Abstract
Glucocorticoids are routinely given in preterm labor and are also elevated by maternal stress; organophosphate exposures are virtually ubiquitous, so coexposures to these two agents are pervasive. We administered dexamethasone to pregnant rats on gestational days 17–19 at a standard therapeutic dose (0.2 mg/kg); offspring were then given chlorpyrifos on postnatal days 1–4, at a dose (1 mg/kg) that produces barely-detectable (<10%) inhibition of brain cholinesterase activity. We evaluated indices for acetylcholine (ACh) synaptic function throughout adolescence, young adulthood and later adulthood, in brain regions possessing the majority of ACh projections and cell bodies; we measured nicotinic ACh receptor binding, hemicholinium-3 binding to the presynaptic choline transporter and choline acetyltransferase activity, all known targets for the adverse developmental effects of dexamethasone and chlorpyrifos given individually. Dexamethasone did not enhance the systemic toxicity of chlorpyrifos, as evidenced by weight gain and measurements of cholinesterase inhibition during chlorpyrifos treatment. Nevertheless, it enhanced the loss of presynaptic ACh function selectively in females, who ordinarily show sparing of organophosphate developmental neurotoxicity relative to males. Females receiving the combined treatment showed decrements in choline transporter binding and choline acetyltransferase activity that were unique (not found with either treatment alone), as well as additive decrements in nicotinic receptor binding. On the other hand, males given dexamethasone showed no augmentation of the effects of chlorpyrifos. Our findings indicate that prior dexamethasone exposure could create a subpopulation that is especially vulnerable to the adverse effects of organophosphates or other developmental neurotoxicants.
Keywords: Chlorpyrifos, Cholinergic neurotransmission, Dexamethasone, Glucocorticoids, Organophosphate pesticides, Preterm delivery
INTRODUCTION
It is now nearly two decades since the National Institutes of Health endorsed glucocorticoids as a consensus treatment for preterm labor occurring between 24 and 34 weeks of gestation, a therapy that prevents 6000 cases of neonatal respiratory distress syndrome and approximately 2000 deaths annually in the U.S. (Gilstrap et al., 1995). As a consequence of this recommendation, 10% of all U.S. newborns, about 400,000 per year, are now exposed to these agents (Matthews et al., 2002). Thus, the promotional effects of glucocorticoids on lung function in the small population of infants who benefit from this therapy need to be balanced against potential adverse effects on the much larger population that receives treatment. The most notable problem is the impact on development of the nervous system. Excess glucocorticoids disrupt fetal brain development, reducing the numbers of neurons and impairing structural assembly and synaptic connectivity, culminating in neuroendocrine, behavioral and cardiovascular disorders (Cavalieri and Cohen, 2006; Drake et al., 2007; Meyer, 1985; Moritz et al., 2005; Pryce et al., 2011; Rokyta et al., 2008; Tegethoff et al., 2009). It is increasingly clear that these factors operate in children exposed prenatally to glucocorticoids (Hirvikoski et al., 2007; Needelman et al., 2008; Newnham, 2001; Peltoniemi et al., 2011) (Crowther et al., 2007).
Although a large research literature exists on the effects of prenatal glucocorticoids on brain development and behavior, relatively little attention has been paid to the question of whether these treatments sensitize the brain to subsequent exposures to neurotoxic chemicals. The increasing number and utilization of industrial chemicals and pesticides are thought to play a large role in the explosive increase in the incidence of neurodevelopmental disorders (Grandjean and Landrigan, 2006; Landrigan, 2010; Landrigan et al., 1994; Szpir, 2006a, b). In a recent in vitro study (Slotkin et al., 2012), we demonstrated that dexamethasone enhanced neural cell loss caused by an organophosphate pesticide (chlorpyrifos); organophosphates are the mostly widely-used class of insecticides, with virtually ubiquitous exposure of the human population (Casida and Quistad, 2004). In addition, dexamethasone exposure completely changed the effects of chlorpyrifos on neurite formation and neurodifferentiation into specific neurotransmitter phenotypes, specifically acetylcholine (ACh) and dopamine (Slotkin et al., 2012). In the current work, we examined whether the outcomes seen in cell cultures occur with exposures to dexamethasone and chlorpyrifos in vivo, using established rat models for these treatments. We administered dexamethasone on gestational days (GD) 17–19, a developmental stage in the rat that corresponds to the stage of brain development in which glucocorticoid therapy is typically given in preterm labor, using a dose (0.2 mg/kg) in the low therapeutic range (Gilstrap et al., 1995). The three-day regimen corresponds to multiple glucocorticoid courses, as used in approximately 85% of all cases (Dammann and Matthews, 2001), and the dose was chosen to produce submaximal effects to allow detection of interactions with chlorpyrifos (Kreider et al., 2005a, 2006; Slotkin et al., 1996, 2006). Chlorpyrifos was given daily on postnatal days (PN) 1–4 at a dose of 1 mg/kg, a regimen that produces barely-detectable inhibition of brain cholinesterase and that disrupts neurobehavioral development, but that is not systemically toxic (Slotkin, 1999, 2004, 2005; Song et al., 1997). This exposure model successfully predicts both the neurobehavioral deficits and abnormalities of brain structure seen in children exposed prenatally to chlorpyrifos (Bouchard et al., 2011; Engel et al., 2011; Rauh et al., 2006, 2011, 2012). Our study thus encompassed four treatment paradigms: control, dexamethasone alone, chlorpyrifos alone, and dexamethasone followed by chlorpyrifos.
We focused our measurements on ACh systems, a known neurotransmitter target for adverse developmental effects of both glucocorticoids and organophosphates (Emgard et al., 2007; Kreider et al., 2005a, b, 2006; Slotkin, 1999, 2004, 2005). We assessed the impact on brain regions comprising the major ACh projections and their corresponding cell bodies, focusing on standard markers of ACh synaptic function: the concentration of α4β2 nicotinic ACh receptors (nAChRs), binding of hemicholinium-3 (HC3) to the presynaptic high-affinity choline transporter, and activity of choline acetyltransferase (ChAT). The α4β2 nAChR is the most abundant nAChR subtype in the mammalian brain (Flores et al., 1992; Happe et al., 1994; Whiting and Lindstrom, 1987; 1988) and underlies the ability of ACh systems to release other neurotransmitters involved in reward, cognition and mood (Buisson and Bertrand, 2001, 2002; Dani and De Biasi, 2001; Fenster et al., 1999; Quick and Lester, 2002). High-affinity choline transporters and ChAT are both constitutive components of ACh nerve terminals but they differ in their regulatory mechanisms and hence in their functional significance. ChAT is the enzyme that synthesizes ACh, but is not regulated by nerve impulse activity, so that its presence provides an index of the development of ACh projections (Happe and Murrin, 1992; Kreider et al., 2005a, 2006; Slotkin, 2004, 2008). In contrast, HC3 binding to the choline transporter is directly responsive to neuronal activity (Klemm and Kuhar, 1979; Simon et al., 1976), so that comparative effects on HC3 binding and ChAT enables the characterization of both the development of innervation and presynaptic impulse activity. Accordingly, in addition to assessing HC3 binding and ChAT activity, we determined the HC3/ChAT ratio as an index of presynaptic activity relative to the number of cholinergic nerve terminals (Abreu-Villaga et al., 2004; Slotkin et al., 1994, 2007). We also contrasted the specific effects on ACh synaptic development with assessments of systemic toxicity (maternal weight gain, litter characteristics, postnatal body and brain region weights), and determinations of whether dexamethasone enhanced the ability of chlorpyrifos to inhibit cholinesterase.
MATERIALS AND METHODS
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague-Dawley rats were shipped by climate-controlled truck (total transit time < 1 h), housed individually and allowed free access to food and water. There were four treatment groups, each comprising 12–14 dams: controls (prenatal saline + postnatal dimethylsulfoxide vehicle), dexamethasone treatment alone (prenatal dexamethasone + postnatal vehicle), chlorpyrifos treatment alone (prenatal saline + postnatal chlorpyrifos), and those receiving the combined treatment (prenatal dexamethasone + postnatal chlorpyrifos). On GD17, 18 and 19, dams received subcutaneous injections of either saline vehicle or 0.2 mg/kg dexamethasone sodium phosphate, a dose at the lower range recommended for therapeutic use in preterm labor (Gilstrap et al., 1995). Parturition occurred during GD22, which was also taken as PNO. After birth, pups were randomized within treatment groups and litter sizes were culled to 10 (5 males and 5 females) to ensure standard nutrition. Control and dexamethasone-treated litters were then assigned to either the vehicle or chlorpyrifos postnatal treatment groups. Chlorpyrifos was dissolved in dimethylsulfoxide to provide consistent absorption (Whitney et al., 1995) and was injected subcutaneously at a dose of 1 mg/kg in a volume of 1 ml/kg once daily on postnatal days 1–4; control animals received equivalent injections of the dimethylsulfoxide vehicle. This regimen has been shown previously to produce developmental neurotoxicity, including robust effects on cholinergic systems, without eliciting growth retardation or any other signs of systemic toxicity (Slotkin, 1999, 2004). Pups were weighed, litters were re-randomized within treatment groups and dams were rotated among litters every few days to distribute differential effects of maternal caretaking equally among all litters, making sure that all the pups in a given litter were from the same treatment group to avoid the possibility that the dams might distinguish among pups with different treatments; cross-fostering, by itself, has no impact on neurochemical or behavioral effects of these treatments (Nyirenda et al., 2001). Animals were weaned on PN21.
On PN4, 2 hr after the last injection, 8 pups from each group were decapitated and whole brain dissected for determination of cholinesterase activity. Subsequently, on PN30, 60, 100 and 150, additional animals were decapitated and brain regions were dissected for determination of cholinergic synaptic markers: frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum, midbrain and brainstem. The two cortical regions were sectioned at the midline and the left half used for the current determinations. The right halves of the cortical regions were reserved for future studies, along with the cerebellum, which is sparse in cholinergic projections. Tissues were frozen in liquid nitrogen and stored at −45° C until assayed. For the cholinergic synaptic markers, each treatment group comprised 12 animals at each age point, equally divided into males and females, with each final litter assignment contributing no more than one male and one female to any of the treatment groups.
Assays
Assays were conducted on each individual tissue, so that each determination represented a value from the corresponding brain region of one animal. For cholinesterase determinations, tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and aliquots of the homogenate were withdrawn for measurement of total protein (Smith et al., 1985) and cholinesterase activity (Ellman et al., 1961). For the latter, the homogenate was diluted in 0.5% Triton ×100, 0.1 M Na2HPO4/KH2PO4 (pH 8) and left on ice for 15 min to allow the Triton ×100 to solubilize membrane-associated cholinesterase. Homogenates were sedimented at 40,000 × g for 15 min and aliquots of the supernatant solution were added to final concentrations of 0.5 mM acetylthiocholine iodide and 0.33 mM 5,5'-dithiobis(2-nitrobenzoic acid) in the same buffer without Triton. Assays were incubated at room temperature for 4, 8, 12, 16 and 20 min, and the enzyme activity was assessed from the linear portion of the time course, reading the absorbance at 415 nm. The assay was standardized by using mercaptoethanol standards and calculated relative to total protein.
For cholinergic synaptic markers, tissues were thawed in 79 volumes of ice-cold 10 mM sodium-potassium phosphate buffer (pH 7.4) and homogenized with a Polytron (Brinkmann Instruments, Westbury, NY). Duplicate aliquots of the homogenate were assayed for ChAT using established procedures (Qiao et al., 2003, 2004). Each tube contained 50 μM [14C]acetyl-coenzyme A as a substrate and activity was determined as the amount of labeled ACh produced relative to tissue protein (Smith et al., 1985). For measurements of HC3 binding, the cell membrane fraction was prepared from an aliquot of the same tissue homogenate by sedimentation at 40,000 × g for 15 min. The pellet was resuspended and washed, and the resultant pellet was assayed with established procedures (Qiao et al., 2003, 2004), using a ligand concentration of 2 nM [3H]HC3 with or without 10 μM unlabeled HC3 to displace specific binding. Determinations of nAChR binding were carried out in another aliquot, each assay containing 1 nM [3H]cytisine with or without 10 μM nicotine to displace specific binding (Slotkin et al., 2008). Binding for both ligands was calculated relative to the membrane protein concentration.
Data analysis
Data were compiled as means and standard errors. Because we evaluated three neurochemical measures that were all related to ACh synapses, the initial comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among regions and measures) incorporating all the variables and measurements so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. The variables in the global test were prenatal treatment (saline control, dexamethasone), postnatal treatment (dimethylsulfoxide control, chlorpyrifos), brain region, age and sex. There were three dependent measures (nAChR binding, HC3 binding, ChAT); in the global ANOVA, these were nested as repeated measures, since all three determinations were derived from the same sample. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control. Further, because of the repeated-measures design, terms were generated for the interaction of the variables with the repeated dependent measures (e.g. interaction of treatment × measure, or of treatment × measure × other variables), which then connote differences in the impact of treatment among the three dependent measures (hereafter, designated simply as “measures”), necessitating separate consideration of each of the three different biochemical endpoints. As permitted by the interaction terms, individual groups that differed from control were identified with Fisher's Protected Least Significant Difference Test. However, where treatment effects were not interactive with other variables, we report only the main treatment effects without performing lower-order analyses of individual values. Similar multivariate ANOVA designs were used to analyze maternal weight gain, litter characteristics, postnatal weights and cholinesterase activity. Significance was assumed at the level of p < 0.05.
To enable ready visualization of treatment effects across different regions, ages and measures, some results are given as the percent change from control values, but statistical procedures were always conducted on the original data. In those circumstances, the tables in the Supplement provide a listing of all the original values.
The design of the studies required two different ways of regarding treatment variables. To characterize the effects of dexamethasone alone, chlorpyrifos alone, or the combined treatment versus controls or versus each other, the four treatment groups were first considered as a one-dimensional factor in the statistical design. To determine whether the effects of dexamethasone and chlorpyrifos were interactive, the treatment factors were changed to a two-dimensional design. In this formulation, synergistic or less-than-additive effects appear as significant interactions between the two treatment dimensions, whereas simple, additive effects do not show significant interactions.
Materials
Animals were purchased from Charles River Laboratories (Raleigh, NC) and chlorpyrifos was obtained from Chem Service (West Chester, PA). PerkinElmer Life Sciences (Boston, MA) was the source for radioligands: [3H]HC3 (specific activity, 125 Ci/mmol), [3H]cytisine (specific activity 35 Ci/mmol) and [14C]acetyl-coenzyme A (specific activity 6.7 mCi/mmol). All other reagents were from Sigma Chemical Co. (St. Louis, MO).
RESULTS
Maternal, litter and growth effects
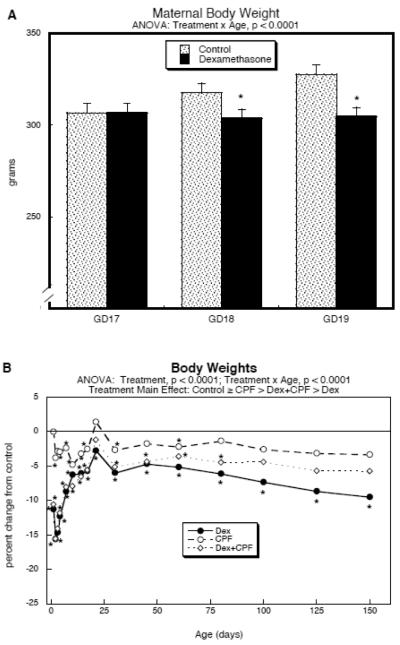
Administration of dexamethasone on GD17–19 attenuated maternal weight gain, leading to about a 10% deficit as compared to controls (Fig. 1A). However, there were no significant differences in litter size at parturition (control, 12.5 ± 0.5 pups, n=14 litters; dexamethasone, 11.9 ± 0.3 pups, n=12 litters), although the percentage of male pups decreased (controls, 56 ± 4%; dexamethasone 44 ± 4, p < 0.05). Prenatal dexamethasone treatment elicited growth retardation in the offspring (main treatment effect, p < 0.0001), commencing at about a 10–15% deficit in the immediate postnatal period, recovering to a 5–10% reduction by the end of the first week and maintained in that range through PN150 (Fig. 1B; original values with standard errors shown in Supplementary Table 1). Postnatal chlorpyrifos exposure elicited a small overall decrement in body weights that was not significantly different from control values but was statistically distinguishable from the larger deficits caused by prenatal dexamethasone (p < 0.0001). Somewhat surprisingly, although the group receiving combined exposure to dexamethasone and chlorpyrifos had weight deficits relative to controls (main treatment effect, p < 0.0001), they eventually recovered to a greater extent than did animals given dexamethasone alone (p < 0.0001). When the statistical model was switched to two treatment dimensions we identified a significant interaction of dexamethasone × chlorpyrifos (p < 0.0001), indicating that the combined treatment showed a significantly smaller weight effect than would have been expected from simple additive effects of dexamethasone and chlorpyrifos.
Figure 1.
Treatment effects on body weights. (A) Maternal weights during GD17-19 dexamethasone treatment (mean ± SE, n=12–14); GD17 is the starting weight (before the first dexamethasone injection). (B) Postnatal weights of offspring (mean values only, n≥14 per sex for each treatment at each age point), shown as the percent change from control values (see original values in Supplementary Table 1; SE values were omitted for clarity, but are provided in Supplementary Table 1). Multivariate ANOVAs are shown at the top of each panel and asterisks denote individual time points where the treatment groups differ from the corresponding control values. For (B), the main treatment effects were all significant from each other in pairwise comparisons (p < 0.0001) with the exception of Control vs. CPF. Results from males and females were combined in (B) because of the absence of a treatment × sex interaction; the percent change was calculated relative to the controls of the corresponding sex to avoid adding inherent male-female weight differences into the variability. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos.
In contrast to the robust effects on body weight, brain region weights did not show any main treatment effects but did display a significant treatment × age interaction (p < 0.04; Supplementary Table 2). The age-dependent difference was limited to a single age point (PN150, main treatment effect, p < 0.002). At that age, the group receiving the combined dexamethasone and chlorpyrifos treatment showed a significant reduction relative to all other groups (p < 0.02 vs. control, p < 0.0007 vs. dexamethasone, p < 0.0005 vs. chlorpyrifos), an effect that was distinguishable from simple additivity of the effects of the individual agents (dexamethasone × chlorpyrifos interaction, p < 0.002). The magnitude of the effects on brain region weights was small: across all regions and both sexes, there was a net increase of 1% caused by dexamethasone, a net increase of 2% caused by chlorpyrifos, but a net decrease of 3% from the combination.
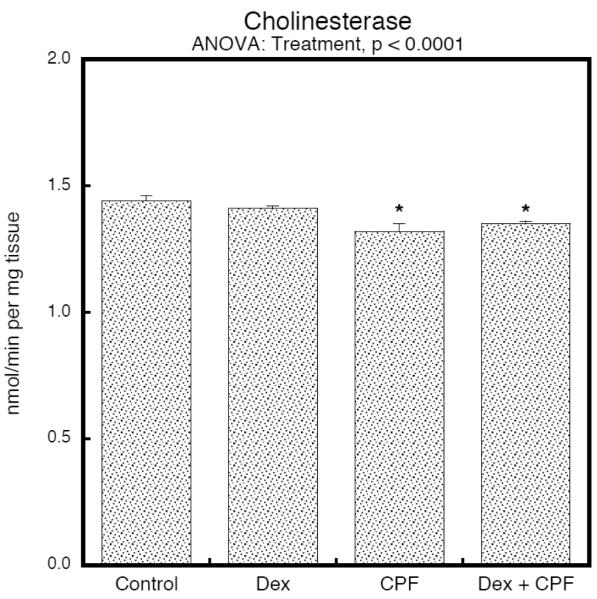
Cholinesterase activity
To determine whether dexamethasone could affect chlorpyrifos pharmacokinetics and the resultant inhibition of cholinesterase, we evaluated brain cholinesterase activity on PN4, 2 hr after the last injection, corresponding to the time of peak inhibition (Dam et al., 2000). In keeping with earlier studies (Song et al., 1997), the postnatal chlorpyrifos regimen used here produced barely-detectable inhibition of brain cholinesterase, amounting to no more than a 5–10% reduction (Fig. 2). By itself, dexamethasone had no significant effect on cholinesterase, nor did it alter the response to chlorpyrifos; note that results are shown combined for males and females because of the absence of a treatment × sex interaction.
Figure 2.
Treatment effects on cholinesterase activity in whole brain on PN4, 2 hours after the final injection of CPF or vehicle (mean ± SE, n=8). ANOVA is shown at the top of the panel and asterisks denote the groups that differ significantly from the control value. Inhibition by CPF was not significantly changed by prenatal dexamethasone treatment (no difference between CPF and Dex+CPF). Values were combined for males and females because of the absence of a treatment × sex interaction. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos.
Global statistical analyses of cholinergic biomarkers
We used a global ANOVA to evaluate all factors (prenatal treatment, postnatal treatment, sex, brain region, age) and all three dependent measures (nAChR binding, HC3 binding, ChAT activity; repeated measures) in a single test, and we identified main treatment effects (p < 0.0001) as well as interactions of treatment × sex (p < 0.006), treatment × region (p < 0.01), treatment × measure (p < 0.0005), treatment × sex × measure (p < 0.003) and treatment × region × measure (p < 0.0007). In light of the sex-dependence of the treatment effects, we then subdivided the data for males and females and found that the treatment effects were sustained: males, p < 0.0001 for the main treatment effect, p < 0.03 for treatment × region, p < 0.0001 for treatment × measure, p < 0.009 for treatment × region × measure; females, p < 0.0002 for treatment. When we evaluated the dexamethasone and chlorpyrifos treatments as two dimensions, we further found a dexamethasone × chlorpyrifos interaction (p < 0.007) that was similarly sex-dependent (dexamethasone × chlorpyrifos × sex, p < 0.007), indicating that the results for the group receiving the combined treatments did not reflect simple, additive effects of dexamethasone and chlorpyrifos. Based on these global results, we analyzed the data separated into the different measures and divided for males and females, and then looked for treatment effects and interactions of treatment with the remaining variables (region, age). However, for each set of measures, we again preceded the lower-order tests by ANOVA for all the factors (treatment, sex, region, age) in a single test to ensure that further subdivisions were justified.
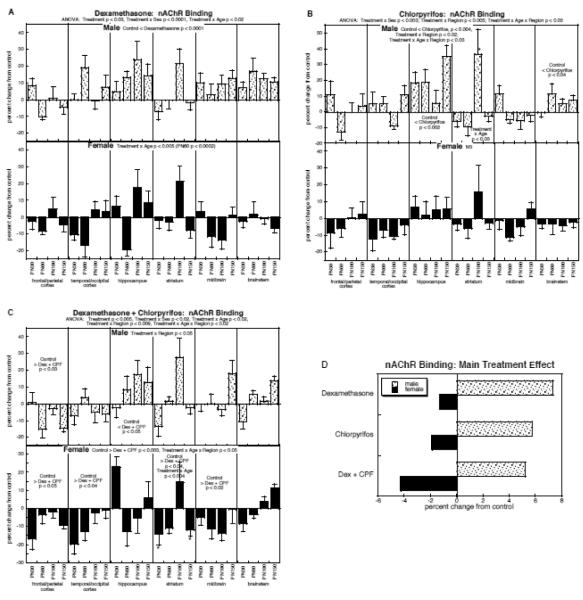
nAChR binding
For nAChR binding, ANOVA across all factors identified a main treatment effect (p < 0.0003) that depended on all the other variables: p < 0.002 for treatment × sex, p < 0.02 for treatment × age, p < 0.04 for treatment × region, p < 0.04 for treatment × age × region. The treatment effects were sustained after subdivision of the data into males and females: males, p < 0.0001 for treatment, p < 0.04 for treatment × region; females, p < 0.02 for treatment. Accordingly, we examined each treatment separately for effects in males and females.
By itself, prenatal dexamethasone exposure evoked a significant overall elevation in nAChR binding in males (main treatment effect, p < 0.0001) but more variable effects in females, which showed only an age-dependent effect, with significant differences restricted to a decrease at PN60 (Fig. 3A). Postnatal chlorpyrifos treatment likewise showed preferential effects in males (Fig. 3B); there was an overall elevation (main treatment effect, p < 0.004), superimposed on regionally-selective effects (treatment × region, p < 0.02). The largest and most consistent increases were in the hippocampus, with smaller but significant overall increases found also in the brainstem; the striatum showed age-dependent effects, reflecting a large but transient effect on PN60. In contrast to the effects of chlorpyrifos in males, the females showed no significant main treatment effect or treatment interactions with the other variables.
Figure 3.
Effects of dexamethasone (A), chlorpyrifos (B), and combined treatment (C) on nAChR binding (mean ± SE, n=6 for each sex in each treatment group), shown as the percent change from control values (see original values in Supplementary Table 3). Because the global ANOVA showed a significant treatment × sex interaction (p < 0.002), values were separated for males and females. Multivariate ANOVA for each treatment appears at the top of the panels; because each treatment showed a significant treatment × sex interaction. For dexamethasone (A), males showed only a main treatment effect, so no lower-order tests were performed; females showed an interaction of treatment × age and the age at which differences were significant is indicated. For chlorpyrifos (B), males showed a significant treatment × region interaction, so lower-order tests were conducted for each region, followed by tests of individual ages (asterisks) where a region showed a significant treatment × age interaction; females were not significant in the multivariate ANOVA, so no lower-order tests were evaluated. For combined treatment (C), both sexes showed interactions of treatment × region, so lower-order tests were run for each region, followed again by tests of individual age points (asterisks) where a region showed a significant treatment × age interaction. Panel (D) shows the simple main treatment effects for each sex, collapsed across all the other variables. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos; NS, not significant.
Combined exposure to both dexamethasone and chlorpyrifos produced effects that were distinct from those seen with either treatment alone (Fig. 3C). In males, there was a treatment × region interaction (p < 0.05), reflecting a switch from predominant increases seen with the individual treatments, to a more mixed regional pattern with the combined treatment. nAChR binding showed a net decrease in the frontal/parietal cortex, whereas values were increased in the hippocampus; however, the magnitude of the hippocampal effect was significantly smaller than would have been expected from additive effects of the two individual treatments (dexamethasone × chlorpyrifos interaction, p < 0.0001). In contrast, for females, combined exposure to dexamethasone and chlorpyrifos produced an augmented effect compared to either treatment alone, with emergence of a significant overall reduction (main treatment effect, p < 0.003) that was interactive with both age and region (treatment × age × region, p < 0.05); consistent deficits were found in the frontal/parietal cortex, temporal/occipital cortex and midbrain, whereas age-dependent changes were seen in the striatum. Accordingly, although either treatment alone spared females relative to males, that protection was lost in the group receiving both dexamethasone and chlorpyrifos.
To illustrate the differences in the main treatment effect among the three groups, we calculated the mean effect on nAChR binding, collapsing the values across all the interactive variables (Fig. 3D). This simplified picture dilutes the effects seen in specific regions or at particular ages by averaging them with the regions or ages for which there was no effect or an opposite effect, so that the absolute magnitude becomes smaller. Despite these limitations, there was an obvious sex difference in the overall patterns, with males showing treatment-related increases in binding whereas females showed net decreases, reflecting the overall treatment × sex interaction seen in the global ANOVA. For males, the combined treatment with dexamethasone and chlorpyrifos produced an increase similar to the effect of chlorpyrifos alone, clearly less than would have been expected from simple additivity of the individual treatment effects (dexamethasone × chlorpyrifos interaction, p < 0.0001). For females, the combined treatment was significantly worse than either treatment alone (p < 0.003 vs. control, p < 0.05 vs. dexamethasone, p < 0.05 vs. chlorpyrifos); however, the net effect was not distinguishable from the additive actions of the two agents (no dexamethasone × chlorpyrifos interaction), each of which showed a slight decrease.
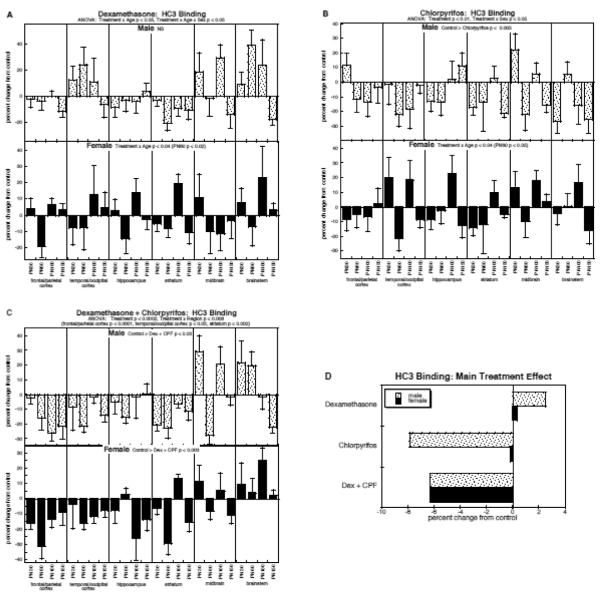
HC3 binding
The overall ANOVA for HC3 binding showed main effects of treatment (p < 0.0001) and interactions of treatment with sex and region (p <0.04 for treatment × sex, p < 0.009 for treatment × region). Again, the main treatment effects were maintained when data were subdivided into males (p < 0.0003) and females (p < 0.01). It should be noted that our inclusion of cell body regions that have low HC3 binding values (midbrain, brainstem) introduced higher variability than would have been seen if we had restricted our measurements to regions enriched in cholinergic nerve terminals (Supplementary Table 4) but this did not obscure the main treatment effects, and hence these values were retained in the analysis.
By itself, dexamethasone had variable effects on HC3 binding across the different regions and ages, resulting in no net differences for males and only a transient, significant decrease (PN60) in females (Fig. 4A). On the other hand, in males, chlorpyrifos elicited a strong overall reduction in HC3 binding (main treatment effect, p < 0.005), without significant distinctions among regions and ages (Fig. 4B); females were spared, again showing significance at one age point (PN60), at which there was an overall decrease. The combined treatment did not appreciably change the pattern in males from that seen with chlorpyrifos alone, again showing a net reduction in HC3 binding (Fig. 4C), concentrated mostly in the regions containing cholinergic nerve terminals (frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum). For females given the combined treatment, we again saw a loss of the “protected” status as compared to males; exposure to both dexamethasone and chlorpyrifos evoked a highly significant net decrease in HC3 binding, an effect that was not obtained with either treatment alone, and that was as large as the deficits seen in males (no treatment × sex interaction).
Figure 4.
Effects of dexamethasone (A), chlorpyrifos (B), and combined treatment (C) on HC3 binding (mean ± SE, n=6 for each sex in each treatment group), shown as the percent change from control values (see original values in Supplementary Table 4). Because the global ANOVA showed a significant treatment × sex interaction (p < 0.04), values were separated for males and females. Multivariate ANOVA for each treatment appears at the top of the panels. For dexamethasone (A), males showed no significant treatment effect or interactions of treatment with the other variables, so no lower-order tests were performed; females showed an interaction of treatment × age and the age at which differences were significant is indicated. For chlorpyrifos (B), males showed only a significant main treatment effect, so no lower-order tests were conducted; females again showed an interaction of treatment × age and the age at which differences were significant is indicated. For combined treatment (C), the multivariate ANOVA showed a significant treatment × region interaction, and lower-order tests for each region appear above the panel; however, the treatment × region interaction was not significant after subdivision by sex, so only main treatment effects are shown within the panels for males and females. Panel (D) shows the simple main treatment effects for each sex, collapsed across all the other variables. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos; NS, not significant.
As before, we collapsed the main treatment effects across all the interactive variables to illustrate these points (Fig. 4D). For males, dexamethasone had a small, nonsignificant promotional effect on HC3 binding, whereas chlorpyrifos evoked a major decrement that was not substantially changed by coexposure to both agents. For females, however, neither treatment alone had a significant overall effect but combined treatment produced a highly significant reduction in HC3 binding (p < 0.01 vs. control) that was distinguishable from either dexamethasone (p < 0.04) or chlorpyrifos singly (p < 0.04), and clearly unique as compared to the two individual effects (dexamethasone × chlorpyrifos, p < 0.02).
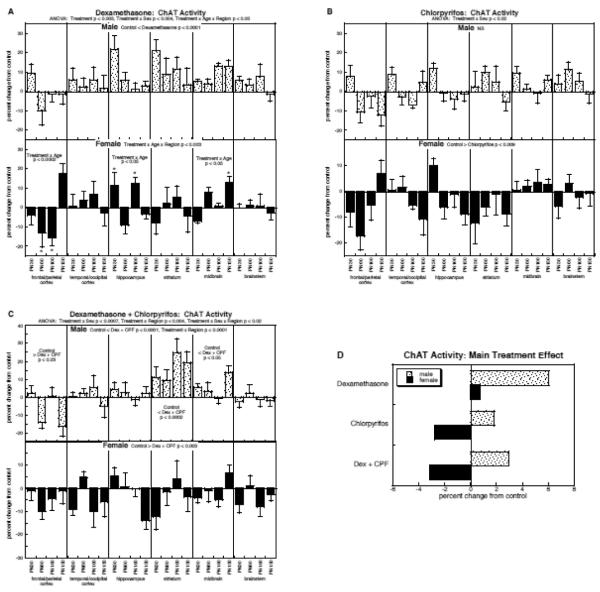
ChAT activity
For ChAT, the multivariate ANOVA confirmed a main treatment effect (p < 0.0003) that was interactive with all the other variables (p < 0.006 for treatment × sex, p < 0.02 for treatment × region, p < 0.05 for treatment × region × age). The treatment effects were again maintained when the data were subdivided for males (p < 0.0008 for treatment, p < 0.006 for treatment × region) and females (p < 0.003 for treatment).
By itself, dexamethasone elicited a net increase in ChAT in males (main treatment effect, p < 0.0001) but a mixed pattern in females, characterized by changes restricted to specific regions and ages (Fig. 5A). Chlorpyrifos evoked a slight overall increase in ChAT in males that was not statistically significant, but in contrast, females showed a net decrease in activity (main effect, p < 0.009; Fig. 5B). With the combined treatment, males again showed a net increase in activity, akin to that seen with dexamethasone alone, with the addition of regional differences that were not seen with the glucocorticoid treatment (Fig. 5C). For females, exposure to both dexamethasone and chlorpyrifos elicited a highly significant net reduction in ChAT (main effect, p < 0.003).
Figure 5.
Effects of dexamethasone (A), chlorpyrifos (B), and combined treatment (C) on ChAT activity (mean ± SE, n=6 for each sex in each treatment group), shown as the percent change from control values (see original values in Supplementary Table 5). Because the global ANOVA showed a significant treatment × sex interaction (p < 0.006), values were separated for males and females. Multivariate ANOVA for each treatment appears at the top of the panels. For dexamethasone (A), males showed only a main treatment effect, so no lower-order tests were performed; females showed an interaction of treatment × age × region, so lower order tests were conducted for each region, and then for each age point (asterisks) in regions showing a treatment × age interaction. For chlorpyrifos (B), males showed no significant treatment effect or interaction of treatment with other variables, so no lower-order tests were conducted; females showed only a main treatment effect, so again, no lower-order tests were run. For combined treatment (C), males showed both a main treatment effect and a treatment × region interaction, so lower-order tests were carried out for each region, but not for each age point (no interaction of treatment × age); females showed only a main treatment effect (no interactions, so no lower-order tests). Panel (D) shows the simple main treatment effects for each sex, collapsed across all the other variables. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos; NS, not significant.
Collapsing the treatment effects across age and region provide a striking illustration of the sex differences in treatment response (Fig. 5D). For males, the main effect of either treatment alone was an increase in ChAT activity, much more so for dexamethasone (significant increase) than for chlorpyrifos (nonsignificant increase; p < 0.0003 for chlorpyrifos vs. dexamethasone); with the combined treatment, there was still a significant increase but the magnitude of the effect was similar to that of chlorpyrifos alone, and distinctly smaller than that seen just with dexamethasone (p < 0.03). Accordingly, there was a significant interaction between the two treatments (dexamethasone × chlorpyrifos. p < 0.03), reflecting less-than-additive effects. For females, the interaction was entirely opposite. By itself, dexamethasone evoked a slight, nonsignificant increase whereas chlorpyrifos elicited a significant net decrease; the combined treatment actually showed a worsened decrease, more than would be expected from additive effects of the two individual treatments (dexamethasone × chlorpyrifos. p < 0.05).
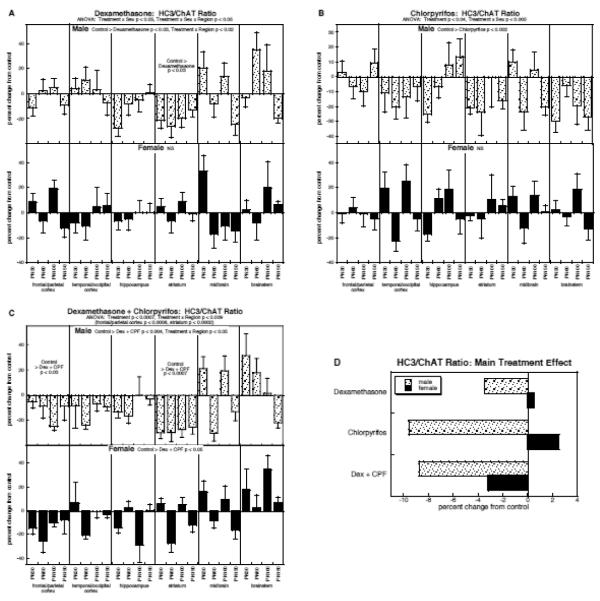
HC3/ChAT ratio
Multivariate ANOVA again confirmed significant treatment effects and interactions for the HC3/ChAT ratio: p < 0.006 for the main treatment effect, p < 0.03 for treatment × sex, p < 0.003 for treatment × region. Dexamethasone by itself produced a net decrease in the ratio in males, with the most consistent effect in the striatum (Fig. 6A); again, the inclusion of cell body regions with low HC3 binding values increased the variability of the global test but did not obscure the significant treatment effect. Females did not show a significant change in HC3/ChAT in response to prenatal dexamethasone. A similar pattern was seen for chlorpyrifos, namely a net reduction in males but no significant change in females, but the magnitude of the effect in males was significantly greater (p < 0.005) than with dexamethasone (Fig. 6B). With combined exposure, males showed decrements akin to those obtained with chlorpyrifos; however, but females showed a marked difference from the individual effects, with emergence of a significant overall decrement that was not seen with either treatment alone (Fig. 6C).
Figure 6.
Effects of dexamethasone (A), chlorpyrifos (B), and combined treatment (C) on the HC3/ChAT ratio (mean ± SE, n=6 for each sex in each treatment group), shown as the percent change from control values (see original values in Supplementary Table 6). Because the global ANOVA showed a significant treatment × sex interaction (p < 0.03), values were separated for males and females. Multivariate ANOVA for each treatment appears at the top of the panels. For dexamethasone (A), males showed both a main treatment effect and a treatment × region interaction, so lower-order tests were carried out for each region, but not for each age point (no interaction of treatment × age); females showed no significant effects, so lower order tests were not conducted. For chlorpyrifos (B), males showed only a main treatment effect and females showed significant effects or interactions, so no lower-order tests were conducted for either sex. For combined treatment (C), the multivariate ANOVA showed a significant treatment × region interaction, and lower-order tests for each region appear above the panel; males showed a significant main treatment effect as well as a treatment × region interaction, enabling lower-order tests for each region (but not for each age, since there was no treatment × age interaction), whereas females showed only a main treatment effect (no interactions, so no lower-order tests). Panel (D) shows the simple main treatment effects for each sex, collapsed across all the other variables. Abbreviations: Dex, dexamethasone; CPF, chlorpyrifos; NS, not significant.
These patterns were further clarified by examining the main treatment effects, collapsed across all regions and ages (Fig. 6D). For males, chlorpyrifos or the combined treatment produced the greatest deficits, substantially greater than for dexamethasone alone and not distinguishably different from additive actions (no dexamethasone × chlorpyrifos interaction). For females, the two individual treatments tended to elicit net increases (both nonsignificant), whereas the combined exposure elicited a significant overall decrease (p < 0.05); this unique effect statistically distinct from additive effects, which would have predicted an increase, not a decrease in the ratio (dexamethasone × chlorpyrifos, p < 0.05).
DISCUSSION
Results obtained in this study show that prenatal exposure to dexamethasone sensitizes the developing brain to subsequent injury by chlorpyrifos in a sex-dependent manner, enhancing the long-term deficits of ACh function primarily in females. Indeed, whereas females were ordinarily less affected than males by either dexamethasone or chlorpyrifos alone, their “protected” status was compromised by the combined exposure. This involved two types of interaction: dexamethasone and chlorpyrifos showed additive effects for nAChR binding, whereas for the other variables, the interactions were either synergistic or unique to the combination; in the latter instance, the combination displayed effects not seen with either agent alone, or even in a direction opposite to the individual effects. Taken together, these findings indicate that prior dexamethasone treatment could create a subpopulation that is especially vulnerable to the adverse effects of organophosphates or other developmental neurotoxicants.
Before exploring the interactions of dexamethasone and chlorpyrifos, it is important to note that sex-selective effects were prominent for the long-term effects on ACh function after exposure to either dexamethasone or chlorpyrifos alone. For nAChR binding, the effects were actually opposite in the two sexes, with large increments observed after dexamethasone or chlorpyrifos alone in males, but only small decrements in females. For HC3 binding, males showed an overall reduction evoked by chlorpyrifos but females did not; likewise, for ChAT, either dexamethasone or chlorpyrifos evoked increases in males, but in females there was no change (dexamethasone) or decreases (chlorpyrifos). The combination of effects on HC3 and ChAT produced clearly distinct patterns for the HC3/ChAT ratio, the index of nerve impulse activity per nerve terminal: dexamethasone or chlorpyrifos caused robust decreases in this index in males, but no effect (dexamethasone) or a slight increase (chlorpyrifos) in females. It is thus evident that males are affected by either agent to a much greater extent, with the net adverse effect reflecting a loss of presynaptic activity; indeed, the prominent upregulation of nAChRs in males could represent a compensatory increase in postsynaptic sensitivity in response to decreased presynaptic input. Although we do not yet know the mechanism underlying these stark sex differences for these particular agents, the outcome is entirely consistent with the general resistance of the female brain to neurotoxic injury. Estrogen itself is neuroprotective (Hilton et al., 2004; Suzuki et al., 2006), neuronal replacement is enhanced by activation of estrogen receptors (Tanapat et al., 1999), and estrogen reduces neuroinflammatory responses, promotes neurotrophic factor release and preserves mitochondrial function after neuronal injury (Arevalo et al., 2012; Arnold and Beyer, 2009).
It is therefore particularly notable that, with sequential exposure to dexamethasone followed by chlorpyrifos, it was only in females that we saw synergistic interactions, indicating that their protected status was compromised by glucocorticoid treatment. In the case of HC3 binding, females receiving the combined treatment showed deficits indistinguishable from those of males; for ChAT, the females had a large deficit, implying loss of ACh terminals, as compared to an increase in males; for the HC3/ChAT ratio, females given both dexamethasone and chlorpyrifos showed a decrease, the same direction of change as in males, whereas they showed increases for the separate treatments. We also found evidence for a loss of postsynaptic reactivity in females given the combined treatment, with additively greater reductions in nAChR binding; again, this was not seen in males. In fact, whereas males showed compensatory nAChR changes (upregulation in the face of decreased presynaptic input), the receptor decrement in females would augment presynaptic deficits. The conclusion is thus inescapable, that females bear the brunt of the major adverse effects of prenatal dexamethasone on subsequent postnatal neurotoxicity of chlorpyrifos, directed toward ACh systems. The enhanced effects cannot be attributed to systemic toxicity, since we did not find any increase in chlorpyrifos-induced cholinesterase inhibition in the group exposed to dexamethasone, nor did the combined treatment produce any greater postnatal growth impairment; indeed, chlorpyrifos appeared to reduce the growth impairment evoked by dexamethasone, evidenced by a significantly smaller weight reduction in the group receiving the combined treatment. Although the interaction for growth effects may seem surprising, it is important to note that eary-life organophosphate exposure can be obesogenic (Slotkin, 2010). Prolonged developmental exposures to chlorpyrifos or other organophosphates lead to elevated weight gain, particularly in males (Lassiter and Brimijoin, 2008; Lassiter et al., 2008; Meggs and Brewer, 2007); however, in our study, which utilized only a brief chlorpyrifos exposure, we did not note a sex-selective growth effect (no treatment × sex interaction for body weights).
Although males did not show synergistic effects after combined exposure to dexamethasone and chlorpyrifos, comparison to the individual agents was still informative. For nAChR binding, the double treatment had effects that were less-than-additive, distinctly smaller than would have been expected from the summed, individual effects of dexamethasone and chlorpyrifos. For HC3 binding, the combination showed simple addition of the two treatment effects, whereas for ChAT, the effect was less than that seen for dexamethasone alone. Consequently, the HC3/ChAT ratio, the index of presynaptic activity per nerve terminal, also showed smaller effects than anticipated from the individual treatments. This points to the idea that, for males, dexamethasone may actually be slightly protective against the adverse effects of chlorpyrifos on ACh function. Indeed, glucocorticoids act simultaneously as agents that can sensitize some types of neurotoxicity but that can also protect against other types, particularly those that involve neuroinflammation (Abraham et al., 2001; Chumas et al., 1993). In our in vitro studies that paralleled the current work, we found that, in dexamethasone-primed cells, chlorpyrifos actually enhanced neurodifferentiation into the ACh phenotype, whereas by itself, it suppressed this phenotype (Slotkin et al., 2012); additionally, at low concentration of chlorpyrifos, dexamethasone protected against cell loss (Slotkin et al., 2012). The current in vivo findings are consistent with this duality, with the important addition that the relative balance of injurious and protective effects of dexamethasone is sex-specific. Nevertheless, it is not clear that this relative protection is biologically important. Although some of the effects of the combined treatment were less than would have been expected from additive damage by the two agents, in no case was the actual effect of dexamethasone + chlorpyrifos significantly less than that of chlorpyrifos alone; the apparent protection could represent a “ceiling” effect, where the damage in males is already maximal and cannot be further increased above the level caused by one agent. We are currently conducting behavioral studies to determine if there is a functional correlate that would indicate a true protection by dexamethasone in males.
There are two additional issues that point to future work. First, although brain region weights were generally unaffected by any of the treatments throughout adolescence and early adulthood, we eventually saw the emergence of a statistically significant decrement by PN150; the effect was synergistically significant for the combined treatment (dexameathasone × chlorpyrifos interaction) and was not found with either agent alone, nor did it display interactions with region or sex. Global reductions in brain region weights and volumes are a hallmark of aging, and previously, we found that early-life exposure to organophosphates hastens aging-related impairment of cognitive and emotional function (Levin et al., 2010). The late-emerging deficits in brain region weights could thus indicate that prenatal dexamethasone treatment worsens the age-associated decline caused by subsequent organophosphate exposure; clearly, future work should examine this possibility. The second issue is the potential for glucocorticoids to sensitize the brain to some neurotoxicant effects while at the same time, to protect against other effects. This is important in two regards. First, prenatal glucocorticoid exposures entail not only therapeutic application in preterm labor, but also endogenous hormone release from maternal stress. Simultaneous stress clearly affects the neurotoxicity of organophosphates in adults (Basha and Poojary, 2011, 2012) but comparable studies have not been undertaken for developmental neurotoxicity. Nevertheless, we have indirect evidence for a role of stress in the developmental neurotoxicity of organophosphates from our earlier studies with prenatal chlorpyrifos exposure that examined dose-response relationships straddling the threshold for impaired maternal weight gain (Qiao et al., 2002). A dose that did not reduce maternal growth evoked clear-cut neurobehavioral deficits in the offspring, whereas the adverse effects were actually reduced at a slightly higher dose that did reduce weight gain (Levin et al., 2002). Originally, we attributed this “inverted-U” dose-response relationship to the inhibition of cholinesterase at the higher dose, given the positive neurotrophic effects of acetylcholine in the developing brain (Hohmann, 2003; Lauder and Schambra, 1999). However, the current study points to the additional possibility of neuroprotection from glucocorticoids released by maternal stress. If this is so, there are much larger implications to our current finding. Standard testing for developmental neurotoxicity requires that the dose range be extended past the point of maternal toxicity, at which stage stress responses are likely to be evoked. As shown here, this could produce a reduction in some aspects of neurotoxicity. Consequently, inverted-U dose-response relationships for developmental neurotoxicity may be much more common than suspected. Future work should address maternal stress and endocrine status during toxicant exposure as a contributor to neurobehavioral outcomes.
A critical issues in toxicology is to determine the factors that render specific subpopulations vulnerable to a given toxicant. Whereas many studies have focused on differences in genetic background that govern susceptibility, such as paraoxonase polymorphisms for organophosphate toxicity (Costa et al., 2003; Povey, 2010), the current results point to equally important contributions of chemical or clinical background. If our findings extend to the human population, we can expect that the offspring of women given glucocorticoids for preterm labor, or for whom there was significant maternal stress during pregnancy, will display enhanced sensitivity to organophosphates and potentially other developmental neurotoxicants commonly found in the environment; we further predict that these interactions will be more prominent in female offspring. Our choice of dexamethasone as used in preterm labor thus sets the stage for extension to studies in children who mothers received this treatment (approximately 10% of the population), information that should be readily available from their medical records; evaluations can be performed using existing databases of studies in children with documented prenatal organophosphate exposure (Bouchard et al., 2011; Engel et al., 2011; Rauh et al., 2006, 2011, 2012). The present study provides a proof-of-principle that these interactions are likely to influence the susceptibility to organophosphate-induced developmental neurotoxicity.
Supplementary Material
Highlights
Glucocorticoids (stress, preterm labor) and organophosphate coexposure is common
We gave dexamethasone to rats prenatally, followed by neonatal chlorpyrifos
Dexamethasone exacerbated chlorpyrifos-induced cholinergic defects in females
Glucocorticoids may create a subpopulation that is vulnerable to neurotoxicants
Acknowledgement
Research was supported by NIH ES010356.
Abbreviation
- ACh
acetylcholine
- ANOVA
analysis of variance
- ChAT
choline acetyltransferase
- GD
gestational day
- HC3
hemicholinium-3
- nAChR
nicotinic acetylcholine receptor
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA), Pardieck Law (Seymour, IN), Tummel & Casso (Edinburg, TX) and the Shanahan Law Group (Raleigh NC).
REFERENCES
- Abraham IM, Harkany T, Horvath KM, Luiten PGM. Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? J Neuroendocrinol. 2001;13:749–60. doi: 10.1046/j.1365-2826.2001.00705.x. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaça Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–90. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, Garcia-Segura LM. Selective oestrogen receptor modulators decrease the inflammatory response of glial cells. J Neuroendocrinol. 2012;24:183–90. doi: 10.1111/j.1365-2826.2011.02156.x. [DOI] [PubMed] [Google Scholar]
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Basha PM, Poojary A. Chlorpyrifos induced region specific vulnerability in rat CNS and modulation by age and cold stress: an interactive study. Neurochem Res. 2011;36:241–9. doi: 10.1007/s11064-010-0311-3. [DOI] [PubMed] [Google Scholar]
- Basha PM, Poojary A. Oxidative macromolecular alterations in the rat central nervous system in response to experimentally co-induced chlorpyrifos and cold stress: a comparative assessment in aging rats. Neurochem Res. 2012;37:335–48. doi: 10.1007/s11064-011-0617-9. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003185. doi:10.1289/ehp,1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–29. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol Sci. 2002;23:130–6. doi: 10.1016/S0165-6147(00)01979-9. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–98. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Cavalieri RL, Cohen WR. Antenatal steroid therapy: have we undervalued the risks? J Matern Fetal Neonat Med. 2006;19:265–9. doi: 10.1080/14767050600676075. [DOI] [PubMed] [Google Scholar]
- Chumas PD, Del Bigio MR, Drake JM, Tuor UI. A comparison of the protective effect of dexamethasone to other potential prophylactic agents in a neonatal rat model of cerebral hypoxia-ischemia. J Neurosurg. 1993;79:414–20. doi: 10.3171/jns.1993.79.3.0414. [DOI] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Furlong CE. Polymorphisms of paraoxonase (PON1) and their significance in clinical toxicology of organophosphates. J Toxicol Clin Toxicol. 2003;41:37–45. doi: 10.1081/clt-120018269. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson J, et al. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. New Eng J Med. 2007;357:1179–89. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev Brain Res. 2000;121:179–87. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dammann O, Matthews SG. Repeated antenatal glucocorticoid exposure and the developing brain. Pediatr Res. 2001;50:563–4. doi: 10.1203/00006450-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Dani JA, De Biasi M. Cellular mechanisms of nicotine addiction. Pharmacol Biochem Behav. 2001;70:439–46. doi: 10.1016/s0091-3057(01)00652-9. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–32. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Anders V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Emgard M, Paradisi M, Pirondi S, Fernandez M, Giardino L, Calza L. Prenatal glucocorticoid exposure affects learning and vulnerability of cholinergic neurons. Neurobiol Aging. 2007;28:112–21. doi: 10.1016/j.neurobiolaging.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003183. doi:10.1289/ehp,1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RAJ. Upregulation of surface α4 β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J Neurosci. 1999;19:4804–14. doi: 10.1523/JNEUROSCI.19-12-04804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. A subtype of nicotinic cholinergic receptor in rat brain is composed of α4 and β2 subunits and is upregulated by chronic nicotine treatment. Mol Pharmacol. 1992;41:31–7. [PubMed] [Google Scholar]
- Gilstrap LC, Christensen R, Clewell WH, D'Alton ME, Davidson EC, Escobedo MB, et al. Effect of corticosteroids for fetal maturation on perinatal outcomes. J Am Med Assoc. 1995;273:413–8. [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Happe HK, Murrin LC. High-affinity choline transport regulation by drug administration during postnatal development. J Neurochem. 1992;58:2053–9. doi: 10.1111/j.1471-4159.1992.tb10946.x. [DOI] [PubMed] [Google Scholar]
- Happe HK, Peters JL, Bergman DA, Murrin LC. Localization of nicotinic cholinergic receptors in rat brain: autoradiographic studies with [3H]cytisine. Neuroscience. 1994;62:929–44. doi: 10.1016/0306-4522(94)90484-7. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Dev Brain Res. 2004;150:191–8. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Nordenstrom A, Lindholm T, Lindblad F, Ritzen EM, Wedell A, et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J Clin Endocrinol Metab. 2007;92:542–8. doi: 10.1210/jc.2006-1340. [DOI] [PubMed] [Google Scholar]
- Hohmann CF. A morphogenetic role for acetylcholine in mouse cerebral neocortex. Neurosci Biobehav Rev. 2003;27:351–63. doi: 10.1016/s0149-7634(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Klemm N, Kuhar MJ. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979;32:1487–94. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Aldridge JE, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Disruption of rat forebrain development by glucocorticoids: critical perinatal periods for effects on neural cell acquisition and on cell signaling cascades mediating noradrenergic and cholinergic neurotransmitter/neurotrophic responses. Neuropsychopharmacology. 2005a;30:1841–55. doi: 10.1038/sj.npp.1300743. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Levin ED, Seidler FJ, Slotkin TA. Gestational dexamethasone treatment elicits sex-dependent alterations in locomotor activity, reward-based memory and hippocampal cholinergic function in adolescent and adult rats. Neuropsychopharmacology. 2005b;30:1617–23. doi: 10.1038/sj.npp.1300716. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets and sex selectivity. Neuropsychopharmacology. 2006;31:12–35. doi: 10.1038/sj.npp.1300783. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–25. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Graham DG, Thomas RD. Environmental neurotoxic illness: research for prevention. Environ Health Perspect. 1994;102(Suppl. 2):117–20. doi: 10.1289/ehp.94102117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassiter TL, Brimijoin S. Rats gain excess weight after developmental exposure to the organophosphorothionate pesticide, chlorpyrifos. Neurotoxicol Teratol. 2008;30:125–30. doi: 10.1016/j.ntt.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, et al. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008;116:1456–62. doi: 10.1289/ehp.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder JM, Schambra UB. Morphogenetic roles of acetylcholine. Environ Health Perspect. 1999;107(Suppl. 1):65–9. doi: 10.1289/ehp.99107s165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–41. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Timofeeva OA, Yang L, Ryde IT, Wrench N, Seidler FJ, et al. Early postnatal parathion exposure in rats causes sex-selective cognitive impairment and neurotransmitter defects which emerge in aging. Behav Brain Res. 2010;208:319–27. doi: 10.1016/j.bbr.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development, and life after birth. Endocr Res. 2002;28:709–18. doi: 10.1081/erc-120016991. [DOI] [PubMed] [Google Scholar]
- Meggs WJ, Brewer KL. Weight gain associated with chronic exposure to chlorpyrifos in rats. J Med Toxicol. 2007;3:89–93. doi: 10.1007/BF03160916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985;65:946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Boon WM, Wintour EM. Glucocorticoid programming of adult disease. Cell Tissue Res. 2005;322:81–8. doi: 10.1007/s00441-005-1096-6. [DOI] [PubMed] [Google Scholar]
- Needelman H, Evans M, Roberts H, Sweney M, Bodensteiner JB. Effects of postnatal dexamethasone exposure on the developmental outcome of premature infants. J Child Neurol. 2008;23:421–4. doi: 10.1177/0883073807309232. [DOI] [PubMed] [Google Scholar]
- Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol. 2001;28:957–61. doi: 10.1046/j.1440-1681.2001.03559.x. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids: fetal effect or maternal influence? J Endocrinol. 2001;170:653–60. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2011;90:719–27. doi: 10.1111/j.1600-0412.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- Povey AC. Gene-environmental interactions and organophosphate toxicity. Toxicology. 2010;278:294–304. doi: 10.1016/j.tox.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Aubert Y, Maier C, Pearce PC, Fuchs E. The developmental impact of prenatal stress, prenatal dexamethasone and postnatal social stress on physiology, behaviour and neuroanatomy of primate offspring: studies in rhesus macaque and common marmoset. Psychopharmacology. 2011;214:33–53. doi: 10.1007/s00213-010-1989-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Abreu-Villaça Y, Tate CA, Cousins MM, Slotkin TA. Chlorpyrifos exposure during neurulation: cholinergic synaptic dysfunction and cellular alterations in brain regions at adolescence and adulthood. Dev Brain Res. 2004;148:43–52. doi: 10.1016/j.devbrainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ Health Perspect. 2002;110:1097–103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–44. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–78. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. 7-Year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003160. doi:10.1289/ehp. 1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, et al. Brain anomalies in children exposed to a common organophosphate pesticide. Proc Natl Acad Sci. 2012;109:7871–6. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokyta R, Yamamotova A, Slamberova R, Franek M, Vaculin S, Hruba L, et al. Prenatal and perinatal factors influencing nociception, addiction and behavior during ontogenetic development. Physiol Res. 2008;57(Suppl. 3):S79–S88. doi: 10.33549/physiolres.931602. [DOI] [PubMed] [Google Scholar]
- Simon JR, Atweh S, Kuhar MJ. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976;26:909–22. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? Reprod Toxicol. 2010;31:297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Barnes GA, McCook EC, Seidler FJ. Programming of brainstem serotonin transporter development by prenatal glucocorticoids. Dev Brain Res. 1996;93:155–61. doi: 10.1016/0165-3806(96)00027-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect. 2008;116:340–8. doi: 10.1289/ehp.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Chlorpyrifos developmental neurotoxicity: interaction with glucocorticoids in PC12 cells. Neurotoxicol Teratol. 2012;34:505–12. doi: 10.1016/j.ntt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Kreider ML, Tate CA, Seidler FJ. Critical prenatal and postnatal periods for persistent effects of dexamethasone on serotonergic and dopaminergic systems. Neuropsychopharmacology. 2006;31:904–11. doi: 10.1038/sj.npp.1300892. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at six months of age. Neuropsychopharmacology. 2007;32:1082–97. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Nemeroff CB, Bissette G, Seidler FJ. Overexpression of the high affinity choline transporter in cortical regions affected by Alzheimer's Disease: evidence from rapid autopsy studies. J Clin Invest. 1994;94:696–702. doi: 10.1172/JCI117387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–74. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–15. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Szpir M. New thinking on neurodevelopment. Environ Health Perspect. 2006a;114:A101–A7. doi: 10.1289/ehp.114-a100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpir M. Tracing the origins of autism: a spectrum of new studies. Environ Health Perspect. 2006b;114:A412–A7. doi: 10.1289/ehp.114-a412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegethoff M, Pryce CR, Meinlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocrine Rev. 2009;30:753–89. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Purification and characterization of a nicotinic acetylcholine receptor from rat brain. Proc Natl Acad Sci. 1987;84:595–9. doi: 10.1073/pnas.84.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PR, Lindstrom J. Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci. 1988;8:3395–404. doi: 10.1523/JNEUROSCI.08-09-03395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.