Abstract
Prior studies have reported instances of both intact (i.e. (Ozonoff & Strayer, 2001) and impaired (i.e. Bennetto, Pennington, & Rogers, 1996) working memory (WM) performance in people with autism spectrum disorder (ASD). In order to investigate the relation between autistic traits that extend into the normal population and WM, 104 normal college-aged students who varied in their levels of autistic traits were tested. The loading of ASD-associated traits in the normal population leads to differing predictions about WM performance. ASD traits related to a local processing style (or ‘attention to detail’) might enhance WM while ASD-associated traits related to difficulty switching attention and reorienting focus (or ‘social interaction’) might impair WM performance. To assess these predictions, participants filled out the Autism Spectrum Quotient (AQ; Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001) and performed a working memory task with both visual and verbal variants. AQ scores were then broken into ‘attention to detail’ and ‘social interaction’ factors, as proposed by Hoekstra and colleagues (Hoekstra, Bartels, Cath, & Boomsma, 2008). The results showed AQ scores did not predict verbal WM performance but they did predict visual WM performance. The social interaction and attention to detail factors of the AQ had opposing relationships with visual WM performance: a higher level of social difficulty was associated with significantly poorer visual WM performance while a higher level of attention to detail was associated with enhanced visual WM performance. Further investigation of the relation between AQ and WM using the original five-factor model proposed by Baron-Cohen and colleagues (2001) revealed an association between impoverished imagination and visual WM overall.
Autism spectrum disorder (ASD) is a pervasive developmental disorder characterized by impairments in social functioning, verbal and non-verbal communication, and restricted, inflexible behaviors and interests (American Psychological Association, 2007). There is also evidence of cognitive and perceptual impairments. People with ASD have deficits in expressive grammar, figurative language and pragmatic aspects of language (Landa & Goldberg, 2005; Salter, Seigal, Claxton, Lawrence, & Skuse, 2008). People with ASD tend to exhibit abnormal visual attention (Sasson, Turner-Brown, Holtzclaw, Lam, & Bodfish, 2008) perhaps due to a strong local processing bias (also called the ‘weak central coherence theory’ of ASD; Frith, 1989) compared to typically developing controls. Furthermore, people with ASD have been shown to exhibit abnormal EEG patterns while engaging in a simple visual perception task, suggesting reduced efficiency of visual integration (Milne, Scope, Pascalis, Buckley, & Makeig, 2009). Of central interest to this report are differences in working memory (WM) function.
WM capacity has been shown to influence a large variety of basic cognitive processes such as reading (Daneman & Carpenter, 1980), attention (Unsworth & Spillers, 2010), intelligence (Unsworth & Engle, 2007) and reasoning ability (Kyllonen & Christal, 1990). Working memory abilities have also been related to social comprehension as measured by theory-of-mind tasks (Carlson, Moses, & Breton, 2002). Not only is WM fundamental to cognition and related to diverse social and cognitive skills - including executive functions known to be perturbed in ASD (see Killiany, Moore, Rehbein, & Moss, 2005; Ozonoff, South, & Provencal, 2007 for further discussion of these deficits) - but deficits in WM would potentially have far-flung consequences for thinking and behavior. Indeed, there is speculation that some of the symptoms associated with ASD could be explained in terms of degraded memory functioning (Killiany, et al., 2005). At first glance the link between WM and the striking social deficits present in people with ASD may be unclear. However, recent reports have shown an association between poor social cognition and impaired spatial reasoning abilities in normally functioning adults (Shelton, Clements-Stephens, Lam, Pak, & Murray, 2012) as well as in a sample of children diagnosed with ASD (Hamilton, Brindley, & Frith, 2009), so it is reasonable to think that impoverished social skills might relate to dysfunction in other cognitive domains as well.
Working Memory and Autism
Only a small number of studies have examined visual WM is the ASD population. One of the only studies to examine ‘object WM’ – that is, WM for colors, oriented lines, shapes, or objects – found that the ASD group was relatively impaired on a delayed-non-match-to-sample task for novel objects (Dawson, Meltzoff, Osterling, & Rinaldi, 1998). A larger number of studies have examined spatial WM in ASD participants. Several studies from the Minshew laboratory have reported spatial WM deficits in ASD participants (Williams, Goldstein, Carpenter, & Minshew, 2005; Williams, Goldstein, & Minshew, 2006). For example, Williams and colleagues tested adults and children with ASD on various WM tasks (Williams, et al., 2005). When compared to control participants, both ASD groups performed normally on verbal and auditory WM tasks but abnormally on spatial WM tasks. However, other studies have reported that spatial WM is normal in ASD (Griffith & Pennington, 1999; Ozonoff & Strayer, 2001) or even enhanced compared to controls (Soulieres, Zeffiro, Girard, & Mottron, 2011).
Verbal WM has generally been reported to be intact in ASD (Koshino, et al., 2005; Mottron, Morasse, & Belleville, 2001; Williams, et al., 2005; Williams, et al., 2006), although one recent finding stands apart from these previous reports. Poirier and colleagues (2011) tested ASD and control groups on three verbal WM tasks. The authors found verbal WM deficits in the ASD group across different tasks that could be pin-pointed to problems in retaining and/or manipulating the temporal order of verbal material (Poirier, Martin, Gaigg, & Bowler, 2011). One important study conducted by Minshew and colleagues (2006) attempted to characterize memory functioning in a sample of 38 children and teens with autism on a standardized neuropsychological memory battery compared to individually-matched normal controls. Participants completed the Wide Range Assessment of Memory and Learning (WRAML), which assess verbal and visual memory as well as learning. The ASD group was found to have lower scores on all three visuospatial subtests contained in the WRAML compared to controls. Spatial WM was shown to be particularly impaired. ASD participants also exhibited impoverished performance compared to controls on verbal subtests (sentence and story memory) that tap into WM abilities (Williams, et al., 2006). Based on these findings, the authors concluded that children with ASD have difficulty encoding complex information, regardless of the modality, rather than a deficit in recall per se (Williams, et al., 2006).
There are a number of explanations for the discrepant findings reported in the literature. First, it has been suggested that the developmental trajectory for people with ASD is perturbed, such that at some points in development, WM may appear to be impaired, and at other points may appear relatively unimpaired (Russo, et al., 2007). However there is currently little evidence to support this view. Second, ASD-linked WM deficits may only be apparent in the most challenging tasks (Minshew & Goldstein, 2001; Ozonoff & Strayer, 2001) or the most complex stimuli (Williams, et al., 2006). Third, it is possible that in the studies reporting null results, the control “normal” population had somewhat elevated levels of autistic traits plus the concomitant cognitive deficits, thus making it difficult to achieve statistical significance when comparing them to the ASD group. This is a particularly important consideration given that ASD traits vary continuously in the normal population, as reviewed below.
Autistic Traits in the General Population
The population diagnosed with ASD is heterogeneous in terms of both disease severity and symptom presentation (Killiany, et al., 2005). Indeed, the severity of ASD symptoms reflects a continuum extending into the general population (Constantino & Todd, 2003). The continuum is smooth, with no evidence of a bimodal distribution separating individuals meeting diagnostic criteria for autism from the general population (Happe, Ronald, & Plomin, 2006). Clinically normal individuals who score highly on the autism-spectrum quotient (AQ) questionnaire perform poorly in tasks on which ASD patients are impaired (Baron-Cohen, et al., 2001). For example, the Reading the Mind in the Eyes task has been shown to be inversely correlated with AQ scores even in the unaffected population (Baron-Cohen, et al., 2001). Furthermore, it has previously been shown that parent ratings of normal children on the Social Responsiveness Scale (Constantino, 2002) were continuously distributed in the normal population. This scale includes items that tap into reciprocal social behavior, social use of language and other behaviors characteristic of children with ASD (Constantino, 2002). Constantino and Todd conclude that social deficits characteristic of ASD are common in the normal population (2003).
Autistic traits in the general population also correlate with performance on non-social cognitive and perceptual tasks. Some of the most consistent findings have been observed in visual attention tasks. For instance, Grinter and colleagues reported that normal individuals who scored highly on the AQ (i.e. reported a higher level of traits related to ASD) were faster to identify embedded figures than low AQ scores and had poorer global form and motion thresholds than low-scoring participants (Grinter, et al., 2009). This finding can be interpreted in terms of an ASD cognitive style biased towards local, rather than global, processing (Happe, 1999). The local processing cognitive style has been shown to extend into normally functioning parents and siblings of children with ASD (Briskman, Frith, & Happe, 2001; Happe, 1999; Happe, Briskman, & Frith, 2001), again indicating that a trait thought to be characteristic of ASD appears with a high frequency in the normal population.
These findings lead to the prediction that autistic traits in the general population will correlate with performance on higher-level cognitive tasks that load highly on visual attention subsystems. Visual WM performance is highly reliant on visual attention (Luck & Vogel, 1997). For instance, curing participants’ attention to a visual stimulus increases accuracy for visual features, such as color, of the cued item compared to uncued items (Schmidt, Vogel, Woodman, & Luck, 2002). These attentional shifts can be directed via either top-down (Palmer, 1990; Sperling, 1960) or bottom-up (Woodman, Vecera, & Luck, 2003) mechanisms. In both cases, early biases in attention result in greater accuracy for representations stored in VWM (Palmer, 1990; Schmidt, et al., 2002; Woodman, et al., 2003). Furthermore, children with ASD have previously been shown to have aberrant visual attention as assessed by scan paths, that is consistent with the local processing bias – they visually explore the scene less and perseverated on one item at the expense of others (Sasson, et al., 2008). This finding suggests that they may have difficulty directing visual attention via top-down mechanisms.
In order to extend previous work examining WM abilities in clinical ASD, we were interested in demonstrating the extent to which sub-clinical autistic traits might correlate with performance on visual WM tasks. Participants were expected to vary in their levels of autistic traits (Baron-Cohen, et al., 2001); this was assessed by administering the AQ. We also predicted that autistic traits would be inversely related to performance on visual but not verbal WM tasks. This prediction was based on findings showing processing differences in visual perception and attention in the ASD population, and also on our literature review that revealed more consistent visual WM deficits than verbal WM deficits in the ASD populations (see Table 1). We assessed visual (i.e. novel shapes) and verbal WM (i.e. words)1 in a group of students at a large public university who were of normal intelligence and without ASD diagnoses.
Table 1.
A review of studies investigating effects of ASD on working memory/short-term memory.
| Reference | Patient population, N |
Control population, N |
WM Task | Findings in patients with ASD |
|---|---|---|---|---|
| Bennetto et al., 1996 | Children and teens with ASD, 19 |
Learning disordered children and teens, 19 |
|
Impaired sentence span. Impaired counting span. |
| Dawson et al., 1998 | Children with ASD, 20 |
Normal children, 20 Children with Down syndrome, 19 |
|
Impaired delayed non-match to sample. |
| Griffith et al., 1999 | Young children with ASD, 18 |
Normal young children, 17 |
|
-all normal- |
| Koshino et al., 2005 | Adults with ASD, 14 |
Normal adults, 14 |
|
-all normal- |
| Minshew et al., 2001 | Teens and young adults with ASD, 52 |
Normal teens and young adults, 40 |
|
Normal letter sequence. Impaired word sequences and oral directions. |
| Mottron et al., 2001 | Children, teens and adults with ASD, 14 |
Normal children, teens and adults, 14 |
|
Intact free recall. Reduced advantage in recall for semantic cues. |
| Ozonoff et al., 2001 | Children and teens with ASD, 25 |
Normal children and teens, 15 Children and teens with Tourette syndrome, 15 |
|
-all normal- |
| Pourier et al., 2011 | Adults with ASD, 16 |
Normal adults, 16 |
|
Impaired performance on digit span forward and backward. |
| Soulieres et al., 2011 | Teens and adults with ASD, 23 |
Normal teens and adults, 14 |
|
Enhanced block design. |
| Southwick et al., 2011 | Children, teens and adults with ASD, 50 |
Normal children, teens and adults, 36 |
|
Impaired on all subtests. |
| Steele et al., 2007 | Children, teens and adults with ASD, 29 |
Normal children, teens and adults, 29 |
|
Impaired performance on spatial span. |
| Williams et al., 2005 | Children with ASD, 24 |
Normal children, 44 |
|
Normal verbal and auditory WM. Impaired spatial WM. |
| Williams et al., 2005 | Adults with ASD, 31 |
Normal adults, 25 |
|
Normal verbal and auditory WM. Impaired spatial WM. |
| Williams et al., 2006 | Children and teens with ASD, 38 |
Normal children and teens, 38 |
|
Normal digit span, and auditory WM. Impaired spatial WM. |
Methods
Participants
844 participants completed the AQ questionnaire online. Of these 844, 104 participants self-selected to additionally complete the in-person portion of the study. However, participants were not obligated to participate in the in-person portion of the study. Of the 104 participants who completed the full study, none reported having a personal history of ASD diagnosis. Participants enrolled in the full study were also screened for psychiatric disorders via self-report. Eleven participants reported having psychiatric diagnoses: 8 reported depression, 3 reported anxiety, 1 reported having obsessive compulsive disorder, 1 reported an eating disorder and 1 participant reported bipolar disorder. The average age of participants who completed the full experiment (n=104) was 20.3 years, average self-reported college grade point average was 3.6, and 24% of the participants were male. For the online only portion of the study, participants were not required to provide demographic information. Participants received payment or course credit for both partial (i.e. AQ only) and full (i.e. AQ and onsite visit, described in more detail below) participation, their choice. All materials and procedures were reviewed and approved by the Temple University Institutional Review Board.
Part 1: Online Questionnaire
The online portion of the study consisted of the Autism-Spectrum Quotient (AQ). This questionnaire is comprised of 50 statements about traits and tendencies that participants respond to using a Likert-type scale. The AQ is a widely-used assessment instrument designed to probe for traits associated with autism in the normal, healthy population (Baron-Cohen, et al., 2001). The maximum score on this scale is 50 points. Scores greater than 32 are considered in the clinically relevant ASD range (Baron-Cohen, et al., 2001). The AQ taps into both social functioning and attention to detail (Hoekstra, et al., 2008), two important facets of autistic traits that may explain memory patterns in participants along the continuum. In addition, the four lower-order factors of the AQ that comprise the factor of social interaction (social skill, attention switching, communication and imagination; Baron-Cohen, et al., 2001) were explored to assess the relative contributions of each of these facets of social interaction to WM performance.
Part 2: Working Memory Task, Onsite
The WM task had four components: a verbal order task, a verbal recognition task, an object order task, and an object recognition task. In the verbal task, participants were required to remember 6 words. Words were drawn from different parts of speech (nouns, adjectives, verbs and adverbs) and word length was between 4-8 letters. Words were presented in black, Courier New size 18 font on a white background. Each trial was initiated by a 1000 ms fixation. Words were presented sequentially at fixation for 1000 ms each. After a delay interval of 1000 ms, the probe task commenced. On order trials, the task was to judge which of two presented words was shown first in the series. On recognition trials, the task was to judge whether or not the probe word had been present in the stimulus set. After a keyboard response was entered, participants pressed the space bar to commence to the next trial. Accuracy, not speed, was emphasized in the instructions.
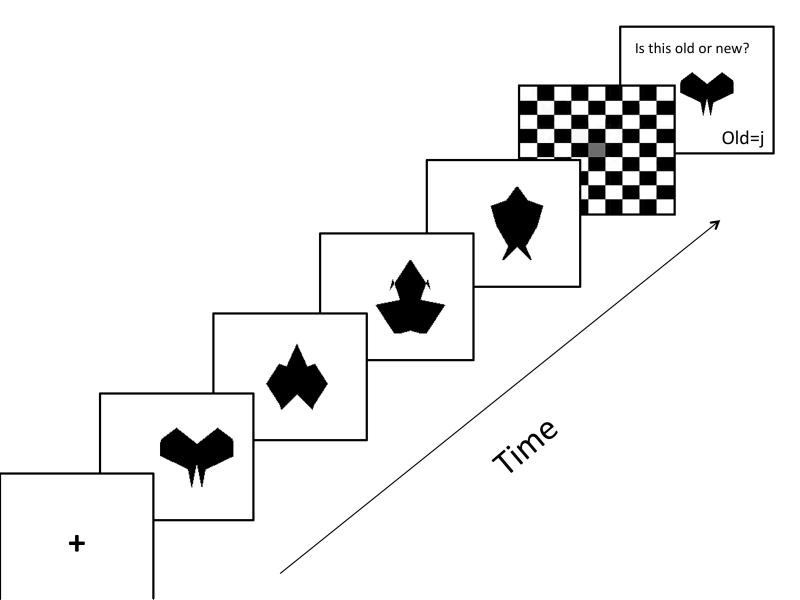
The object portion of the WM task had a similar design to the verbal portion with a few exceptions. Visual stimuli consisted of 37 novel shapes used in prior studies of visual WM (Jiang, Olson, & Chun, 2000). Novel shapes were used, rather than pictures of objects or colors that would have been easy to name, in order to minimize the contribution of verbal WM to this task. Shapes were presented in black against a white background. This set size was used because previous research indicates a capacity limit of 4 items for visual information (Cowan, 2001; Luck & Vogel, 1997). The same timing parameters and probe tasks were used as described in the verbal portion. See Figure 1 for a graphic depiction of a visual recognition trial.
Figure 1.
Schematic illustration of the visual WM task design. Each slide was shown for 1000 ms, except the response slide, which required a response to move on to the next trial.
There were 45 trials of each type for a total of 180 trials. In total, the task took 25-30 minutes, depending on participant response times.
Statistical Analyses
Composite AQ scores were broken down into two distinct factors: ‘social interaction’ and ‘attention to detail’ (Hoekstra, et al., 2008). The ten items in the attention to detail factor of the AQ have to do with a preference for details and patterns. This is distinct from attention switching, which was found to be more related to social problems. The social interaction factor taps approach to social interaction, difficulties with communication, and empathy for others (Hoekstra, et al., 2008). Squared semipartial correlations are reported in order to assess the extent to which unique portions of the variance accounted for by the overall model are attributed to either of the two factors. Additionally, the original 5-factor structure of the AQ proposed by Baron-Cohen et al. (2001) was tested in order to discern the relative contributions of lower-order factors contained in the social interaction factor identified by Hoekstra and colleagues (2008). For both the two and five-factor regression models, factors were entered simultaneously. For these analyses, data from 100 participants are considered, as four participant’s individual responses to items on the AQ were lost and thus scores for each individual factor could not be computed. In both the two-factor and the five-factor models, these data are derived from the overall AQ score (so total AQ score is implicit in all regression models). Because our interest was not in total AQ score per se, but rather the composition of items with respect to lower order factors comprising the total score, total AQ is not directly interrogated by the regression models.
For the WM task, proportion correct was calculated for the order trials and corrected recognition (hits minus false alarms) was computed for the recognition trials. Multiple regression was employed to determine the effect of each of the AQ factors on WM performance. The overall model is discussed in terms of joint significance of the AQ factors. Furthermore, significant contributions of each factor to the model are discussed.
Since we ran a number of different regression models examining the effect of both the two-factor and the five-factor model on visual and verbal recognition and recall, the issue of multiple comparisons and Type I error is raised. In experimental designs many researchers prefer to adjust the significance level of findings to maintain a familywise error rate of .05 (Maxwell & Delaney, 2004, p. 291). However, in multiple regression, multiple comparisons adjustment are often not used because the effect of each predictor on each outcome constitutes a family analogous to those in factorial ANOVA. Thus, we follow the conventional practice of not making special adjustments to our p-values, as we believe that doing so would result in overly conservative significance thresholds.
Results
In our normal college-age participants that completed both parts of the study, AQ scores ranged from 7 to 31 (M score = 16.85, SD = 6.17). Importantly, none of the AQ scores in our normal sample met or exceeded the cut-off score of 32. Scores on the AQ have previously been shown to be normally distributed in both males and females (Baron-Cohen, et al., 2001). However we found no effect of gender on AQ scores (M females= 17.12, SD females = 6.53; M males= 15.87, SD males = 5.29, t(102)= .859, p = .39). Given that our gender distribution was skewed towards females (24% of our sample completing the full study were males), our sample may not have had enough power to detect differences in scores by gender. AQ scores did not correlate with participants’ GPA (GPA data available for 89 participants; r = −.136, p = .205).
Two-factor model of the AQ (Hoekstra, et al., 2008)
To investigate whether personality and cognition factors as measured on the AQ have a relationship to memory performance, a series of regressions were conducted. The two AQ factors, social interaction and attention to detail, were entered simultaneously as predictors in each of the following models, thus the full model allows us to explore the effect of AQ score overall as well as look at unique contributions of each factor of the AQ with respect to variance accounted for by the model. Jointly, the AQ factors did not predict WM accuracy on the verbal recognition task (M = .872, SD= .135 R2 = .021, F(2, 97) = 1.046, p = .355)2 or the verbal order task (M = .786, SD = .117, R2 = .029, F(2, 97) = 1.467, p = .236).
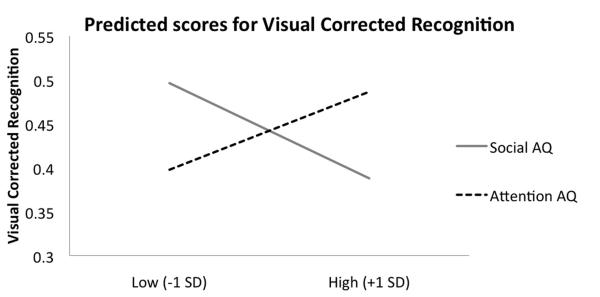
In contrast, both AQ factors jointly predicted performance on the visual portion of the task (M = .690, SD = .107, R2 = .086, F(2, 97) = 4.544, p =. 013). This effect appears to be driven mainly by the visual recognition task (M = .721, SD = .122, R2 = .073, F(2, 97) = 3.827, p =. 025). In this model, each factor predicted a unique portion of the variance accounted for, such that higher social AQ was associated with poorer performance (β = −.233, t(99) = −2.347, p =.021, sr2= .076), and higher attention AQ was associated with better performance, although this effect was marginally significant (β = .182, t(99) = 1.835, p =.070, sr2= .020; see Figure 2 for a graphical depiction of the directionality of the effects). The overall model also predicted performance on the visual order task (M = .657, SD = .114, R2 = .060, F(2, 97) = 3.082, p = .050), such that only the social factor negatively affected the ability to successfully complete this task (β = −.235, t(99) = −2.352, p = .021, sr2= .054; attention factor β = .118, t(99) = 1.177, p = .242, sr2= .013).
Figure 2.
Predicted visual recognition scores given a 1-standard deviation increase in social or attention AQ factors. This figure illustrates how these factors differentially predict visual recognition performance: that higher attention to details predicts better visual WM, but higher social abnormalities predict worse visual WM.
Because corrected recognition is computed by combining hit rates (i.e. correctly labeling an old item as old) and false alarm rates (i.e. incorrectly labeling a new item as old), we investigated whether one response type was driving the effect observed in the visual recognition performance. Again, both the attention and the social factors of the AQ were entered simultaneously into the model. This analysis showed that the ability of the AQ factors to jointly predict corrected recognition was actually due to the hit rates, not false alarm rates. The overall model significantly predicted hit rates (M = .760, SD = .133, R2 = .073, F(2, 97) = 3.814, p = .025) with a positive effect of the attention factor (β = .198, t(99) = 1.998, p = .049, sr2= .038) and a negative effect of the social factor (β = −.219, t(99) = −2.213, p = .029, sr2= .047). Both factors of the AQ did not jointly predict false alarm rates (M = .126, SD = .185, R2 = .029, F(2, 97) = 1.460, p = .237).
Five-factor model of the AQ (Baron-Cohen, et al., 2001)
The same analyses as above were conducted with the original 5-factor model of the AQ proposed by Baron-Cohen and colleagues (2001). The original ‘attention to detail’ factor identified as standing apart from the social factor by Hoekstra et al. (2008) was entered into the model, but will not be discussed in detail as these data have already been presented. Instead, we will focus on the relative contribution of lower order factors making up the social interaction factor: imagination, communication, attention switching, and social skill.
As above, the 5 AQ factors did not predict WM accuracy on the verbal recognition task (R2 = .042, F(5, 94) = .892, p = .532) or the verbal order task (R2 = .099, F(5, 94) = 2.070, p = .076). Importantly, none of the 5 factors were significant predictors for verbal recognition (all p-values > .231) or verbal order (all p-values > .079).
In contrast, the 5 factors predicted performance on the visual portion of the task (R2 = .120, F(5, 94) = 2.564, p = .032). Here, the significant effect for visual recognition drops out (R2 = .089, F(5, 94) = 1.842, p = .112). However, exploration of the contribution of individual factors is warranted. Only the Imagination subscale was revealed as a marginally significant negative predictor of visual recognition performance (β = −.029, t(99) = −1.797, p =.076, sr2= .031; all other lower-order social factors p > .396). The overall 5-factor model marginally predicted performance on the visual order task (R2 = .107, F(5, 94) = 2.224, p = .056), such that only the Imagination factor negatively affected the ability to successfully complete this task (β = −.268, t(99) = −2.577, p = .012, sr2= .063; all other factors p > .262.).
Again, we investigated whether one response type was particularly related to the visual recognition performance. In contrast to the two-factor structure of the AQ, the overall model did not predict hit rates (R2 = .079, F(5, 94) = 1.606, p = .166). There were no significant effects of any single factor beyond the attention to detail data described in the two-factor model (all p-values > .219). The 5-factor model of the AQ did not predict false alarm rates overall (R2 = .054, F(5, 99) = 1.076, p = .379) nor did any of the individual factors predict a significant portion of the variance (all p-values > .124).
Bivariate Correlations: AQ Factors and WM Performance
It is important to further explore bivariate correlations between the 2-factor and the 5-factor structure of the AQ, as these traits are highly interrelated in diagnosed ASD. Data for the 5-factor structure are presented in tabular format (see table 2). Importantly, our data support the Hoekstra 2-factor model (2008), as the attention to detail factor seems to stand apart from scores on other factors. Similarly, scores on the social skill measure correlate with scores on the attention switching, communication and imagination subscales, indicating that these factors, as in Hokestra et al. (2008) appear to ‘hang together’. With respect to the two-factor structure, attention to detail and social interaction were not significantly correlated with one another (r=.166, p = .098). Data representing correlations between memory scores and AQ factors are presented in table 3.
Table 2.
Bivariate Correlations Between Factors of the AQ
| Attention to Detail |
Social Skill |
Attention Switching |
Communication | Imagination | |
|---|---|---|---|---|---|
| Attention to Detail |
– | – | – | – | – |
| Social Skill | .113 | – | – | – | – |
| Attention Switching |
.152 | .339** | – | – | – |
| Communication | .170 | .548** | .367** | – | – |
| Imagination | .030 | .201* | .332** | .153 | – |
Note: denotes p < .05
denotes p < .01
Table 3.
Bivariate Correlations Between Factored AQ and Memory Performance
| Visual TO | Verbal TO | Visual Corrected Recognition |
Verbal Corrected Recognition |
|
|---|---|---|---|---|
| Attention to Detail | .078 | −.168 | .143 | −.081 |
| Social Skill | −.157 | .073 | −.147 | −.128 |
| Attention Switching | −.123 | −.171 | −.126 | −.099 |
| Communication | −.057 | .091 | −.092 | −.008 |
| Imagination | −.292* | −.138 | −.223* | −.132 |
| Higher order factor: Social Interaction |
−.215* | −.062 | −.202* | −.132 |
Note: TO= Temporal Order;
denotes p < .05
denotes p < .01
Discussion
The results of our study show that sub-clinical autistic traits in a normal college-aged sample were related to visual working memory performance for novel objects. Autistic traits were assessed by the AQ which can be broken down into a social factor and an attention to detail factor (Hoekstra, et al., 2008) as well as a five-factor structure tapping individuals’ abilities in the domains of attention to detail, attention switching, communication, imagination and social skills (Baron-Cohen, et al., 2001).
An interesting dissociation was found: on the visual recognition task, high scores on the attention factor of the AQ predicted enhanced visual WM, while high scores on the social factor of the AQ predicted poorer visual WM (see Figure 2). This pattern was specifically related to increased sensitivity, as measured by hit rates (i.e. correctly responding that an old item is old), rather than to differences in false alarm rates (i.e. incorrectly responding old when an item was in fact new). There were no significant effects of autistic traits on verbal WM performance.
These findings were explored in more detail by using the five-factor structure of the AQ. Our data indicate that within the higher-order factor of social interaction the factor of imagination is specifically related to visual WM impairment. Questions making up the imagination subscale include “I find it difficult to imagine what it would be like to be someone else” and “When I’m reading a story, I find it difficult to work out the characters’ intentions.” In other words, a high score on the imagination subscale indicates poor imagination. The five-factor structure of the AQ did not show a >particular effect on hits or false alarm performance. Instead, we can take the association of the imagination subscale as being globally related to impoverished mental imagery for novel visual stimuli. Because the items were presented in a serial fashion, participants in both the recognition and the order judgments had to imagine the shapes they had been shown in order to successfully complete the visual task. It is plausible that subjects who find it difficult to create mental images in everyday life would find it difficult (or at least, unnatural) to create mental images of the novel shapes used in our task.
More generally, our findings resonate with prior findings from the Minshew lab showing that children and adults with ASD have intact verbal WM but impaired visuospatial WM (Minshew & Goldstein, 2001). Our results extend their findings by showing that in the normal population, visual but not verbal WM performance was related to the degree of self-reported autistic symptoms. Although the verbal/visual distinction has an elegant simplicity, it may be more accurate to characterize this along a complexity dimension. Minshew and colleagues have proposed that regardless of the domain, memory deficits in ASD can be predicted by the complexity of the to-be-remembered stimuli (Williams, et al., 2006). As novel visual shapes might be considered to be more complex than the verbal stimuli used in our experiment, future research in this vein should strive to equate verbal and visual stimuli for difficulty to discern the particular memory pattern associated with stimulus domain (verbal or visual) versus stimulus complexity (simple to complex) in both clinical and non-clinical samples. On a related note, it is also possible that ASD traits correlate with low-level visual encoding deficits (Boucher, 1981). In the future, eye-tracking methods could be useful in evaluating the degree to which this possibility might explain the variation of memory abilities in normal participants, and how that might relate to AQ factors found to be of importance in predicting performance on visual memory tasks.
The ‘weak central coherence’ theory of ASD (Frith, 1989) suggests that a key characteristic of ASD is a local-processing bias, which is akin to the AQ factor attention to detail. Thus, it makes sense that having a high score on this factor may help WM performance for novel objects. The object stimuli used in our task were very similar to one another – they were all black, lacking internal features, all about the same size, and all bilaterally symmetrical. To distinguish one from the other, one must attend to and encode visual details into memory. However, our data indicate that having the trait of high attention to detail is only helpful for some WM tasks as this factor did not predict verbal WM performance or visual WM performance on the order task.
Interestingly, a high score on the social interaction factor was related to impaired visual WM performance. This factor contains data from a variety of questions: questions about social skills, communication, imagination and attention switching/ability to refocus attention. Surprisingly, the attention switching subscale was not implicated in the ability to complete this task. The factor that stood apart from the others when assessing the lower-order factors comprising social interaction was the imagination component. However, not all regression models that were significant in the two-factor structure came out in testing the five-factor structure, indicating that there was some additive, non-significant effect associated with the additional items comprising the social interaction factor with respect to performance on some of the memory measures explored here.
Our findings provide a plausible explanation for why some studies of WM in ASD have failed to find statistically significant effects (see Table 1). Our data indicate that the composition of the sample populations with respect to AQ scores - or some equivalent measure of ASD - is very important. On the one hand, it is possible that by chance, one might select a normal control group that has somewhat elevated AQ scores and thus, the concomitant social and cognitive deficits. This would make it nearly impossible to achieve statistical significance when comparing their performance to that of the ASD group. On the other hand, it is possible that by chance, one might select an ASD sample population that has AQ scores characterized by a greater weighting towards the attention to detail factor, rather than the social interaction factor. As shown by our data, this would tend to wash out any group differences in visual WM scores. As such, researchers may wish to assess ASD symptoms in their normal healthy control population due to the fact that these traits vary continuously in normals (von dem Hagen, et al., 2011), as well as examining the differential predictions about WM that result from this 2-factor structure of the AQ.
Last, it is worth noting that our effects were strong in a female-dominated sample (76% of study participants). Similar findings have been reported in several other studies (see Bayliss & Kritikos, 2011; Bayliss & Tipper, 2005). These findings indicate that an autistic-processing style, more than gender, influences individual differences in the domains of memory and attention. In fact, our female participants exhibit a numerically higher AQ score compared to males. It is possible that this finding may be driven more by the relatively larger variance captured in our sample of females in comparison to males simply based on gender distributions. However, as much previous research has focused on ASD associated traits being ‘extreme male’ forms of normally occurring personality traits (i.e. Baron-Cohen, 2002) gender differences in the normal population as they relate to both AQ and WM abilities may warrant more systematic study.
Our findings contribute to the extant literature on the variance of ASD-related traits in the normal population (see Bayliss & Kritikos, 2011; Bayliss & Tipper, 2005 for other examples). Furthermore, we demonstrate that factors of the AQ may contribute differently to memory profiles. It is possible that employing the breakdown of AQ scores according to one or both of the factor structures presented here in clinical populations of people with ASD may provide additional insight into the controversy regarding WM abilities; however, one would expect scores on all factors to exhibit relatively less variance in a clinical sample.
Implications
This research is the first of its kind to demonstrate that memory patterns in healthy, normal subjects vary according to ASD-associated traits, specifically with respect to two different factor structures of the AQ. Findings presented here should motivate future researchers using ASD samples to assess ASD-associated trait loading in their typically developing control populations to account for the relation between traits in normals and cognitive functioning.
Caveats
An important caveat to note is that it although our statistics were performed in such a way that may suggest that AQ scores precipitate differences in visual WM scores (i.e. AQ factors were entered into the regression model as predictors), it is quite possible that in fact WM abilities might precipitate response patterns on the AQ. Importantly, the directionality of the relation between visual WM and AQ scores cannot be determined by the data presented here.
Another important point is that participants were not formally screened for psychiatric disorders or ASD. It is possible that collecting this information via self-report methods might encourage participants to under-report the incidence of these factors.
Lastly, it is unclear if autistic traits in the general population manifest the same behavioral profile as they do in those with a diagnosis of ASD. Although previous research suggests that traits are measured similarly in people in the normal population as well as those with ASD (Wakabayashi, Baron-Cohen, & Wheelwright, 2006), this question is in need of further research.
Conclusions
The results of our study lend further support to the idea that ASD-associated traits in clinically normal individuals accounts for a portion of the variance in cognition-specifically WM. In addition, we show that the profile of AQ scores based on a two-factor structure predicts differential performance on a visual WM task. Deficits in visual WM associated with social dysfunction were examined further and appear to be directly related to difficulty with mental imagery. Future research in normal individuals may benefit from using factored AQ scores in order to disambiguate cognitive outcomes as they relate to trait loading on these factors.
Acknowledgments
The research found here was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, or the National Institutes of Health.
Footnotes
The terms “short-term memory” (STM) and “working memory” (WM) refer to different things; the former emphasizes the storage aspect of memory, and the latter emphasizes the manipulation of information held in memory. Although the distinction between storage and manipulation is of theoretical interest, in practice, the terms STM and WM have often been used somewhat interchangeably.
Responses to individual items for the AQ were lost for 4 participants; as such the scores for the individual AQ factors could not be calculated. Data presented in this section represents n=100.
References
- American Psychological Association . APA Dictionary of Psychology. 1 ed American Psychological Association; Washington, DC: 2007. [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. TRENDS in Cognitive Sciences. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and methemeticians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bayliss A, Kritikos A. Brief report: Perceptual load and the Autism Spectrum in typically developed individuals. Journal of Autism and Developmental Disorders. 2011:1–6. doi: 10.1007/s10803-010-1159-8. [DOI] [PubMed] [Google Scholar]
- Bayliss A, Tipper S. Gaze and arrow cueing of attention reveals individual differences along the autism spectrum as a function of target context. British Journal of Psychology. 2005;96:95–114. doi: 10.1348/000712604X15626. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Pennington B, Rogers S. Intact and impaired memory functions in autism. Child Development. 1996;67:1816–1835. [PubMed] [Google Scholar]
- Boucher J. Memory for recent events in autistic children. Journal of Autism & Developmental Disorders. 1981;11:293–302. doi: 10.1007/BF01531512. [DOI] [PubMed] [Google Scholar]
- Briskman J, Frith U, Happe F. Exploring the Cognitive Phenotype of Autism: Weak Central Coherence in Parents and Siblings of Children with Autism: II. Real-life Skills and Preferences. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(03):309–316. [PubMed] [Google Scholar]
- Carlson S, Moses L, Breton C. How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant and Child Development. 2002;11:73–92. [Google Scholar]
- Constantino J. The Social Responsiveness Scale. Western Psychological Services; Los Angeles, CA: 2002. [Google Scholar]
- Constantino J, Todd R. Autistic traits in the general population. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Daneman M, Carpenter P. Individual differences in working memory and reading. Verbal Learning and Verbal Behavior. 1980;19(4):450–466. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological Correlates of Early Symptoms of Autism. Child Development. 1998;69(5):1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Frith U. Autism: Explaining the enigma. Blackwell: 1989. [Google Scholar]
- Griffith EM, Pennington BF. Executive Functions in Young Children with Autism. Child Development. 1999;70(4):817. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Grinter E, Maybery M, Van Beek P, Pellicano E, Badcock J, Badcock D. Global Visual Processing and Self-Rated Autistic-like Traits. Journal of Autism and Developmental Disorders. 2009;39(9):1278–1290. doi: 10.1007/s10803-009-0740-5. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Brindley R, Frith U. Visual perspective taking impairment in children with autistic spectrum disorder. Cognition. 2009;113:37–44. doi: 10.1016/j.cognition.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Happe F. Autism: Cognitive deficit or cognitive style? Trends in Cognitive Sciences. 1999;3(6):216–222. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Happe F, Briskman J, Frith U. Exploring the Cognitive Phenotype of Autism: Weak Central Coherence in Parents and Siblings of Children with Autism: I. Experimental Tests. Journal of Child Psychology and Psychiatry. 2001;42(3):299–307. [PubMed] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience. 2006;9(10):1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Bartels M, Cath D, Boomsma D. Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): A study in Dutch population and patient groups. Journal of Autism and Developmental Disorders. 2008;38:1555–1566. doi: 10.1007/s10803-008-0538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Olson IR, Chun MM. Organization of visual short-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(3):683–702. doi: 10.1037//0278-7393.26.3.683. [DOI] [PubMed] [Google Scholar]
- Killiany R, Moore T, Rehbein L, Moss M. Memory and executive functions in autism. In: Bauman M, Kemper T, editors. The neurobiology of autism. 2 ed Johns Hopkins University Press; Baltimore: 2005. pp. 59–64. [Google Scholar]
- Koshino H, Carpenter P, Minshew N, Cherkassky V, Keller T, Just M. Functional connectivity in an fMRI working memory task in high functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Kyllonen P, Christal R. Reasoning ability is (little more than) working-memory capacity?! Intelligence. 1990;14:389–433. [Google Scholar]
- Landa RJ, Goldberg MC. Language, Social, and Executive Functions in High Functioning Autism: A Continuum of Performance. Journal of Autism & Developmental Disorders. 2005;35(5):557–573. doi: 10.1007/s10803-005-0001-1. [DOI] [PubMed] [Google Scholar]
- Luck S, Vogel E. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzig data: A model comparison perspective. Second ed Lawrence Erlbaum Associates; Mahwah, NJ: 2004. [Google Scholar]
- Milne E, Scope A, Pascalis O, Buckley D, Makeig S. Independent Component Analysis Reveals Atypical Electroencephalographic Activity During Visual Perception in Individuals with Autism. Biological Psychiatry. 2009;65(1):22–30. doi: 10.1016/j.biopsych.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Minshew N, Goldstein G. The pattern of intact and impaired memory functions in autism. The Journal of Child Psychology and Psychiatry. 2001;42(8):1095–1101. doi: 10.1111/1469-7610.00808. [DOI] [PubMed] [Google Scholar]
- Mottron L, Morasse L, Belleville S. A study of memory functioning in individuals with autism. The Journal of Child Psychology and Psychiatry. 2001;42(2):253–260. [PubMed] [Google Scholar]
- Ozonoff S, South M, Provencal S. Executive functions in autism. In: Perez J, Gonzalez P, Comi M, Nieto C, editors. New developments in autism: The future is today. Jessica Kingsley Publishers; London, Philadelphia: 2007. pp. 185–213. [Google Scholar]
- Ozonoff S, Strayer D. Further evidence of intact working memory in autism. Journal of Autism and Developmental Disorders. 2001;31(3):257–263. doi: 10.1023/a:1010794902139. [DOI] [PubMed] [Google Scholar]
- Palmer J. Attentional limits on the perception and memory of visual information. Journal of Experimental Psychology: Human Perception and Performance. 1990;16(2):332–350. doi: 10.1037//0096-1523.16.2.332. [DOI] [PubMed] [Google Scholar]
- Poirier M, Martin JS, Gaigg SB, Bowler DM. Short-term memory in autism spectrum disorder. Journal of Abnormal Psychology. 2011;120(1):247–252. doi: 10.1037/a0022298. [DOI] [PubMed] [Google Scholar]
- Russo N, Flanagan T, Iarocci G, Berringer D, Zelazo P, Burack J. Deconstructing executive deficits among persons with autism: Implications for cognitive neuroscience. Brain and Cognition. 2007;65:77–86. doi: 10.1016/j.bandc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Salter G, Seigal A, Claxton M, Lawrence K, Skuse D. Can autistic children read the mind of an animated triangle? Autism. 2008;12(4):349–371. doi: 10.1177/1362361308091654. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Turner-Brown LM, Holtzclaw TN, Lam KSL, Bodfish JW. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1(1):31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Vogel E, Woodman G, Luck S. Voluntary and automatic attentional control of visual working memory. Attention, Perception, & Psychophysics. 2002;64(5):754–763. doi: 10.3758/bf03194742. [DOI] [PubMed] [Google Scholar]
- Shelton A, Clements-Stephens A, Lam W, Pak D, Murray A. Should social savvy equal good spatial skills? The interaction of social skills with spatial perspective-taking. Journal of Experimental Psychology: General. 2012;141(2):199–205. doi: 10.1037/a0024617. [DOI] [PubMed] [Google Scholar]
- Soulieres I, Zeffiro T, Girard M, Mottron L. Enhanced mental image mapping in autism. Neuropsychologia. 2011;49:848–857. doi: 10.1016/j.neuropsychologia.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Sperling G. The information available in brief visual presentations. Psychological Monographs: General and Applied. 1960;74(11):1–29. Whole No. 498. [Google Scholar]
- Unsworth N, Engle R. The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review. 2007;114(1):104–132. doi: 10.1037/0033-295X.114.1.104. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Spillers G. Working memory capacity: Attention control, secondary memory, or both? A direct test of the dual-component model. Journal of Memory and Language. 2010;62:392–406. [Google Scholar]
- von dem Hagen E, Nummenmaa L, Yu R, Engell A, Ewbank M, Calder A. Autism spectrum traits in the typical population predict structure and function in the posterior temporal sulcus. Cerebral Cortex. 2011;21:493–500. doi: 10.1093/cercor/bhq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi A, Baron-Cohen S, Wheelwright S. Are autistic traits an independent personality dimension? A study of the Autism-Spectrum Quotient (AQ) and the NEO-PI-R. Personality and Individual Differences. 2006;41(5):873–883. [Google Scholar]
- Williams D, Goldstein G, Carpenter P, Minshew N. Verbal and spatial working memory in autism. Journal of Autism and Developmental Disorders. 2005;35(6):747–756. doi: 10.1007/s10803-005-0021-x. [DOI] [PubMed] [Google Scholar]
- Williams D, Goldstein G, Minshew N. The profile of memory function in children with autism. Neuropsychology. 2006;20(1):21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman G, Vecera S, Luck S. Perceptual organization influences visual working memory. Psychonomic Bulletin & Review. 2003;10(1):80–87. doi: 10.3758/bf03196470. [DOI] [PubMed] [Google Scholar]