Abstract
In healthy forests, vertebrate frugivores move seeds from intact to degraded forests, aiding in the passive regeneration of degraded forests. Yet vertebrate frugivores are declining around the world, and little is known about the impact of this loss on regeneration of degraded areas. Here, we use a unique natural experiment to assess how complete vertebrate frugivore loss affects native seed rain in degraded forest. All native vertebrate frugivores (which were primarily avian frugivores) have been functionally extirpated from the island of Guam by the invasive brown tree snake (Boiga irregularis), whereas the nearby island of Saipan has a relatively intact vertebrate frugivore community. We captured seed rain along transects extending from intact into degraded forest and compared the species richness, density and condition of the seed rain from native bird-dispersed tree species between the two islands. Considering seeds from native bird-dispersed species, approximately 1.66 seeds landed per 26 days in each square meter of degraded forest on Saipan, whereas zero seeds landed per 26 days per square meter in degraded forest on Guam. Additionally, on Saipan, 69% of native bird-dispersed seeds in intact forest and 77% of seeds in degraded forest lacked fleshy fruit pulp, suggesting ingestion by birds, compared to 0% of all seeds on Guam. Our results show an absence of seed rain in degraded forests on Guam, correlated with the absence of birds, whereas on Saipan, frugivorous birds regularly disperse seeds into degraded forests, providing a mechanism for re-colonization by native plants. These results suggest that loss of frugivores will slow regeneration of degraded forests on Guam.
Introduction
Between one-third and one-half of Earth's land surface has been heavily influenced by humans [1], and 60% of tropical forestland (approximately 850 million hectares) is classified as secondary or degraded [2]. Degraded forests tend to harbour lower biological diversity, sequester less carbon, and differ in function and productivity when compared with nearby undisturbed forests [3]–[5]; however, specific restoration activities by humans in degraded forests can reverse these impacts and result in increased species diversity, ecosystem functioning, and carbon sequestration [5]–[7]. Some of this human-driven tropical forest restoration is achieved through active planting of native tree seedlings [8], but the majority of tropical forest regeneration takes place in a passive way, occurring via natural forest succession [9]. Passive regeneration is less resource and labor intensive than active restoration, but its success depends on a variety of factors, such as forest type, land-use history, and distance to nearest intact forest [10]. Thus, a key challenge for ecologists is to determine (1) the conditions under which passive regeneration can occur, and (2) which conservation measures most effectively assist passive regeneration [10].
Vertebrate frugivores assist in passive regeneration by transporting seeds to degraded areas from nearby intact forest [11]. Since approximately 90% of tropical forest tree species have fleshy fruits adapted for vertebrate dispersal [12], and vertebrate dispersers around the globe are under threat from overhunting, habitat fragmentation and invasive species [13], there is a critical need to understand the impact of frugivore loss on both the passive regeneration and active restoration of degraded forest.
Frugivores play an important role in the regeneration of degraded forests. Primates [14], [15], lizards [16], [17], bats [18], and birds [18]–[21] have all been shown to move seeds from primary to degraded habitat. Many frugivores travel long distances with seeds [22] and do not show an aversion to travelling through degraded forest areas [11], [23]; some bird species even show a preference for feeding, perching and roosting, and therefore defecating seeds, near gaps or edges [24]–[26]. In particular, vertebrate frugivores are important for moving the seeds of woody pioneer species and deep-forest, large-seeded species into degraded landscapes [27]–[31]. Several studies in regions with healthy frugivore communities have directly monitored seed rain and seedling recruitment in degraded areas adjacent to intact forests and have shown that vertebrate frugivores do move seeds, sometimes from far away, into degraded areas, often acting as the major (or only) source of native seed rain (e.g. [18], [19], [32]–[35]). The importance of vertebrate seed dispersal has been recognized by the implementation of restoration techniques which encourage dispersal, such as the planting of fruiting trees and ‘tree islands’ within degraded areas [36]–[38].
Despite the evidence that vertebrates play a role in regeneration of secondary forests, conservation or restoration of vertebrate frugivores is frequently ignored as a strategy for restoration of degraded lands (e.g. [39], [40]). The worldwide decline of vertebrate frugivore populations [41]–[43] may have negative impacts on forest regeneration [44]–[48], yet the magnitude of the effect of frugivore loss on seed dispersal to degraded forest is unknown. Here, we use a unique natural experiment to examine the impact of complete loss of vertebrate frugivores on seed dispersal from intact to degraded forests. Such natural experiments enable scientists to answer questions that cannot be addressed with a manipulative experiment for logistical or ethical reasons [49]; for example, removing all frugivores from large spatial areas is not feasible, thus a natural experiment provides a unique opportunity for understanding the magnitude of the seed dispersal provided by frugivores into degraded forest areas. While it is unlikely that forests around the world will lose all frugivores, examining the impact of such a dramatic change provides a critical view of the worst-case scenario.
The brown tree snake (Boiga irregularis) was accidentally introduced to the island of Guam in the late 1940s, causing a widespread loss of Guam's native forest bird species by the mid to late 1980s [50], [51]. Prior to the introduction of the brown tree snake, Guam's native forest avifauna consisted of 12 species, of which six were frugivorous. Predation by the brown tree snake led to the extirpation of five frugivorous species and the functional extirpation of the sixth [51]. Unlike many other places around the world that have lost native frugivores, Guam's forests have not been colonized by introduced avian frugivores that could play a similar ecological role to the extirpated species. The nearby island of Saipan provides a strong comparison to Guam, as it has similar forests, but in contrast to the silent forests of Guam, Saipan has no known snake population and healthy bird populations [52].
We investigated whether the species richness and density of bird-dispersed seeds varies between degraded forests on Saipan (with birds) and Guam (no birds). We also asked whether the proportion of seeds lacking fleshy fruit pulp (primarily due to handling by birds) differs between forests with birds (Saipan) and those without (Guam). Ours is the first study of which we are aware that utilizes a comparison between similar forests with and without avian frugivores to investigate the regeneration potential of degraded forests.
Materials and Methods
Ethics Statement
All field studies described here were conducted with the use of the necessary field permits. We obtained permission for the use of our study sites from the Government of Guam Forestry Division (for study sites on Guam), and from the Commonwealth of the Northern Mariana Islands Division of Fish and Wildlife (for study sites on Saipan). The field studies did not involve any protected or endangered species.
Site Description
This study was conducted on the Micronesian Islands of Guam (13°27′N, 144°46′E) and Saipan (15°11′N, 145°44′E). Both islands are at the southern end of the Mariana Island chain (Figure 1), and have climates with an average annual temperature around 27°C. The tropical climate of Guam averages 2586 mm of rainfall per year [53]. Saipan receives 1900–2300 mm of precipitation per year [54].
Figure 1. Map of the Mariana Islands.
The locations of Guam and Saipan, the two islands used in this study, are shown.
The primary forest type on Guam and Saipan is karst forest, which grows on a rugged karst limestone substrate; there are approximately 40 tree and shrub species in this forest type, but 8–12 species dominate. Large swaths of karst forest were destroyed on both Guam and Saipan in the 1930s and 40s as a result of World War II, and clearing has continued for development since then. When land is cleared, the rugged karst substrate is lost, and replaced by a topographically homogenous, red dirt substrate, which is typically colonized by the non-native leguminous tree, Leucaena leucocephala (Family: Fabaceae). Today, both islands have large areas of degraded forest composed of nearly monotypic stands of L. leucocephala with a sparse understory [55], [56]. Only 13% of Guam's remaining land area is intact karst forest, while 25% is degraded forest [55]. On Saipan, a smaller island with a proportionally larger impact of WWII, less than one percent of the total land area is intact karst forest, with 61% covered by degraded forest [56].
In this study, we define degraded forests as forests dominated by L. leucocephala. Degraded forest has a low (4–8 m in height) canopy that allows significant light to penetrate to the forest floor, whereas the intact karst forest is taller (7–13 m in height), creating a multi-storied, low-light environment. Narrow bands of transitional forest, characterized by a mixture of L. leucocephala and native forest trees, form the ecotone between degraded and intact forest.
Seed dispersers
Birds and bats are the primary seed dispersers in the Mariana Islands. Prior to the introduction of the brown tree snake, six frugivorous birds dispersed seeds on Guam: the White-throated Ground-dove (Gallicolumba xanthonura), Mariana Fruit-dove (Ptilinopus roseicapilla), Mariana Crow (Corvus kubaryi), Guam Rail (Gallirallus owstoni), Bridled White-eye (Zosterops conspicillatus) and Micronesian Starling (Aplonis opaca) [51]. Only one frugivorous bird species, the Micronesian Starling, remains on the island of Guam, with a localized population likely numbering less than 500 individuals (J. Quitagua, L. Obra, D. Vice, pers.comm.). We have not seen Micronesian Starlings at any of the sites used for this study on Guam. Four of the six species formerly found on Guam are also native to Saipan (all except Mariana Crow and Guam Rail). In addition, the partially frugivorous Golden White-eye (Cleptornis marchei) is present on Saipan. Historically, the native Marianas Fruit Bat (Pteropus mariannus) was an important frugivore in forests on Guam and Saipan; however, populations of bats on both islands are functionally absent due to hunting and, on Guam, predation by the brown tree snake [57].
No non-native species have taken over the entire functional role of the native seed dispersers on Guam, although a small number of tree species may be dispersed by invasive mammals. One non-native avian frugivore, the Philippine Turtle-dove (Streptopelia bitorquata), is found on both Guam and Saipan, typically along roadsides; it is rarely seen in intact karst forest on Guam, and thus is unlikely to be playing a significant role in seed dispersal. Introduced rats, primarily Rattus diardii (sensu [58]), are present in forests on both Guam and Saipan [59], and introduced pigs (Sus scrofa) and Philippine deer (Rusa marianna) are present on Guam. While all three species consume fruit and seeds, their role in seed dispersal is unclear. In other locations, invasive rat species such as Rattus diardii and Rattus rattus are primarily considered seed predators [60], [61] and rat eradication projects have resulted in increased seedling recruitment [62]. However, invasive rats occasionally disperse seeds [63] and may be effective dispersers for very small seeds [64]; their role in the Marianas is unstudied. Germination experiments from scat samples of deer and pigs on Guam show that deer are not effective dispersers, while pigs disperse seeds from at least three tree species, Morinda citrifolia (native), Ficus prolixa (native), and Carica papaya (naturalized non-native), as well as several non-native herbaceous weed species (A. Gawel, unpub. data). In this study, we capture seed rain exclusively from flying frugivores in order to quantify the effect of native frugivore loss on seed rain into degraded forests, and thus, we do not measure dispersal by feral pigs or rats.
Site selection
We selected three sites on each island where degraded forest bordered intact karst forest. Transitional zones between the intact and degraded forests were no more than 10 m wide at any site. On Guam, the three sites were located on the northern half of the island, where intact karst forest is prevalent along cliff lines. On Saipan, we selected a northern, a central and a southern site, also where intact forest remains along cliff lines. Sites were separated from each other by a minimum of 5 km.
Bird-dispersed tree species
Our study focused on seeds from native, bird-dispersed tree species. We designated a tree species as ‘bird-dispersed’ if its seed is covered by fleshy fruit pulp, birds have been observed consuming either the flesh or the entire fruit, and each seed is small enough to be consumed or carried by the largest frugivorous bird species that once occurred on the island. Additionally, we have found seeds from two common species, Premna obtusifolia and Psychotria mariana, in bird scat, providing additional support for their status as ‘bird-dispersed’. Table 1 includes a summary of the bird species seen consuming fruit from each tree species. All recorded observations of avian frugivory in the Marianas come from observations of birds rather than trees, thus we do not have any quantitative estimate of the proportion of fruit crops consumed by birds.
Table 1. Presence and dispersers of bird-dispersed tree species and their seeds on Guam and Saipan.
| Family | Species | Bird species seen consuming fruit1 | Guam | Saipan | ||||
| Site 1 | Site 2 | Site 3 | Site 1 | Site 2 | Site 3 | |||
| Euphorbiaceae | Melanolepis multiglandulosa | BRWE, WTGD, GOWE, MFD, MIST | Traps | Both | * | Both | ||
| Verbenaceae | Premna obtusifolia | WTGD, BRWE, GOWE, MFD, MACR, MIST | Both | Both, Fruit | Both, Fruit | Both | Traps | Both |
| Rubiaceae | Psychotria mariana | GOWE2 | Traps | Traps | Both | Traps | Traps | |
Disperser identity is based on bird observations reported in Craig [95] and Jenkins [96]. No tree species were present in intact karst forest survey transects but not in seed trap contents. A tree species that was present in both intact karst forest survey and in seed trap contents is indicated below by ‘Both’. ‘Traps’ indicates a tree species was present in seed trap contents, but was not present in intact karst forest surveys. ‘Fruit’ indicates that the species was seen fruiting during the forest surveys; if a species was found in seed traps (‘Both’ or ‘Traps’), we also assume that it fruited during the study.
MFD = Mariana Fruit-dove, WTGD = White-throated Ground-dove, BRWE = Bridled White-eye, GOWE = Golden White-eye, MACR = Mariana Crow, MIST = Micronesian Starling. We lack information on fruit in the diet of the Guam Rail.
Other bird species likely disperse Psychotria mariana, but systematic observations of fruiting trees have not been conducted.
Seed traps
At each site, we set up seed traps in intact karst forest and along three parallel transects from the intact forest/degraded forest boundary into degraded forest. Circular seed traps (0.5 m2) were constructed using polyvinyl hoops with screen door netting added to make a basket, and suspended from trees at a height of 1.3 m. By hanging the traps, we measured only dispersal by volant frugivores, and not by terrestrial frugivores. Beginning with a trap in the transitional forest, three parallel transects were established a minimum of 10 m apart from one another, and a trap was placed every 10 m along each transect into degraded forest out to a distance of 100 m. To sample the intact forest seed rain, four additional seed traps were set up in the adjacent intact karst forest 10 m from the start of each transect. These four traps were arranged in a 5-m by 5-m square, with a trap at each corner; we placed traps in this arrangement because a small number of transects contained a small cliff or other boundary within the native forest which prevented us from creating 40-meter straight line seed trap arrays. The square array of seed traps at the base of each transect ensured all native forest areas were sampled using the same method. Since the forest canopy is low, seed traps spaced 5 meters apart are unlikely to be underneath the same tree canopy and thus can be considered independent samples. We considered seed traps to be ‘intact forest traps’ if they were within intact forest or in the transitional zone because native seeds could fall into any of these traps without the help of birds. Traps were designated as ‘degraded forest traps’ if they were located in degraded forest (i.e. traps at 10–100 m from the intact/degraded forest boundary).
After 26 days, seed trap contents were collected and dried in a drying oven to aid in leaf litter removal. All seeds from bird-dispersed tree species were counted and divided into “whole fruit” and “de-pulped” categories based on the presence or absence of a fleshy fruit covering.
Forest comparisons
Since this was a large-scale comparative study between two islands, and our main comparison is between degraded forests on Guam and Saipan, we could not control for island-specific differences in species richness of the forests [65], [66]. However, we took several steps to ensure that differences in degraded forest seed rain truly reflected differences in movement from intact to degraded forest, not differences in intact karst forest or degraded forest diversity. As described above, we sampled seed rain in intact karst forest in addition to degraded forest because intact forest is the most likely source of seeds in degraded forest traps. We qualitatively compared intact karst forest composition between Guam and Saipan using three parallel straight-line 25-m transects, separated by more than 5 m, in intact karst forest at each site. On each transect, we recorded the presence or absence of bird-dispersed tree species (Table 1, Table S1). The degraded forest at all sites on both islands was dominated by L. leucocephala, however a few sites contained remnant trees of native bird-dispersed species as well. We recorded the location of remnant trees in degraded forest and took them into account in our analyses, as our objective was to assess movement of seeds from intact karst forest, not dispersal within degraded forest.
Analyses
We investigated whether forest type (intact vs. degraded) and island (Guam vs. Saipan) affected the species richness and density of native bird-dispersed seeds, and the proportion of native de-pulped seeds. We only used data on native bird-dispersed tree species in our analyses, to evaluate the role of birds in the regeneration of native tree species in degraded forest. Thus, we excluded data from two non-native, bird-dispersed naturalized species, Carica papaya and Triphasia trifolia, and one non-native, wind-dispersed invasive species, L. leucocephala, despite the presence of seeds in the traps. We did not use distance from the intact forest edge as a continuous explanatory variable for the analyses because preliminary analyses indicated that response variables were not a simple linear (or log-linear) function of distance (Figure S1). The sampling unit for the analyses was the individual trap, blocked by site and forest type.
To account for the few remnant native trees in degraded forest, we excluded seeds from traps in degraded forest within 10 m of a fruiting remnant native tree. The only native species we found fruiting in the degraded forest was Premna obtusifolia, and only eight of the 180 degraded forest traps (4%) were located within 10 m of these fruiting adults. Five of these eight traps were located on Guam, where their contribution would have increased the density and richness of native bird-dispersed seeds in degraded forest, making their omission a conservative approach. Results were qualitatively similar even when including these traps.
To determine whether the species richness and density of native bird-dispersed seeds found in degraded forest seed traps was greater on Saipan than Guam, we used generalized linear mixed effects models with a Poisson error distribution [67]. We used the number of species per trap as the response, and island, forest type, and an island by forest type interaction as fixed effects and site as a random effect. We also tried a binomial error distribution for the species richness analysis, with the proportion of native bird-dispersed species observed in each trap out of the total number of bird-dispersed species found in all traps as the response. This analysis yielded qualitatively similar results; however, we chose not to use this model structure because we do not with certainty know the actual number of native bird-dispersed species present in our sites. Though we performed transect surveys of our study sites, there were bird-dispersed species in our study areas that were not fruiting at the time of the experiment, and thus did not appear in our seed traps (Table S1).
For each analysis, we fit five models; (1) null (intercept only); (2) island effects only; (3) forest type effects only; (4) island and forest type effects; and (5) island, forest type, and their interaction. We identified the best fitting model with Akaike's Information Criterion (AIC) values. We chose the simplest model (i.e. fewest explanatory variables) within two AIC units of the best-fitting model [68]. If birds play an important role in dispersing native tree seeds to degraded forests, the full model with an interaction between island and forest type will best fit observed data, with the interaction coefficient showing that the difference in the species richness or density of native seeds in degraded versus intact karst forest is smaller on Saipan than on Guam.
We conducted additional analyses to test whether seed species richness or density is significantly greater in degraded forests on Saipan than on Guam, as hypothesized. We fit a simpler model using degraded forest data only, and tested for island effects using a similar generalized linear model structure. We then selected the best fitting model using AIC values, as described above. The best-fit model for seed density showed overdispersion, which we corrected for by adding an observation-level random effect [69].
We used two separate analyses to determine whether the proportion of bird-dispersed seeds with the pulp removed (likely by birds) differed between the two islands, and between intact and degraded forest on Saipan. We used generalized mixed effects models for both analyses, specifying a binomial error distribution and site as a random effect. We used AIC values to identify best fitting models. We expected to find a higher proportion of seeds with pulp removed on Saipan than on Guam (i.e. best-fitting models include island), and a higher proportion of seeds with pulp removed in degraded than in intact karst forest on Saipan.
All statistical analyses were performed using R version 2.13.0 [70] with the lme4 package (published March 8, 2011).
Results
The species composition of the intact forests on Guam and Saipan were qualitatively similar as shown by transect surveys (Table 1). Similarly, seed rain in intact forest on both islands contained seeds from three native, bird-dispersed tree species: Melanolepis multiglandulosa, Premna obtusifolia, and Psychotria mariana (Table 1). In addition, nearly all traps on both islands contained seeds from the non-native species Leucaena leucocephala, although the seed rain in degraded forest differed between islands (summarized in Table 2). All seeds from native, bird-dispersed tree species found in traps in degraded forest on Saipan were from three species: Melanolepis multiglandulosa, Premna obtusifolia, and Psychotria mariana.
Table 2. Summary of seed trap contents on each island, and in each forest type.
| Forest type | Guam | Saipan | |
| # (%) of traps with seeds from native bird-dispersed tree species | Intact | 17 (37.8%) | 32 (71.1%) |
| Degraded | 51 (5.6%) | 412 (45.6%) | |
| # (%) of traps with Leucaena | Intact | 26 (57.8%) | 22 (48.9%) |
| Degraded | 88 (97.8%) | 83 (92.2%) |
In total, we had 45 traps on each island in intact forest, and 90 traps per island in degraded forest.
All five of these traps were under either native or naturalized remnant fruiting trees and contained whole fruits covered with fleshy fruit pulp.
Three of these traps were under native or naturalized remnant fruiting trees.
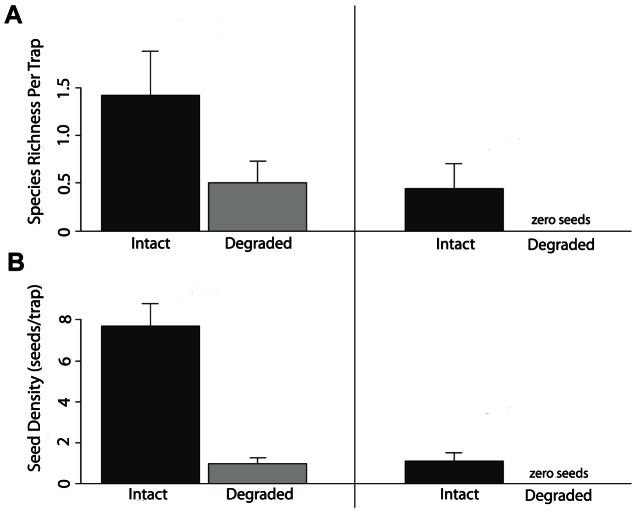
After excluding seeds captured in traps located underneath remnant native trees in degraded forest on each island, significantly more native, bird-dispersed species were captured per square meter of degraded forest on Saipan than on Guam, where none were caught. Based on coefficients from the best-fit statistical model, which incorporates the random effects of transects within sites, 1.66 seeds of native bird-dispersed tree species fall every 26 days in each square meter of degraded forest on Saipan. The species richness and density of seeds from native bird-dispersed tree species are best explained by models including island, forest type and their interaction (Table 3). Native seed species richness was greater on Saipan than on Guam, and greater in intact than in degraded forests (Figure 2A). The density of seeds from native bird-dispersed trees was higher on Saipan than on Guam and lower in degraded forests than intact karst forests (Figure 2B).
Table 3. Comparison of statistical models containing different combinations of Island and Forest type as explanatory variables for the richness of (column 1) and density (column 2) of seeds that come from native bird-dispersed species.
| Model | Native richness | Seed density | ||
| AIC Values | Δ AIC | AIC Values | Δ AIC | |
| Full model | 176.47 | 865.46 | ||
| Island, Forest type | 192.69 | 16.22 | 872.31 | 6.85 |
| Forest type | 207.05 | 30.58 | 883.03 | 17.57 |
| Island | 243.56 | 67.09 | 1349.03 | 483.57 |
| Null (intercept) | 257.92 | 81.45 | 1359.75 | 494.29 |
AIC values of best fitting models are in bold.
Figure 2. Mean (A) species richness and (B) density of native bird-dispersed seeds on Guam and Saipan.
Dark grey bars indicate intact karst forest, while light grey bars indicate degraded forest. Error bars represent standard error. Means were calculated using raw data on native bird-dispersed seeds per trap (0.5 m2); in degraded forests, we used only seeds found in traps with no remnant native trees nearby.
The proportion of seeds from bird-dispersed tree species that were de-pulped was lower on Guam than on Saipan. On Guam, zero seeds were de-pulped; on Saipan, 68.5% of seeds in intact forest, and 76.3% of seeds in degraded forest, were de-pulped. Because there were no native bird-dispersed seeds away from remnant trees and no de-pulped seeds in degraded forest traps on Guam, we were unable to fit a full model with island, forest type, and their interaction to these data. Instead, we compared the proportion of seeds that were de-pulped out of all seeds found on each island. The model containing island as a predictor fit better than the model without island, indicating that proportion of seeds with pulp removed differs greatly between Guam and Saipan (delta AIC = 14.47). The proportion of bird-dispersed seeds with pulp removed was slightly higher in degraded forest than intact karst forest on Saipan (0.77 vs. 0.69), but the AIC value for the model including forest type as an explanatory variable was within 1.5 AIC units of the null model (including only an intercept), indicating that forest type does not significantly improve model fit.
Discussion
Avian frugivores act as critical links between intact karst forest and degraded forest in the Mariana Islands. We found increased species richness and density in the seed rain landing in degraded forest on Saipan compared with Guam, likely due to the absence of frugivorous birds in the forests on Guam. Zero seeds/m2 from bird-dispersed tree species were dispersed into degraded forests on Guam, indicating a loss of seed dispersal services on this island. In contrast, approximately 1.66 native bird-dispersed seeds/m2 are dispersed into degraded forest on Saipan per 26 days. While this is a small number of seeds, these seeds provide the potential for regeneration of native trees within the degraded forest. There are few wind-dispersed tree species in the Marianas, and Mariana Fruit Bats are functionally extinct on Guam and Saipan, leaving birds and gravity as the primary modes of dispersal. This is the second study to document the loss of an ecosystem function once provided by birds on Guam; the previous demonstration focused on birds as pollinators [71].
Vertebrate dispersers play a key role in catalyzing regeneration of native forest. In systems with intact frugivore communities, vertebrate dispersal from native to degraded forest is evident from the abundance of seeds and seedlings from fleshy-fruited species found in degraded forest [20], [29], [33], [72]. A study of forests on the island of Saipan suggests that vertebrate dispersal is important for bringing native seeds to degraded forest because most of the native seeds and seedlings sampled in degraded forest had small, fleshy fruits typically dispersed by birds [73]. In our study, the native species dispersed into seed traps in degraded forest on Saipan were, in descending order of abundance, Premna obtusifolia, Psychotria mariana, and Melanolepis multiglandulosa. These species produce large fruit crops, and birds have been observed eating all three species. M. multiglandulosa is a pioneer species in this system, as it is only found in gaps and edges of forests.
Initial dispersal events from intact to degraded forests are important because they can initiate the regeneration of degraded areas. Once a native fleshy-fruited tree becomes established in degraded forest, it provides habitat, food, and perching and roosting sites for frugivores, which in turn will bring more seeds from the nearby intact forest [28], [36], [74], [75]. Without birds bringing in early pioneer species, we predict that regeneration of native species in degraded forest will progress more slowly on Guam than on Saipan.
While the focus of this study is on seed dispersal into degraded forests, we also found significant differences in seed rain in intact karst forests (Figure 2). Seed traps placed in intact forest on Guam collected a lower richness and density of seeds from native bird-dispersed species than on Saipan. Using transect surveys (Table 1), we showed that the forests on Guam and Saipan were qualitatively similar in species composition; thus, we believe that these differences in abundance and diversity reflect the lack of dispersal by birds in Guam's forests, rather than differences in species composition or inadequate sampling. Frugivorous birds often consume seeds from one tree, then perch on another and defecate, leading to a higher richness of seeds within a single seed trap. On Guam, the only seeds found in forest traps likely originated from tree species with canopies overhanging the traps since no volant dispersal agents are present; this potentially explains the lower richness and density of seeds in traps placed in Guam's intact karst forests compared to traps on Saipan (Figure 2).
In addition to moving seeds into degraded forests, birds may influence the likelihood that seeds germinate. When birds ingest fruit, they remove the fleshy fruit pulp and scarify the seed, processes that enhance germination in some species, but do not affect or even reduce seed germination in others [76]–[81]. A recent study of 15 New Zealand tree species found a statistically significant but biologically unimportant effect of birds on the proportion of seeds that germinated, suggesting that germination of most tree species would be unaffected by the loss of ingestion by birds [82]. In our study, none of the native bird-dispersed seeds found on Guam had their fleshy fruit removed, as was expected since birds are absent. On Saipan, however, nearly two-thirds of all bird-dispersed native seeds found in traps lacked fruit pulp. In a separate study, germination experiments in an outdoor nursery for two of the common bird-dispersed species, Premna obtusifolia and Psychotria mariana showed increased germination of handled seeds over whole fruit (Rogers, unpub.). Thus, the loss of birds on Guam may affect germination due to the loss of seed handling for at least these two species, regardless of whether the seeds land in degraded or intact karst forest. However, this positive effect of bird handling may not be a general phenomenon in Mariana plants, and thus requires additional study. Un-dispersed seeds may also experience lower levels of germination due to increased natural enemy attack near the parent trees (i.e. Janzen-Connell effects) [83].
There are at least two aspects of the present study that limit our capacity to predict the full impact of birds on degraded forest regeneration. First, we did not assess rates of germination from the seed bank in degraded forest. It is possible that native species were at one point deposited in the seed bank by birds, but are waiting for the right conditions to germinate [84]. However, it is unlikely that native seeds would still be viable if they fell prior to land clearing (10–80 years ago) or were brought to the degraded forests when bird dispersers were present on the island (>25 years ago), since many tropical seeds are viable for short periods of time [27], [85]. Second, we did not assess rates of germination and survival of seeds and seedlings in degraded forest, so it is possible that bird dispersal of native seeds into degraded forest does not lead to regeneration of those species. There are several lines of indirect evidence that suggest that at least some native seeds, once dispersed into degraded forests, can germinate and survive. On Saipan, native seedlings have been observed growing in the understory of many L. leucocephala-dominated forests [73], [86]. In addition, we have observed forests on Saipan that have a soil substrate similar to that indicative of cleared forest, but that support a forest cover similar to intact forest with only a few large adult L. leucocephala trees. These areas were likely bulldozed many years ago changing the substrate from karst to soil, then were initially colonized by L. leucocephala, which has since been gradually replaced by native forest species, resulting in a forest with a similar composition and diversity to intact karst forest. Finally, L. leucocephala does not appear to invade intact karst forest successfully, perhaps because it does not compete well with native trees in undisturbed forest [86]. If L. leucocephala is indeed a poor competitor with native trees, then karst forest regeneration in areas currently dominated by L. leucocephala may be successful without labor-intensive management of L. leucocephala once native seedlings get established.
Bird populations worldwide are in decline due to habitat loss, climate change, introduced disease, and invasive species [41], [47]. Nearly one-quarter of all frugivorous birds are prone to extinction [42], and the decline will impact the ecosystem services they provide [22], [47], [87]–[89]. Birds provide an important ecosystem service when they move seeds from native to degraded forest. In the Marianas, the intact karst forest harbours greater tree species diversity than degraded forest, including many species of cultural importance (e.g. Intsia bijuga, Premna obtusifolia, Eugenia spp.), and provides habitat for wildlife (e.g. coconut crabs, Mariana Fruit Bats, Micronesian Starlings). In addition, intact forests are highly productive, and often store more carbon than degraded forests due to the abundance of large, old-growth trees as opposed to small, successional trees found in degraded forests, as well as a higher canopy with understory, mid-story and canopy tree levels [6], [7], [90]; we suspect this is also the case in the Marianas. Thus, if seed dispersal by birds does speed up regeneration of degraded forest, frugivorous birds deserve recognition for providing a key ecosystem service for the people of the Mariana Islands.
Land managers should explicitly consider the role of seed dispersers in degraded forest regeneration. In locations where frugivore populations are healthy, conservation of intact forest and frugivore communities will ensure that passive regeneration of degraded forest is not limited by dispersal. In locations where frugivore communities are reduced or absent, conservation of intact forest, restoration of frugivore communities, and intensive management may all be needed to restore or replace the ecosystem services provided by frugivores. Conservation of remnant intact forest patches should be prioritized because these remnants serve as a seed source for degraded forests; forest regeneration is most likely to occur in areas where both older remnant forest stands and seed dispersers—such as avian frugivores—persist nearby [91], [92]. Restoration of frugivore communities should also restore the ecological functions they provide (e.g. seed dispersal). In cases where frugivores are absent or reduced in abundance and their restoration is impossible, management efforts will need to include active planting of seeds and seedlings in degraded forest. There has been little effort to restore native karst forest speces to areas currently covered with degraded forest in the Marianas, except for a few small-scale plantings by volunteers across the islands. Our results suggest that these efforts are necessary on Guam because birds no longer provide seed dispersal to degraded forests. In addition, managers should consider re-introducing frugivorous birds within snake exclosures in order to restore seed dispersal services. Reintroduction of native frugivores should be a top priority restoration strategy, since non-native frugivores may not replace the ecological functions of native frugivores [93]. Finally, in situations where populations of native frugivores cannot be conserved, introduction of analogue frugivores should be considered, in order to at least partially maintain the ecosystem services they provide [94]. On Guam, this could mean introducing analogue disperser species that are able to co-exist with brown tree snakes.
The island of Guam provides the worst-case scenario for the impact of frugivore loss on seed rain in degraded forest, and shows a stark view of the disruption of ecosystem services provided by frugivores, evident here as the complete cessation of seed rain into degraded forests. The unequivocal absence of adequate frugivore populations on Guam both demands immediate action, and provides an opportunity for managers to test and advance new methods for addressing seed dispersal disruptions.
Supporting Information
Seed rain in degraded forest with respect to distance from intact karst forest boundary.
(TIFF)
Summary of tree species found in forest surveys or seed traps, with biogeographic status.
(DOC)
Acknowledgments
We thank Elizabeth Hooshiar for guidance, Kaitlin Mattos for organizational skills, Jenny Howard for field help, Eric Cook for seed identification assistance and Ross Miller at the University of Guam for providing vehicles and lab space. Dennis Hansen, Ailene Kane Ettinger, Susan Waters, and two anonymous reviewers provided helpful comments on an earlier version of the manuscript.
Funding Statement
Funding for this research was provided by the National Science Foundation Research Experience for Undergraduates supplement to DEB-0816465. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of Earth's ecosystems. Science 277: 494–499. [Google Scholar]
- 2.ITTO (2002) ITTO guidelines for the restoration, management and rehabilitation of degraded and secondary tropical forests. ITTO Policy Development Series.
- 3. De Jong W, Chokkalingam JS (2001) Tropical secondary forests in Asia: introduction and synthesis. J Trop For Sci 13: 563–576. [Google Scholar]
- 4. Chokkalingam U, De Jong W (2001) Secondary forest: a working definition and typology. International Forestry Review 3: 19–26. [Google Scholar]
- 5. Fearnside P, Laurance WF (2004) Tropical deforestation and greenhouse-gas emissions. Ecological Applications 14: 982–986. [Google Scholar]
- 6. Laurance WF (1997) Biomass collapse in Amazonian forest fragments. Science 278: 1117–1118 doi:10.1126/science.278.5340.1117 [Google Scholar]
- 7. Nascimento HEM, Laurance WF (2004) Biomass dynamics in Amazonian forest fragments. Ecological Applications 14: S127–S138. [Google Scholar]
- 8. Parrotta JA, Turnbull JW, Jones N (1997) Catalyzing native forest regeneration on degraded tropical lands. For Ecol Manage 99: 1–7. [Google Scholar]
- 9. Lundberg J, Moberg F (2003) Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6: 87–98 doi:10.1007/s10021-002-0150-4 [Google Scholar]
- 10. Holl KD, Aide TM (2011) When and where to actively restore ecosystems? Forest Ecology and Management 261: 1558–1563 doi:10.1016/j.foreco.2010.07.004 [Google Scholar]
- 11. Wunderle JM (1997) The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. For Ecol Manage 99: 223–235 doi:10.1016/S0378-1127(97)00208-9 [Google Scholar]
- 12. Howe HF, Smallwood J (1982) Ecology of seed dispersal. Annual Review of Ecology and Systematics 13: 201–228. [Google Scholar]
- 13. Farwig N, Berens DG (2012) Imagine a world without seed dispersers: A review of threats, consequences and future directions. Basic and Applied Ecology 13: 109–115 doi:10.1016/j.baae.2012.02.006 [Google Scholar]
- 14. Culot L, Muñoz Lazo FJJ, Huynen M-C, Poncin P, Heymann EW (2010) Seasonal variation in seed dispersal by Tamarins alters seed rain a secondary rain forest. International Journal of Primatology 31: 553–569 doi:10.1007/s10764-010-9413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira ACM, Ferrari SF (2000) Seed dispersal by black-handed tamarins, Saguinus midas niger (Callitrichinae, Primates): implications for the regeneration of degraded forest habitats in eastern Amazonia. Journal of Tropical Ecology 16: 709–716. [Google Scholar]
- 16. Rodríguez-Pérez J, Traveset A (2010) Seed dispersal effectiveness in a plant–lizard interaction and its consequences for plant regeneration after disperser loss. Plant Ecology 207: 269–280 doi:10.1007/s11258-009-9671-7 [Google Scholar]
- 17. Traveset A, González-Varo JP, Valido A (2012) Long-term demographic consequences of a seed dispersal disruption. Proceedings of the Royal Society B 279: 3298–3303 doi:10.1098/rspb.2012.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duncan RS, Chapman CA (1999) Seed dispersal and potential forest succession in abandoned agriculture in tropical Africa. Ecological Applications 9: 998–1008. [Google Scholar]
- 19. Pejchar L, Pringle RM, Ranganathan J, Zook JR, Duran G, et al. (2008) Birds as agents of seed dispersal in a human-dominated landscape in southern Costa Rica. Biological Conservation 141: 536–544 doi:10.1016/j.biocon.2007.11.008 [Google Scholar]
- 20. Debussche M, Isenmann P (1994) Bird-dispersed seed rain and seedling establishment in patchy Mediterranean vegetation. Oikos 69: 414–426. [Google Scholar]
- 21. Neilan W, Catterall CP, Kanowski J, Mckenna S (2006) Do frugivorous birds assist rainforest succession in weed dominated oldfield regrowth of subtropical Australia? Biological Conservation 129: 393–407. [Google Scholar]
- 22. Whelan CJ, Wenny DG, Marquis RJ (2008) Ecosystem services provided by birds. Ann NY Acad Sci 1134: 25–60 doi:10.1196/annals.1439.003 [DOI] [PubMed] [Google Scholar]
- 23. Gillies CS, St. Clair CC (2010) Functional responses in habitat selection by tropical birds moving through fragmented forest. J Appl Ecol 47: 182–190 doi:10.1111/j.1365-2664.2009.01756.x [Google Scholar]
- 24. Levey DJ (1987) Seed size and fruit-handling techniques of avian frugivores. American Naturalist 129: 471–485. [Google Scholar]
- 25. Malmborg PK, Willson MF (1988) Foraging ecology of avian frugivores and some consequences for seed dispersal in an Illinois woodlot. The Condor 90: 173–186. [Google Scholar]
- 26. Brothers TS, Spingarn A (1992) Fragmentation and alien plant invasion of central Indiana forests. Conservation Biology 6: 91–100. [Google Scholar]
- 27. Uhl C (1987) Factors controlling succession following slash-and-burn agriculture in Amazonia. Journal of Ecology 75: 377–407. [Google Scholar]
- 28. Vieira ICG, Uhl C, Nepstad D (1994) The role of the shrub Cordia multispicata Cham. as a “succession facilitator” in an abandoned pasture, Paragominas, Amazônia. Vegetatio 115: 91–99. [Google Scholar]
- 29. Da Silva JMC, Uhl C, Murray G (1996) Plant succession, landscape management, and the ecology of frugivorous birds in abandoned Amazonian pastures. Conservation Biology 10: 491–503. [Google Scholar]
- 30. Kitamura S, Suzuki S, Yumoto T, Poonswad P, Chuailua P, et al. (2004) Dispersal of Aglaia spectabilis, a large-seeded tree species in a moist evergreen forest in Thailand. Journal of Tropical Ecology 20: 421–427 doi:10.1017/S0266467404001555 [Google Scholar]
- 31. Galetti M, Keuroghlian A, Hanada L, Morato MI (2001) Frugivory and seed dispersal by the Lowland Tapir (Tapirus terrestris) in Southeast Brazil. Biotropica 33: 723–726. [Google Scholar]
- 32. Teegalapalli K, Hiremath AJ, Jathanna D (2009) Patterns of seed rain and seedling regeneration in abandoned agricultural clearings in a seasonally dry tropical forest in India. Journal of Tropical Ecology 26: 25 doi:10.1017/S0266467409990344 [Google Scholar]
- 33. Ingle NMR (2003) Seed dispersal by wind, birds, and bats between Philippine montane rainforest and successional vegetation. Oecologia 134: 251–261. [DOI] [PubMed] [Google Scholar]
- 34. Del Castillo RF, Ríos M a P (2008) Changes in seed rain during secondary succession in a tropical montane cloud forest region in Oaxaca, Mexico. Journal of Tropical Ecology 24: 433–444 doi:10.1017/S0266467408005142 [Google Scholar]
- 35. Au AYY, Corlett RT, Hau BCH (2006) Seed rain into upland plant communities in Hong Kong, China. Plant Ecology 186: 13–22 doi:10.1007/s [Google Scholar]
- 36. Cole RJ, Holl KD, Zahawi RA (2010) Seed rain under tree islands planted to restore degraded lands in a tropical agricultural landscape. Ecological Applications 20: 1255–1269. [DOI] [PubMed] [Google Scholar]
- 37. Kelm DH, Wiesner KR, Von Helversen O (2008) Effects of artificial roosts for frugivorous bats on seed dispersal in a neotropical forest pasture mosaic. Conservation Biology 22: 733–741 doi:10.1111/j.1523-1739.2008.00925.x [DOI] [PubMed] [Google Scholar]
- 38. Bianconi GV, Suckow UMS, Cruz-Neto AP, Mikich SB (2010) Use of fruit essential oils to assist forest regeneration by bats. Restoration Ecology 20: 211–217 doi:10.1111/j.1526-100X.2010.00751.x [Google Scholar]
- 39.Lamb D, Gilmour D (2003) Rehabilitation and restoration of degraded forests. Report to IUCN and WWF. Gland, Switzerland,
- 40. Holl KD, Loik ME, Lin EHV, Samuels IA (2000) Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restoration Ecology 8: 339–349 doi:10.1046/j.1526-100x.2000.80049.x [Google Scholar]
- 41. Pimm S, Raven P, Peterson A, Sekercioglu CH, Ehrlich PR (2006) Human impacts on the rates of recent, present, and future bird extinctions. Proc Natl Acad Sci USA 103: 10941–10946 doi:10.1073/pnas.0604181103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekercioglu CH, Daily GC, Ehrlich PR (2004) Ecosystem consequences of bird declines. PNAS 101: 18042–18047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peres CA, Palacios E (2007) Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal-mediated seed dispersal. Biotropica 39: 304–315 doi:10.1111/j.1744-7429.2007.00272.x [Google Scholar]
- 44. Sharam GJ, Sinclair ARE, Turkington R (2009) Serengeti birds maintain forests by inhibiting seed predators. Science 325: 51 doi:10.1126/science.1173805 [DOI] [PubMed] [Google Scholar]
- 45. Nuñez-Iturri G, Olsson O, Howe HF (2008) Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biological Conservation 141: 1536–1546. [Google Scholar]
- 46. Nuñez-Iterri G, Howe HF (2007) Bushmeat and the fate of trees with seeds dispersed by large primates in a lowland rain forest in Western Amazonia. Biotropica 39: 348–354. [Google Scholar]
- 47. Sekercioglu CH (2006) Increasing awareness of avian ecological function. Trends Ecol Evol 21: 464–471 doi:10.1016/j.tree.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 48. Holbrook KM, Loiselle BA (2009) Dispersal in a Neotropical tree, Virola flexuosa (Myristicaceae): Does hunting of large vertebrates limit seed removal/? Ecology 90: 1449–1455. [DOI] [PubMed] [Google Scholar]
- 49. Diamond J (1983) Ecology: Laboratory, field and natural experiments. Nature 304: 586–587 doi:10.1038/304586a0 [Google Scholar]
- 50. Savidge JA (1987) Extinction of an island forest avifauna by an introduced snake. Ecology 68: 660–668. [Google Scholar]
- 51. Wiles GJ, Bart J, Beck Jr RE, Aguon CF (2003) Impacts of the Brown Tree Snake: patterns of decline and species persistence in Guam's avifauna. Conservation Biology 17: 1350–1360. [Google Scholar]
- 52. Camp RJ, Pratt TK, Marshall AP, Amidon F, Williams LL (2009) Recent status and trends of the land bird avifauna on Saipan, Mariana Islands, with emphasis on the endangered Nightingale Reed-warbler Acrocephalus luscinia . Bird Conservation International 19: 323 doi:10.1017/S0959270909008417 [Google Scholar]
- 53.Lander MA, Guard CP (2003) Creation of a 50-year rainfall database, annual rainfall climatology, and annual rainfall distribution map for Guam. Technical Report 102. Water and Environmental Research.Mangilao, Guam. Institute of the Western Pacific, University of Guam.
- 54.Lander MA (2004) Rainfall climatology for Saipan: distribution, return-periods, El Niño, tropical cyclones, and long-term variations. Technical Report 103. Water and Environmental Research.Mangilao, Guam. Institute of the Western Pacific, University of Guam.
- 55.Liu Z, Fischer L (2006) Guam vegetation mapping using very high spatial resolution imagery: methodology. McClellan, CA.
- 56.Liu Z, Fischer L (2006) Commonwealth of the Northern Mariana Islands vegetation mapping using very high spatial resolutionary imagery: methodology. McClellan, CA.
- 57.US Fish and Wildlife Service (2009) Draft revised recovery plan for the Mariana Fruit Bat or Fanihi (Pteropus mariannus mariannus). Portland, OR.
- 58. Robins JH, Hingston M, Matisoo-Smith E, Ross HA (2007) Identifying Rattus species using mitochondrial DNA. Molecular Ecology Notes 7: 717–729. [Google Scholar]
- 59. Wiewel AS, Yackel Adams AA, Rodda GH (2009) Distribution, density, and biomass of introduced small mammals in the Southern Mariana Islands. Pacific Science 63: 205–222. [Google Scholar]
- 60. Chimera CG, Drake DR (2010) Could poor seed dispersal contribute to predation by introduced rodents in a Hawaiian dry forest? Biological Invasions 13: 1029–1042 doi:10.1007/s10530-010-9887-4 [Google Scholar]
- 61. Meyer J-Y, Butaud J-F (2008) The impacts of rats on the endangered native flora of French Polynesia (Pacific Islands): drivers of plant extinction or coup de grâce species? Biological Invasions 11: 1569–1585 doi:10.1007/s10530-008-9407-y [Google Scholar]
- 62. Bellingham PJ, Towns DR, Cameron EK, Davis JJ, Wardle DA, et al. (2010) New Zealand island restoration: seabirds, predators, and the importance of history. New Zealand Journal of Ecology 34: 115–136. [Google Scholar]
- 63. Shiels AB, Drake DR (2010) Are introduced rats (Rattus rattus) both seed predators and dispersers in Hawaii? Biological Invasions 13: 883–894 doi:10.1007/s10530-010-9876-7 [Google Scholar]
- 64. Williams PA, Karl BJ, Bannister P, Lee WG (2000) Small mammals as potential seed dispersers in New Zealand. Austral Ecology 25: 523–532. [Google Scholar]
- 65. Sagarin R, Pauchard A (2010) Observational approaches in ecology open new ground in a changing world. Frontiers in Ecology and the Environment 8: 379–386. [Google Scholar]
- 66. Hewitt JE, Thrush SF, Dayton PK, Bonsdorff E (2007) The effect of spatial and temporal heterogeneity on the design and analysis of empirical studies of scale-dependent systems. American Naturalist 169: 398–408 doi:10.1086/510925 [DOI] [PubMed] [Google Scholar]
- 67.Crawley MJ (2007) The R Book. Chichiester, U.K.: John Wiley and Sons.
- 68.Burnham KP, Anderson DR (2002) Model selection and multimodel interference: a practical information-theoretic approach. New York: Springer.
- 69. Browne WJ, Subramanian SV, Jones K, Goldstein H (2005) Variance partitioning in multilevel logistic models that exhibit overdispersion. Journal of the Royal Statistical Society A 168: 599–613. [Google Scholar]
- 70. R Development Core Team R (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing 1: 409. [Google Scholar]
- 71. Mortensen HS, Dupont YL, Olesen JM (2008) A snake in paradise: disturbance of plant reproduction following extirpation of bird flower-visitors on Guam. Biological Conservation 141: 2146–2154 doi:10.1016/j.biocon.2008.06.014 [Google Scholar]
- 72. Herrera CM, Jordano P, Lopez-Soria L, Amat JA (1994) Recruitment of a mast-fruiting, bird-dispersed tree: bridging frugivore activity and seedling establishment. Ecological Monographs 64: 315–344. [Google Scholar]
- 73. Craig RJ (1993) Regeneration of native Mariana forest in disturbed habitats. Micronesia 26: 99–108. [Google Scholar]
- 74. Gorchov DL, Cornejo F, Ascorra C, Jaramillo M (1993) The role of seed dispersal in the natural regeneration of rain forest after strip-cutting in the Peruvian Amazon. Vegetatio 107/108: 339–349. [Google Scholar]
- 75. McClanahan TR, Wolfe RW (1993) Accelerating forest succession in a fragmented landscape: the role of birds and perches. Conservation Biology 7: 279–288. [Google Scholar]
- 76. Traveset A, Bermejo T, Wilson MF (2001) Effect of manure composition on seedling emergence and growth of two common shrub species of Southeast Alaska. Plant Ecology 155: 29–34. [Google Scholar]
- 77.Traveset A, Verdu M (2002) A meta-analysis of the effect of gut treatment on seed germination. In: Levey DJ, Silva WR, Galetti M, editors. Seed dispersal and frugivory: Ecology, evolution and conservation. Wallingford, UK: CABI International. 339–350.
- 78.Traveset A, Robertson AW, Rodriguez-Perez J (2007) A review on the role of endozoochory on seed germination. In: Dennis AJ, Schupp EW, Green RA, Westcott DA, editors. Seed dispersal: Theory and its application in a changing world. CABI Publishing. 78–103.
- 79. Kelly D, Ladley JJ, Robertson AW (2004) Is dispersal easier than pollination? Two tests in New Zealand Loranthaceae. New Zealand Journal of Botany 42: 89–103. [Google Scholar]
- 80. Samuels IA, Levey DJ (2005) Effects of gut passage on seed germination: do experiments answer the questions they ask? Functional Ecology 19: 365–368. [Google Scholar]
- 81.Robertson AW, Trass A, Ladley JJ, Kelly D (2006) Assessing the benefits of frugivory for seed germination: the importance of the deinhibition effect. Functional Ecology 20.. [Google Scholar]
- 82. Kelly D, Ladley JJ, Robertson AW, Anderson SH, Wotton DM, et al. (2010) Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit-dispersal in New Zealand. New Zealand Journal of Ecology 34: 66–85. [Google Scholar]
- 83. Wotton DM, Kelly D (2011) Frugivore loss limits recruitment of large-seeded trees. Proceedings of the Royal Society B 278: 3345–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vazquez-Yanes C, Orozco-Segovia A (1993) Patterns of seed longevity and germination in the tropical rainforest. Annu Rev Ecol Syst 24: 69–87. [Google Scholar]
- 85. Nepstad DC, Uhl C, Pereira A, Da Silva JMC (1996) A comparative study of tree establishment in abandoned pasture and mature forest of eastern Amazonia. Oikos 76: 25–39. [Google Scholar]
- 86. Craig RJ (1992) Territoriality, habitat use and ecological distinctness of an endangered Pacific Island reed-warbler. J Field Ornithol 63: 436–444. [Google Scholar]
- 87. Chapin FSI, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, et al. (2000) Consequences of changing biodiversity. Nature 405: 234–242. [DOI] [PubMed] [Google Scholar]
- 88. Luck GW, Daily GC, Ehrlich PR (2003) Population diversity and ecosystem services. Trends Ecol Evol 18: 331–336 doi:10.1016/S0169-5347(03)00100-9 [Google Scholar]
- 89. Wenny DG, Devault TL, Johnson MD, Kelly D, Sekercioglu CH, et al. (2011) The need to quantify ecosystem services provided by birds. The Auk 128: 1–14. [Google Scholar]
- 90. Lewis SL, Lopez-Gonzalez G, Sonké B, Affum-Baffoe K, Baker TR, et al. (2009) Increasing carbon storage in intact African tropical forests. Nature 457: 1003–1006 doi:10.1038/nature07771 [DOI] [PubMed] [Google Scholar]
- 91. Guariguata MR, Ostertag R (2001) Neotropical secondary forest succession: changes in structural and functional characteristics. For Ecol Manage 148: 185–206 doi:10.1016/S0378-1127(00)00535-1 [Google Scholar]
- 92. Chazdon RL, Peres CA, Dent D, Sheil D, Lugo AE, et al. (2009) The potential for species conservation in tropical secondary forests. Conservation Biology 23: 1406–1417 doi:10.1111/j.1523-1739.2009.01338.x [DOI] [PubMed] [Google Scholar]
- 93.Kelly D, Robertson AW, Ladley JJ, Anderson SH, McKenzie RJ (2006) The relative (un)importance of introduced animals as pollinators and dispersers of native plants. In: Allen RB, Lee WG, editors. Biological Invasions in New Zealand. Berlin: Springer. 227–245.
- 94. Griffiths CJ, Hansen DM, Jones CG, Zuël N, Harris S (2011) Resurrecting extinct interactions with extant substitutes. Current Biology 21: 762–765 doi:10.1016/j.cub.2011.03.042 [DOI] [PubMed] [Google Scholar]
- 95.Craig R (1996) Seasonal population surveys and natural history of a Micronesian bird community. The Wilson Bulletin 108: : 246–267. Available:. [Google Scholar]
- 96.Jenkins J (1983) Native forest birds of Guam. Ornithological Monographs 31.. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Seed rain in degraded forest with respect to distance from intact karst forest boundary.
(TIFF)
Summary of tree species found in forest surveys or seed traps, with biogeographic status.
(DOC)