PCR) analysis
Total RNA was prepared from irradiated KAS6/1 cells using the RNeasy Plus Mini kit according to manufacturer’s protocol (QIAGEN, Valencia, CA). First strand cDNA was synthesized using the SuperScript III First-strand Synthesis System (Invitrogen, Carlabad, CA). The following primer sequences were used; MICA: forward primer 5′ TTG AGC CGC TGA GAG GGT GGC 3′, and reverse primer 5′ GGG AGA GGA AGA GCT CCC CAT C 3′, MCIB: forward primer 5′ GCC CCC TGA CCC CTT GTT CC 3′, and reverse primer 5′ GGG CTG GTC AAC TTG GCG AAA 3′. For internal control, primers specific for human GAPDH were used: forward 5′ CCT CCC GCT TCG CTC TCT GC 3′ and reverse 5′ GGG TGG CAG TGA TGG CAT GG 3′.
Western blotting
For western blotting, aliquots (20–50 μl) of homogenized cell lysates were subjected to 10% SDS-PAGE and then transferred onto a nitrocellulose membrane (Amersham, Piscataway, NJ). MICA and MICB proteins were detected with a polyclonal rabbit anti-human MICA antibody or a monoclonal mouse anti-human MICB antibody respectively (Abcam, Cambridge, MA).
In vivo experiments
All procedures involving animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Foundation. Four to six-week-old female ICR-SCID mice from Taconic (Germantown, NY) were given whole body irradiation (2 Gy) 24 hours before intravenous tail vein or subcutaneous injection of KAS-6/1 cells (6×106 cells/100 μl). In the therapy experiment, tumor bearing mice were randomized into treatment groups and given the test article intravenously via the tail vein. The mice received either saline, CIK cells alone or MV infected CIK cells. In experiments involving irradiation, mice received whole body irradiation (2 Gy) one day before infusion of test articles. Mice were observed daily and were euthanized when any of the following signs was observed: tumor burden more than 10% of body weight, if tumor ulcerated or if mice developed paralysis from the disseminated myeloma disease, became inactive or lost more than 20% of body weight. At the end of the study (day 90), all remaining mice were euthanized. Kaplan–Meier survival curves were generated and analyzed by logrank test.
Statistical Analysis
Statistical significances of differences were evaluated by the Student t test or logrank test using either Prism 5 (GraphPad software) or JMP 9 software. A probability level of P≤ 0.05 was considered to be statistically significant.
RESULTS
Infection of CIK cells by MV
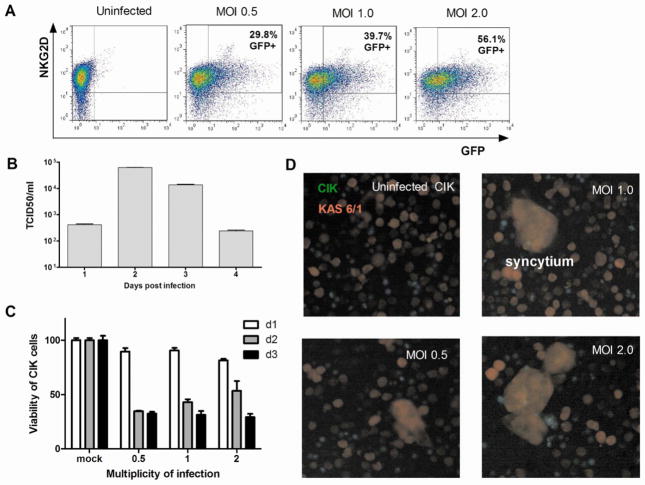
Cytokine induced killer cells were generated from Ficoll gradient purified human peripheral blood lymphocytes (PBL) using a cocktail of IFN-γ, OKT3 and IL-2 as described previously [4, 6, 31]. At the end of three weeks, expression of NKG2D by CIK cells was confirmed by flow cytometry with an APC-conjugated anti-NKG2D antibody (Fig. 1A). Uninfected CIK cells expressed high levels of NKG2D with mean fluorescence intensity (MFI) of 103. The CIK cells were infected with MV expressing GFP (MV-GFP) at MOI of 0.5, 1.0, or 2.0 for 2 hours. After the virus inoculum was removed, the cells were cultured for 48 hours in media containing a fusion inhibitory peptide (+FIP) that prevents intercellular fusion (syncytia formation) to enable quantitation by flow cytometry (Fig. 1A). MV-infected CIK cells still expressed high levels of NKG2D receptor which is important in mediating cellular cytotoxicity. NKG2D expression level on MV infected cells was comparable to uninfected cells, with NKG2D MFI ranging from 132 (MOI 0.5) to 86.9 (MOI 2.0). There was a corresponding increase in the numbers of GFP positive cells (~30% to 56%) with increase in MOI from 0.5 to 2.0 (Fig. 1A). Measles virus was able to propagate in the infected CIK cells and viral progeny yield was maximal at day 2 (Fig. 1B). Viability of MV infected CIK cells was determined at days 1, 2, and 3 post infection. Cell viability decreased to 80% on day 1 and to 50% by day 2 (Fig. 1C). It is apparent that decrease in viral yield by day 3 is a result of death of the infected CIK cells.
Figure 1. Infection of CIK cells with measles virus.
(A) Dual color flow cytometry showing NKG2D expression in MV-GFP infected CIK cells. Quantitation of MV infection in CIK cells was performed by flow cytometry for GFP positive cells at 24, 48 and 72 h post infection at various multiplicities of infection (MOI, ratio of virus:cell). (B) Replication of MV-Luc in CIK cells as measured by amount of progeny virus produced post infection (MOI 1.0). Error bars represent S.D. (n=3 replicates). (C) Viability of CIK cells at different days post MV-NIS infection. (D) Transfer of MV from infected CIK cells to myeloma KAS-6/1 cells by heterologous intercellular fusion. At 18 hours post infection, MV-Luc infected CIK cells (labeled green with CellTracker Green CMFDA) were mixed with DsRed-expressing KAS-6/1 target cells at a 1:1 ratio. The co-culture was photographed 48h later using a fluorescence microscope (200X magnification). Syncytia were seen in co-cultures of infected CIK with KAS-6/1 cells due to heterofusion of cells.
Infected CIK cells could potentially transfer the viral infection to the myeloma cells through heterologous intercellular fusion. This transfer was assessed in a co-culture experiment where MV-luciferase (MV-Luc) infected CIK cells labeled green with CellTrackerGreen CMFDA were mixed with DsRed-expressing KAS 6/1 target cells at a ratio of 1:1 (Fig. 1D). By 48 hours after co-culture, significant measles virus-dependent heterocellular fusion was observed between the green CIK cells and red KAS-6/1 cells, resulting in the formation of large syncytia that were orange in color when viewed through the dsRed/GFP dual filter (Fig. 1D). In contrast, no heterofusion was seen if uninfected CIK cells were mixed with KAS-6/1 cells.
Uninfected and MV-infected CIK cells are highly cytotoxic against KAS-6/1 myeloma cells
Next, we examined the cytotoxic activity of CIK cells against KAS-6/1 cells in vitro. Target KAS-6/1 cells stably expressing firefly luciferase (Fluc) were mixed with MV-infected or uninfected CIK effector cells at an effector:target (E:T) ratio of 1:1. Luciferase activity, a measure of the number of viable target KAS-6/1-Fluc cells, was determined 24, 48, and 72 hours after cell mixing. Uninfected CIK cells showed strong cytotoxic activity against the KAS-6/1 myeloma cells (Fig. 2A). MV-infected CIK cells were considerably more potent. In contrast, no anti-KAS-6/1 activity was seen when the cells were co-cultured with another myeloma cell line, MM1, instead of CIK cells (Fig. 2B). However, MV-infected MM1 cells were able to heterofuse with the KAS-6/1 cells and induced significant cell death (Fig. 2B).
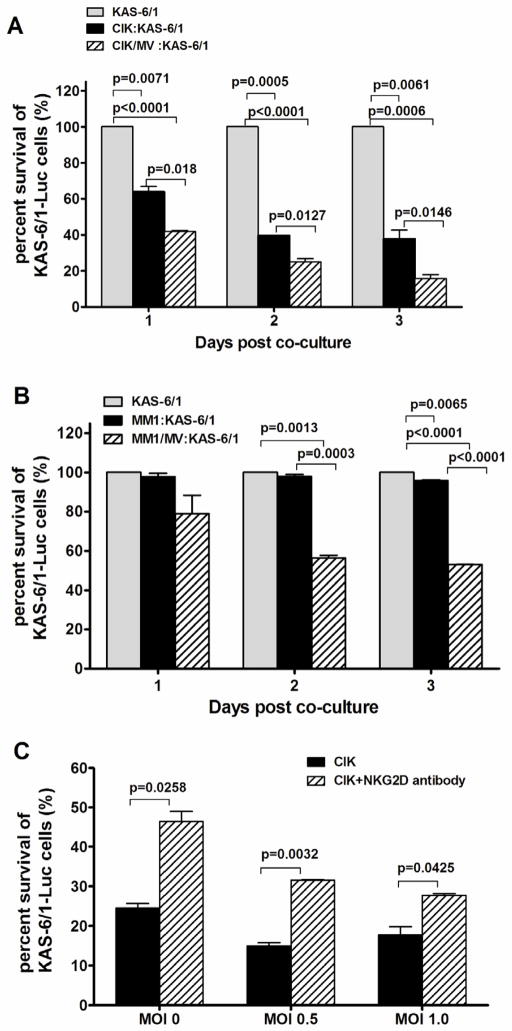
Figure 2.
Cytotoxic activity of CIK and MV-infected CIK (CIK/MV, MOI 1.0) cells on KAS-6/1 myeloma cells. KAS-6/1 cells stably expressing firefly luciferase (KAS-6/1-Fluc) were co-cultured with uninfected or MV-infected (A) CIK cells or (B) MM1 myeloma cell line at 1:1 ratio. Killing of KAS-6/1-Fluc cells was measured by quantitation of luciferase activity at different time points after co-culture. (C) CIK killing of myeloma cells was mediated in part through the NKG2D receptor. Uninfected or MV-infected CIK cells (MOI 0.5 and 1.0) were premixed with an NKG2D-blocking antibody and co-cultured with KAS-6/1-Fluc cells at a ratio of 1:1. Myeloma cell death was quantitated at 24 hours post co-culture by measuring luciferase activity.
Anti-NKG2D blocking antibody attenuates the cytotoxicity of CIK and MV/CIK cells
The exact mechanism involved in CIK tumor recognition and killing is not completely known but the NKG2D molecule, which is expressed on the surface of CIK cells and interacts with MHC-unrestricted ligands on tumor cells, is known to be a key determinant of killing specificity. As shown in figure 2C, addition of a monoclonal antibody that blocks NKG2D resulted in 50% reduction of the KAS-6/1 killing by uninfected CIK cells. Likewise, when MV-infected CIK cells were pretreated with the anti-NKG2D blocking antibody, there was a 50% increase in viable KAS-6/1 target cells, suggesting that NKG2D also contributed significantly to the observed cytotoxicity in this co-culture (Fig. 2C).
Impact of stromal cells on CIK and CIK/MV killing of MM cells
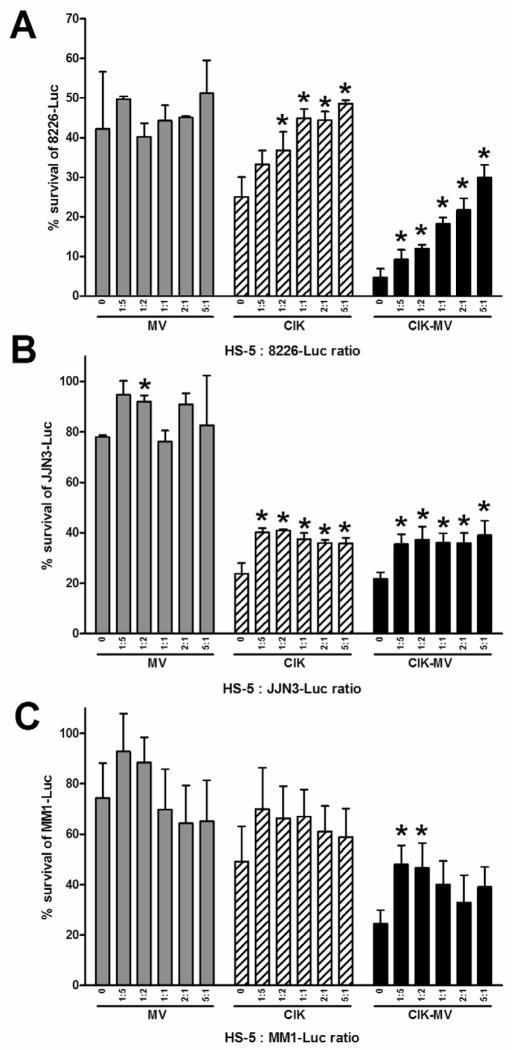
The human healthy bone marrow derived stroma cell line, HS-5, secretes a variety of cytokines and supports proliferation of myeloma cells and may inhibit CIK/MV therapy of myeloma cells [33, 34]. Hence, we co-cultured a panel of myeloma cell lines RPMI 8226, MM1 and JJN-3 with HS-5 stromal cells. The HS-5 to myeloma cell ratio tested were 1:5, 1:2, 1:1, 2:1 and 5:1. The culture was then exposed to MV, CIK cells or MV infected CIK cells. As shown in figure 3, in the presence of increasing numbers of HS-5 stromal cells, there was minimal impact on MV oncolysis in all 3 myeloma cell lines. However, co-culture of myeloma cells with increasing numbers of HS-5 cells resulted in significant inhibition (P < 0.05) of CIK or CIK/MV mediated killing of the myeloma cells. This inhibition was most profoundly seen in RPMI 8226 where increasing numbers of HS-5 stromal cells resulted in a corresponding increase in inhibition of myeloma cell killing (Fig. 3). In the absence of HS-5 cells, more than 90% of RPMI cells were killed by CIK/MV treatment but co-culture with HS-5 cells (5:1 ratio) resulted in a significant reduction to 70% cells killed.
Figure 3.
The impact of healthy bone marrow derived stromal cells on virus or CIK killing of myeloma cells. Normal human bone marrow stromal HS-5 cells were co-cultured with myeloma cells stably expressing firefly luciferase (RPMI 8226, JJN-3 or MM1) at various cell ratios. The co-cultures were exposed to MV, CIK cells or MV infected CIK cells. Viability of myeloma-Luc cells was determined by measurement of luciferase activity. Bars represent the mean ± SEM of 3 independent experiments. Asterisk represents a significant difference (P < 0.05) between the treatment group and the respective control (no HS-5 cells).
Measles infected CIK cells have superior activity in an orthotopic myeloma model
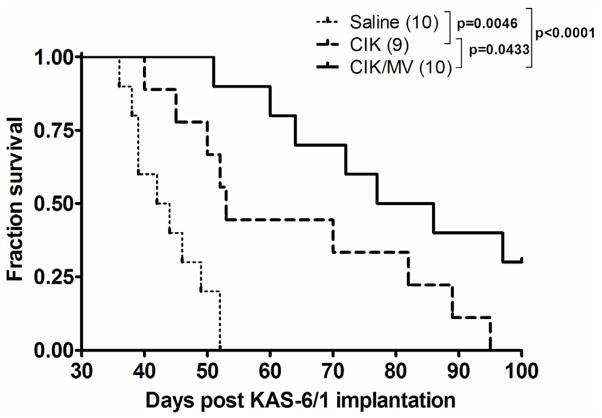
To evaluate the anti-tumor activity of CIK or CIK/MV cells, SCID mice bearing established systemic human myeloma KAS-6/1 disease were given 2 doses (3 weeks apart) of saline, 3×106 CIK cells or 3×106 MV-NIS infected (MOI 1.0) CIK cells. Treatment began 30 days post IV infusion of the KAS-6/1 myeloma cells and overall survivals were plotted in a Kaplan-Meier curve (Fig. 4). Median survivals for the saline control group, CIK and CIK/MV groups were 43 days, 53 days and 82 days, respectively. Treatment of mice with either CIK or CIK/MV significantly prolonged survival compared to the saline control animals. The P value for saline versus CIK is 0.0046 and for saline versus CIK/MV is 0.0001. The CIK/MV cells were superior compared CIK cells at prolonging the survival of mice bearing systemic myeloma disease (P=0.0433).
Figure 4.
Kaplan-Meier survival curves of mice with disseminated myeloma that received 2 doses (3 weeks apart) of saline, 3×106 CIK or MV-infected CIK (CIK/MV, MOI 1.0) cells. The P values comparing differences between treatment groups are shown.
Induction of NKG2D ligands by ionizing radiation in KAS-6/1 myeloma cells
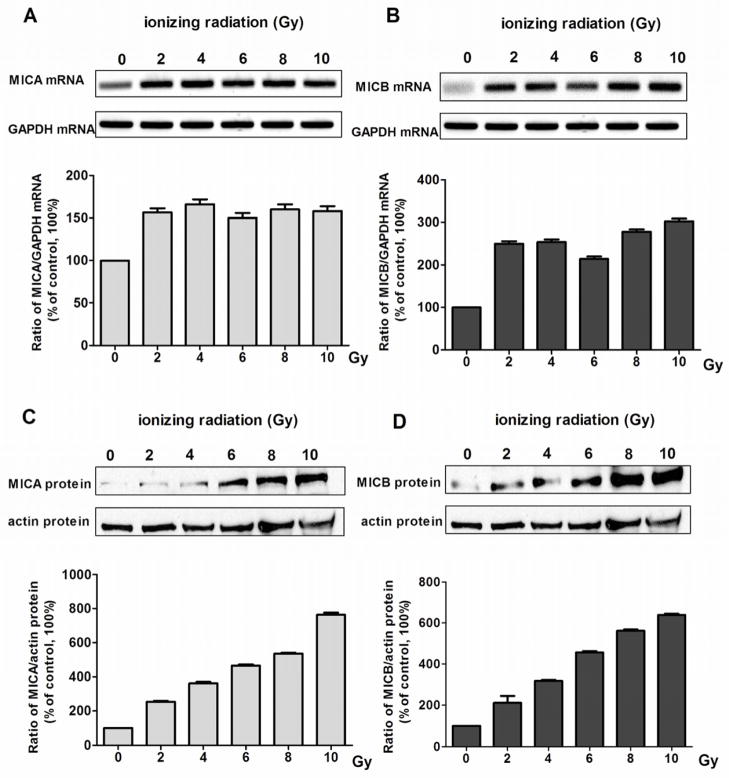
Next, we investigated if ionizing radiation can increase the expression of NKG2D ligands in human KAS-6/1 cells and consequently enhance myeloma cell susceptibility to CIK-mediated cytotoxicity. KAS-6/1 cells received various amounts of irradiation (2 to 10 Gy) and 24 hours later, were analyzed for MICA and MICB mRNA and protein levels. Ionizing radiation increased the levels of MICA and MICB mRNA (Fig. 5A, 5B RT-PCR) and protein (Fig. 5C, 5D Western blotting) in KAS-6/1 cells. Compared to non-irradiated cells, there was a clear dose dependent increase in the levels of these NKG2D ligands with the amount of irradiation given.
Figure 5.
Dose dependent increase in NKG2D ligands, MICA and MICB, in irradiated KAS-6/1 cells. Levels of MICA and MICB ligands in non-irradiated or irradiated KAS-6/1 cells were measured by (A, B) RT-PCR for mRNA levels and by (C, D) immunoblotting for protein levels Relative intensities of MICA and MICB bands were normalized to GAPDH or beta-actin. The normalized MICA or MICB level in control non-irradiated cells was considered to be 100%. Bars represent the mean ± SD of 3 independent experiments.
Combination of CIK/MV cells with ionizing radiation is superior to either alone
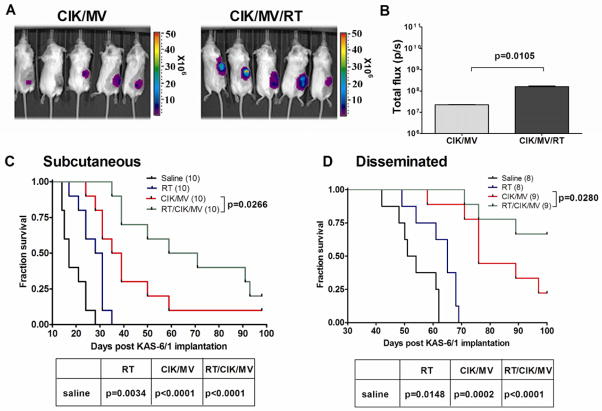
We next evaluated if irradiation of KAS 6/1 tumors would result in enhanced trafficking of CIK cells and delivery of MV to the tumors. Subcutaneous KAS-6/1 xenografts were irradiated at 2 Gy and 24 hours later, mice received MV-Luc infected CIK cells intravenously. As shown in Fig. 6, imaging for bioluminescence signals and quantitation of luciferase activity (photon counts) indicated that irradiation resulted in a significantly increased MV-Luc signal in the tumors.
Figure 6. Combination of CIK/MV cells with ionizing radiation is superior to either therapy alone.
(A) Bioluminescent images of mice after intravenous injection of MV-Luc-infected CIK into mice with subcutaneous KAS-6/1 xenografts. (B) Quantitation of levels of virally encoded luciferase activity in tumors of mice (n=3). Kaplan–Meier survival curves of mice with (C) subcutaneous or (D) disseminated myeloma, and that were given saline, irradiation (2 Gy), MV-NIS infected CIK cells (CIK/MV) or CIK/MV and XRT (2 Gy).
Subsequently, the antitumor activity of saline alone, irradiation (XRT) alone, MV-infected CIK alone or a combination of MV-infected CIK with XRT, were compared in both the subcutaneous and disseminated models of KAS-6/1 human myeloma disease. The oncolytic MV, MV-NIS, that is currently undergoing Phase I clinical testing in patients with relapsed refractory myeloma, was used for these therapy studies.
Mice with established subcutaneous KAS-6/1 xenografts were given intravenous therapy via the tail vein with 2 doses of 3×106 CIK/MV cells (MOI 1.0). Survival of mice in the various treatment groups was plotted in a Kaplan-Meier curve. As shown in Fig. 6C, the median survival for saline control was 17 days, 2 Gy XRT was 30 days, CIK/MV was 37 days, and CIK/MV/XRT was 65 days. The P values comparing survival curves for saline treated versus XRT is 0.0034, for saline versus CIK/MV is <0.0001, for saline versus CIK/MV/XRT is <0.0001 and for CIK/MV versus CIK/MV/XRT is 0.0266.
The antitumor activities of the various treatment options were next tested in the disseminated KAS-6/1 myeloma model. To enable non-invasive tracking of tumor growth, mice were given KAS-6/1 cells stably expressing Gaussia luciferase, which is a secreted reporter protein [35]. Regular sampling of the blood from mice via the tail vein allows measurement of Gaussia luciferase activity and estimation of tumor burden [26]. When Gaussia luciferase activity measured 10,000 IU to 50,000 IU/5μl of blood, mice were randomized into groups and given therapy. As shown in Fig. 6D, the median survival of mice treated with saline was 53 days, with XRT was 65 days and with CIK/MV was 76 days. In the CIK/MV/XRT group, median survival was >100 days. The P values comparing survival curves for saline treated versus XRT is 0.0148, saline versus CIK/MV is 0.0002, saline versus CIK/MV/RT is 0.0001, CIK/MV versus CIK/MV/XRT is 0.028. Thus, survival of mice treated with CIK/MV/RT was significantly prolonged compared to CIK/MV in both subcutaneous and disseminated KAS-6/1 myeloma model.
DISCUSSION
Multiple myeloma remains an incurable plasma cell malignancy and innovative therapeutic approaches are urgently needed [36–39]. In addition to small molecules, immunotherapy using adoptively transferred immune cells, cancer vaccines and virotherapy using tumor selective oncolytic viruses are being pursued in clinical trials [17, 40–43]. Cytokine induced killer cells are polyclonal T effector cells endowed with potent MHC-unrestricted antitumor activity against hematological and solid malignancies [6, 13, 44–46]. These cells can be generated from peripheral blood lymphocytes by addition of IFN-gamma on the first day to activate the monocytes, followed by anti-CD3 antibody and IL-2 the next day, and subsequent periodic addition of IL-2 to sustain T cell proliferation [13]. In our study, more than 90% of cells in the bulk culture were CD3+ and 25% were CD3+CD56+ at three weeks post culture initiation. Tumor cell killing (autologous and allogeneic origin) is largely mediated by the MHC non-restricted CD3+CD56+ cells in these cultures [6, 13]. About 80% of the cells in the bulk culture also express the natural killer (NK) cell receptor, NKG2D, which is important for tumor recognition [6, 47]. Use of CIK or natural killer (NK) cells for myeloma treatment is highly promising [48–50]. Killing of human myeloma U266 cells by CIK cells is mediated by NKG2D recognition of its ligands, MICA and MICB, expressed on the myeloma cells [51, 52]. Final killing of the tumor cells is mediated by perforin and granzyme [53]. Interestingly, bortezomib, a very effective antimyeloma drug, has been shown to down regulate cell surface expression of HLA class I, thereby sensitizing myeloma cells to NK cell lysis [54]. In this study, we investigated the potential of CIK cells as a single agent or in combination with measles virotherapy. We showed that CIK cells have anti-myeloma activity against the Mayo patient derived KAS-6/1 myeloma cell line [55]. At a E:T ratio of 1:1, 40% of KAS-6/1 cells were killed by day 2. Killing of KAS-6/1 cells was mediated through interaction with NKG2D receptors as addition of an anti-NKG2D blocking antibody inhibited CIK mediated cell killing by 50%. In vivo, CIK therapy (2 doses, 5×107 cells/kg/dose) significantly prolonged survival of mice with disseminated KAS-6/1 myeloma disease compared to saline controls. Co-culture of myeloma cells with the human bone marrow stromal HS-5 cells did not negatively impact on MV killing of the myeloma cells but inhibited CIK and CIK/MV killing. This is particularly evident in RPMI 8226 cells where there was a clear dose dependent inhibition on CIK or CIK/MV killing of myeloma cells.
Cytokine induced killer cells have entered clinical testing including in patients with renal, colorectal cancer and lymphoma [6, 13, 16]. No major adverse events were reported and a small number of patients have achieved disease stabilization or partial to complete responses. A larger number of studies have been performed in China, with encouraging clinical results [6, 14]. A recent review indicated that about 936 patients in 24 trials were treated with CIK cells, and in five studies, CIK cells were co-cultured with dendritic cells [14]. The number of CIK cells used ranged from 6×106 to 1.5×1010 per patient. Out of the reported 563 patients, there were 40 complete responses, 126 partial responses, 135 disease stabilization and 58 progressive disease [14].
To further enhance the potency of CIK immunotherapy, other strategies involving active immunotherapy are being pursued to achieve long-term tumor control through induction of tumor specific effector and memory T cells. These include the use of DC tumor vaccines and DC-activated CIK obtained by co-culture of CIK with DC that have been pulsed with tumor lysates or antigens [16, 51, 56]. Thorne et al. reported that synergistic killing of tumor cells was achieved by combining oncolytic virotherapy with CIK immunotherapy in immunocompetent and immunocomprised mice [57]. In this approach, CIK killing of the tumor cells is augmented by the oncolytic activity of replication competent vaccinia virus [58]. We have previously reported successful delivery of oncolytic measles viruses to tumors using cell carriers such as lymphokine activated T cells [59], monocyte cell line [60], CD14+ primary monocytes [61], adipose tissue derived mesenchymal stromal cells [30] and the MM1 myeloma tumor cell line [26, 62]. While these cell carriers can traffick to tumors, none of them has direct antitumor activity. In this respect, CIK cells are more attractive as cell carriers for myeloma because they are biologically active against the tumor. In addition to being highly susceptible to MV infection, and able to transfer MV infection to myeloma cells, we confirmed that the levels of NKG2D, the major cell-surface receptor involved in CIK cell-mediated cytotoxicity, remained high in MV infected CIK cells. Essentially, the function of the CIK cells is unaffected by viral infection during this period. In vivo, MV-infected CIK cells were superior to CIK cells alone at prolonging the survival of mice with disseminated myeloma (Fig. 4). We also compared the tumor killing activity of myeloma cell line MM1 versus CIK cells as virus carriers. At day 3, more than 80% of KAS-6/1 cells were killed by MV-infected CIK cells compared to only 50% by MV infected MM1 cells (E:T ratio 1:1). Viral infection has been demonstrated to increase NKG2D ligand expression on infected cell surface [57, 63]. However, in our case, MV infection did not appear to increase the level of NKG2D on the CIK cells (Fig 1).
Since myeloma is a radiosensitive tumor, we also explored the feasibility of combing XRT with CIK/MV dual therapy. The combination of MV-infected CIK cells with XRT significantly prolonged the survival of mice with subcutaneous or disseminated human myeloma compared to CIK/MV. Recent studies using irradiation and adoptive immunotherapy showed that irradiation of malignant tumor cells enhanced killer cell-mediated cytotoxicity [64, 65]. The NKG2D ligands MICA and MICB are known to be induced by ionizing radiation [66] which may enhance trafficking of the CIK cells to tumors and/or killing by CIK cells. Indeed, we demonstrated here that the mRNA and protein levels of MICA and MICB were increased with increasing doses of irradiation given to KAS-6/1 cells. The consequence of increased MICA and MICB on KAS-6/1 cells may also contribute to the enhanced delivery of MV-Luc to the tumors, resulting in enhanced therapeutic outcome of MV/CIK therapy in both the subcutaneous and disseminated models of myeloma.
In summary, we demonstrated that here CIK cells can act as efficient vehicles to deliver oncolytic MV systemically to subcutaneous and disseminated human myeloma tumors in mice. Compared to the other cell carriers that we have tested, the cytolytic CIK cells may have an additional advantage as virus carriers due to their intrinsic antimyeloma activity. Low dose radiation also increased levels of MICA/B on myeloma cells, resulting in enhanced therapeutic outcome. Since CIK therapy is already a clinical reality, we envisage that clinical translation of this promising delivery platform is highly feasible and worth pursuing in future studies.
Acknowledgments
This study was supported by grants from the NIH/NCI (CA129193, CA129966, CA136547, Mayo Clinic SPORE in Ovarian Cancer P50CA136393). We thank Dr. Allan B Dietz (Mayo Clinic Human Cell Therapy Laboratory) for the kind gift of CD3 sorted T cells.
Footnotes
Authorship Contributions
CSL, LS and YWC designed, performed, analyzed the experiments and wrote the manuscript, SJR and KWP designed the experiments, interpreted the data, wrote and edited the manuscript. KWP supervised the study.
Conflict-of-interest disclosure
SJR and KWP are co-founders of Imanis Life Sciences (Rochester, MN).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nature reviews Cancer. 2012;12:335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- 2.Chesi M, Bergsagel PL. Many multiple myelomas: making more of the molecular mayhem. Hematology Am Soc Hematol Educ Program. 2011;2011:344–353. doi: 10.1182/asheducation-2011.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1679–1687. doi: 10.1016/j.bbmt.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86:1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 6.Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. Journal of Cancer. 2011;2:363–368. doi: 10.7150/jca.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zafirova B, Wensveen FM, Gulin M, Polic B. Regulation of immune cell function and differentiation by the NKG2D receptor. Cellular and molecular life sciences: CMLS. 2011;68:3519–3529. doi: 10.1007/s00018-011-0797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 9.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 10.Obeidy P, Sharland AF. NKG2D and its ligands. Int J Biochem Cell Biol. 2009;41:2364–2367. doi: 10.1016/j.biocel.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Eagle RA, Trowsdale J. Promiscuity and the single receptor: NKG2D. Nat Rev Immunol. 2007;7:737–744. doi: 10.1038/nri2144. [DOI] [PubMed] [Google Scholar]
- 12.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linn YC, Hui KM. Cytokine-induced NK-like T cells: from bench to bedside. J Biomed Biotechnol. 2010;2010:435745. doi: 10.1155/2010/435745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li XD, Xu B, Wu J, et al. Review of Chinese clinical trials on CIK cell treatment for malignancies. Clin Transl Oncol. 2012;14:102–108. doi: 10.1007/s12094-012-0768-4. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Zhang Z, Tang L, et al. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: a systematic review and pooled analysis. Cytotherapy. 2012;14:483–493. doi: 10.3109/14653249.2011.649185. [DOI] [PubMed] [Google Scholar]
- 16.Thanendrarajan S, Nowak M, Abken H, Schmidt-Wolf IG. Combining cytokine-induced killer cells with vaccination in cancer immunotherapy: more than one plus one? Leuk Res. 2011;35:1136–1142. doi: 10.1016/j.leukres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eager RM, Nemunaitis J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011;18:305–317. doi: 10.1038/cgt.2011.7. [DOI] [PubMed] [Google Scholar]
- 19.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8:12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 20.Lech PJ, Russell SJ. Use of attenuated paramyxoviruses for cancer therapy. Expert Rev Vaccines. 2010;9:1275–1302. doi: 10.1586/erv.10.124. [DOI] [PubMed] [Google Scholar]
- 21.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 22.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 23.Muhlebach MD, Mateo M, Sinn PL, et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature. 2011;480:530–533. doi: 10.1038/nature10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noyce RS, Richardson CD. Nectin 4 is the epithelial cell receptor for measles virus. Trends Microbiol. 2012 doi: 10.1016/j.tim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Blechacz B, Splinter PL, Greiner S, et al. Engineered measles virus as a novel oncolytic viral therapy system for hepatocellular carcinoma. Hepatology. 2006;44:1465–1477. doi: 10.1002/hep.21437. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Russell SJ, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers RM, Greiner SM, Harvey ME, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82:700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dingli D, Peng KW, Harvey ME, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 29.Duprex WP, McQuaid S, Hangartner L, Billeter MA, Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. Journal of virology. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mader EK, Maeyama Y, Lin Y, et al. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 32.Hadac EM, Peng KW, Nakamura T, Russell SJ. Reengineering paramyxovirus tropism. Virology. 2004;329:217–225. doi: 10.1016/j.virol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Tamura H, Ishibashi M, Yamashita T, et al. Marrow stromal cells induce B7-H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2012 doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 34.Scuto A, Krejci P, Popplewell L, et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25:538–550. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapoor P, Rajkumar SV. Update on risk stratification and treatment of newly diagnosed multiple myeloma. Int J Hematol. 2011;94:310–320. doi: 10.1007/s12185-011-0947-z. [DOI] [PubMed] [Google Scholar]
- 37.Engelhardt M, Kleber M, Udi J, et al. Consensus statement from European experts on the diagnosis, management, and treatment of multiple myeloma: from standard therapy to novel approaches. Leuk Lymphoma. 2010;51:1424–1443. doi: 10.3109/10428194.2010.487959. [DOI] [PubMed] [Google Scholar]
- 38.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 39.Johnson SK, Heuck CJ, Albino AP, et al. The use of molecular-based risk stratification and pharmacogenomics for outcome prediction and personalized therapeutic management of multiple myeloma. Int J Hematol. 2011;94:321–333. doi: 10.1007/s12185-011-0948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stief AE, McCart JA. Oncolytic virotherapy for multiple myeloma. Expert Opin Biol Ther. 2008;8:463–473. doi: 10.1517/14712598.8.4.463. [DOI] [PubMed] [Google Scholar]
- 41.Mitsiades CS, Davies FE, Laubach JP, et al. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1916–1923. doi: 10.1200/JCO.2010.34.0760. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblatt J, Avigan D. Cellular immunotherapy for multiple myeloma. Best Pract Res Clin Haematol. 2008;21:559–577. doi: 10.1016/j.beha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Szmania S, Tricot G, van Rhee F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk Lymphoma. 2006;47:2037–2048. doi: 10.1080/10428190600742292. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–187. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Shi J, Tricot G, Szmania S, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–653. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. Embo J. 2004;23:255–259. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi M, Zhang B, Tang ZR, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10:1146–1151. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin J, Zhu H, Lu X, et al. Autologous cytokine-induced killer cells in the treatment of multiple myeloma concomitant with lung cancer and paraneoplastic dermatoses. Intern Med. 2010;49:2341–2346. doi: 10.2169/internalmedicine.49.3996. [DOI] [PubMed] [Google Scholar]
- 50.Su X, Zhang L, Jin L, Ye J, Guan Z, Chen R. Coculturing dendritic cells with zoledronate acid efficiently enhance the anti-tumor effects of cytokine-induced killer cells. J Clin Immunol. 2010;30:766–774. doi: 10.1007/s10875-010-9434-1. [DOI] [PubMed] [Google Scholar]
- 51.Marten A, Renoth S, von Lilienfeld-Toal M, et al. Enhanced lytic activity of cytokine-induced killer cells against multiple myeloma cells after co-culture with idiotype-pulsed dendritic cells. Haematologica. 2001;86:1029–1037. [PubMed] [Google Scholar]
- 52.Lu X, Zhu A, Cai X, et al. Role of NKG2D in cytokine-induced killer cells against multiple myeloma cells. Cancer Biol Ther. 2012;13:623–629. doi: 10.4161/cbt.19850. [DOI] [PubMed] [Google Scholar]
- 53.Verneris MR, Baker J, Edinger M, Negrin RS. Studies of ex vivo activated and expanded CD8+ NK-T cells in humans and mice. J Clin Immunol. 2002;22:131–136. doi: 10.1023/a:1015415928521. [DOI] [PubMed] [Google Scholar]
- 54.Shi J, Tricot GJ, Garg TK, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–1317. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia. 1996;10:866–876. [PubMed] [Google Scholar]
- 56.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–1502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 58.Thorne SH, Contag CH. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene therapy. 2008;15:753–758. doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- 59.Ong HT, Hasegawa K, Dietz AB, Russell SJ, Peng KW. Evaluation of T cells as carriers for systemic measles virotherapy in the presence of antiviral antibodies. Gene therapy. 2007;14:324–333. doi: 10.1038/sj.gt.3302880. [DOI] [PubMed] [Google Scholar]
- 60.Iankov ID, Blechacz B, Liu C, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 61.Peng KW, Dogan A, Vrana J, et al. Tumor-associated macrophages infiltrate plasmacytomas and can serve as cell carriers for oncolytic measles virotherapy of disseminated myeloma. Am J Hematol. 2009;84:401–407. doi: 10.1002/ajh.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munguia A, Ota T, Miest T, Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene therapy. 2008;15:797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- 63.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 64.Ishikawa E, Tsuboi K, Saijo K, Takano S, Ohno T. X-irradiation to human malignant glioma cells enhances the cytotoxicity of autologous killer lymphocytes under specific conditions. Int J Radiat Oncol Biol Phys. 2004;59:1505–1512. doi: 10.1016/j.ijrobp.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 65.Sumareva R, Ukrainsky G, Kiremidjian-Schumacher L, et al. Effect of combined adoptive immunotherapy and radiotherapy on tumor growth. Radiat Oncol Investig. 1999;7:22–29. doi: 10.1002/(SICI)1520-6823(1999)7:1<22::AID-ROI3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 66.Kim JY, Son YO, Park SW, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Experimental & molecular medicine. 2006;38:474–484. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]